Use of Proline to Induce Salt Stress Tolerance in Guava
Abstract
:1. Introduction
2. Results
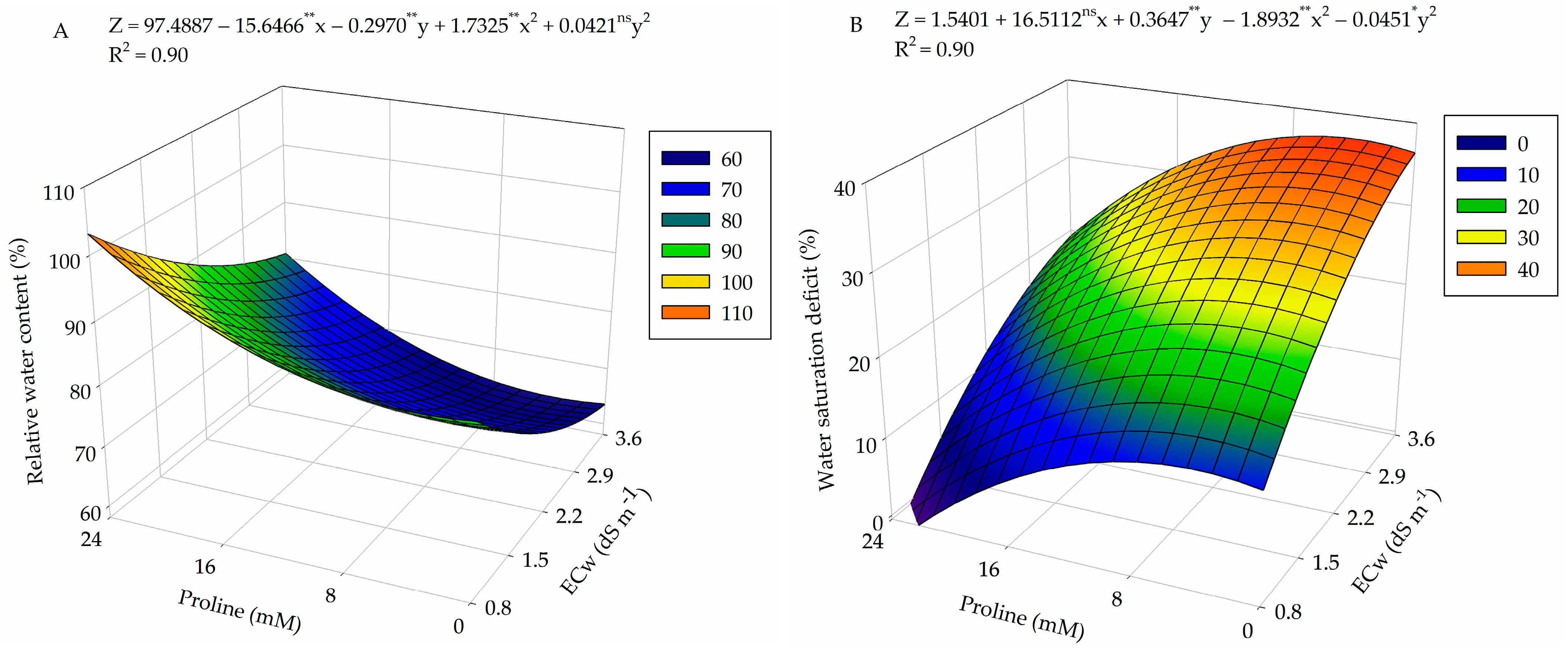
2.1. Relative Water Content, Electrolyte Leakage in the Leaf Blade, and Water Saturation Deficit
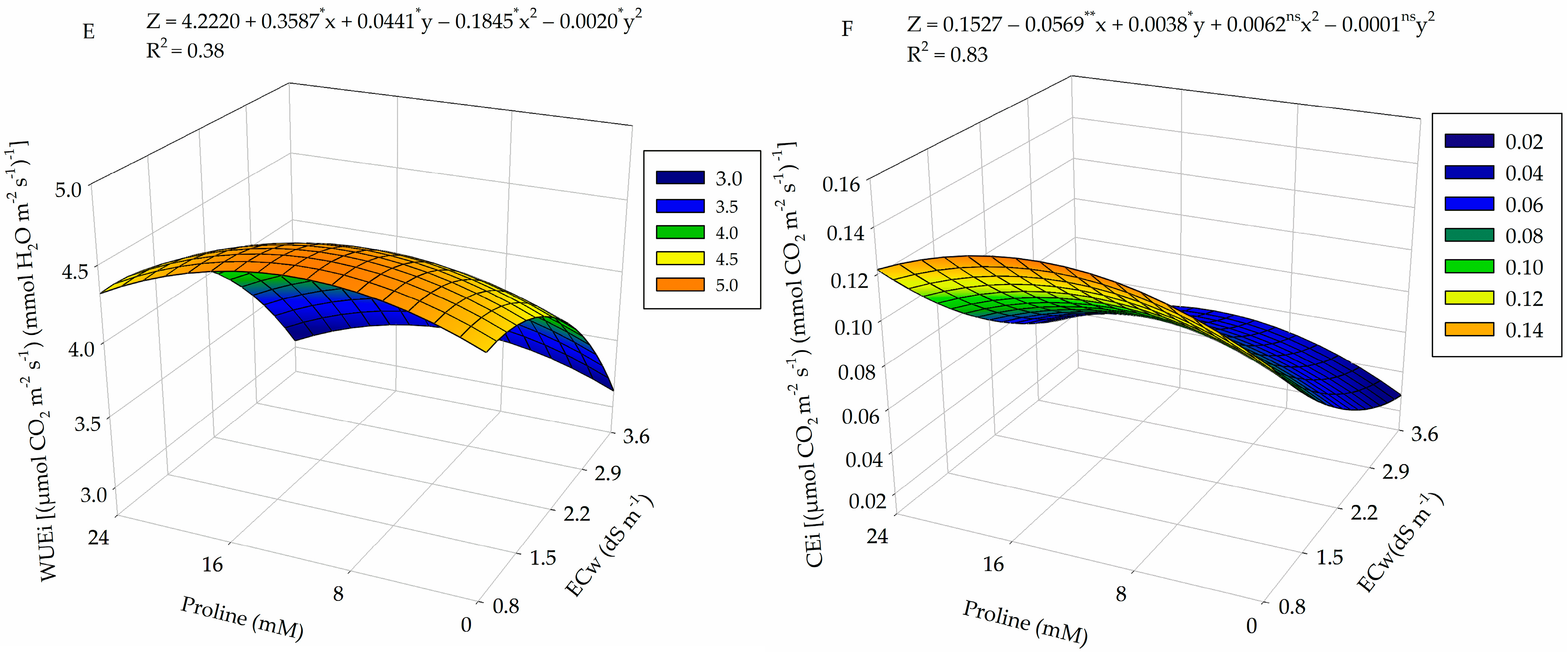
2.2. Gas Exchange
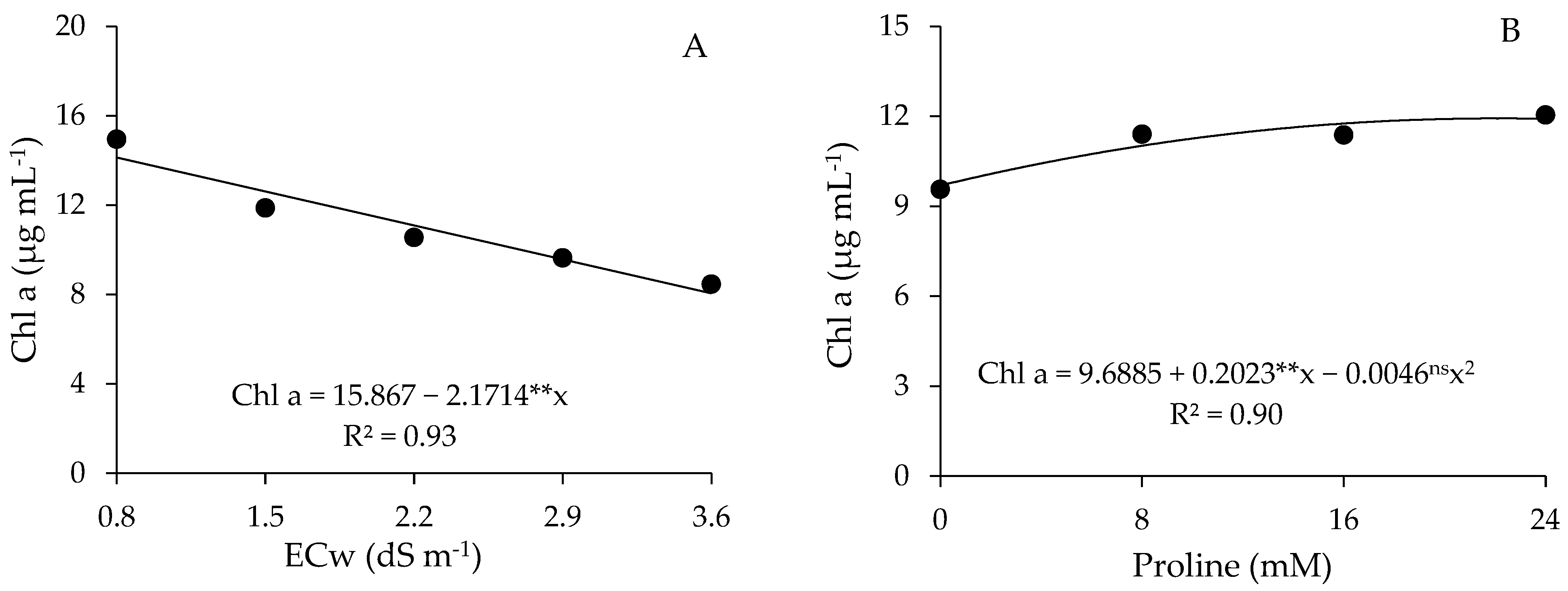
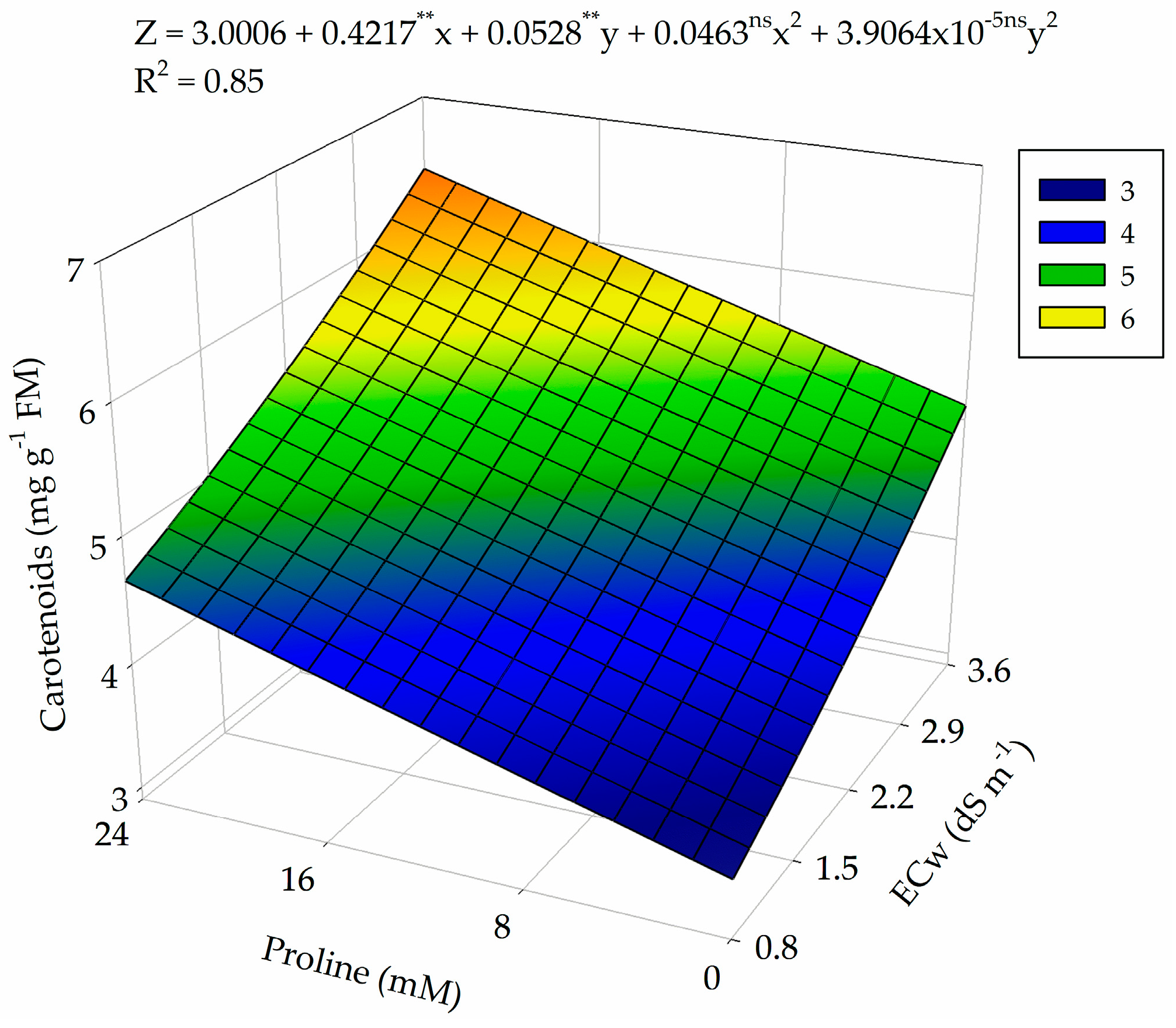
2.3. Photosynthetic Pigments
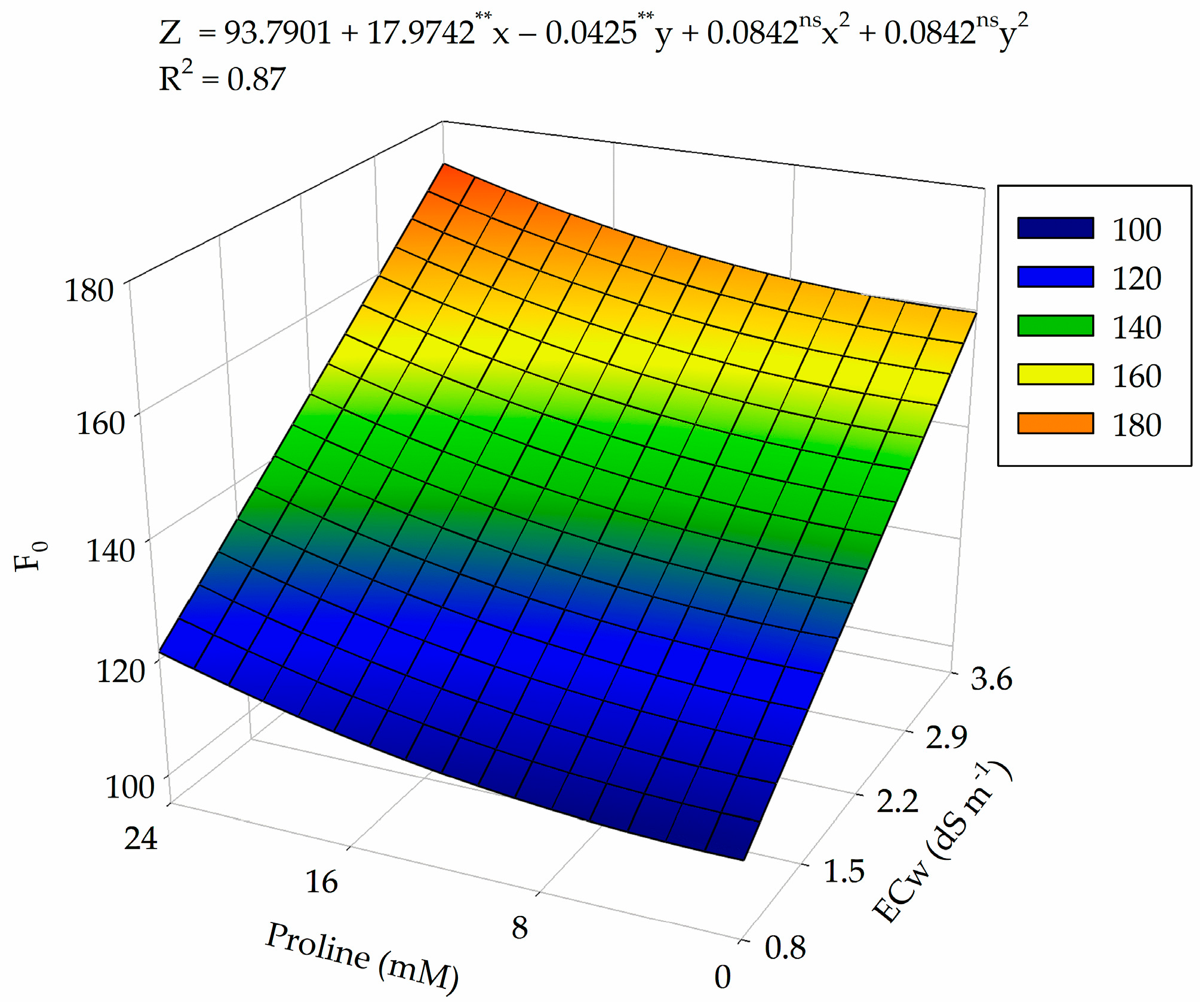
2.4. Photochemical Efficiency
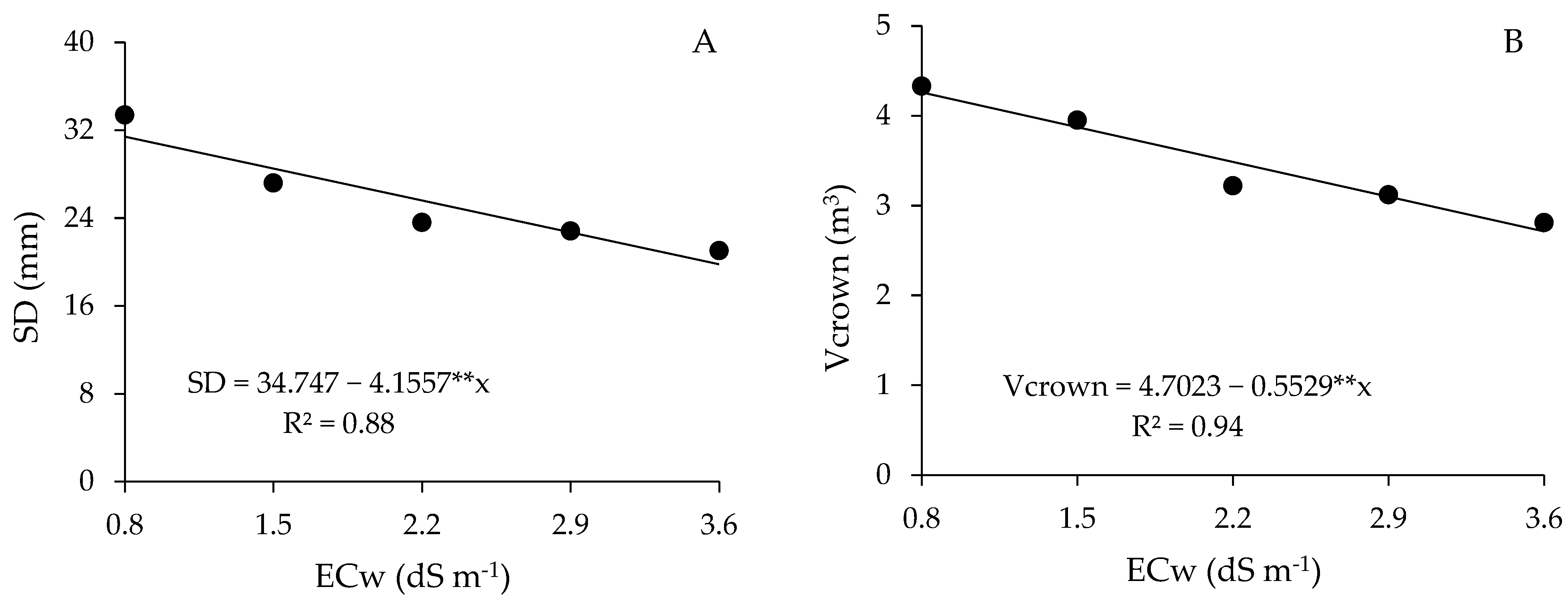
