CaMAPK1 Plays a Vital Role in the Regulation of Resistance to Ralstonia solanacearum Infection and Tolerance to Heat Stress
Abstract
:1. Introduction
2. Results
2.1. The Sequence Analysis of CaMAPK1
2.2. The Expression Profile of CaMAPK1 in Response to R. solanacearum, Heat Stress, and Exogenous Applied Phytohormones
2.3. Analysis of the Promoter Activity of CaMAPK1 against R. solanacearum and Exogenous Hormones
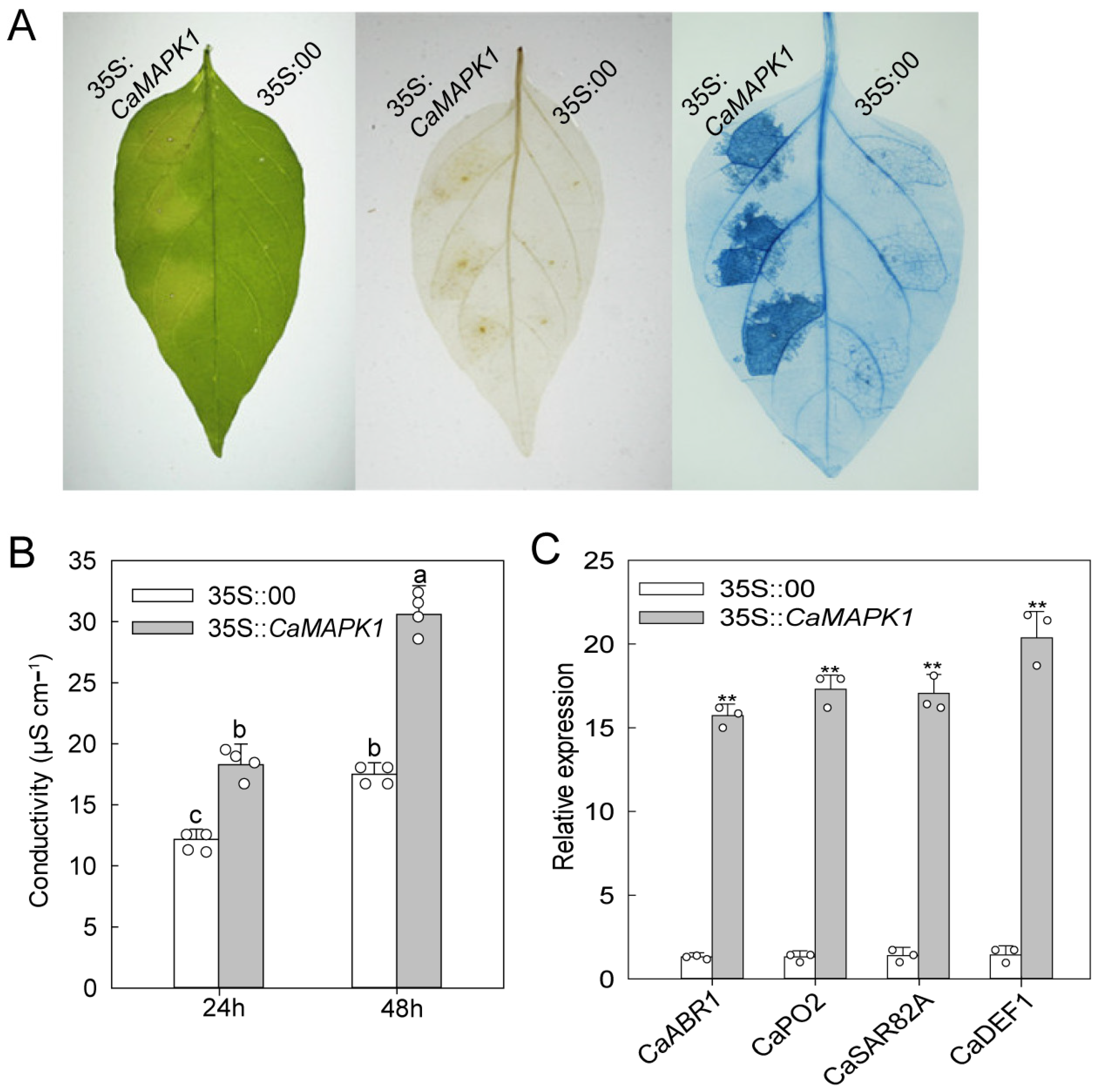
2.4. Transient Expression of CaMAPK1 Induces Cell Death and Defense Responses
2.5. Knock-Down of CaMAPK1 Attenuated the Resistance of Pepper against R. solanacearum Infection
2.6. Silencing of CaMAPK1 Attenuated Thermotolerance of Pepper Plants
3. Discussion
3.1. CaMAPK1 Acts as a Positive Regulator of Defense Response and Cell Death in Pepper Plants
3.2. CaMAPK1 Participates in the Regulation of Thermotolerance of Pepper Plants
3.3. CaMAPK1 May Contribute to the Immunity against R. solanacearum Mediated by SA, JA, and ABA
4. Materials and Methods
4.1. Plant Materials and Pathogen Inoculation
4.2. Heat Stress Treatment
4.3. Quantitative Real-Time PCR
4.4. Protein Extraction and Immunoblot Assay
4.5. Subcellular Localization
4.6. Agrobacterium-Mediated Transient Expression
4.7. Virus-Induced Gene Silencing in Pepper Plants
4.8. Histochemical Staining
4.9. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Thomma, B.P.; Nurnberger, T.; Joosten, M.H. Of PAMPs and effectors: The blurred PTI-ETI dichotomy. Plant Cell 2011, 23, 4–15. [Google Scholar] [CrossRef]
- Jones, J.D.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef]
- Ngou, B.P.M.; Jones, J.D.G.; Ding, P. Plant immune networks. Trends Plant Sci. 2022, 27, 255–273. [Google Scholar] [CrossRef]
- Yuan, M.; Ngou, B.P.M.; Ding, P.; Xin, X.F. PTI-ETI crosstalk: An integrative view of plant immunity. Curr. Opin. Plant Biol. 2021, 62, 102030. [Google Scholar] [CrossRef]
- Koster, P.; DeFalco, T.A.; Zipfel, C. Ca2+ signals in plant immunity. EMBO J. 2022, 41, e110741. [Google Scholar] [CrossRef]
- DeFalco, T.A.; Zipfel, C. Molecular mechanisms of early plant pattern-triggered immune signaling. Mol. Cell 2021, 81, 3449–3467. [Google Scholar] [CrossRef]
- Nakagami, H.; Pitzschke, A.; Hirt, H. Emerging MAP kinase pathways in plant stress signalling. Trends Plant Sci. 2005, 10, 339–346. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, S. MAPK cascades in plant disease resistance signaling. Annu. Rev. Phytopathol. 2013, 51, 245–266. [Google Scholar] [CrossRef]
