Photosynthetic Acclimation of Larch to the Coupled Effects of Light Intensity and Water Deficit in Regions with Changing Water Availability
Abstract
:1. Introduction
2. Materials and Methods
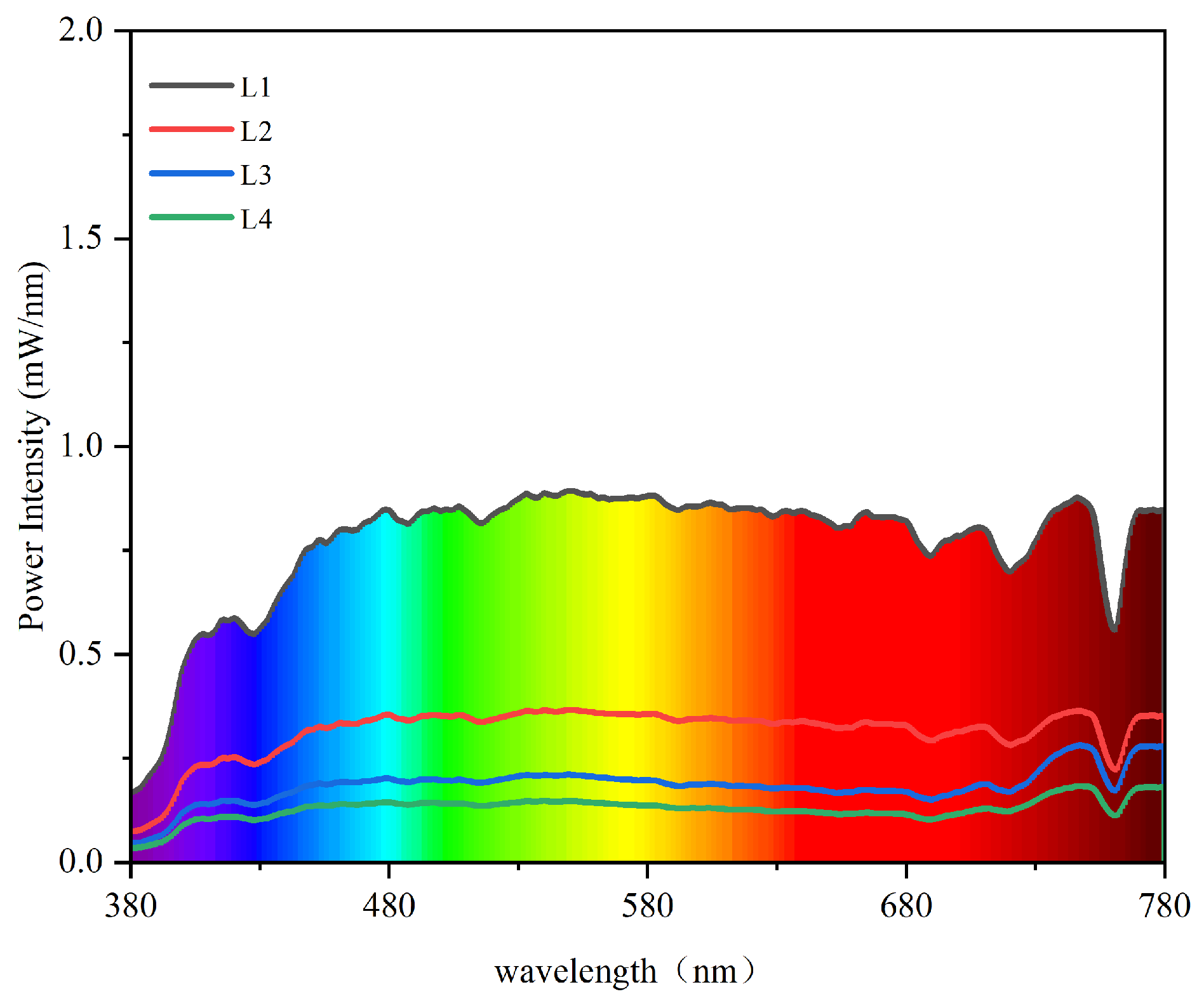
2.1. Plant Materials and Treatments
2.2. Measurement of Growth Response
2.3. Gas Exchange Measurements
2.4. Chlorophyll Contents Determination
2.5. Chlorophyll Fluorescence Characteristics Analysis
2.6. Determination of the Total Contents of Flavonoids and Phenols
2.7. LC-MS Analysis of Phenolic Compounds
2.8. Statistical Analysis
3. Results and Analysis
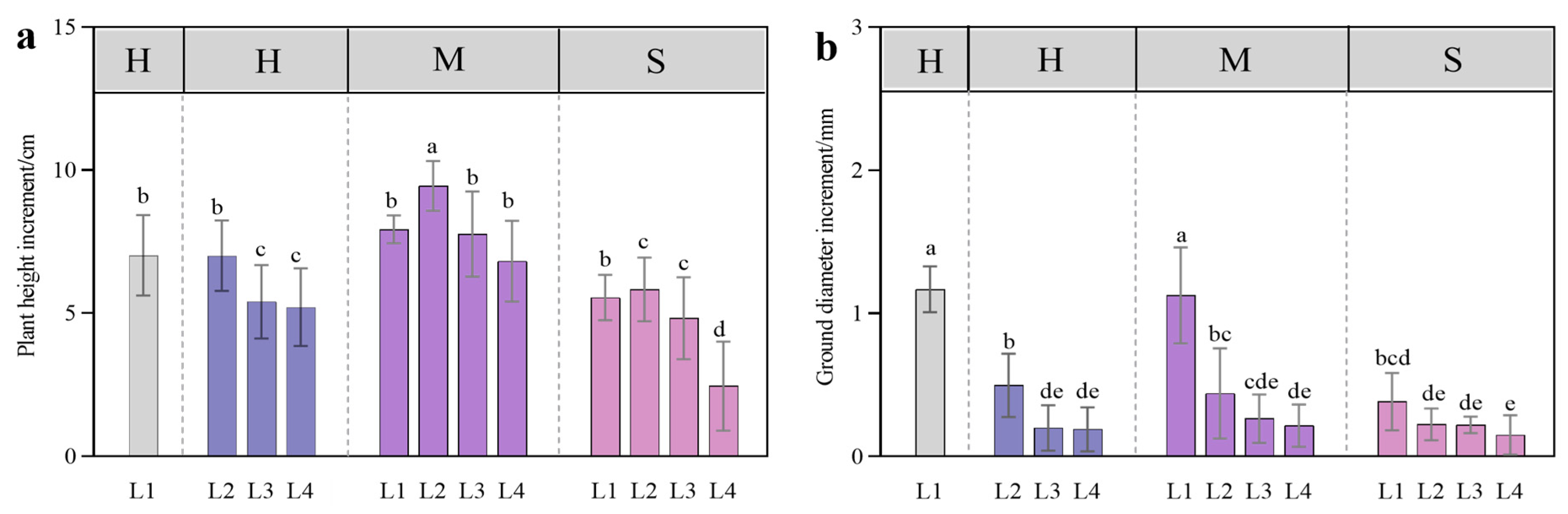
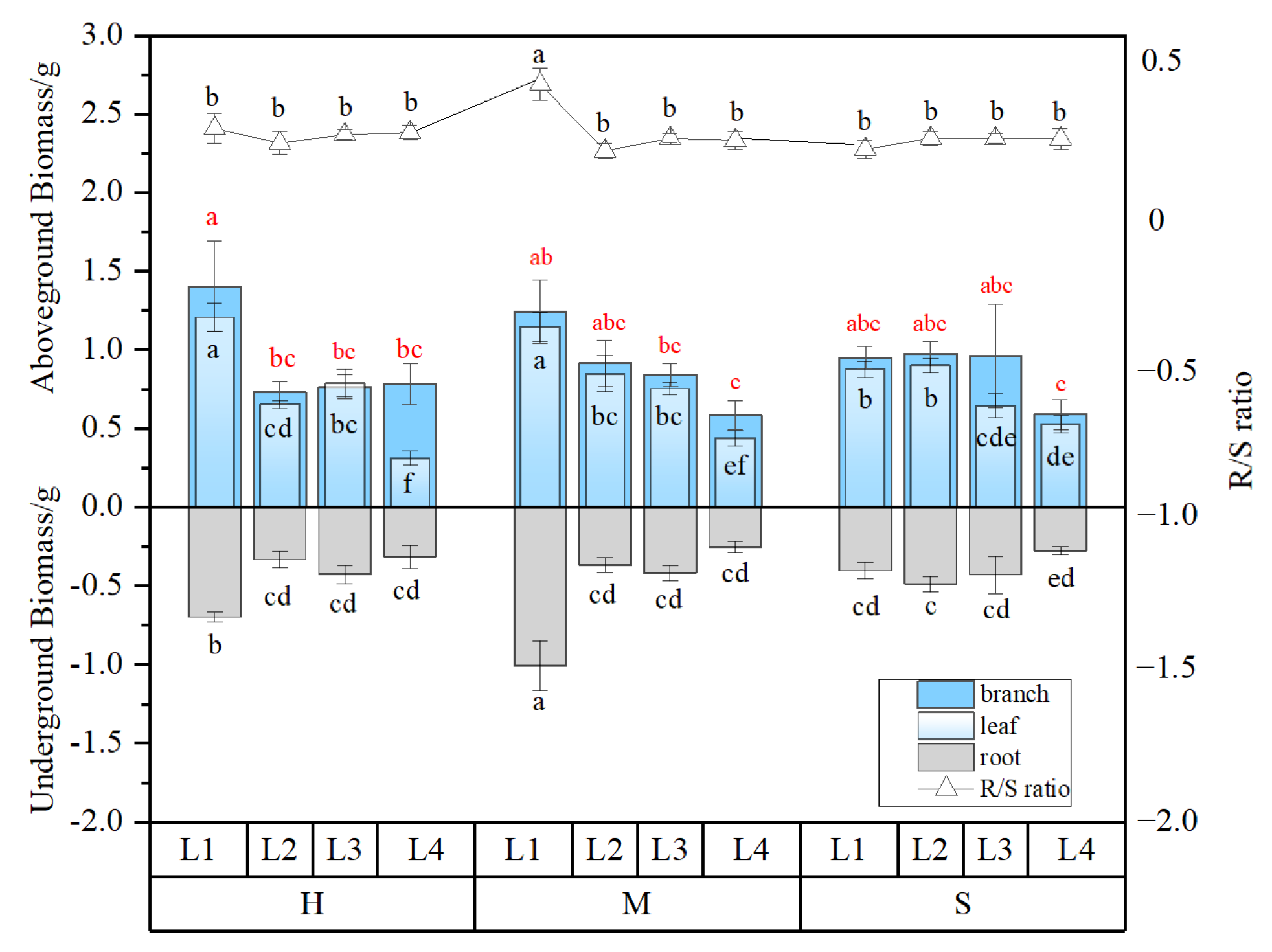
3.1. Plant Growth Response to Coupled Light Intensity and Water Conditions
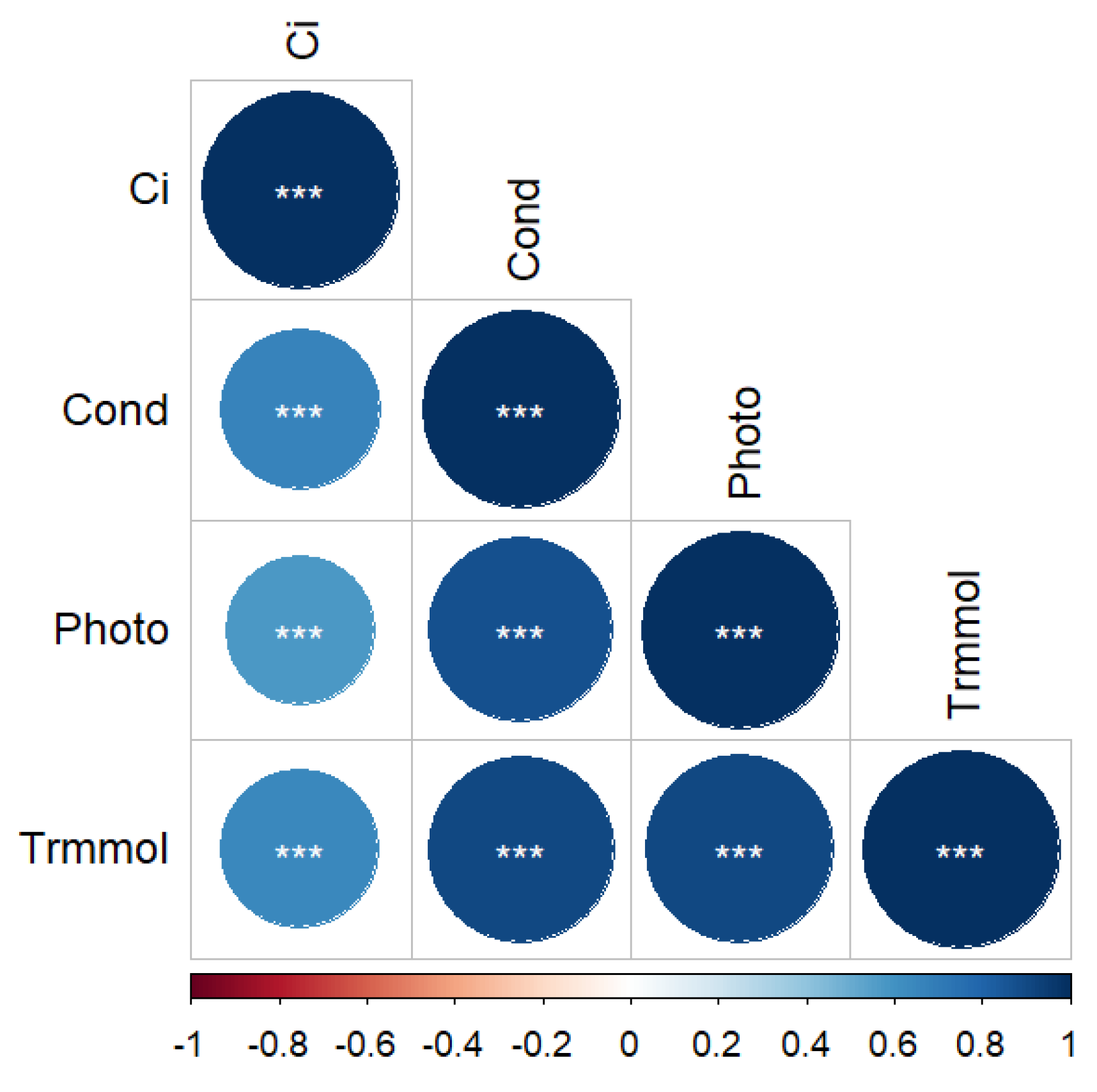
3.2. Gas Exchange Response to Coupled Light Intensity and Water Conditions
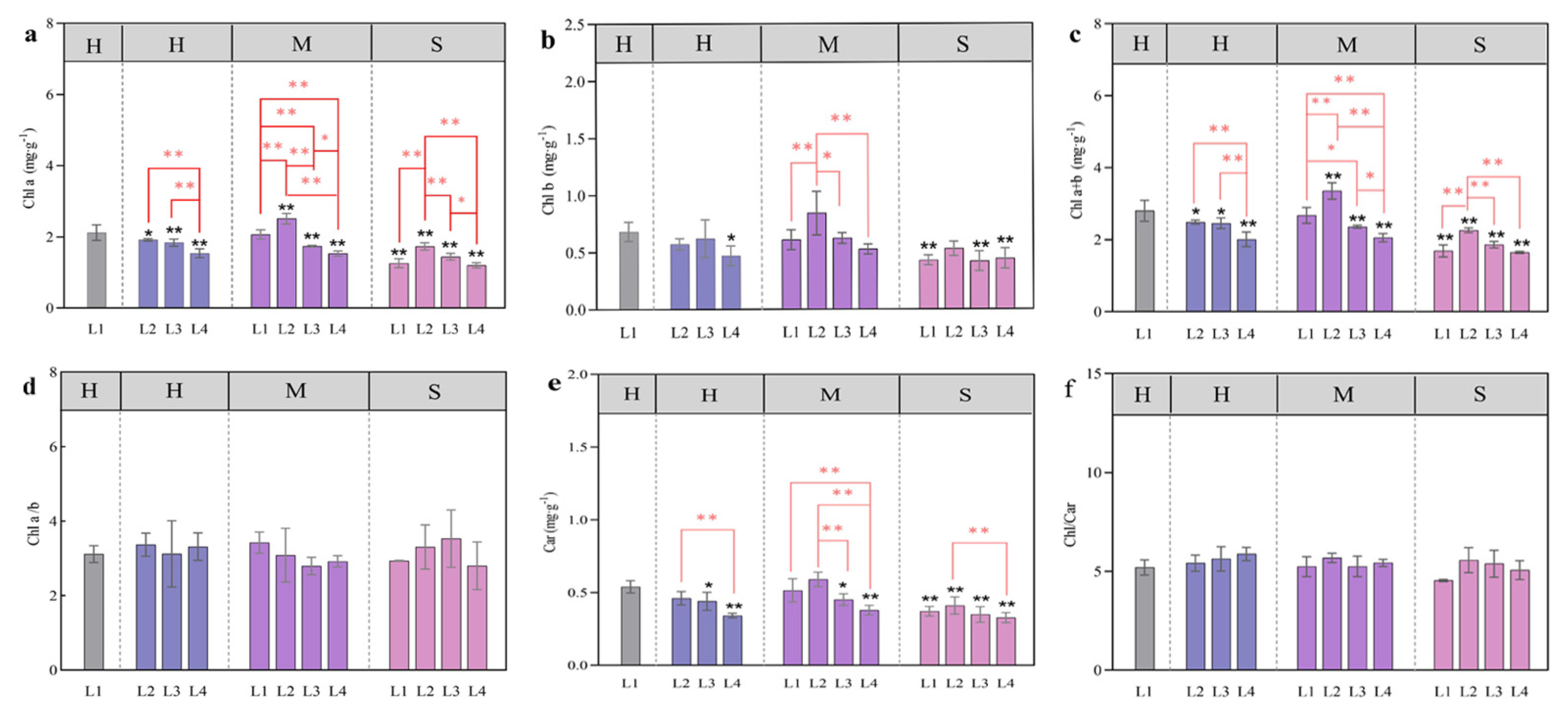
3.3. Changes in the Photosynthetic Pigment Content in Response to Coupled Light Intensity and Water Conditions
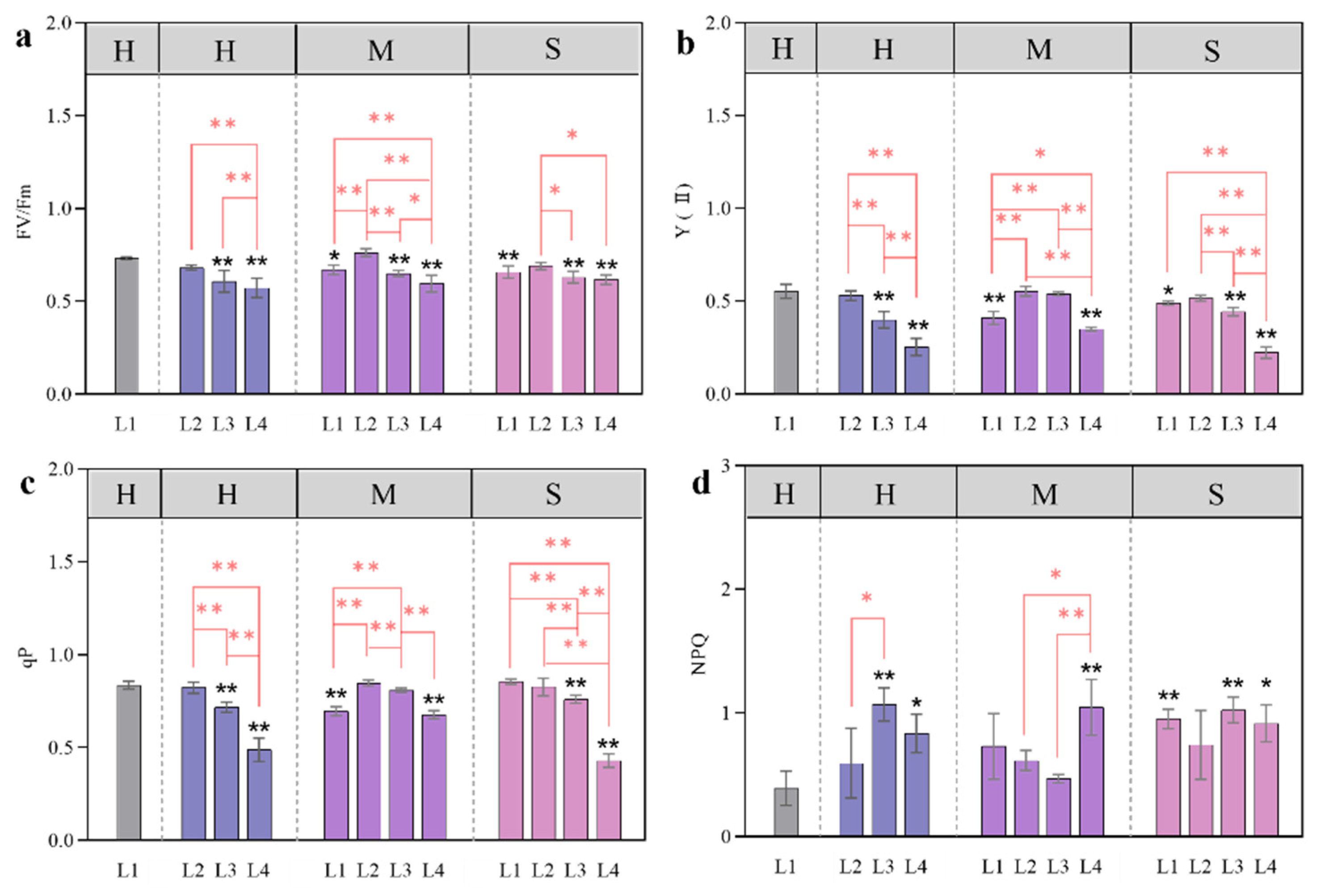
3.4. Changes in Chlorophyll Fluorescence in Response to Coupled Light Intensity and Water Conditions
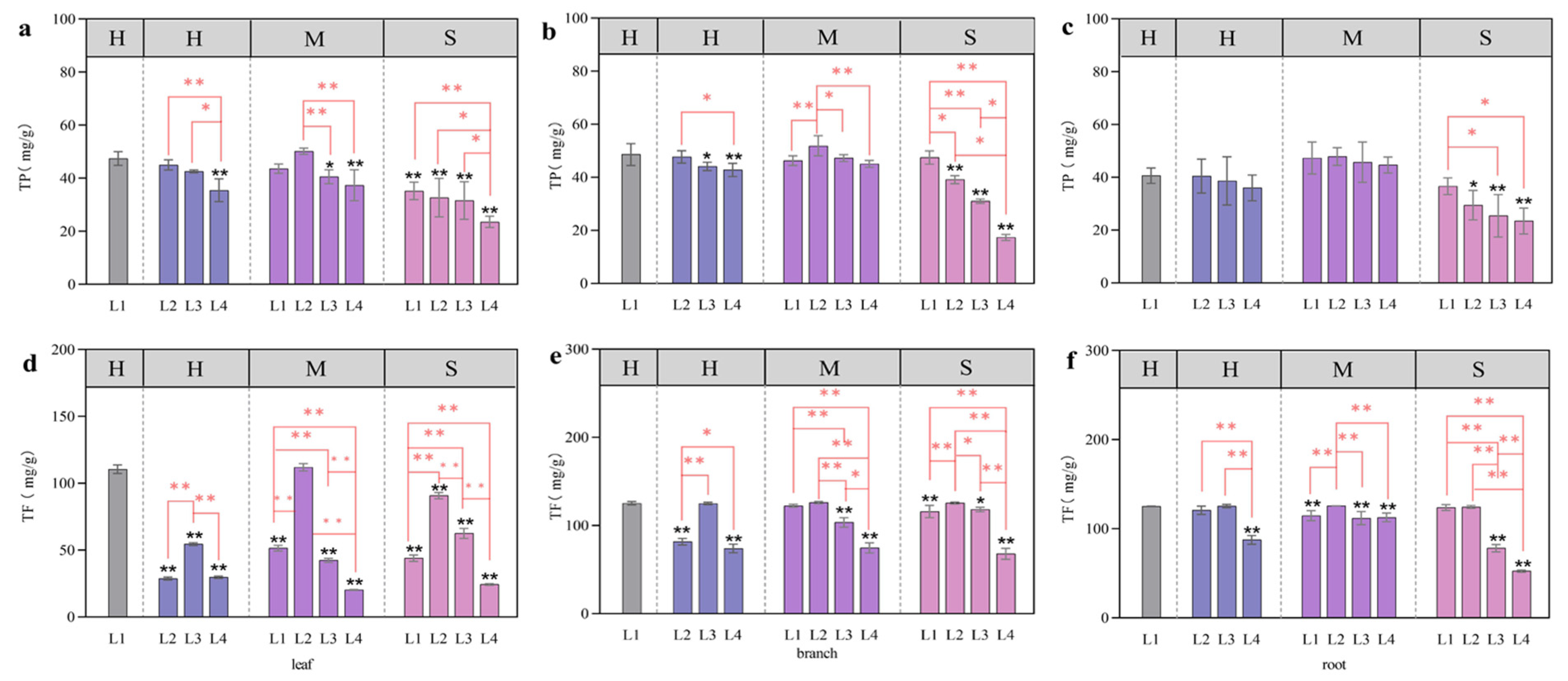
3.5. Total Phenols and Total Flavonoids Associated with the Coupling of Light Intensity and Water Conditions
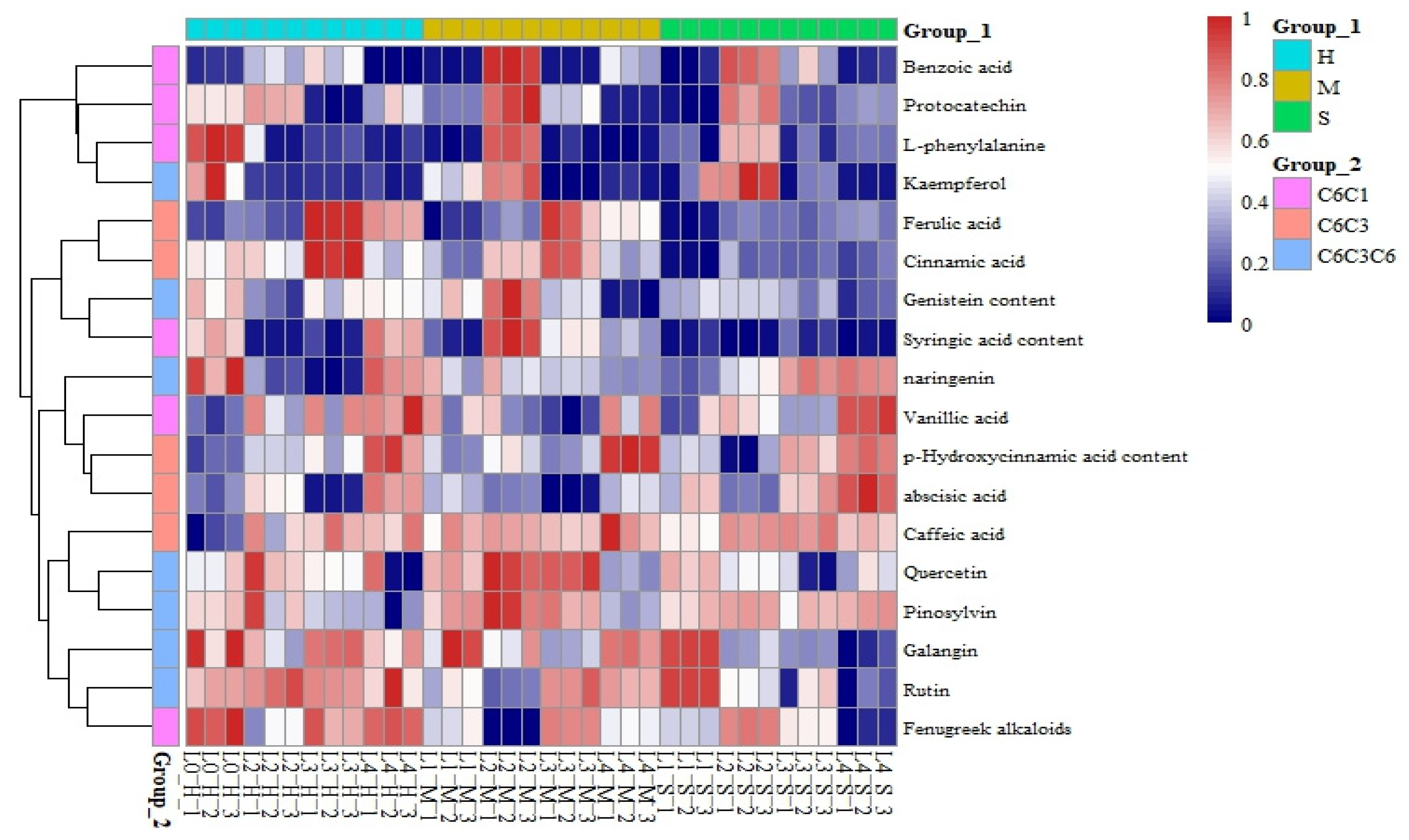
3.6. Responses of the Phenolic Compound Content to Coupled Light Intensity and Water Conditions
4. Discussion
4.1. Effects of Light and Water on Plant Growth and Photosynthesis
4.2. Effects of Light and Water on Photosynthetic Pigments and Chlorophyll Fluorescence
4.3. Effects of Light and Water on Total Phenols and Total Flavonoids
4.4. Effects of Light and Water on Phenolic Compounds
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Gauthier, S.; Bernier, P.; Kuuluvainen, T.; Shvidenko, A.Z.; Schepaschenko, D.G. Boreal forest health and global change. Science 2015, 349, 819–822. [Google Scholar] [CrossRef] [PubMed]
