Abstract
The timing of potato tuberization is affected by potato ripeness, environmental factors, and polygene regulation. The accurate control of the transition to tuberization has both scientific and practical production value, but the key factors regulating this transition remain unclear. This study grafted an early-maturing potato variety (Favorita) scion to the late-maturing Qingshu 9 variety and demonstrated that a heterologous early-maturing scion can induce early potato formation on a late-maturing rootstock. The transcriptome of functional leaves and stolons of grafted plants was comprehensively analyzed and 593 differentially expressed genes (DEGs) were identified, including 38 transcription factors. Based on gene molecular function analysis and previous reports, we propose that PIF5, bHLH93, CBF3, ERF109, TCP19, and YABBY1 are the key DEGs that induce tuber formation in early- and late-maturing potatoes. The YABBY1 gene was subjected to functional verification. The leaf area of StYABBY1-overexpressing plants was smaller than the wild type and no potato tubercles were formed, while an RNA interference plant line showed no change in leaf area and formed tubers, indicating that StYABBY1 has a role in leaf size regulation and tuber formation.
1. Introduction
Potato (Solanum tuberosum L.) is the world’s fourth-largest staple crop after wheat, rice, and maize [1]. It is widely grown in over 150 countries and is a staple food for more than 1.3 billion people due to its adaptability, nutritional content, and low production costs [2]. Differences between potato varieties in flowering time, tuberization time, leaf senescence, and life cycle duration are the most important agronomic traits for distinguishing potato maturity [3]. However, the key factors regulating potato setting time remain unclear.
Grafting is an ancient agricultural production technique with a 4000-year history in China. It is widely used in research into scion material exchange and signaling between rootstocks [4,5,6]. The rootstock can control a plant’s structure by strengthening the scion, inducing early flowering, and enhancing pest and disease resistance and soil adaptability. RNA, proteins, hormones, and even chloroplasts and nuclear genomes can be transported from rootstock to scion [7,8,9]. The key genes for potato tuber formation (StBEL5, StPOTH1, and StSP6A) can be transferred downward to the stolon tip through the grafting junction to regulate tuber formation [10,11,12]. The miRNA172 molecule controls potato tuber formation and can be transported to stolons over long distances through grafted junctions, regulating the expression of the target tuber development gene StBEL5 and promoting tuber enlargement [13]. Long distance-transported miRNAs respond to various physiological processes arising from grafting and can regulate plant growth and development and response to environmental stress by regulating the expression of transcription factors and target genes. Recent studies have shown that siRNA acts as a silencing signal in grafted plants, moving within the plant and mediating transcriptional [14] and post-transcriptional gene silencing [15]. Thus, it serves as a systemic signal that can epigenetically modify the recipient genome.
Transcriptome analysis in grafted plants can reveal the specific genes involved in regulating physiological responses induced by grafting, as well as elucidating differentially expressed genes (DEGs), the main metabolic pathways affecting plant growth and development, and the molecular mechanisms of plant trait changes [16,17,18]. Recent studies have shown that the leaf growth rate of grafted commercial potato varieties Hopehely and White Lady is directly proportional to the time of tuber formation and that differences in leaf metabolites are evident [19]. The grafting of early-maturing (Z5) and late-maturing (Z18) varieties of the Zhongshu 5 potato revealed the molecular mechanism of plant maturation and identified the related long-distance signaling molecules [20]. The present study grafted early- and late-maturing potato varieties homologously (self-grafting) and heterologously (onto each other). A transcriptome analysis of the grafted plants revealed DEGs and the main metabolic pathways that induce tuber formation in these early- and late-maturing varieties. StYABBY1 was selected for functional verification from the key DEGs—overexpression resulted in smaller leaves and no potato tuber formation. This study provides new information for research into the genetic regulation of setting time in early- and late-maturing potato varieties.
2. Results
2.1. Heterologous Early-Maturing Scions Induce Potato Formation in Late-Maturing Rootstocks
The early-maturing variety Favorita (F) in the potato-setting stage was selected as the scion and the late-maturing variety Qingshu 9 (Q) in the budding stage was selected as the rootstock for heterologous grafting (Figure 1a). This FQ combination (Figure 1d) exhibited earlier tuber formation than ungrafted, control plants (CK, all of which had stolons; Figure 1b) or QQ grafted plants (late-maturing scion grafted onto late-maturing rootstock; three out of seven of these plants had stolons; Figure 1c). Thus, heterologous early-maturing scions play an important role in inducing early potato formation in late-maturing rootstocks.

Figure 1.
Phenotypes of reciprocally grafted potato plants after 18 d growth: (a) late-maturing variety Qingshu 9 (Q) in the budding stage and early-maturing variety Favorita (F) in the tuberization stage; (b) control variety Qingshu 9 (CK); (c) self-grafted Qingshu 9 (QQ); and (d) Favorita scion heterografted onto Qingshu 9 rootstock (FQ).
2.2. Transcriptome Sequencing
To investigate why early-maturing scions induce early tuber formation on late-maturing rootstocks, rootstocks were grafted at the early stage of potato formation. The BGISEQ sequencing platform was used for the transcriptome sequencing of 24 functional leaves and stolons of grafted plants. Samples produced an average of 46 M data, dropping to 44.30 M after filtering. The Q30 quality score was consistently above 91%. HISAT2 (v2.1.0) software was used to align clean reads to the reference genome GCF_000226075.1_SolTub_3.0 sequence. The average mapping rates of the FF, QQ, FQ, and QF leaves and stolons ranged from 72.2% to 82.0% (Table 1), showing that sequencing results were reliable and suitable for analysis.

Table 1.
Transcriptome sequencing quality statistics.
2.3. Identification of DEGs
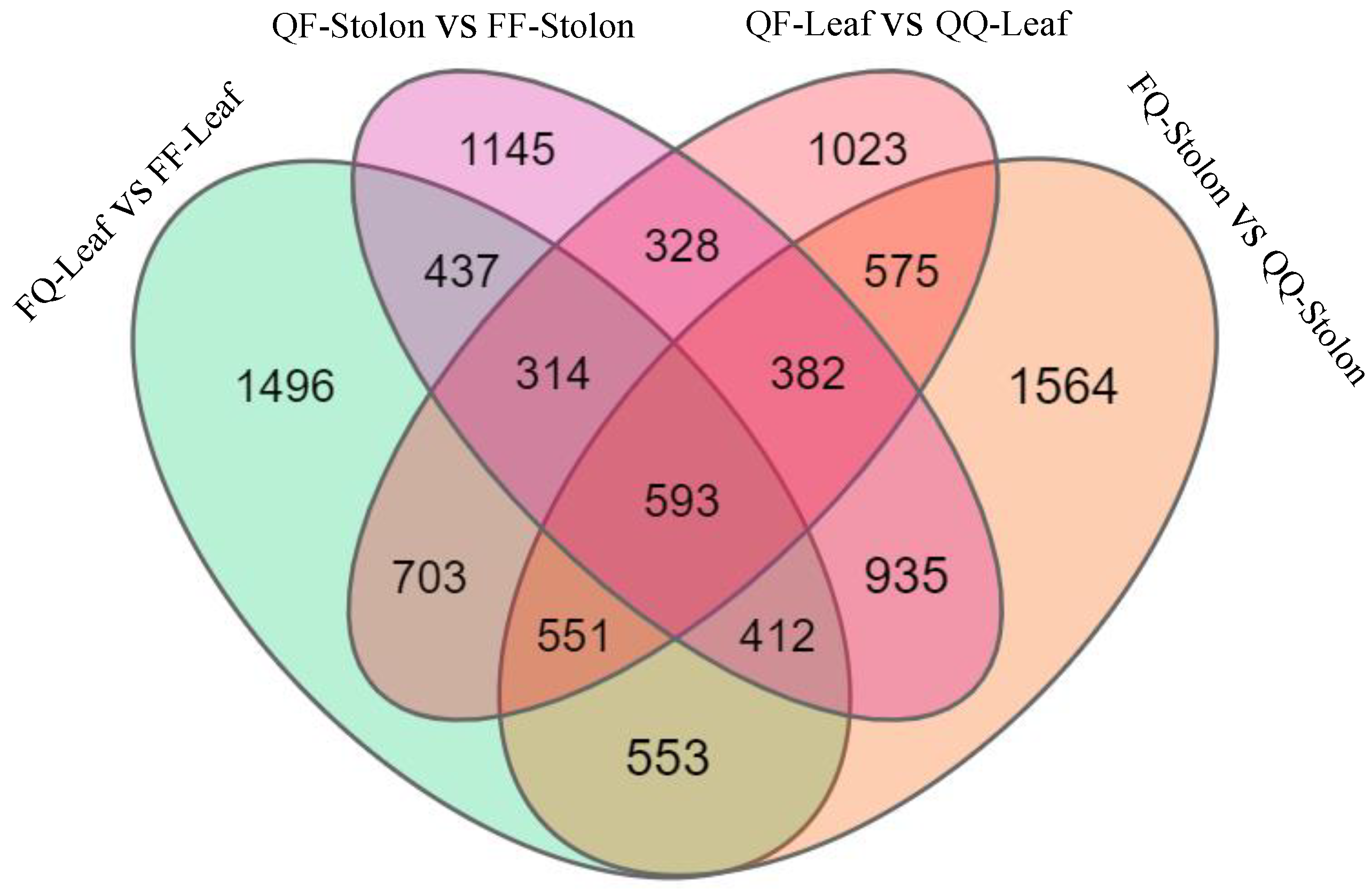
The transcriptome sequencing of the 24 samples of leaves and stolons from heterologous (FQ, QF) and self-grafted (QQ, FF) plants was used to determine the DEGs involved in regulating tuber formation induced by grafting in early- and late-maturing varieties. The screening criteria were log2 (fold change) ≥ 0 and p < 0.5. The late-maturing variety Q exhibited 1496 genes in its rootstock leaves and 1145 genes in its scion creeping stem. The early-maturing variety F exhibited 1023 genes in its rootstock leaves and 1564 genes in its scion creeping stem. A total of 593 genes were upregulated in QF-leaf vs. QQ-leaf, FQ-stolon vs. QQ-stolon, FQ-leaf vs. FF-leaf, and QF-stolon vs. FF-stolon (Figure 2).