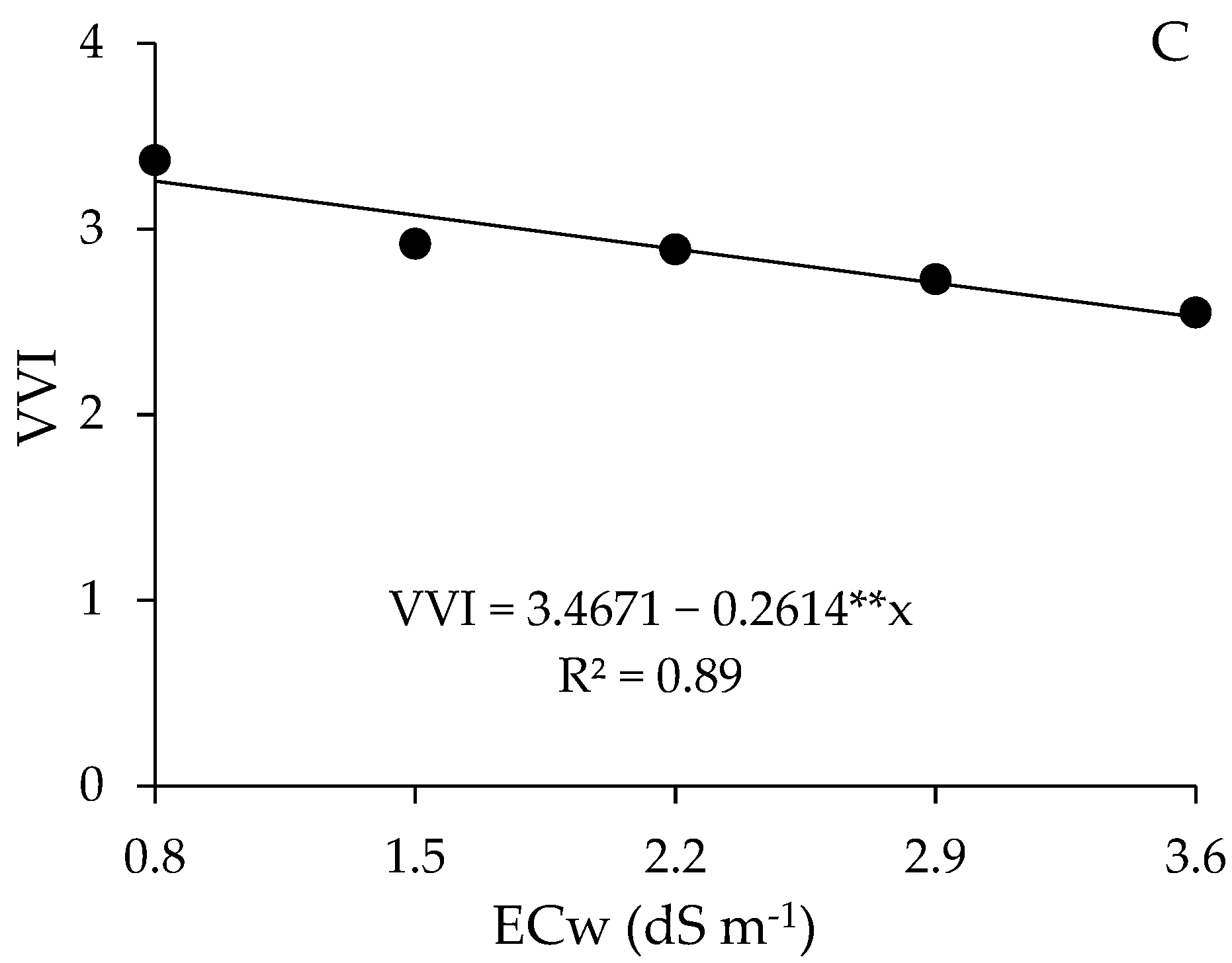
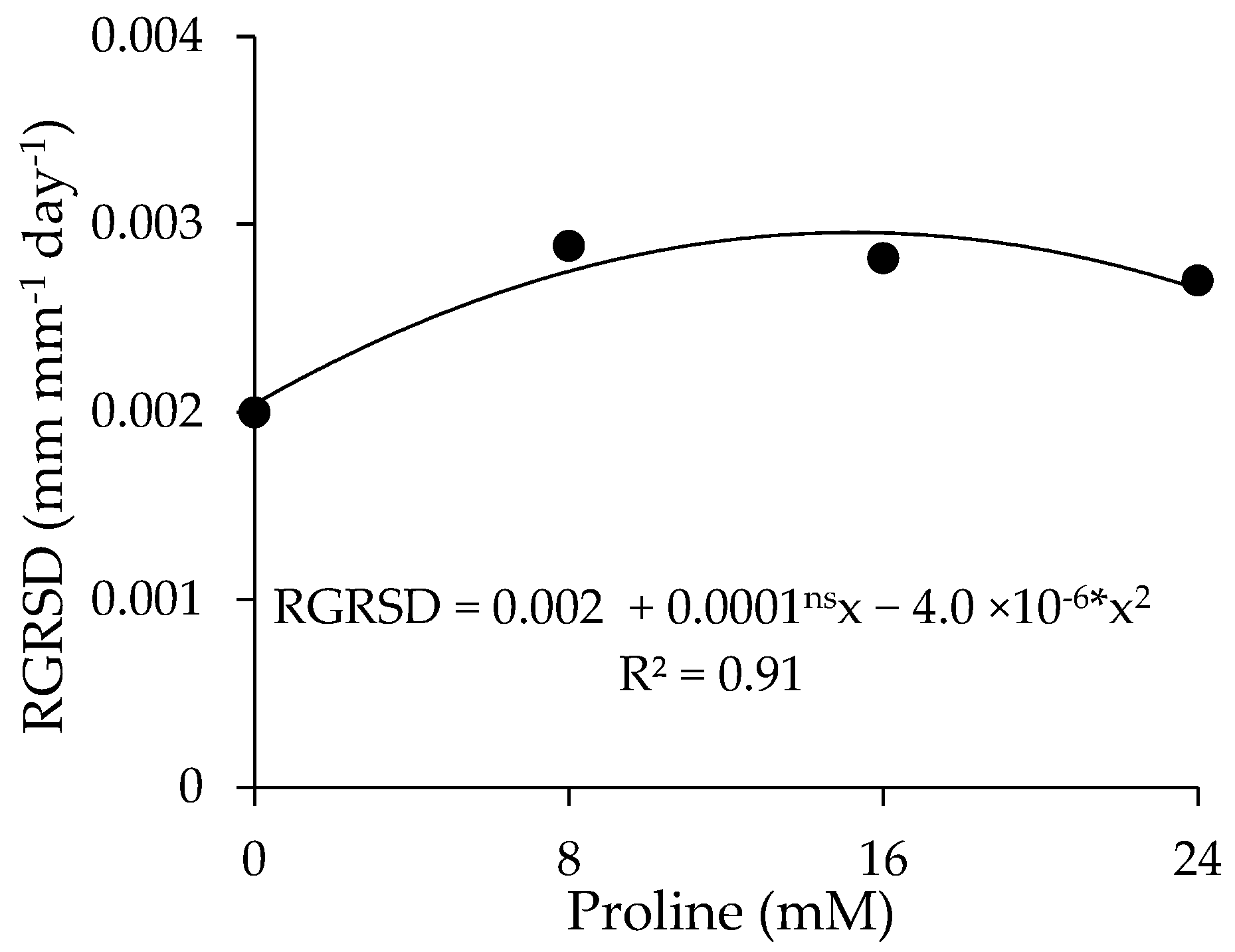
2.5. Growth Parameters
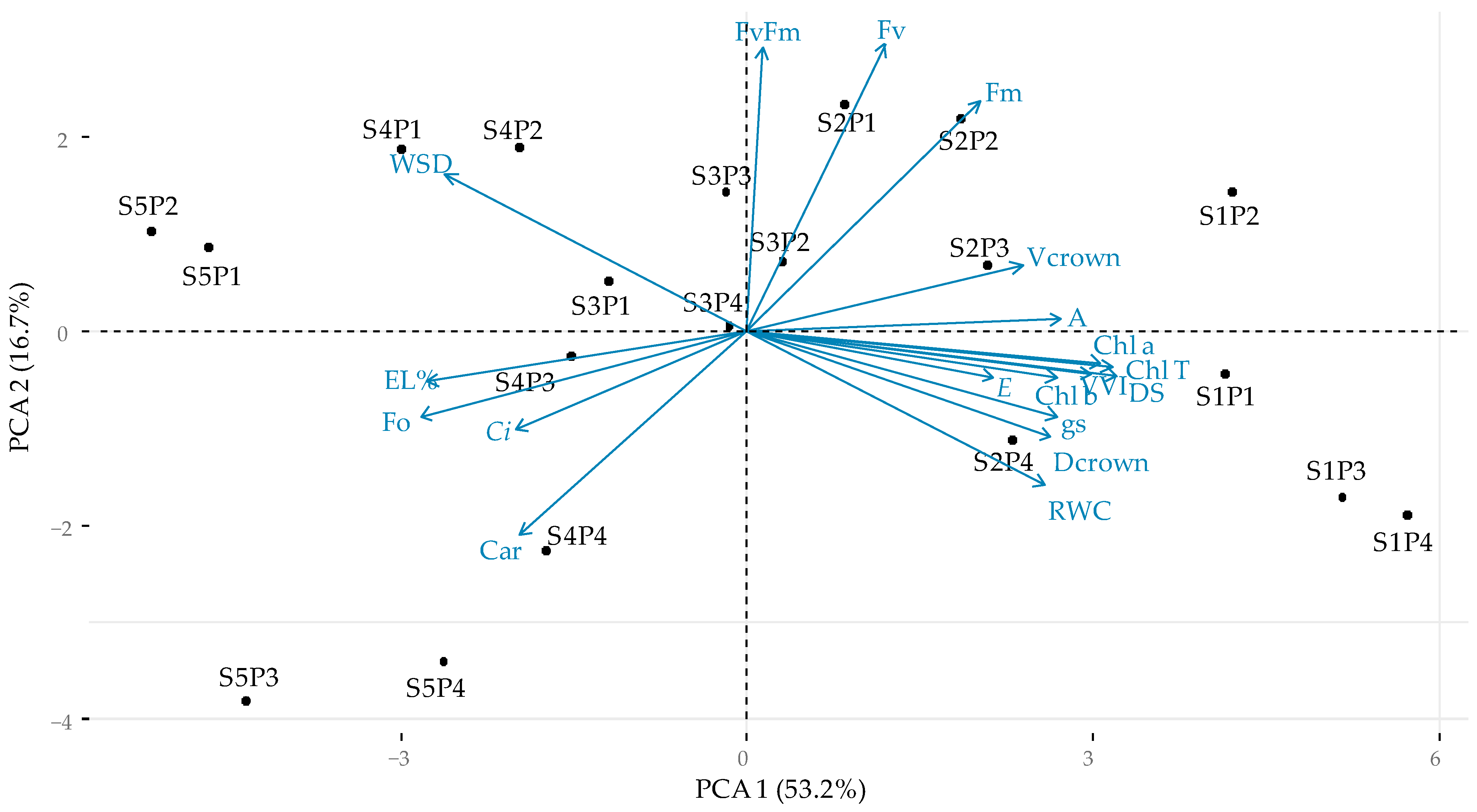
2.6. Principal Component Analysis
3. Discussion
4. Materials and Methods
4.1. Experimental Area Location and Characterization
4.2. Experimental Design and Treatments
4.3. Experiment Setup and Conduct
4.4. Preparation of Waters and Irrigation Management
4.5. Preparation and Application of Proline Concentrations
4.6. Crop Management
4.7. Variables Analyzed
4.7.1. Relative Water Content, Electrolyte Leakage in the Leaf Blade, and Water Saturation Deficit
4.7.2. Gas Exchange
4.7.3. Photosynthetic Pigments
4.7.4. Photochemical Efficiency
4.7.5. Growth
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Diaz-Garcia, L.; Padilla-Ramírez, J.S. Development of single nucleotide polymorphism markers and genetic diversity in guava (Psidium guajava L.). Plants People Planet 2023, 5, 58–69. [Google Scholar] [CrossRef]
- Arango, V.A.V.; Martínez, A.A.B.; Santos, L.E.O.; Zapata, J.L.H. Caracterización del epicarpio de guayaba (Psidium guajava L.) como alternativa natural para uso en productos alimenticios processados. Biotecnol. Sect. Agropecu. Agroindustrial 2020, 18, 26–36. [Google Scholar] [CrossRef]
- IBGE. Instituto Brasileiro de Geografia e Estatística. Produção Agrícola—Lavoura Permanente, Safra—2022. Available online: https://cidades.ibge.gov.br/brasil/pesquisa/15/11954 (accessed on 18 January 2024).