- Pitzschke, A.; Schikora, A.; Hirt, H. MAPK cascade signalling networks in plant defence. Curr. Opin. Plant Biol. 2009, 12, 421–426. [Google Scholar] [CrossRef]
- Dadras, A.; Furst-Jansen, J.M.R.; Darienko, T.; Krone, D.; Scholz, P.; Sun, S.; Herrfurth, C.; Rieseberg, T.P.; Irisarri, I.; Steinkamp, R.; et al. Environmental gradients reveal stress hubs pre-dating plant terrestrialization. Nat. Plants 2023, 9, 1419–1438. [Google Scholar] [CrossRef]
- Desikan, R.; Hancock, J.T.; Ichimura, K.; Shinozaki, K.; Neill, S.J. Harpin induces activation of the Arabidopsis mitogen-activated protein kinases AtMPK4 and AtMPK6. Plant Physiol. 2001, 126, 1579–1587. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhu, Q.; Tan, Y.; Deng, M.; Zhang, L.; Cao, Y.; Guo, X. Mitogen-activated protein kinases MPK3 and MPK6 phosphorylate receptor-like cytoplasmic kinase CDL1 to regulate soybean basal immunity. Plant Cell 2024, 36, 963–986. [Google Scholar] [CrossRef] [PubMed]
- Ayatollahi, Z.; Kazanaviciute, V.; Shubchynskyy, V.; Kvederaviciute, K.; Schwanninger, M.; Rozhon, W.; Stumpe, M.; Mauch, F.; Bartels, S.; Ulm, R.; et al. Dual control of MAPK activities by AP2C1 and MKP1 MAPK phosphatases regulates defence responses in Arabidopsis. J. Exp. Bot. 2022, 73, 2369–2384. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, S. Mitogen-activated protein kinase cascades in plant signaling. J. Integr. Plant Biol. 2022, 64, 301–341. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ding, Y.; Shi, Y.; Zhang, X.; Zhang, S.; Gong, Z.; Yang, S. MPK3- and MPK6-Mediated ICE1 Phosphorylation Negatively Regulates ICE1 Stability and Freezing Tolerance in Arabidopsis. Dev. Cell 2017, 43, 630–642.e634. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Wang, P.; Si, T.; Hsu, C.C.; Wang, L.; Zayed, O.; Yu, Z.; Zhu, Y.; Dong, J.; Tao, W.A.; et al. MAP Kinase Cascades Regulate the Cold Response by Modulating ICE1 Protein Stability. Dev. Cell 2017, 43, 618–629.e615. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Wu, Q.; Wang, M.; Chen, D.; Li, J.; Shen, J.; Hou, S.; Zhang, P.; Qin, L.; Acharya, B.R.; et al. Maize MITOGEN-ACTIVATED PROTEIN KINASE 20 mediates high-temperature-regulated stomatal movement. Plant Physiol. 2023, 193, 2788–2805. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, J.; Genin, S.; Magori, S.; Citovsky, V.; Sriariyanum, M.; Ronald, P.; Dow, M.; Verdier, V.; Beer, S.V.; Machado, M.A.; et al. Top 10 plant pathogenic bacteria in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 614–629. [Google Scholar] [CrossRef] [PubMed]