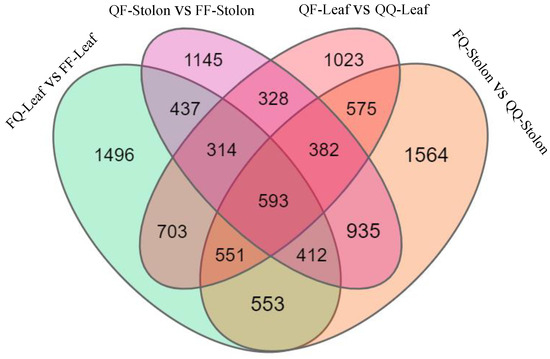
Figure 2.
Venn diagram of differentially expressed genes.
2.4. GO Enrichment of DEGs and KEGG Pathway Analysis
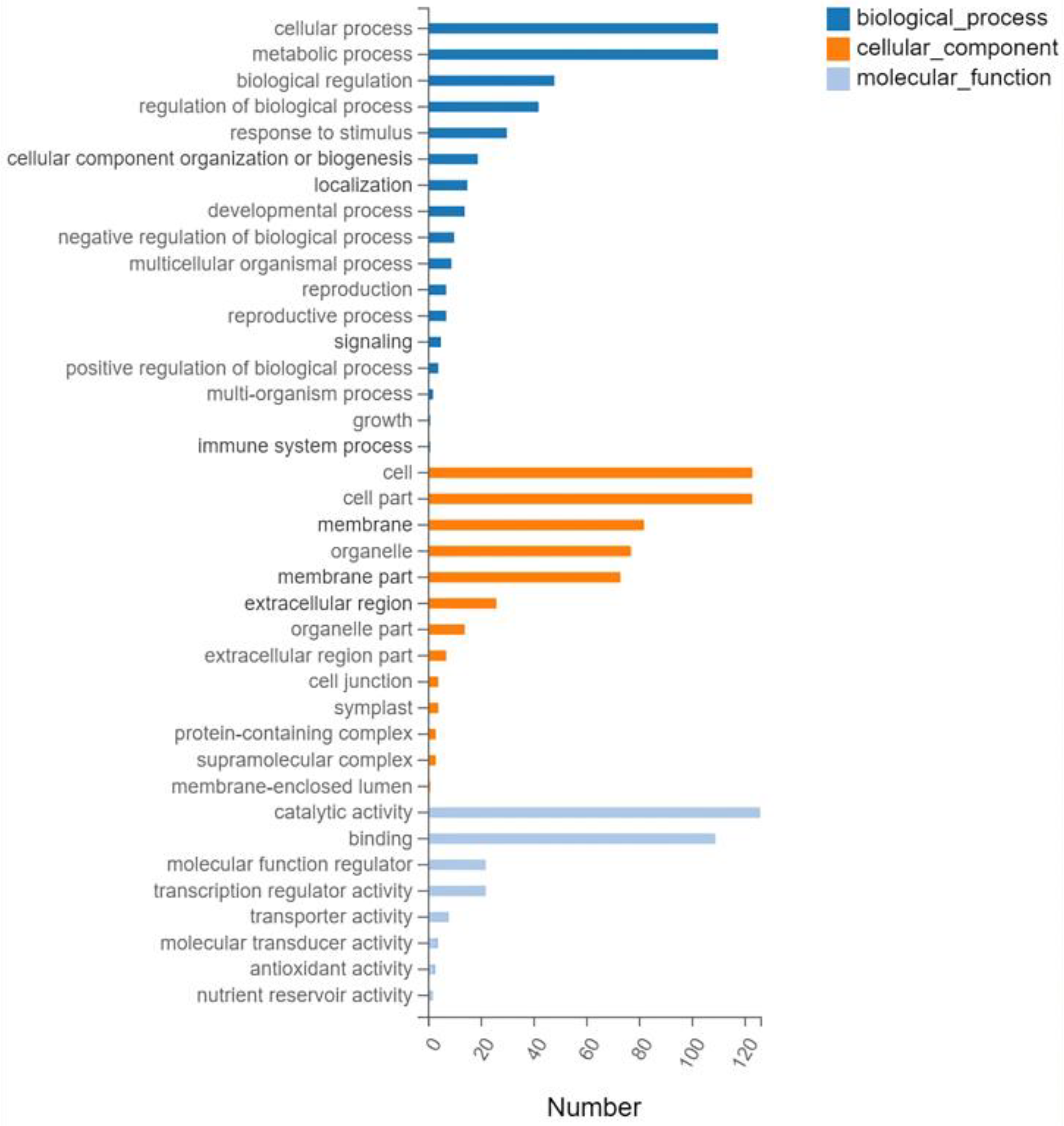
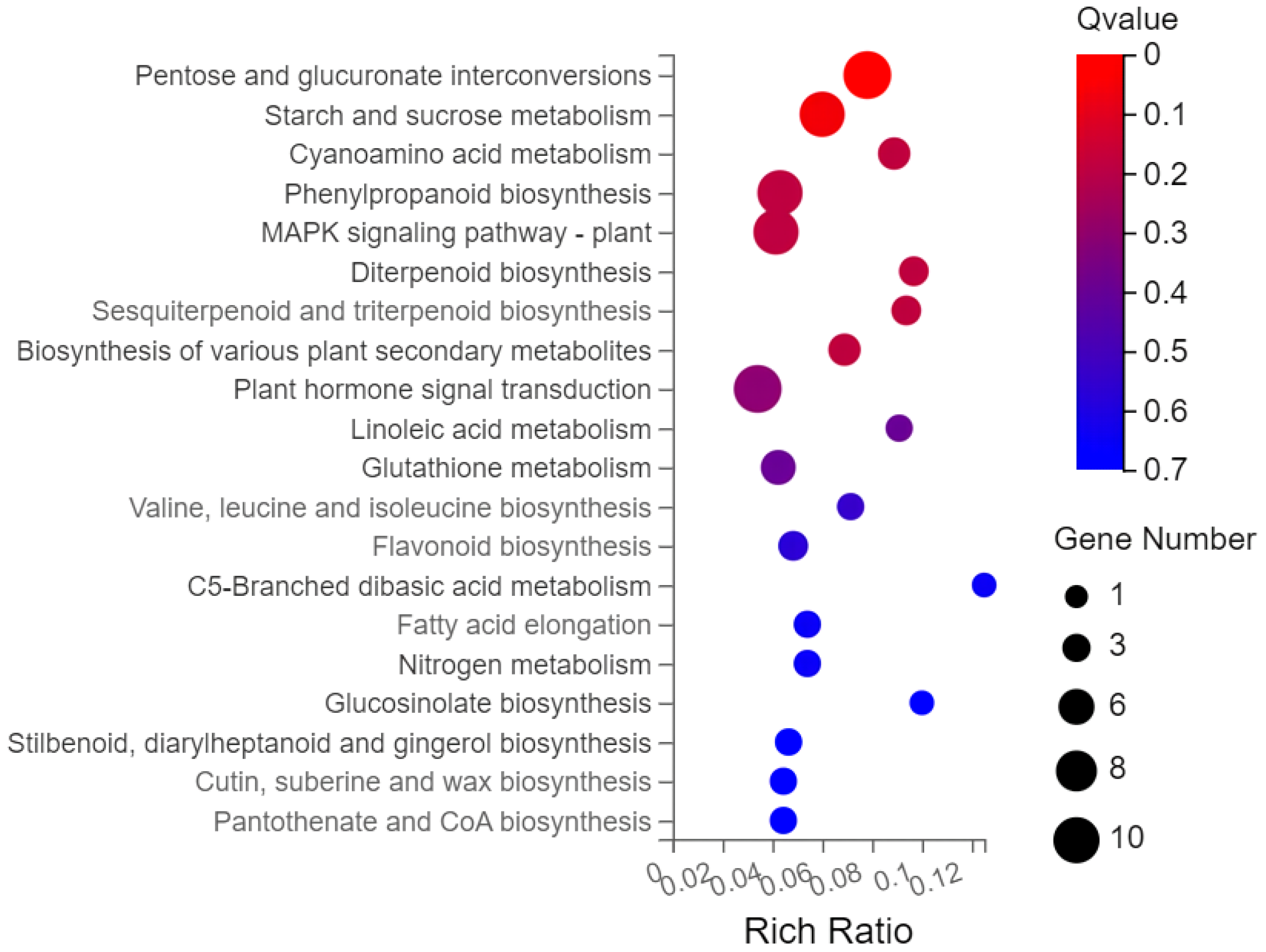
Gene ontology (GO) analysis was performed on the 593 screened DEGs (Figure 3). There were significant enrichments in 17 biological processes, 13 cellular components, and 8 molecular functions. Cellular processes and metabolic processes showed the highest degree of enrichment among the biological processes (110 genes each). The most enriched cellular component genes were related to cells (123), cell components (123), membranes (82), and organelles (77). The most enriched major molecular functions were catalytic activity (126 genes), binding (109), molecular function regulator (22), and transcription regulator activity (22). The Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis of the 593 DEGs showed enrichment predominantly in the interconversion of pentose and glucuronic acid, starch and sucrose metabolism, phenylpropanoid biosynthesis, plant hormone signal transduction, and plant MAPK signaling pathways, in addition to other pathways (Figure 4).
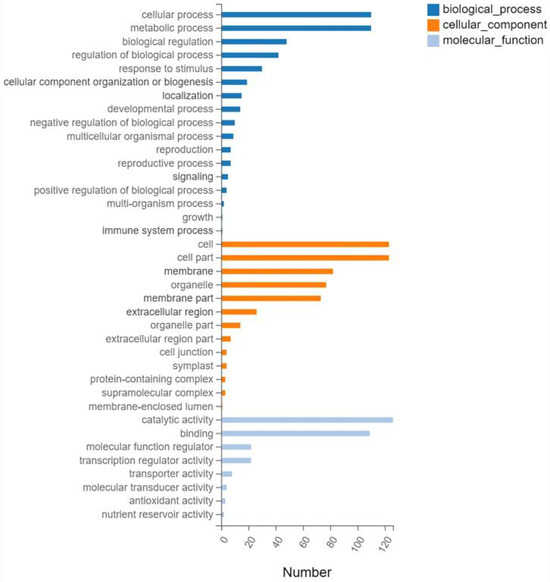
Figure 3.
Gene ontology classifications.