- Castro, J.M.C.; Ribeiro, J.M. Pesquisa e Desenvolvimento para a Cultura da Goiabeira: A Contribuição da Embrapa Semiárido, 1st ed.; Embrapa Semiárido: Petrolina, Brazil, 2020; 82p. [Google Scholar]
- Melo, E.N.; Nobre, R.G.; Pinheiro, F.W.A.; Souza, L.P.; Lima, G.S.; Gheyi, H.R.; Elias, J.J.; Silva, W.L. Evaluation of West Indian cherry (Malpighia emarginata) rootstock under saline water irrigation and nitrogen fertilization. Aust. J. Crop Sci. 2018, 12, 1034–1040. [Google Scholar] [CrossRef]
- Nunes, K.G.; Costa, R.N.T.; Cavalcante, I.N.; Gondim, R.S.; Lima, S.C.R.V.; Mateos, L. Groundwater resources for agricultural purposes in the Brazilian semi-arid region. Rev. Bras. Eng. Agrícola Ambient. 2022, 26, 915–923. [Google Scholar] [CrossRef]
- Ondrasek, G.; Rathod, S.; Manohara, K.K.; Gireesh, C.; Anantha, M.S.; Sakhare, A.S.; Parmar, B.; Yadav, B.K.; Bandumula, N.; Raihan, F.; et al. Salt stress in plants and mitigation approaches. Plants 2022, 11, 717. [Google Scholar] [CrossRef]
- Silva, E.M.; Gheyi, H.R.; Nobre, R.G.; Lima, G.S.; Soares, L.A.A.; Bonifácio, B.F. Saline waters and nitrogen/potassium fertilization combinations on physiological aspects and production of West Indian cherry. Rev. Ambiente Água 2021, 16, e2780. [Google Scholar] [CrossRef]
- Nóbrega, J.S.; Gomes, V.R.; Soares, L.A.A.; Lima, G.S.; Silva, A.A.R.; Gheyi, H.R.; Torres, R.A.F.; Silva, F.J.L.; Silva, T.I.; Costa, F.B.; et al. Hydrogen peroxide alleviates salt stress effects on gas exchange, growth, and production of naturally colored cotton. Plants 2024, 13, 390. [Google Scholar] [CrossRef]
- Negrão, S.; Schmöckel, S.M.; Tester, M. Evaluating physiological responses of plants to salinity stress. Ann. Bot. 2017, 119, 1–11. [Google Scholar] [CrossRef]
- Kumar, N.; Haldarb, S.; Saikia, R. Root exudation as a strategy for plants to deal with salt stress: An updated review. Environ. Exp. Bot. 2023, 216, 105518. [Google Scholar] [CrossRef]
- Ferreira, J.T.A.; Lima, G.S.; Silva, S.S.; Soares, L.A.A.; Fátima, R.T.; Nóbrega, J.S.; Gheiy, H.R.; Almeira, F.A.; Mendonça, A.J.T. Hydrogen peroxide in the induction of tolerance of guava seedlings to salt stress. Semin. Ciências Agrárias 2023, 44, 739–754. [Google Scholar] [CrossRef]
- Bezerra, I.L.; Gheyi, H.R.; Nobre, R.G.; Barbosa, J.L.; Fátima, R.T.; Elias, J.J.; Azevedo, F.L. Physiological alterations and production of guava under water salinity and nitrogen fertilizer application. Semin. Ciências Agrárias 2018, 39, 1945–1956. [Google Scholar] [CrossRef]
- Orsini, F.; Pennisi, G.; Mancarella, S.; Al-Nayef, M.; Sanoubar, R.; Nicola, S.; Gianquinto, G. Hydroponic lettuce yields are improved under salt stress by utilizing white plastic film and exogenous applications of proline. Sci. Hortic. 2018, 233, 283–293. [Google Scholar] [CrossRef]
- Noreen, S.; Faiz, S.; Akhter, M.S.; Shah, K.H. Influence of foliar application of osmoprotectants to ameliorate salt stress in sunflower (Helianthus annuus L.). Sarhad J. Agric. 2019, 35, 1316–1325. [Google Scholar] [CrossRef]
- Santos, L.F.S.; Lima, G.S.; Lima, V.L.A.; Silva, A.A.R.; Silva, A.A.R.; Nóbrega, J.S.; Capitulino, J.D.; Borborema, L.D.A.; Souza, A.R.; Caetano, E.J.M. Foliar application of proline on the mitigation of salt stress in the physiological indices of sour passion fruit. Semin. Ciências Agrárias 2023, 44, 1637–1660. [Google Scholar] [CrossRef]
- Nounjan, N.; Chansongkrow, P.; Charoensawan, V.; Siangliw, J.L.; Toojinda, T.; Chadchawan, S.; Theerakulpisut, P. High performance of photosynthesis and osmotic adjustment are associated with salt tolerance ability in rice carrying drought tolerance QTL: Physiological and co-expression network analysis. Front. Plant Sci. 2018, 9, e1135. [Google Scholar] [CrossRef]
- Mansour, M.M.F.; Ali, E.F. Glycinebetaine in saline conditions: An assessment of the current state of knowledge. Acta Phys. Plant. 2017, 39, 56. [Google Scholar] [CrossRef]
- Veloso, L.L.S.A.; Nobre, R.G.; Souza, C.M.A.; Fátima, R.T.; Souza, L.P.; Elias, J.J.; Azevêdo, F.L.; Santos, J.B. Morphophysiology of guava cv. Paluma with water of different salt concentrations and proline doses. Semin. Ciências Agrárias 2018, 39, 1877–1886. [Google Scholar] [CrossRef]
- Gohari, G.; Panahirad, S.; Sepehri, N.; Akbari, A.; Zahedi, S.M.; Jafari, H.; Dadpour, M.R.; Fotopoulos, V. Enhanced tolerance to salinity stress in grapevine plants through application of carbon quantum dots functionalized by proline. Environ. Sci. Pollut. Res. 2021, 28, 42877–42890. [Google Scholar] [CrossRef]
- El-Moukhtari, A.; Cabassa-Hourton, C.; Farissi, M.; Savouré, A. How does proline treatment promote salt stress tolerance during crop plant development? Front. Plant Sci. 2020, 11, e1127. [Google Scholar] [CrossRef]
- Silva, F.A.; Pereira, F.H.F.; Campos Júnior, J.E.; Nóbrega, J.S.; Dias, M.S. Aplicação foliar de prolina no crescimento e fisiologia do milho verde cultivado em solo salinizado. Colloq. Agrar. 2020, 16, 23–34. [Google Scholar] [CrossRef]
- Lu, Y.; Fricke, W. Salt Stress—Regulation of root water uptake in a whole-plant and diurnal context. Int. J. Mol. Sci. 2023, 24, 8070. [Google Scholar] [CrossRef] [PubMed]
- Lima, G.S.; Pinheiro, F.W.A.; Souza, W.B.B.; Soares, L.A.A.; Gheyi, H.R.; Nobre, R.G.; Queiroga, R.C.F.; Fernandes, P.D. Physiological indices of sour passion fruit under brackish water irrigation strategies and potassium fertilization. Rev. Bras. Eng. Agrícola Ambient. 2023, 27, 383–392. [Google Scholar] [CrossRef]