- Triviño, M.; Potterf, M.; Tijerín, J.; Ruiz-Benito, P.; Burgas, D.; Eyvindson, K.; Blattert, C.; Mönkkönen, M.; Duflot, R. Enhancing resilience of boreal forests through management under global change: A review. Curr. Landsc. Ecol. Rep. 2023, 8, 103–118. [Google Scholar] [CrossRef]
- Pohjanmies, T.; Triviño, M.; Le Tortorec, E.; Mazziotta, A.; Snäll, T.; Mönkkönen, M. Impacts of forestry on boreal forests: An ecosystem services perspective. Ambio 2017, 46, 743–755. [Google Scholar] [CrossRef]
- Huang, C.; Li, S.; He, H.S.; Liang, Y.; Xu, W.R.; Wu, M.M.; Wu, Z.W.; Huang, C.; Chen, F.S. Effects of forest management practices on carbon dynamics of China’s boreal forests under changing climates. J. Environ. Manag. 2023, 335, 9. [Google Scholar] [CrossRef] [PubMed]
- Astrup, R.; Bernier, P.Y.; Genet, H.; Lutz, D.A.; Bright, R.M. A sensible climate solution for the boreal forest. Nat. Clim. Change 2018, 8, 11–12. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, W.J.; Ballantyne, A.; He, H.S.; Wang, X.; Liu, S.; Ciais, P.; Wimberly, M.C.; Piao, S.; Yu, K. Forest disturbance decreased in China from 1986 to 2020 despite regional variations. Commun. Earth 2023, 4, 15. [Google Scholar] [CrossRef]
- Dong, L.-B.; Ma, R.; Tian, D.-Y.; Wang, T.; Liu, Z.-G. Structure and dynamics of co-dominant species in different succession stages of natural forests in Daxing’an Mountains. Chin. J. Ofapplied Ecol. 2022, 33, 2077–2087. [Google Scholar]
- Dulamsuren, C. Organic carbon stock losses by disturbance: Comparing broadleaved pioneer and late-successional conifer forests in Mongolia’s boreal forest. For. Ecol. Manag. 2021, 499, 8. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Xu, J.L.; Jiang, Y.; Mandra, T.; Rademacher, T.T.; Xue, F.; Dong, M.Y.; Pederson, N. Higher plasticity of water uptake in spruce than larch in an alpine habitat of North-Central China. Agric. For. Meteorol. 2021, 311, 15. [Google Scholar] [CrossRef]