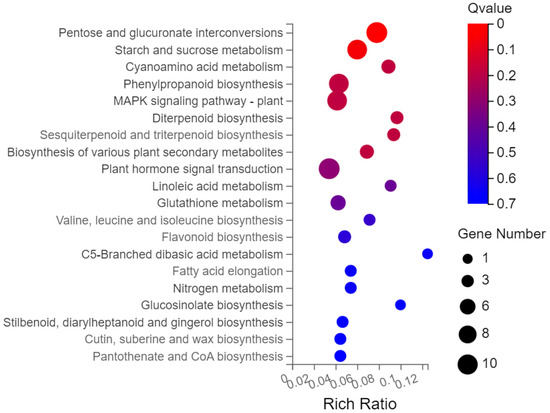
Figure 4.
KEGG enrichment analysis bubble chart.
2.5. Identification of Transcription Factor Gene Families
Transcription factors (TFs) are a class of proteins with particular structures that play crucial roles in regulating plant growth and development. Changes in 38 TF genes were identified among the DEGs (classified in Supplementary Materials File S2 Excels). These included the bHLH TF phytochrome interacting factor 5 (PIF5), which is involved in photomorphogenesis, shade response, flowering time, and leaf senescence [21,22,23,24], and bHLH93, which regulates flowering time in Arabidopsis [25]. CBF3 promotes leaf senescence and natural dormancy in fruit trees, improves plant resistance, and inhibits plant growth [26,27]. ERF109 regulates jasmonic acid signaling and auxin biosynthesis during lateral root formation in Arabidopsis [26,27,28]. TCP19 plays a redundant role in controlling leaf senescence [29], while the ectopic expression of YABBY1 in rice plants leads to abnormal flowering period [30]. Based on transcriptome sequencing data, StYABBY1 is highly expressed in potato leaves (Supplementary Materials File S1 Section 2). There are few reports on the functions of YABBY TFs in potato, so further exploration of the role of YABBY1 is warranted.