- Jacinto Júnior, S.G.; Moraes, J.G.L.; Silva, F.D.B.; Silva, B.N.; Sousa, G.G.; Oliveira, L.L.B.; Mesquita, R.O. Respostas fisiológicas de genótipos de fava (Phaseolus lunatus L.) submetidas ao estresse hídrico cultivadas no Estado do Ceará. Rev. Bras. Meteorol. 2019, 34, 413–422. [Google Scholar] [CrossRef]
- Sarabi, B.; Fresneau, C.; Ghaderi, N.; Bolandnazar, S.; Streb, P.; Badec, F.W.; Citerne, S.; Tangama, M.; David, A.; Ghashaghaie, J. Stomatal and non-stomatal limitations are responsible in down-regulation of photosynthesis in melon plants grown under the saline condition: Application of carbon isotope discrimination as a reliable proxy. Plant Phys. Biochem. 2019, 141, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Nobre, R.G.; Rodrigues Filho, R.A.; Lima, G.S.; Linhares, E.R.L.; Soares, L.A.A.; Silva, L.A.; Teixeira, A.D.S.; Macumbi, M.J.V. Gas exchange and photochemical efficiency of guava under saline water irrigation and nitrogen-potassium fertilization. Rev. Bras. Eng. Agrícola Ambient. 2023, 27, 429–437. [Google Scholar] [CrossRef]
- Figueiredo, F.R.A.; Fátima, R.T.; Nóbrega, J.S.; Silva, T.I.; Ferreira, J.T.A.; Ribeiro, J.E.S.; Leal, M.P.S.; Soares, L.A.A.; Dias, T.J. Morphophysiology and gas exchange of pomegranate under salt stress and foliar application of nitrogen. Rev. Bras. Eng. Agrícola Ambient. 2023, 27, 958–965. [Google Scholar] [CrossRef]
- Souza, L.P.; Lima, G.S.; Gheyi, H.R.; Fátima, R.T.; Silva, A.A.R.; Nobre, R.G.; Soares, L.A.A.; Lacerda, C.N. Morphophysiology and production of West Indian cherry under salt stress and nitrogen-phosphorus-potassium fertilization. Rev. Bras. Eng. Agrícola Ambient. 2023, 27, 539–549. [Google Scholar] [CrossRef]
- Capitulino, J.D.; Lima, G.S.; Azevedo, C.A.V.; Silva, A.A.R.; Arruda, T.F.L.; Soares, L.A.A.; Gheyi, H.R.; Fernandes, P.D.; Farias, M.S.S.; Silva, F.A.; et al. Influence of foliar application of hydrogen peroxide on gas exchange, photochemical efficiency, and growth of soursop under salt stress. Plants 2023, 12, 599. [Google Scholar] [CrossRef]
- Zheng, J.L.; Zhao, L.Y.; Wu, C.W.; Shen, B.; Zhu, A.Y. Exogenous proline reduces NaCl-induced damage by mediating ionic and osmotic adjustment and enhancing antioxidant defense in Eurya emarginata. Acta Physiol. Plant. 2015, 37, 181. [Google Scholar] [CrossRef]
- El-Banna, M.F.; Masa, A. Exogenous application of proline mitigates deteriorative effects of salinity stress in NFT closed-loop system: An ultrastructural and physio-biochemical investigation on hydroponically grown tomato (Solanum lycopersicon L.). Sci. Hortic 2024, 330, 113061. [Google Scholar] [CrossRef]
- Torres, R.A.F.; Lima, G.S.; Paiva, F.J.S.; Soares, L.A.A.; Silva, F.A.; Silva, L.A.; Oliveira, V.K.N.; Mendonça, A.J.T.; Roque, I.A.; Silva, S.T.A. Physiology and production of sugar-apple under water stress and application of proline. Braz. J. Biol. 2023, 83, e273404. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, S.M.; Abolhassani, M.; Hadian-Deljou, M.; Feyzi, H.; Akbary, A.; Rasouli, F.; Koçak, M.Z.; Kulak, M.; Gohari, G. Proline-functionalized graphene oxide nanoparticles (GO-Pro NPs): A new engineered nanoparticle to ameliorate salinity stress on grape (Vitis vinifera L. cv Sultana). Plant Stress 2023, 7, 100128. [Google Scholar] [CrossRef]
- Shahverdi, M.A.; Omidi, H.; Tabatabaei, S.J. Stevia (Stevia rebaudiana Bertoni) responses to NaCl stress: Growth, photosynthetic pigments, diterpene glycosides and ion content in root and shoot. J. Saudi Soc. Agric. Sci. 2019, 18, 355–360. [Google Scholar] [CrossRef]
- Xavier, A.V.O.; Lima, G.S.; Gheyi, H.R.; Silva, A.A.R.; Soares, L.A.A.; Lacerda, C.N. Gas exchange, growth and quality of guava seedlings under salt stress and salicylic acid. Rev. Ambiente Água 2022, 17, e2816. [Google Scholar] [CrossRef]
- Soares, L.A.A.; Oliveira, S.G.; Lima, G.S.; Fernandes, P.D.; Araújo, R.H.C.R.; Fernandes, E.A. Physiological changes of pomegranate seedlings under salt stress and nitrogen fertilization. Rev. Bras. Eng. Agrícola Ambient. 2021, 25, 453–459. [Google Scholar] [CrossRef]
- El-Betalgi, H.S.; Mohamed, H.I.; Sofy, M.R. Role of ascorbic acid, glutathione and proline applied as singly or in sequence combination in improving chickpea plant through physiological change and antioxidant defense under different levels of irrigation intervals. Molecules 2020, 25, 1702. [Google Scholar] [CrossRef]
- El-Shawa, G.M.R.; Rashwan, E.M.; Abdelaal, K.A.A. Mitigating salt stress effects by exogenous application of proline and yeast extract on morphophysiological, biochemical and anatomical characters of calendula plants. Sci. J. Flowers Ornam. Plants 2020, 7, 461–482. [Google Scholar] [CrossRef]
- Merwad, A.R.M.; Desoky, E.S.M.; Rady, M.M. Response of water deficit stressed Vigna unguiculata performances to silicon, proline or methionine foliar application. Sci. Hortic. 2018, 228, 132–144. [Google Scholar] [CrossRef]
- Monteiro, J.G.; Cruz, F.J.R.; Nardin, M.B.; Santos, D.M.M. Crescimento e conteúdo de prolina em plântulas de guandu submetidas a estresse osmótico e à putrescina exógena. Pesqui. Agropecu. Bras. 2014, 49, 18–25. [Google Scholar] [CrossRef]