- Ge, Z.X.; Man, X.L.; Cai, T.J.; Duan, B.X.; Xiao, R.H.; Xu, Z.P. Environmental Factors at Different Canopy Heights Had Significant Effects on Leaf Water-Use Efficiency in Cold-Temperate Larch Forest. Sustainability 2022, 14, 5126. [Google Scholar] [CrossRef]
- Gao, R.M.; Shi, X.D.; Wang, J.R. Comparative studies of the response of larch and birch seedlings from two origins to water deficit. N. Z. J. Forest. Sci. 2017, 47, 11. [Google Scholar] [CrossRef]
- Yan, Q.L.; Gang, Q.; Zhu, J.J.; Sun, Y.R. Variation in survival and growth strategies for seedlings of broadleaved tree species in response to thinning of larch plantations: Implication for converting pure larch plantations into larch-broadleaved mixed forests. Environ. Exp. Bot. 2016, 129, 108–117. [Google Scholar] [CrossRef]
- Poorter, L.; Kitajima, K. Carbohydrate storage and light requirements of tropical moist and dry forest tree species. Ecology 2007, 88, 1000–1011. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Wang, B.; Han, S.; Liu, Y.; Ma, X.; Zhang, C.; Tie, N. Photosynthetic Characteristics of Larix gmelinii Seedlings under Different Light Intensity. Appl. Ecol. Environ. Res. 2023, 21, 4117–4134. [Google Scholar] [CrossRef]
- Becker, P. Competition in the regeneration niche between conifers and angiosperms: Bond’s slow seedling hypothesis. Funct. Ecol. 2000, 14, 401–412. [Google Scholar] [CrossRef]
- Bai, X.P.; Zhang, X.L.; Li, J.X.; Duan, X.Y.; Jin, Y.T.; Chen, Z.J. Altitudinal disparity in growth of Dahurian larch (Larix gmelinii Rupr.) in response to recent climate change in northeast China. Sci. Total Environ. 2019, 670, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wei, F.L.; Fu, B.J.; Zhang, W.M.; Sun, C.L. Ecosystems in China have become more sensitive to changes in water demand since 2001. Commun. Earth Environ. 2023, 4, 8. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, Y.; Li, Z.; Han, S.; Wang, X. Climate change increased the intrinsic water use efficiency of Larix gmelinii in permafrost degradation areas, but did not promote its growth. Agric. For. Meteorol. 2022, 320, 108957. [Google Scholar] [CrossRef]