2.6. Sequence Analysis and Subcellular Localization of Potato StYABBY1
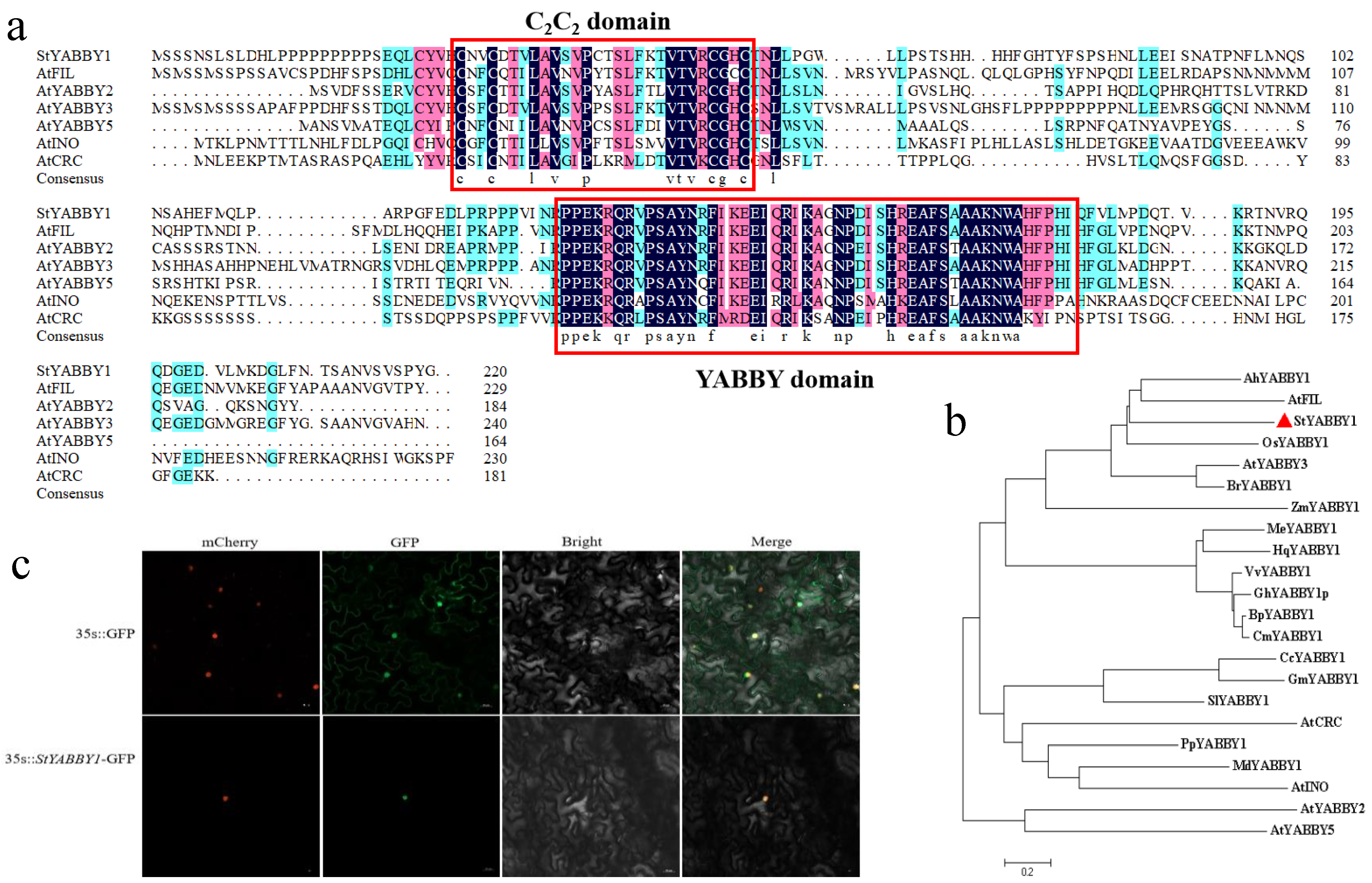
StYABBY1 is located on chromosome 1 of the potato genome. The open reading frame was 660 bp, encoding 219 amino acids, and its molecular weight was 24,454.66 kDa. Amino acid sequence comparison with six subfamilies of Arabidopsis thaliana showed that StYABBY1 had a complete C2C2 zinc finger structure at the N terminus and a helix-loop-helix YABBY domain similar to the HMG structure at the C terminus (Figure 5a). Phylogenetic tree analysis showed that potato StYABBY1 was located in the same evolutionary branch as the Arabidopsis AtFIL, peanut AhYABBY1, and rice OsYABBY1 genes, and the evolutionary process was relatively conservative (Figure 5b). The tobacco transformation system was used to study the subcellular localization of StYABBY1. StYABBY1-GFP was prepared and introduced into tobacco leaves. Fluorescence microscopy showed that it co-localized with the nuclear marker mCherry in the nucleus, while control 35S::GFP was expressed in the entire epidermal cell nuclear membrane (Figure 5c).
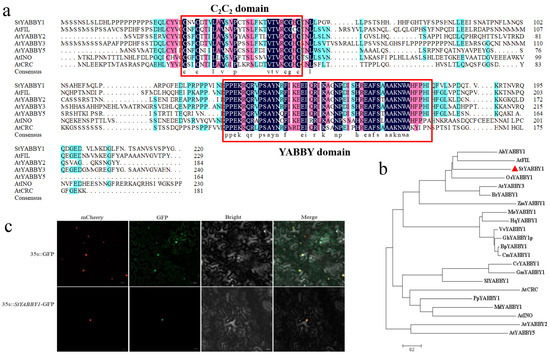
Figure 5.
StYABBY1 gene sequence analysis and subcellular localization. (a) Multiple sequence alignment of StYABBY1 and Arabidopsis StYABBY proteins; (b) phylogenetic tree of StYABBY1 and YABBY1 proteins in other species; and (c) subcellular localization of StYABBY1 protein in tobacco (Nicotiana benthamiana) epidermal cells.
2.7. StYABBY1 Overexpression Produces Smaller Leaves and Affects Tuber Formation
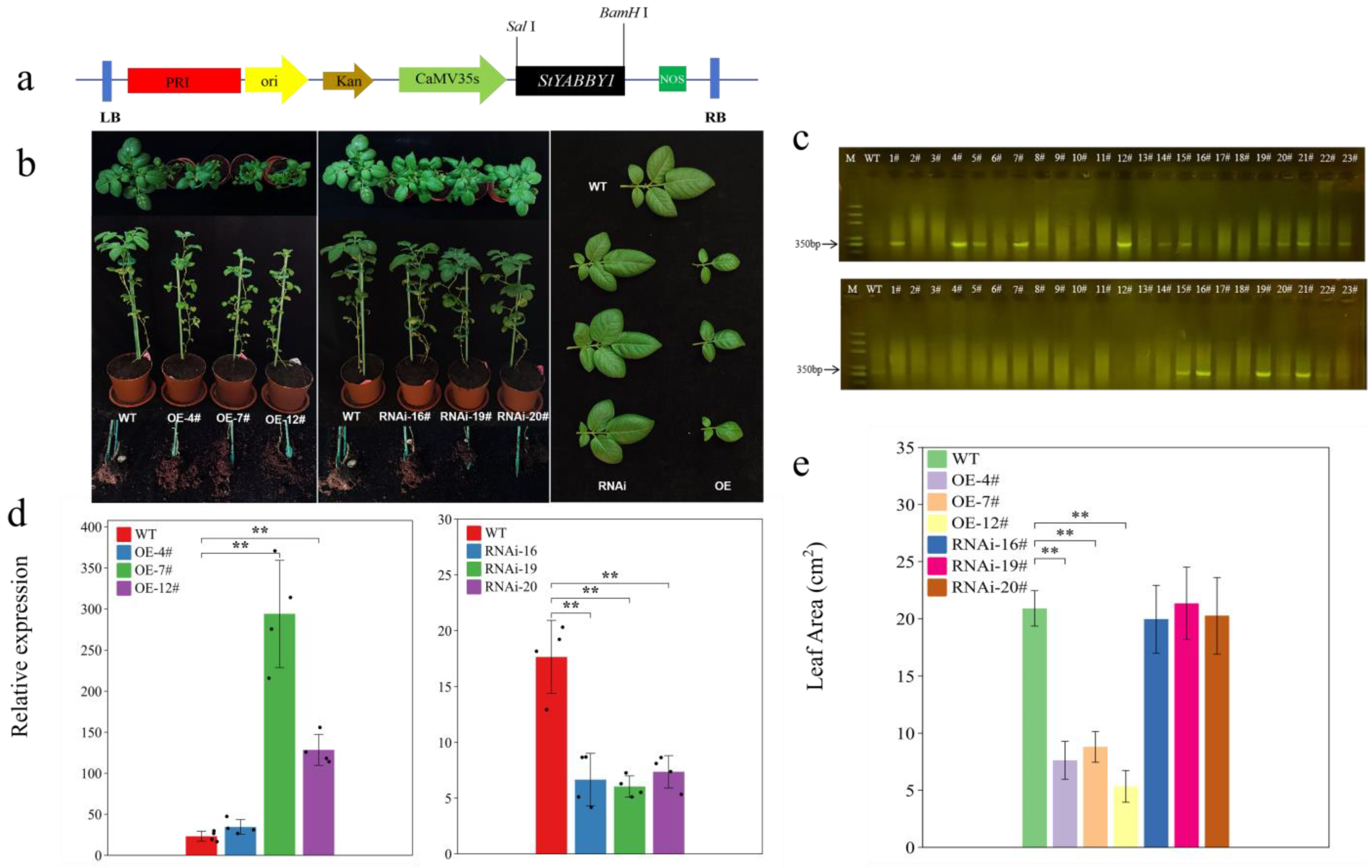
Transgenic potato plants were produced using StYABBY1 overexpression (OE) and RNA interference (RNAi) vectors to clarify the biological function of StYABBY1 (Figure 6a). Resistance gene primers were designed and screened to identify transgenic plants by PCR (Figure 6c). To analyze the expression level of StYABBY1 in various overexpression and interference plants, three overexpression lines were selected for RNA extraction and quantitative reverse transcription PCR with fluorescence detection. The expression of StYABBY1 increased 1.49 to 12.74-fold in leaves of the overexpression lines compared to the wild type (WT), while expression in leaves of the interference plants fell by 2.39 to 2.92-fold (Figure 6d), demonstrating the success of both StYABBY1 overexpression and RNA interference in these transgenic lines. Phenotypic analysis showed that the leaf area of StYABBY1 overexpression plants was smaller than that of the wild type, while no tuber tubes were formed. By contrast, the RNAi line plants had tubers, but their leaf area was unaffected (Figure 6b,e). It can, therefore, be inferred that the StYABBY1 gene plays an important role in potato leaf development and tuber formation.