- Group, M. Mitogen-activated protein kinase cascades in plants: A new nomenclature. Trends Plant Sci. 2002, 7, 301–308. [Google Scholar] [CrossRef]
- Liu, Z.; Shi, L.; Liu, Y.; Tang, Q.; Shen, L.; Yang, S.; Cai, J.; Yu, H.; Wang, R.; Wen, J.; et al. Genome-wide identification and transcriptional expression analysis of mitogen-activated protein kinase and mitogen-activated protein kinase kinase genes in Capsicum annuum. Front. Plant Sci. 2015, 6, 780. [Google Scholar] [CrossRef]
- Huh, S.U.; Lee, G.J.; Jung, J.H.; Kim, Y.; Kim, Y.J.; Paek, K.H. Capsicum annuum transcription factor WRKYa positively regulates defense response upon TMV infection and is a substrate of CaMK1 and CaMK2. Sci. Rep. 2015, 5, 7981. [Google Scholar] [CrossRef]
- Kim, M.; Jeong, S.; Lim, C.W.; Lee, S.C. Mitogen-Activated Protein Kinase CaDIMK1 Functions as a Positive Regulator of Drought Stress Response and Abscisic Acid Signaling in Capsicum annuum. Front. Plant Sci. 2021, 12, 646707. [Google Scholar] [CrossRef]
- Du, H.; Yang, J.; Chen, B.; Zhang, X.; Xu, X.; Wen, C.; Geng, S. Dual RNA-seq Reveals the Global Transcriptome Dynamics of Ralstonia solanacearum and Pepper (Capsicum annuum) Hypocotyls during Bacterial Wilt Pathogenesis. Phytopathology 2022, 112, 630–642. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.; Tenhaken, R.; Dixon, R.; Lamb, C. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 1994, 79, 583–593. [Google Scholar] [CrossRef]
- Maimbo, M.; Ohnishi, K.; Hikichi, Y.; Yoshioka, H.; Kiba, A. Induction of a small heat shock protein and its functional roles in Nicotiana plants in the defense response against Ralstonia solanacearum. Plant Physiol. 2007, 145, 1588–1599. [Google Scholar] [CrossRef]
- Guo, M.; Zhai, Y.F.; Lu, J.P.; Chai, L.; Chai, W.G.; Gong, Z.H.; Lu, M.H. Characterization of CaHsp70-1, a pepper heat-shock protein gene in response to heat stress and some regulation exogenous substances in Capsicum annuum L. Int. J. Mol. Sci. 2014, 15, 19741–19759. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.J.; Lee, D.E.; Shin, D.H.; Kim, K.U.; Kim, H.Y.; Ohashi, Y.; Han, O.; Baik, M.G.; Back, K. Molecular cloning and cultivar specific expression of MAP kinases from Capsicum annuum. Mol. Cells 2001, 11, 48–54. [Google Scholar] [CrossRef]