- Wang, W.-J.; He, H.S.; Yu, G.; Wen-Xin, L.I.; Zhong-Hua, Z.; Yuan-Gang, Z.U. Methodological Comparison of Chlorophyll and Carotenoids Contents of Plant Species Measured by DMSO and Acetone-extraction Methods. Bull. Bot. Res. 2009, 29, 224–229. [Google Scholar]
- Wolfe, K.; Wu, X.; Liu, R.H. Antioxidant Activity of Apple Peels. J. Agric. Food Chem. 2003, 51, 609–614. [Google Scholar] [CrossRef]
- Pastrana-Bonilla, E.; Akoh, C.C.; Sellapan, S.; Krewer, G. Phenolic Content and Antioxidant Capacity of Muscadine Grapes. J. Agric. Food Chem. 2003, 51, 5497–5503. [Google Scholar] [CrossRef]
- Liu, Y. Comparative metabolomics study on the metabolic basis of two varieties of astragalus in response to UV-B and drought stress. Northeast. For. Univ. 2018, 176, 104094. [Google Scholar]
- Lin, J.; Zhang, R.; Hu, Y.; Song, Y.; Hänninen, H.; Wu, J. Interactive effects of drought and shading on Torreya grandis seedlings: Physiological and growth responses. Trees 2019, 33, 951–961. [Google Scholar] [CrossRef]
- Guo, X.; Guo, W.H.; Luo, Y.J.; Tan, X.F.; Du, N.; Wang, R.Q. Morphological and biomass characteristic acclimation of trident maple (Acer buergerianum Miq.) in response to light and water stress. Acta Physiol. Plant. 2013, 35, 1149–1159. [Google Scholar] [CrossRef]
- Chen, B.X.; Li, Y.B.; Liu, G.L.; Fan, S.H.; Yang, H.J.; Su, H.R. Effects of the interaction between shade and drought on physiological characteristics in Calamus viminalis seedlings. Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 305–317. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhao, F.; Zhang, H.H.; Zhu, Z.L. Responses of the growth, photosynthetic characteristics, endogenous hormones and antioxidant activity of Carpinus betulus L. seedlings to different light intensities. Front. Plant Sci. 2022, 13, 18. [Google Scholar] [CrossRef]