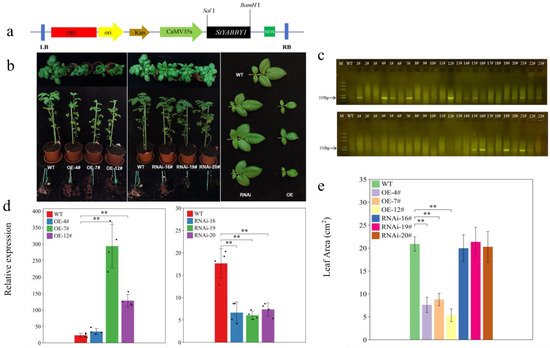
Figure 6.
Effects of overexpressing StYABBY1 on the leaves of potato plants. (a) Schematic diagram of pRI101-StYABBY1 vector; (b) phenotypes of overexpression and RNAi transgenic plants at 60 d; (c) screening for kanamycin resistance by PCR electrophoresis; (d) relative expression of StYABBY1 gene in transgenic plants; and (e) leaf area of transgenic plants.Note: # Number suffix, ** (p < 0.05).
3. Discussion
Grafting experiments have been previously used to identify mobile signals that induce tuberization [31]. This study conducted grafting experiments to identify the key DEGs responsible for tuber induction in early- and late-maturing potato varieties and validated the biological functions of candidate genes.
Heterografting and reciprocal grafting were performed on the tetraploid early-maturing potato variety Favorita and the late-maturing variety Qingshu 9 under normal growth conditions. Heterografting revealed the effect of early-maturing scions on the tuberization of late-maturing rootstocks: the rootstocks were induced to tuberize earlier. The phenotypic changes induced by grafting resulted from the interaction between scion and rootstock. This is consistent with previous reports that tobacco flowering scions grafted onto potatoes promoted tuberization in the rootstock, suggesting that flowering and tuberization signals are similar [32]. The main drivers of potato tuber formation are the homologs of the flowering gene FT, StSP6A, and StCO [33]. Under appropriate conditions, StSP6A is activated and expressed in the leaves, and then the protein is transported to the tip of the stolons and induces tuber formation [11]. This study provides new insight into differences in the timing of tuberization between early- and late-maturing varieties by investigating the interaction between scion and rootstock.
Most early-maturing potato varieties exhibit small above-ground plants, large leaves, early flowering, and early tuber formation compared to late-maturing varieties. Genetic studies of potato maturation indicate that these traits are controlled by multiple genes. The main site of action—StCDF1, located on chromosome 5—contains 12 unique alleles. StCDF1.1 is the allele for late maturation, while StCDF1.2 and StCDF1.3 are alleles for early maturation [34,35]. Potato maturity is associated with initial tuberization time. Plants that tuberize early usually exhibit early maturation. Only a few signals, such as StSP3D and StSP6A, have been related to potato tuberization [36,37], but whether they positively or negatively affect maturation is currently unclear. This study screened 593 upregulated genes and found that PIF5, bHLH93, CBF3, ERF109, TCP19, and YABBY1 may be key genes in the induction of tuber formation in early- and late-maturing potatoes. The late-maturing StCDF1.1 and early-maturing StCDF1.2 and StCDF1.3 genes were not among these 593 genes. However, the key differential gene CBF3 and the long-distance mobile signal molecules linked to potato maturation (StCBF1 and StCBF2) belong to the same family [20], so the previously unreported key differential gene YABBY1 was selected for further functional and molecular mechanism analysis.
The YABBY transcription factor family has various biological roles in higher plant growth and development. Its members regulate key functions in the development of above-ground lateral organs such as leaves and flowers [38,39], secondary metabolite formation [40,41,42], and the maintenance of meristematic tissue activity [43,44]. In recent years, the YABBY family has been identified and investigated in an increasing number of species, and its functions have been determined. The overexpression of YABBY-homologous genes led to abnormal development of floral organs and leaves in Arabidopsis [43], rice [45], and maize (Zea mays) [44]. The sequence of the StYABBY1 gene was cloned from potatoes in the present study. The gene produced an amino acid sequence containing a complete C2C2 zinc finger and YABBY domains. It belongs to the filamentous flower (FIL) subfamily, which is similar to proteins in Arabidopsis, tomato, and other dicotyledonous species [46,47]. Phylogenetic analysis showed that StYABBY1 is closely homologous in Arabidopsis, peanut, and rice. This high evolutionary conservation suggests that the gene might have similar functions in these plants. OsYAB1 ectopic expression in rice resulted in normal vegetative growth and abnormal flowering periods. The number of stamens and carpels in the spikelets increased compared to wild-type plants [30]. The ectopic expression of TaYAB1, BraYAB1-702, and GmFILa in Arabidopsis led to leaf curling and delayed flowering [48,49,50]. This study showed that StYABBY1 transcription factor is a repressor of leaf growth and tuber formation in potato. These findings confirm that StYABBY1 regulates leaf size and plays important biological roles in potatoes, but its pathways require further study.
4. Materials and Methods
4.1. Plant Materials, Growth Conditions, and Grafting
Late-maturing potato variety Qingshu 9 (Q; growth period 120 d) and early-maturing variety Favorita (F; growth period 60 d) were utilized in heterografting experiments conducted in greenhouses of the Biotechnology Research Institute of the Qinghai Academy of Agriculture and Forestry Sciences (Xining, China). Healthy, undamaged potato tubers were planted on 27 April 2023, in pots of 58 cm height and 40 cm diameter filled with a mixture of nutrient soil and substrate. One hundred pots were prepared for each variety and seedlings were fixed on 6 July 2023, with one plant per pot. Ungrafted Q was used as a control, with the treatments being self-grafted QQ and heterografted FQ (QQ: scion and rootstock from different Qingshu 9 plants; FQ: ungrafted Favorita with Qingshu 9 as the rootstock). Each combination was replicated in 24 randomly arranged pots. Grafting was performed using the cleft method on 15 July 2023: a 3–4 cm vertical incision was made in the rootstock, and the scion was inserted into the incision, fixed immediately with breathable sealing film, and then covered with a self-sealing bag to conserve moisture. The bag was removed after 7 d and the tuberization status was recorded after 18 d of growth.