- Eulgem, T.; Rushton, P.J.; Schmelzer, E.; Hahlbrock, K.; Somssich, I.E. Early nuclear events in plant defence signalling: Rapid gene activation by WRKY transcription factors. EMBO J. 1999, 18, 4689–4699. [Google Scholar] [CrossRef]
- Giuliano, G.; Pichersky, E.; Malik, V.S.; Timko, M.P.; Scolnik, P.A.; Cashmore, A.R. An evolutionarily conserved protein binding sequence upstream of a plant light-regulated gene. Proc. Natl. Acad. Sci. USA 1988, 85, 7089–7093. [Google Scholar] [CrossRef]
- Bienz, M.; Pelham, H.R. Heat shock regulatory elements function as an inducible enhancer in the Xenopus hsp70 gene and when linked to a heterologous promoter. Cell 1986, 45, 753–760. [Google Scholar] [CrossRef]
- Leubner-Metzger, G.; Petruzzelli, L.; Waldvogel, R.; Vogeli-Lange, R.; Meins, F., Jr. Ethylene-responsive element binding protein (EREBP) expression and the transcriptional regulation of class I beta-1,3-glucanase during tobacco seed germination. Plant Mol. Biol. 1998, 38, 785–795. [Google Scholar] [CrossRef]
- Shi, L.; Li, X.; Weng, Y.; Cai, H.; Liu, K.; Xie, B.; Ansar, H.; Guan, D.; He, S.; Liu, Z. The CaPti1-CaERF3 module positively regulates resistance of Capsicum annuum to bacterial wilt disease by coupling enhanced immunity and dehydration tolerance. Plant J. Cell Mol. Biol. 2022, 111, 250–268. [Google Scholar] [CrossRef]
- Guo, M.; Yin, Y.X.; Ji, J.J.; Ma, B.P.; Lu, M.H.; Gong, Z.H. Cloning and expression analysis of heat-shock transcription factor gene CaHsfA2 from pepper (Capsicum annuum L.). Genet. Mol. Res. GMR 2014, 13, 1865–1875. [Google Scholar] [CrossRef]
- Mittler, R.; Finka, A.; Goloubinoff, P. How do plants feel the heat? Trends Biochem. Sci. 2012, 37, 118–125. [Google Scholar] [CrossRef]
- Choi, D.S.; Hwang, B.K. Proteomics and functional analyses of pepper abscisic acid-responsive 1 (ABR1), which is involved in cell death and defense signaling. Plant Cell 2011, 23, 823–842. [Google Scholar] [CrossRef]
- Hong, J.K.; Choi, H.W.; Hwang, I.S.; Kim, D.S.; Kim, N.H.; Choi, D.S.; Kim, Y.J.; Hwang, B.K. Function of a novel GDSL-type pepper lipase gene, CaGLIP1, in disease susceptibility and abiotic stress tolerance. Planta 2008, 227, 539–558. [Google Scholar] [CrossRef]
- Liu, K.; Shi, L.; Luo, H.; Zhang, K.; Liu, J.; Qiu, S.; Li, X.; He, S.; Liu, Z. Ralstonia solanacearum effector RipAK suppresses homodimerization of the host transcription factor ERF098 to enhance susceptibility and the sensitivity of pepper plants to dehydration. Plant J. Cell Mol. Biol. 2024, 117, 121–144. [Google Scholar] [CrossRef]