- Liu, C.; Tian, T.; Li, S.; Wang, F.; Liang, Y. Growth response of Chinese woody plant seedlings to different light intensities. Acta Ecol. Sin. 2018, 38, 518–527. [Google Scholar]
- Haoyun, W.; Feng, W.; Min, L.; Xiaokun, Z.; Changshuang, S.; Guijie, D. Morphological and Physiological Responses of Pinus massoniana Seedlings to Different Light Gradients. Forests 2021, 12, 523. [Google Scholar] [CrossRef]
- Agathokleous, E.; Feng, Z.Z.; Peñuelas, J. Chlorophyll hormesis: Are chlorophylls major components of stress biology in higher plants? Sci. Total Environ. 2020, 726, 9. [Google Scholar] [CrossRef]
- Li, J.; Zeng, S. Response of physiological indicators and photosynthetic characteristics of Larix gmelinii to different light environments. Jiangsu Agric. Sci. 2021, 49, 149–153. [Google Scholar]
- Liu, X. Effects of Shading Treatments on Growth of Larix principis-rupprechtii Seedlings. Shanxi For. Sci. Technol. 2012, 41, 31–33. [Google Scholar]
- Liu, W.; Yang, Q.; Qiu, Z.; Zhao, J. Effects of light intensity and nutrient addition on growth, photosynthetic pigments and nutritional quality of pea seedlings. In Proceedings of the International Symposium on New Technologies for Environment Control, Energy-Saving and Crop Production in Greenhouse and Plant, Jeju, Republic of Korea, 6–11 October 2013; Volume 1037, pp. 391–396. [Google Scholar]
- Chai, S.F.; Tang, J.M.; Mallik, A.; Shi, Y.C.; Zou, R.; Li, J.T.; Wei, X. Eco-physiological basis of shade adaptation of Camellia nitidissima, a rare and endangered forest understory plant of Southeast Asia. BMC Ecol. 2018, 18, 10. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Dong, B.D.; Wang, Y.K.; Qiao, Y.Z.; Shi, C.H.; Jin, L.L.; Liu, M.Y. Photosynthetic base of reduced grain yield by shading stress during the early reproductive stage of two wheat cultivars. Sci. Rep. 2020, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, M.M.T.; Albano-Machado, F.G.; Penha, D.M.; Pinho, M.M.; Natale, W.; de Miranda, M.R.A.; Moura, C.F.H.; Alves, R.E.; Corrêa, M.C.D. Shade improves growth, photosynthetic performance, production and postharvest quality in red pitahaya (Hylocereus costaricensis). Sci. Hortic. 2021, 286, 10. [Google Scholar] [CrossRef]