A reciprocal grafting experiment was conducted in an artificial climate chamber at the Biotechnology Research Institute of the Chinese Academy of Tropical Agricultural Sciences. Healthy, undamaged potato tubers were planted on 27 March 2022, in pots of 18 cm height and 23 cm diameter filled with a mixture of nutrient soil and substrate. The light intensity was 5000 Lx, temperature 23 °C/20 °C, photoperiod 12 h/day, and humidity 45%. After emergence, one plant with 7–10 leaves was cultivated in each pot. Grafting was performed when the plant reached 40–50 cm height. Self-grafted FF and QQ were used as controls (FF: scion and rootstock from different Favorita plants; QQ: scion and rootstock from different Qingshu 9 plants), with the treatments being heterografted FQ and QF (FQ: Favorita as scion, Qingshu 9 as rootstock; QF: Qingshu 9 as scion, Favorita as rootstock). Each of the four grafted combinations was replicated 12 times. Grafting was performed on 10 May 2022, using the cleft method and surviving plants were cultivated until death.
4.2. Sample Collection and Preparation
Functional leaves and stolons of self-grafted FF and QQ and heterografted FQ and QF plants were sampled from the second to third leaves down from the top (three biological replicates of each). Soil and dust were washed off with distilled water before samples were blotted dry with filter paper, cut into small pieces (approximately 50–100 mg), transferred to 5 mL capped centrifuge tubes, immediately frozen with liquid nitrogen, and stored at −80 °C before sending on dry ice to BGI Genomics for transcriptome sequencing.
4.3. RNAseq Sample and Library Preparation
Grafted plant tissue samples of FF-leaf, FF-stolon, QQ-leaf, QQ-stolon, FQ-leaf, FQ-stolon, QF-leaf, and QF-stolon were collected. RNA extraction and transcriptome sequencing of three biological replicates were conducted by BGI Genomics using the BGISEQ sequencing platform.
4.4. Sequence Data Analysis
Raw sequencing data were filtered using SOAPnuke (v.1.5.6) [51] to remove (i) reads containing adapters (adapter contamination), (ii) reads with an unknown base N content greater than 5%, and (iii) low-quality reads (quality value below 15 accounting for more than 20% of the total bases in the read). The analysis and plotting of the clean data were performed using the Dr.Tom multi-omics data mining system (https://biosys.bgi.com). Differential gene analysis was conducted using HISAT2 (v.2.1.0) [52] to align the clean data to the reference genome, and Bowtie2 (v.2.3.4.3) [53] to align the clean data to the reference gene set (provided by the Dr.Tom system). Gene expression quantification used RSEM (v.1.3.1) [54] and heatmaps were drawn using pheatmap (v.1.0.8) [55]. Differential gene detection was performed using DESeq2 (v.1.4.5) [56], applying a Q value threshold of ≤0.05. KEGG and GO enrichment analyses based on hypergeometric distributions were used to explore the functional differential genes associated with phenotypic changes (Q value threshold ≤ 0.05 [57]). Genes meeting this condition were defined as significantly enriched in candidate genes.
4.5. Cloning, Multiple Sequence Alignment, and Phylogenetic Relationships of StYABBY1
Total RNA was extracted from Désirée potato leaves using the RNAprep Pure Plant Total RNA Kit (Tiangen, Beijing, China; www.transgen.com.cn). cDNA was synthesized using the MonScript RT Super Mix with dsDNase protocol (Monad, Wuhan, China; www.monadbiotech.com). The StYABBY1 sequence was downloaded from the Phytozome database (https://phytozome-next.jgi.doe.gov/). The full-length coding sequence (CDS) of StYABBY1 was amplified using gene-specific PCR primers (Supplementary Material File S1 Sections 1–7). Homologous amino acid sequences of StYABBY1 from various species were downloaded from the National Center for Biotechnology Information (NCBI) database. Multiple sequence alignment was performed in DNAMAN 7.0 and a phylogenetic tree was constructed using the neighbor-joining method in mega 6.0.
4.6. Subcellular Localization of StYABBY1
The Agrobacterium strain GV3101 (pSoup-p19) carrying the pCAMBIA1300-StYABBY1-GFP fusion plasmid and the pCAMBIA1300-GFP plasmid were transiently transformed into tobacco (Nicotiana benthamiana) epidermal cells and dark-cultivated for 24 h before transferring to a light incubator for 48–72 h. The green fluorescent protein (GFP) signal was observed using a laser confocal microscope (Zeiss LSM800).
4.7. Potato Genetic Transformation
The CDS of StYABBY1 was cloned from Désirée potato leaves, and Sal1 and BamH1 were chosen as restriction enzyme cutting sites. The pRI101-StYABBY1 vector was constructed and activated, and correctly sequenced clones were extracted and transferred into Agrobacterium GV3101. The complete vector was transformed into potatoes using the Agrobacterium-mediated transformation method as described previously [58].
4.8. Phenotypic Identification of Transgenic Plants
Successful transgenic plants were propagated on standard MS medium supplemented with Timentin (500 μL/100 mL). After 25 d of growth, lids were removed in an LB-QHS artificial climate chamber (temperature 22 °C/16 °C, light intensity 5000 Lx, photoperiod 12 h/12 h, humidity 45%) and, after acclimatizing for 5 d, plants were transplanted into pots containing nutrient soil. Tuberization was recorded after 60 d, the length and width of functional leaves were measured, and leaf area was estimated using a non-destructive estimation model [59].
4.9. Real-Time Quantitative PCR (qRT-PCR) Analysis
Total RNA extraction and cDNA synthesis were performed using the Tiangen extraction kit and the Monad MonScript RT Super Mix with dsDNase kit. Primers were designed using Primer Premier 6.0 software (Supplementary Material File S1 Section S8). The qRT-PCR program was set according to the required annealing temperature and cycle number. The internal reference gene was β-actin. Relative expression levels were determined using the (Ct) 2−ΔΔCt method, with four biological replicates.
4.10. Data Analysis
SPSS 17.0 (IBM) was used for data analysis. Comparisons between wild-type and transgenic lines were made using Student’s t-test, with significance thresholds at p < 0.05 and p < 0.01. Graphs were created using WPS 2019 and GraphPad Prism 9.5 software.
5. Conclusions
This study identified 593 DEGs related to tuberization induction in early- and late-maturing potato varieties, partially corroborating previous reports on the functioning of genes related to tuber formation. Specifically, PIF5, bHLH93, CBF3, ERF109, TCP19, and YABBY1 were identified as potential key genes inducing tuberization. The StYABBY1 gene was cloned from potatoes and encoded a typical C2C2 zinc finger domain and YABBY domain TF of the FIL subfamily. The biological functions of StYABBY1 in regulating leaf size and tuber development were studied, shedding further light on the regulatory mechanisms of tuber formation and suggesting that gene engineering is a possible route to managing potato growth stages and tuberization.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants13131879/s1, Supplementary Materials File S1: 1. Primers used in this study; 2. Cloning sequence of StYABBY1 gene; 3. iRNA target sequence; 4. Multiple sequence alignment of original sequences; 5 Primitive sequence of phylogenetic tree; 6. Original qPCR data of genetically modified plants; 7. Statistical data on leaf area of genetically modified plants; 8. Classification and functional annotation of transcription factors; Supplementary Materials File S2: S1. DEGs-593; S2. DEGs-Gen expression.
Author Contributions
Conceptualization, Y.M. and J.W.; methodology, Y.M.; software, K.D. and L.Z.; validation, Y.M., M.L. and S.W.; formal analysis, J.L.; investigation, Y.M.; resources, W.W.; data curation, Y.M. and W.W.; writing—original draft preparation, Y.M.; writing—review and editing, Y.M.; visualization, F.W.; supervision, F.W.; project administration, F.W.; funding acquisition, J.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the A born commander Scientist Project of Qinghai Province: “Capacity Improvement of Innovation system of potato breeding and seed industry production in plateau” (2023-NK-146); the Kunlun High-end Innovation and Entrepreneurship Talents–Leading Talent Project of Qinghai Province, China; and the Agriculture Research System of MOF and MARA (No. CARS-9).