- Butt, M.; Sattar, A.; Abbas, T.; Sher, A.; Ijaz, M.; Ul-Allah, S.; Shaheen, M.R.; Kaleem, F. Foliage applied proline induces salt tolerance in chili genotypes by regulating photosynthetic attributes, ionic homeostasis, and antioxidant defense mechanisms. Hortic. Environ. Biotechnol. 2020, 61, 693–702. [Google Scholar] [CrossRef]
- Stefanov, M.; Yotsova, E.; Gesheva, M.; Dimitrava, D.; Maskovska, L.; Dancheva, S.; Apostolova, E.L. Role of flavonoids and proline in the protection of photosynthetic apparatus in Paulownia under salt stress. S. Afr. J. Bot. 2021, 139, 246–253. [Google Scholar] [CrossRef]
- Menegazzo, R.F.; Rickli, M.E.; Scanavacca, J.; Lima, J.D.; Silveira, A.C.; Menegazzo, A.W.; Souza, S.G.H.; Lopes, A.D. Resposta diferencial a diferentes classes de herbicidas: Tradescantia pallida (Rose) D. R. Hunt var. purpurea Boom como planta modelo. Res. Soc. Dev. 2021, 10, e6910111452. [Google Scholar] [CrossRef]
- Veloso, L.L.S.A.; Capitulino, J.D.; Lima, G.S.; Azevedo, C.A.V.; Silva, A.A.R.; Gheyi, H.R. Application methods of hydrogen peroxide in soursop seedlings irrigated with saline water. Comun. Sci. 2021, 12, e3288. [Google Scholar] [CrossRef]
- Faseela, P.; Sinisha, A.K.; Brestič, M.; Puthur, J.T. Chlorophyll a fluorescence parameters as indicators of a particular abiotic stress in rice. Photosynthetica 2020, 58, 293–300. [Google Scholar] [CrossRef]
- Lacerda, C.N.; Lima, G.S.; Soares, L.A.A.; Fátima, R.T.; Gheyi, H.R.; Azevedo, C.A. Morphophysiology and production of guava as a function of water salinity and salicylic acid. Rev. Bras. Eng. Agrícola Ambient. 2022, 26, 451–458. [Google Scholar] [CrossRef]
- Queiroz, G.C.M.; Medeiros, J.F.; Silva, R.R.; Morais, F.M.S.; Sousa, L.V.; Souza, M.V.P.; Santos, E.N.; Ferreira, F.N.; Silva, J.M.C.; Clemente, M.I.B.; et al. Growth, solute accumulation, and ion distribution in sweet sorghum under salt and drought stresses in a brazilian potiguar semiarid area. Agriculture 2023, 13, 803. [Google Scholar] [CrossRef]
- Byrt, C.S.; Munns, R.; Burton, R.A.; Gilliham, M.; Wege, S. Root cell wall solutions for crop plants in saline soils. Plant Sci. 2018, 269, 47–55. [Google Scholar] [CrossRef]
- Abrar, M.M.; Sohail, M.; Saqib, M.; Akthar, J.; Abbas, G.; Wahad, H.A.; Mumtaz, M.Z.; Mehmood, K.; Memon, M.S.; Sun, N.; et al. Interactive salinity and water stress severely reduced the growth, stress tolerance, and physiological responses of guava (Psidium guajava L.). Sci. Rep. 2022, 12, 18952. [Google Scholar] [CrossRef]
- Lima, G.S.; Santos, J.B.; Soares, L.A.A.; Gheyi, H.R.; Nobre, R.G. Irrigação com águas salinas e aplicação de prolina foliar em cultivo de pimentão ‘All Big’. Comun. Sci. 2016, 7, 513–522. [Google Scholar] [CrossRef]
- Pommer, C.V.; Murakami, K.R.N.; Watlington, F. Goiaba no mundo. Agronômico 2006, 58, 22–26. [Google Scholar]
- Teixeira, P.C.; Donagema, G.K.; Fontana, A.; Teixeira, W.G. Manual de Métodos de Análise de Solo, 3rd ed.; EMBRAPA: Brasília, Brazil, 2017; 573p. [Google Scholar]
- Richards, L.A. Diagnosis and Improvement of Saline and Alkali Soils; Department of Agriculture: Washington, DC, USA, 1954; 160p. [Google Scholar]
- Cavalcanti, F.D.A.; Santos, J.C.P.; Pereira, J.R.; Leite, J.P.; Silva, M.C.L.; Freira, F.J.; Silva, D.J.; Sousa, A.R.; Messias, A.S.; Farias, C.M.B.; et al. Recomendações de Adubação para o Estado de Pernambuco: 2. Aproximação, 3rd ed.; Instituto Agronômico de Pernambuco-IPA: Recife, Brazil, 2008; 212p. [Google Scholar]
- Weatherley, P.E. Studies in the water relations of the cotton plant. I. The field measurements of water deficits in leaves. New Phytol. 1950, 49, 81–97. [Google Scholar] [CrossRef]
- Scotti-Campos, P.; Pham-Thi, A.T.; Semedo, J.N.; Pais, I.P.; Ramalho, J.C.; Matos, M.C. Physiological responses and membrane integrity in three Vigna genotypes with contrasting drought tolerance. Emir. J. Food Agric. 2013, 25, 1002–1013. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E.; Møller, I.M.; Murphy, A. Fisiologia e Desenvolvimento Vegetal; Artmed: Porto Alegre, Brazil, 2017; 888p. [Google Scholar]
- Cruz, A.C.F.; Santos, R.P.; Iarema, L.; Fernandes, K.R.G.; Kuki, K.N.; Araújo, R.F.; Otoni, W.C. Métodos comparativos na extração de pigmentos foliares de três híbridos de Bixa orellana L. Rev. Bras. Biociências 2007, 5, 777–779. [Google Scholar]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Portella, C.R.; Marinho, C.S.; Amaral, B.D.; Carvalho, W.S.G.; Campos, G.S.; Silva, M.P.S.; Sousa, M.C. Desempenho de cultivares de citros enxertados sobre o trifoliateiro ‘Flying Dragon’ e limoeiro ‘Cravo’ em fase de formação do pomar. Bragantia 2016, 75, 70–75. [Google Scholar] [CrossRef]
- Benincasa, M.M.P. Análise de Crescimento de Plantas, Noções Básicas, 2nd ed.; FUNEP: Jaboticabal, Brazil, 2003; 41p. [Google Scholar]
- Ferreira, D.F. Sisvar: A computer analysis system to fixed effects split plot type designs. Braz. J. Biom. 2019, 37, 529–535. [Google Scholar] [CrossRef]


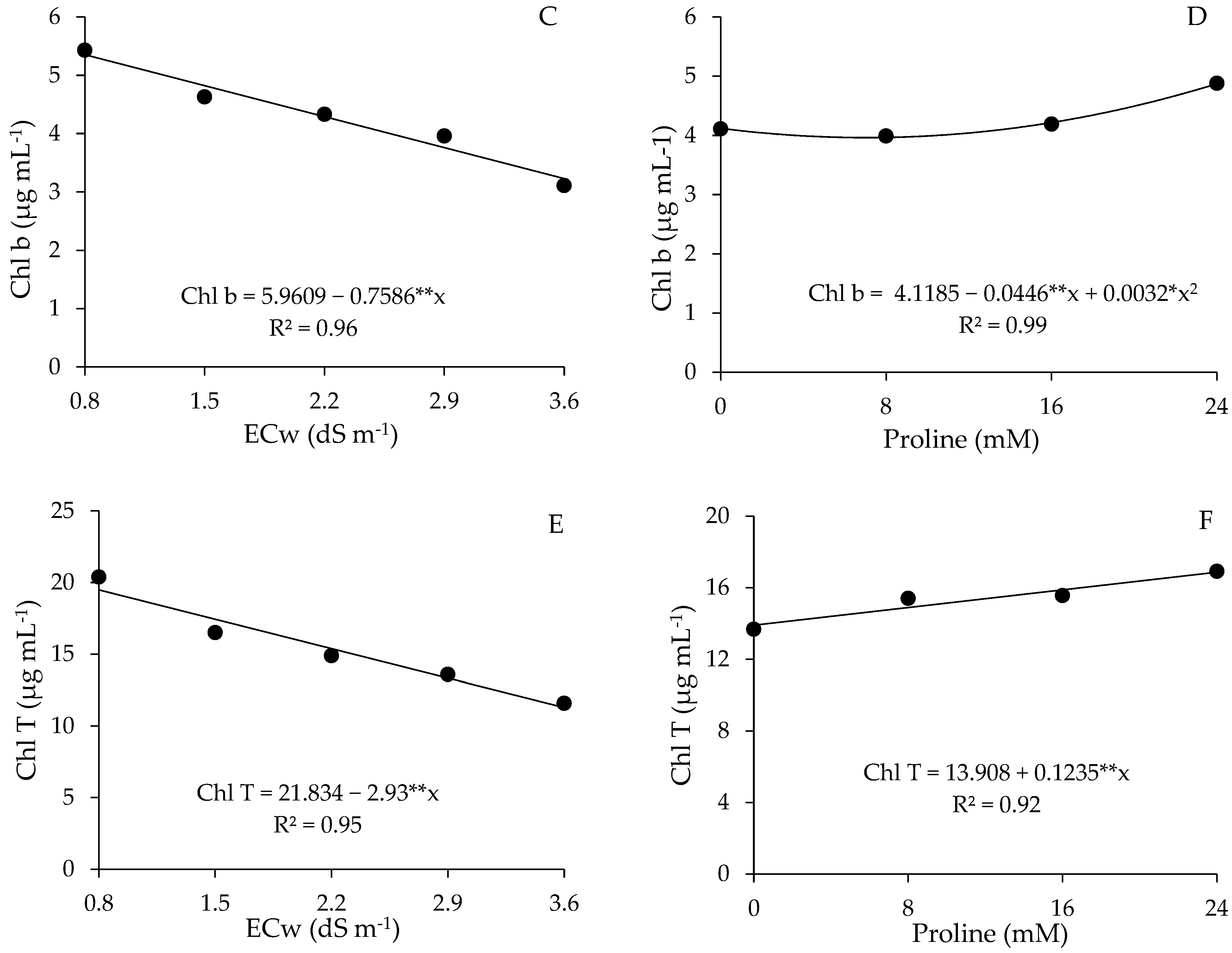



| Source of Variation | DF | Mean Squares | ||
|---|---|---|---|---|
| RWC | WSD | EL% | ||
| Salinity levels (ECw) | 4 | 1022 ** | 1033 ** | 746 ** |
| Linear regression | 1 | 3899 ** | 3934 ** | 2886 ** |
| Quadratic regression | 1 | 138 ns | 144 ns | 73 * |
| Proline (PROL) | 3 | 998 ** | 992 ** | 112 ** |
| Linear regression | 1 | 2500 ** | 2473 ** | 16 ns |
| Quadratic regression | 1 | 491 ** | 500 ** | 164 ** |