- Pashkovskiy, P.P.; Soshinkova, T.N.; Korolkova, D.V.; Kartashov, A.V.; Zlobin, I.E.; Lyubimov, V.Y.; Kreslavski, V.D.; Kuznetsov, V.V. The effect of light quality on the pro-/antioxidant balance, activity of photosystem II, and expression of light-dependent genes in Eutrema salsugineum callus cells. Photosynth. Res. 2018, 136, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-R. A discussion on chlorophyll fluorescence kinetics parameters and their significance. Chin. Bull. Bot. 1999, 16, 444. [Google Scholar]
- Ahmed, I.M.; Nadira, U.A.; Bibi, N.; Cao, F.B.; He, X.Y.; Zhang, G.P.; Wu, F.B. Secondary metabolism and antioxidants are involved in the tolerance to drought and salinity, separately and combined, in Tibetan wild barley. Environ. Exp. Bot. 2015, 111, 1–12. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, J.; Zhang, W.; Pan, X.J. Shading in fruit changes the polyphenol accumulation of pellicle by regulating activity of key enzymes and expression of their gene related polyphenol anabolism of Juglans sigillata Dode. Sci. Hortic. 2024, 329, 11. [Google Scholar] [CrossRef]
- Wu, Y.Q.; Yang, H.; Huang, Z.J.; Lyu, L.; Wu, W.L.; Li, W.L. Effect of Shade Strength on the Fruit Quality of Different Blueberry Cultivars. J. Soil. Sci. Plant Nutr. 2023, 23, 4127–4140. [Google Scholar] [CrossRef]
- Xu, H.L.; Liu, J.M.; Fan, X.F.; Xu, W.; Fan, S.M. Effects of Different Light Intensity on Growth and Total Flavonoids of Tetrastigma hemsleyanum. Mod. Chin. Med. 2020, 22, 1866–1870. [Google Scholar]