Data Availability Statement
All data supporting the conclusions of this article are provided within the article and its Supplementary Information. The transcriptome data used in the current study are available in the National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA) database (https://www.ncbi.nlm.nih.gov/sra) (accession number: PRJNA1102724) (accessed on 25 June 2024).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wang, F.; Xia, Z.; Zou, M.; Zhao, L.; Jiang, S.; Zhou, Y.; Zhang, C.; Ma, Y.; Bao, Y.; Sun, H. The autotetraploid potato genome provides insights into highly heterozygous species. Plant Biotechnol. J. 2022, 20, 1996–2005. [Google Scholar] [CrossRef] [PubMed]
- Stokstad, E. The new potato. Science 2019, 363, 574–577. [Google Scholar] [CrossRef] [PubMed]
- Jing, S.; Sun, X.; Yu, L.; Wang, E.; Cheng, Z.; Liu, H.; Jiang, P.; Qin, J.; Begum, S.; Song, B. Transcription factor StABI5-like 1 binding to the FLOWERING LOCUS T homologs promotes early maturity in potato. Plant Physiol. 2022, 189, 1677–1693. [Google Scholar] [CrossRef] [PubMed]
- Meng, C.; Xu, D.; Son, Y.-J.; Kubota, C. Simulation-based economic feasibility analysis of grafting technology for propagation operation. In Proceedings of the IIE Annual Conference, Orlando, FL, USA, 19–23 May 2012; p. 1. [Google Scholar]
- Haroldsen, V.M.; Szczerba, M.W.; Aktas, H.; Lopez-Baltazar, J.; Odias, M.J.; Chi-Ham, C.L.; Labavitch, J.M.; Bennett, A.B.; Powell, A.L. Mobility of transgenic nucleic acids and proteins within grafted rootstocks for agricultural improvement. Front. Plant Sci. 2012, 3, 39. [Google Scholar] [CrossRef] [PubMed]
- Mudge, K.; Janick, J.; Scofield, S.; Goldschmidt, E.E. A history of grafting. Hortic. Rev. 2009, 35, 437–493. [Google Scholar]
- Xia, C.; Zheng, Y.; Huang, J.; Zhou, X.; Li, R.; Zha, M.; Wang, S.; Huang, Z.; Lan, H.; Turgeon, R. Elucidation of the mechanisms of long-distance mRNA movement in a Nicotiana benthamiana/tomato heterograft system. Plant Physiol. 2018, 177, 745–758. [Google Scholar] [CrossRef] [PubMed]
- Stegemann, S.; Bock, R. Exchange of genetic material between cells in plant tissue grafts. Science 2009, 324, 649–651. [Google Scholar] [CrossRef]
- Liu, N.; Yang, J.; Fu, X.; Zhang, L.; Tang, K.; Guy, K.M.; Hu, Z.; Guo, S.; Xu, Y.; Zhang, M. Genome-wide identification and comparative analysis of grafting-responsive mRNA in watermelon grafted onto bottle gourd and squash rootstocks by high-throughput sequencing. Mol. Genet. Genom. 2016, 291, 621–633. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, B.; Kondhare, K.R.; Hannapel, D.J.; Banerjee, A.K. Mobile RNAs and proteins: Prospects in storage organ development of tuber and root crops. Plant Sci. 2019, 284, 73–81. [Google Scholar] [CrossRef]
- Sharma, P.; Lin, T.; Hannapel, D.J. Targets of the StBEL5 transcription factor include the FT ortholog StSP6A. Plant Physiol. 2016, 170, 310–324. [Google Scholar] [CrossRef]
- Banerjee, A.K.; Chatterjee, M.; Yu, Y.; Suh, S.-G.; Miller, W.A.; Hannapel, D.J. Dynamics of a mobile RNA of potato involved in a long-distance signaling pathway. Plant Cell 2006, 18, 3443–3457. [Google Scholar] [CrossRef] [PubMed]
- Bhogale, S.; Mahajan, A.S.; Natarajan, B.; Rajabhoj, M.; Thulasiram, H.V.; Banerjee, A.K. MicroRNA156: A potential graft-transmissible microRNA that modulates plant architecture and tuberization in Solanum tuberosum ssp. andigena. Plant Physiol. 2014, 164, 1011–1027. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Kasai, A.; Yamada, K.; Li, T.; Harada, T. A mobile signal transported over a long distance induces systemic transcriptional gene silencing in a grafted partner. J. Exp. Bot. 2011, 62, 4561–4570. [Google Scholar] [CrossRef] [PubMed]
- Kasai, A.; Bai, S.; Li, T.; Harada, T. Graft-transmitted siRNA signal from the root induces visual manifestation of endogenous post-transcriptional gene silencing in the scion. PLoS ONE 2011, 6, e16895. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhou, P.; Zhu, W.; Wang, F. De novo comparative transcriptome analysis of genes differentially expressed in the scion of homografted and heterografted tomato seedlings. Sci. Rep. 2019, 9, 20240. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Song, G.Q. Rootstock-to-scion transfer of transgene-derived small interfering RNA s and their effect on virus resistance in nontransgenic sweet cherry. Plant Biotechnol. J. 2014, 12, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Moran, P.J.; Cheng, Y.; Cassell, J.L.; Thompson, G.A. Gene expression profiling of Arabidopsis thaliana in compatible plant-aphid interactions. Arch. Insect Biochem. 2002, 51, 182–203. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, D. Transcriptome analysis of scions grafted to potato rootstock for improving late blight resistance. BMC Plant Biol. 2021, 21, 272. [Google Scholar] [CrossRef]
- Hui, Z.; Xu, J.; Jian, Y.; Bian, C.; Duan, S.; Hu, J.; Li, G.; Jin, L. Identification of long-distance transport signal molecules associated with plant maturity in tetraploid cultivated Potatoes (Solanum tuberosum L.). Plants 2022, 11, 1707. [Google Scholar] [CrossRef]
- Sakuraba, Y.; Jeong, J.; Kang, M.-Y.; Kim, J.; Paek, N.-C.; Choi, G. Phytochrome-interacting transcription factors PIF4 and PIF5 induce leaf senescence in Arabidopsis. Nat. Commun. 2014, 5, 4636. [Google Scholar] [CrossRef]
- Fernández, V.; Takahashi, Y.; Le Gourrierec, J.; Coupland, G. Photoperiodic and thermosensory pathways interact through CONSTANS to promote flowering at high temperature under short days. Plant J. 2016, 86, 426–440. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.N.; Kathare, P.K.; Huq, E. Dynamic regulation of PIF 5 by COP 1–SPA complex to optimize photomorphogenesis in Arabidopsis. Plant J. 2018, 96, 260–273. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Ma, L.; Lv, Y.; Qi, L.; Peng, J.; Li, H.; Zhou, Y.; Song, P.; Duan, J.; Li, J. SALT OVERLY SENSITIVE2 stabilizes phytochrome-interacting factors PIF4 and PIF5 to promote Arabidopsis shade avoidance. Plant Cell 2023, 35, 2972–2996. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N. Role of bHLH93 in Controlling Flowering Time in Arabidopsis Thaliana. Doctoral Dissertation, The University of Texas at Austin, Austin, TX, USA, 2011. [Google Scholar]
- Gilmour, S.J.; Fowler, S.G.; Thomashow, M.F. Arabidopsis transcriptional activators CBF1, CBF2, and CBF3 have matching functional activities. Plant Mol. Biol. 2004, 54, 767–781. [Google Scholar] [CrossRef] [PubMed]