| Interaction (ECw × PROL) | 12 | 126 ** | 127 ** | 63 ** |
| Blocks | 2 | 90 ns | 89 ns | 14 ns |
| Residual | 38 | 45 | 45 | 17 |
| CV (%) | 8.51 | 31.80 | 14.58 | |
| Source of Variation | DF | Mean Squares | |||||
|---|---|---|---|---|---|---|---|
| gs | E | Ci | A | WUEi | CEi | ||
| Salinity levels (ECw) | 4 | 0.044 ** | 4.87 ** | 12106 ** | 196.55 ** | 4.67 ns | 0.0141 ** |
| Linear regression | 1 | 0.124 ** | 17.34 ** | 46256 ** | 775.71 ** | 9.22 ns | 0.0550 ** |
| Quadratic regression | 1 | 0.039 ** | 1.64 ns | 125 ns | 0.43 ns | 6.94 | 0.0010 ns |
| Proline (PROL) | 3 | 0.005 ** | 0.42 ns | 6356 ** | 45.22 * | 0.44 ns | 0.0018 ns |
| Linear regression | 1 | 0.015 ** | 1.09 ns | 5534 ** | 85.86 ** | 0.30 ns | 0.0008 ns |
| Quadratic regression | 1 | 0.0004 ns | 0.17 ns | 9238 ** | 26.00 ns | 0.61 ns | 0.0045 ** |
| Interaction (ECw × PROL) | 12 | 0.005 ** | 2.29 * | 3295 ** | 33.62 ** | 8.38 ** | 0.0020 * |
| Blocks | 2 | 0.004 * | 1.39 ns | 229 ns | 7.11 ns | 1.32 ns | 0.0006 ns |
| Residual | 38 | 0.001 | 0.87 | 769 | 12.09 | 2.50 | 0.0008 |
| CV (%) | 26.17 | 27.42 | 26.17 | 25.76 | 14.64 | 36.12 | |
| Source of Variation | DF | Mean Squares | ||||
|---|---|---|---|---|---|---|
| Chl a | Chl b | Chl a/Chl b | Chl T | Car | ||
| Salinity levels (ECw) | 4 | 74.34 ** | 8.76 ** | 0.45 ns | 132.43 ** | 6.00 ** |
| Linear regression | 1 | 277.3 ** | 33.88 ** | 0.04 ns | 504.99 ** | 23.46 ** |
| Quadratic regression | 1 | 15.16 ** | 0.02 ns | 1.46 * | 14.03 * | 0.10 ns |
| Proline (PROL) | 3 | 17.09 ** | 2.36 ** | 1.73 ** | 26.46 ** | 4.70 ** |
| Linear regression | 1 | 41.21 ** | 4.60 ** | 0.03 ns | 73.22 ** | 13.79 ** |
| Quadratic regression | 1 | 5.11 ns | 2.47 * | 4.48 ** | 0.47 ns | 0.00008 ns |
| Interaction (ECw × PROL) | 12 | 2.97 ns | 0.60 ns | 0.89 ns | 3.84 ns | 1.15 ** |
| Blocks | 2 | 0.21 ns | 0.26 ns | 0.11 ns | 0.02 ns | 0.53 |
| Residual | 38 | 2.12 | 0.46 | 0.29 | 3.03 | 0.40 |
| CV (%) | 13.13 | 15.92 | 20.50 | 11.32 | 13.15 | |
| Source of Variation | DF | Mean Squares | |||
|---|---|---|---|---|---|
| F0 | Fm | Fv | Fv/Fm | ||
| Salinity levels (ECw) | 4 | 5073 ** | 26,326 ** | 25,561 * | 0.014 ns |
| Linear regression | 1 | 20,254 ** | 75,075 ** | 49,227 * | 0.006 ns |
| Quadratic regression | 1 | 3.72 ns | 25,271 * | 50,596 * | 0.051 * |
| Proline (PROL) | 3 | 502 ns | 4951 ns | 16,240 ns | 0.023 ns |
| Linear regression | 1 | 1310 * | 8883 ns | 26,498 ns | 0.030 ns |
| Quadratic regression | 1 | 176 ns | 2554 ns | 5890 ns | 0.005 ns |
| Interaction (ECw × PROL) | 12 | 581 * | 3874 ns | 6013 ns | 0.009 ns |
| Blocks | 2 | 383 ns | 1649 ns | 3257 ns | 0.003 ns |
| Residual | 38 | 256 | 6021 | 7314 | 0.009 |
| CV (%) | 11.54 | 15.55 | 23.65 | 13.78 | |
| Source of Variation | DF | Mean Squares | |||||
|---|---|---|---|---|---|---|---|
| SD | Vcrown | VVI | Dcrown | AGRSD | RGRSD | ||
| Salinity levels (ECw) | 4 | 287.47 ** | 4.75 ** | 1.11 ** | 1.47 ** | 0.0021 ** | 0.000007 ns |
| Linear regression | 1 | 1016.40 ** | 17.91 ** | 4.00 ** | 5.69 ** | 0.0078 ** | 0.000003 ns |
| Quadratic regression | 1 | 115.46 ** | 0.48 ns | 0.14 ns | 0.19 ns | 0.0006 ns | 0.000000 ns |
| Proline (PROL) | 3 | 8.60 ns | 0.61 ns | 0.07 ns | 0.58 ** | 0.0011 * | 0.000003 * |
| Linear regression | 1 | 25.28 ns | 1.42 ns | 0.14 ns | 1.69 ** | 0.0021 * | 0.000003 ns |
| Quadratic regression | 1 | 0.03 ns | 0.35 ns | 0.0001 ns | 0.01 ns | 0.0012 ns | 0.000004 * |
| Interaction (ECw × PROL) | 12 | 14.93 ns | 1.40 ns | 0.30 ns | 0.44 ** | 0.0007 * | 0.000002 ns |
| Blocks | 2 | 12.85 ns | 0.09 ns | 0.07 ns | 0.05 ns | 0.0002 ns | 0.000002 ns |
| Residual | 38 | 8.57 | 0.83 | 0.16 | 0.06 ns | 0.0003 | 0.000008 |
| CV (%) | 11.44 | 26.14 | 14.20 | 15.22 | 35.58 | 34.50 | |
| Chemical characteristics | |||||||||
| pH (H2O) 1:2.5 | OM dag kg−1 | P (mg kg−1) | K+ | Na+ | Ca2+ | Mg2+ | Al3+ +H+ | ESP (%) | ECse (dS m−1) |
| (cmolc kg−1) | |||||||||
| 7.19 | 1.40 | 59.5 | 0.49 | 0.07 | 4.70 | 3.63 | 0 | 0.79 | 0.58 |
| Physical characteristics | |||||||||
| Particle-size fraction (g kg−1) | Textural class | Moisture (kPa) | AW | Total porosity | BD | PD | |||
| Sand | Silt | Clay | 33.43 * | 1519.8 ** | (kg dm3) | ||||
| ........... | dag kg−1 | .......... | % | ||||||
| 735.1 | 201.4 | 60.30 | SL | 15.78 | 6.41 | 9.37 | 55.05 | 1.20 | 2.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, S.T.d.A.; Lima, G.S.d.; Lima, V.L.A.d.; Nóbrega, J.S.; da Silva, S.S.; Ferreira, J.T.A.; Dantas, M.V.; Roque, I.A.; Soares, L.A.d.A.; Torres, R.A.F.; et al. Use of Proline to Induce Salt Stress Tolerance in Guava. Plants 2024, 13, 1887. https://doi.org/10.3390/plants13141887
Silva STdA, Lima GSd, Lima VLAd, Nóbrega JS, da Silva SS, Ferreira JTA, Dantas MV, Roque IA, Soares LAdA, Torres RAF, et al. Use of Proline to Induce Salt Stress Tolerance in Guava. Plants. 2024; 13(14):1887. https://doi.org/10.3390/plants13141887
Chicago/Turabian StyleSilva, Smyth Trotsk de Araújo, Geovani Soares de Lima, Vera Lúcia Antunes de Lima, Jackson Silva Nóbrega, Saulo Soares da Silva, Jean Telvio Andrade Ferreira, Maila Vieira Dantas, Iara Almeida Roque, Lauriane Almeida dos Anjos Soares, Rafaela Aparecida Frazão Torres, and et al. 2024. "Use of Proline to Induce Salt Stress Tolerance in Guava" Plants 13, no. 14: 1887. https://doi.org/10.3390/plants13141887