- Pan, J.Q.; Guo, B.L. Effects of Light Intensity on the Growth, Photosynthetic Characteristics, and Flavonoid Content of Epimedium pseudowushanense BLGuo. Molecules 2016, 21, 1475. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wu, K.; Liu, Y.; Liu, J.; Tang, Z. Effects of light intensity on the growth, photosynthetic characteristics, and secondary metabolites of Eleutherococcus senticosus Harms. Photosynthetica 2020, 58, 881–889. [Google Scholar] [CrossRef]
- Dey, G.; Chakraborty, M.; Mitra, A. Profiling C6-C3 and C6-C1 phenolic metabolites in Cocos nucifera. J. Plant Physiol. 2005, 162, 375–381. [Google Scholar] [CrossRef]
- Yan, J.; Lu, Y.; Yan, S. The effects of irradiance on the production of phenolic compounds and condensed tannins in Larix gmelinii needles. Biol. Plant. 2014, 58, 159–163. [Google Scholar] [CrossRef]

| Light Conditions | Water Conditions | Mark |
|---|---|---|
| natural sunlight | 80% soil saturated water content | L1-H |
| natural sunlight | 60% soil saturated water content | L1-M |
| natural sunlight | 40% soil saturated water content | L1-S |
| 50% shading | 80% soil saturated water content | L2-H |
| 50% shading | 60% soil saturated water content | L2-M |
| 50% shading | 40% soil saturated water content | L2-S |
| 75% shading | 80% soil saturated water content | L3-H |
| 75% shading | 60% soil saturated water content | L3-M |
| 75% shading | 40% soil saturated water content | L3-S |
| 90% shading | 80% soil saturated water content | L4-H |
| 90% shading | 60% soil saturated water content | L4-M |
| 90% shading | 40% soil saturated water content | L4-S |
| Variables | F-Values and Significant Levels | ||
|---|---|---|---|
| Water | Light | Water × Light | |
| plant height increment | 71.99 ** | 24.44 ** | 2.28 * |
| ground diameter increment | 29.92 ** | 82.24 ** | 10.57 ** |
| biomass—branch | 0.10 NS | 5.8 ** | 1.13 NS |
| biomass—root | 0.9 NS | 45.06 ** | 4.30 ** |
| biomass—leaf | 2.5 NS | 18.40 ** | 5.50 ** |
| R/S | 1.2 NS | 2.2 NS | 2.64 * |
| Pn | 413.35 ** | 42.03 ** | 10.36 ** |
| Gs | 46.95 ** | 20.2 ** | 5.47 ** |
| Ci | 13.21 ** | 82.95 ** | 5.74 ** |
| Tr | 1850.53 ** | 504.32 ** | 150.24 ** |