- An, D.; Ma, Q.; Yan, W.; Zhou, W.; Liu, G.; Zhang, P. Divergent regulation of CBF regulon on cold tolerance and plant phenotype in cassava overexpressing Arabidopsis CBF3 gene. Front. Plant Sci. 2016, 7, 231446. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Cai, X.-T.; Zhao, P.-X.; Xu, P.; Xiang, C.-B. Arabidopsis ERF109 regulates auxin transport-related genes in root development. bioRxiv 2019. bioRxiv:725572v1. [Google Scholar]
- Danisman, S.; van Dijk, A.D.; Bimbo, A.; van der Wal, F.; Hennig, L.; de Folter, S.; Angenent, G.C.; Immink, R.G. Analysis of functional redundancies within the Arabidopsis TCP transcription factor family. J. Exp. Bot. 2013, 64, 5673–5685. [Google Scholar] [CrossRef]
- Jang, S.; Hur, J.; Kim, S.-J.; Han, M.-J.; Kim, S.-R.; An, G. Ectopic expression of OsYAB1 causes extra stamens and carpels in rice. Plant Mol. Biol. 2004, 56, 133–143. [Google Scholar] [CrossRef]
- Kondhare, K.R.; Natarajan, B.; Banerjee, A.K. Molecular signals that govern tuber development in potato. Int. J. Dev. Biol. 2020, 64, 133–140. [Google Scholar] [CrossRef]
- Chailakhyan, M.K.; Yanina, L.I.; Devedzhyan, A.G.; Lotova, G.N. Photoperiodism and tuber formation in grafting of tobacco onto potato. Dokl. Akad. Nauk. SSSR 2019, 257, 1276–1280. [Google Scholar]
- Abelenda, J.A.; Navarro, C.; Prat, S. Flowering and tuberization: A tale of two nightshades. Trends Plant Sci. 2014, 19, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Kloosterman, B.; Abelenda, J.A.; Gomez, M.D.M.C.; Oortwijn, M.; de Boer, J.M.; Kowitwanich, K.; Horvath, B.M.; van Eck, H.J.; Smaczniak, C.; Prat, S. Naturally occurring allele diversity allows potato cultivation in northern latitudes. Nature 2013, 495, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Hoopes, G.; Meng, X.; Hamilton, J.P.; Achakkagari, S.R.; Guesdes, F.D.A.F.; Bolger, M.E.; Coombs, J.J.; Esselink, D.; Kaiser, N.R.; Kodde, L. Phased, chromosome-scale genome assemblies of tetraploid potato reveal a complex genome, transcriptome, and predicted proteome landscape underpinning genetic diversity. Mol. Plant 2022, 15, 520–536. [Google Scholar] [CrossRef] [PubMed]
- Navarro, C.; Abelenda, J.A.; Cruz-Oró, E.; Cuéllar, C.A.; Tamaki, S.; Silva, J.; Shimamoto, K.; Prat, S. Control of flowering and storage organ formation in potato by FLOWERING LOCUS T. Nature 2011, 478, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Plantenga, F.D.; Bergonzi, S.; Abelenda, J.A.; Bachem, C.W.; Visser, R.G.; Heuvelink, E.; Marcelis, L.F. The tuberization signal StSP6A represses flower bud development in potato. J. Exp. Bot. 2019, 70, 937–948. [Google Scholar] [CrossRef] [PubMed]
- Bowman, J.L.; Smyth, D.R. CRABS CLAW, a gene that regulates carpel and nectary development in Arabidopsis, encodes a novel protein with zinc finger and helix-loop-helix domains. Development 1999, 126, 2387–2396. [Google Scholar] [CrossRef] [PubMed]
- Siegfried, K.R.; Eshed, Y.; Baum, S.F.; Otsuga, D.; Drews, G.N.; Bowman, J.L. Members of the YABBY gene family specify abaxial cell fate in Arabidopsis. Development 1999, 126, 4117–4128. [Google Scholar] [CrossRef] [PubMed]
- Boter, M.; Golz, J.F.; Giménez-Ibañez, S.; Fernandez-Barbero, G.; Franco-Zorrilla, J.M.; Solano, R. FILAMENTOUS FLOWER is a direct target of JAZ3 and modulates responses to jasmonate. Plant Cell 2015, 27, 3160–3174. [Google Scholar] [CrossRef]
- Wang, Q.; Reddy, V.A.; Panicker, D.; Mao, H.Z.; Kumar, N.; Rajan, C.; Venkatesh, P.N.; Chua, N.H.; Sarojam, R. Metabolic engineering of terpene biosynthesis in plants using a trichome-specific transcription factor Ms YABBY 5 from spearmint (Mentha spicata). Plant Biotechnol. J. 2016, 14, 1619–1632. [Google Scholar] [CrossRef]
- Yang, C.; Ma, Y.; Li, J. The rice YABBY4 gene regulates plant growth and development through modulating the gibberellin pathway. J. Exp. Bot. 2016, 67, 5545–5556. [Google Scholar] [CrossRef]
- Lugassi, N.; Nakayama, N.; Bochnik, R.; Zik, M. A novel allele of FILAMENTOUS FLOWER reveals new insights on the link between inflorescence and floral meristem organization and flower morphogenesis. BMC Plant Biol. 2010, 10, 131. [Google Scholar] [CrossRef]
- Strable, J.; Vollbrecht, E. Maize YABBY genes drooping leaf1 and drooping leaf2 regulate floret development and floral meristem determinacy. Development 2019, 146, dev171181. [Google Scholar] [CrossRef]
- Tanaka, W.; Toriba, T.; Hirano, H.Y. Three TOB 1-related YABBY genes are required to maintain proper function of the spikelet and branch meristems in rice. New Phytol. 2017, 215, 825–839. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Nagasawa, N.; Kawasaki, S.; Matsuoka, M.; Nagato, Y.; Hirano, H.-Y. The YABBY gene DROOPING LEAF regulates carpel specification and midrib development in Oryza sativa. Plant Cell 2004, 16, 500–509. [Google Scholar] [CrossRef]
- Toriba, T.; Harada, K.; Takamura, A.; Nakamura, H.; Ichikawa, H.; Suzaki, T.; Hirano, H.-Y. Molecular characterization the YABBY gene family in Oryza sativa and expression analysis of OsYABBY1. Mol. Genet. Genom. 2007, 277, 457–468. [Google Scholar] [CrossRef]
- Zhao, W.; Su, H.Y.; Song, J.; Zhao, X.Y.; Zhang, X.S. Ectopic expression of TaYAB1, a member of YABBY gene family in wheat, causes the partial abaxialization of the adaxial epidermises of leaves and arrests the development of shoot apical meristem in Arabidopsis. Plant Sci. 2006, 170, 364–371. [Google Scholar] [CrossRef]
- Yang, H.; Shi, G.; Li, X.; Hu, D.; Cui, Y.; Hou, J.; Yu, D.; Huang, F. Overexpression of a soybean YABBY gene, GmFILa, causes leaf curling in Arabidopsis thaliana. BMC Plant Biol. 2019, 19, 234. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-L.; Yang, Z.-P.; Zhang, J.; Zhang, L.-G. Ectopic expression of BraYAB1-702, a member of YABBY gene family in Chinese cabbage, causes leaf curling, inhibition of development of shoot apical meristem and flowering stage delaying in Arabidopsis thaliana. Int. J. Mol. Sci. 2013, 14, 14872–14891. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, Y.; Kristiansen, K.; Wang, J. SOAP: Short oligonucleotide alignment program. Bioinformatics 2008, 24, 713–714. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Kolde, R.; Kolde, M.R. Package ‘pheatmap’. R Package 2015, 1, 790. [Google Scholar]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Storey, J.D.; Bass, A.J.; Dabney, A.; Robinson, D. qvalue: Q-Value Estimation for False Discovery Rate Control. Available online: http://github.com/jdstorey/qvalue (accessed on 29 April 2024).
- Shen, X. Preliminary Studies on Producing New Germplasm by Transforming Cassava MeSUT1 into Potato. Master’s Thesis, Nanjing Agricultural University, Nanjing, China, 2020. [Google Scholar]
- Jerez Mompie, E.; Martín Martín, R.; Díaz Hernández, Y. Estimate of the leave area in two potato varieties (Solanum tuberosum L.) for non destructive methods. Cultiv. Trop. 2013, 35, 57–61. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).