- Dang, F.F.; Wang, Y.N.; Yu, L.; Eulgem, T.; Lai, Y.; Liu, Z.Q.; Wang, X.; Qiu, A.L.; Zhang, T.X.; Lin, J.; et al. CaWRKY40, a WRKY protein of pepper, plays an important role in the regulation of tolerance to heat stress and resistance to Ralstonia solanacearum infection. Plant Cell Environ. 2013, 36, 757–774. [Google Scholar] [CrossRef]
- Mou, S.; He, W.; Jiang, H.; Meng, Q.; Zhang, T.; Liu, Z.; Qiu, A.; He, S. Transcription factor CaHDZ15 promotes pepper basal thermotolerance by activating HEAT SHOCK FACTORA6a. Plant Physiol. 2024, 195, 812–831. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Qiu, A.L.; Shi, L.P.; Cai, J.S.; Huang, X.Y.; Yang, S.; Wang, B.; Shen, L.; Huang, M.K.; Mou, S.L.; et al. SRC2-1 is required in PcINF1-induced pepper immunity by acting as an interacting partner of PcINF1. J. Exp. Bot. 2015, 66, 3683–3698. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Yang, S.; Yan, Y.; Xiao, Z.; Cheng, J.; Wu, J.; Qiu, A.; Lai, Y.; Mou, S.; Guan, D.; et al. CaWRKY6 transcriptionally activates CaWRKY40, regulates Ralstonia solanacearum resistance, and confers high-temperature and high-humidity tolerance in pepper. J. Exp. Bot. 2015, 66, 3163–3174. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Schiff, M.; Dinesh-Kumar, S.P. Virus-induced gene silencing in tomato. Plant J. 2002, 31, 777–786. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, L.; Shi, W.; Qiu, Z.; Yan, S.; Liu, Z.; Cao, B. CaMAPK1 Plays a Vital Role in the Regulation of Resistance to Ralstonia solanacearum Infection and Tolerance to Heat Stress. Plants 2024, 13, 1775. https://doi.org/10.3390/plants13131775
Shi L, Shi W, Qiu Z, Yan S, Liu Z, Cao B. CaMAPK1 Plays a Vital Role in the Regulation of Resistance to Ralstonia solanacearum Infection and Tolerance to Heat Stress. Plants. 2024; 13(13):1775. https://doi.org/10.3390/plants13131775
Chicago/Turabian StyleShi, Lanping, Wei Shi, Zhengkun Qiu, Shuangshuang Yan, Zhiqin Liu, and Bihao Cao. 2024. "CaMAPK1 Plays a Vital Role in the Regulation of Resistance to Ralstonia solanacearum Infection and Tolerance to Heat Stress" Plants 13, no. 13: 1775. https://doi.org/10.3390/plants13131775
APA StyleShi, L., Shi, W., Qiu, Z., Yan, S., Liu, Z., & Cao, B. (2024). CaMAPK1 Plays a Vital Role in the Regulation of Resistance to Ralstonia solanacearum Infection and Tolerance to Heat Stress. Plants, 13(13), 1775. https://doi.org/10.3390/plants13131775