| Chl a | 78.14 ** | 47.42 ** | 10.27 ** |
| Chl b | 12.42 ** | 4.56 * | 2.29 NS |
| Car | 18.93 ** | 15.52 ** | 2.50 NS |
| Chl a+b | 71.66 ** | 38.78 ** | 9.58 ** |
| Chl a/b | 0.35 NS | 0.34 NS | 1 NS |
| Chl/Car | 2.33 NS | 2.65 NS | 1.03 NS |
| Fv/Fm | 1.69 NS | 23.88 ** | 3.67 ** |
| qP | 6.80 ** | 171.37 ** | 25.00 ** |
| NPQ | 2.49 NS | 2.74 ** | 2.77 ** |
| Y(II) | 7.50 ** | 137.40 ** | 15.53 ** |
| TP—leaf | 36.02 ** | 13.6 ** | 0.886 NS |
| TP—branch | 16.85 ** | 9.06 ** | 2.93 * |
| TP—root | 28.04 ** | 2.38 NS | 6.31 NS |
| TF—leaf | 7.94 ** | 293.86 ** | 57.13 ** |
| TF—branch | 0.04 NS | 22.15 ** | 16.74 ** |
| TF—root | 7.96 ** | 13.74 ** | 4.63 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, L.; Song, X.; Shi, Y.; Guan, X.; Tang, H.; Huang, H.; Chen, J.; Zhang, Z.; Tang, Z. Photosynthetic Acclimation of Larch to the Coupled Effects of Light Intensity and Water Deficit in Regions with Changing Water Availability. Plants 2024, 13, 1891. https://doi.org/10.3390/plants13141891
Jin L, Song X, Shi Y, Guan X, Tang H, Huang H, Chen J, Zhang Z, Tang Z. Photosynthetic Acclimation of Larch to the Coupled Effects of Light Intensity and Water Deficit in Regions with Changing Water Availability. Plants. 2024; 13(14):1891. https://doi.org/10.3390/plants13141891
Chicago/Turabian StyleJin, Lu, Xiaoqian Song, Yu Shi, Xin Guan, Huimin Tang, Haiyan Huang, Jiaqi Chen, Zhonghua Zhang, and Zhonghua Tang. 2024. "Photosynthetic Acclimation of Larch to the Coupled Effects of Light Intensity and Water Deficit in Regions with Changing Water Availability" Plants 13, no. 14: 1891. https://doi.org/10.3390/plants13141891
APA StyleJin, L., Song, X., Shi, Y., Guan, X., Tang, H., Huang, H., Chen, J., Zhang, Z., & Tang, Z. (2024). Photosynthetic Acclimation of Larch to the Coupled Effects of Light Intensity and Water Deficit in Regions with Changing Water Availability. Plants, 13(14), 1891. https://doi.org/10.3390/plants13141891







