Cassava Breeding and Cultivation Challenges in Thailand: Past, Present, and Future Perspectives
Abstract
:1. Introduction: Cassava Domestication and Cultivation Worldwide and in Thailand
2. Global Cassava Genetic Resources
3. Special Features of Cassava Physiology, Reproductive Biology, Roots and Starch
4. Cassava Breeding in Thailand
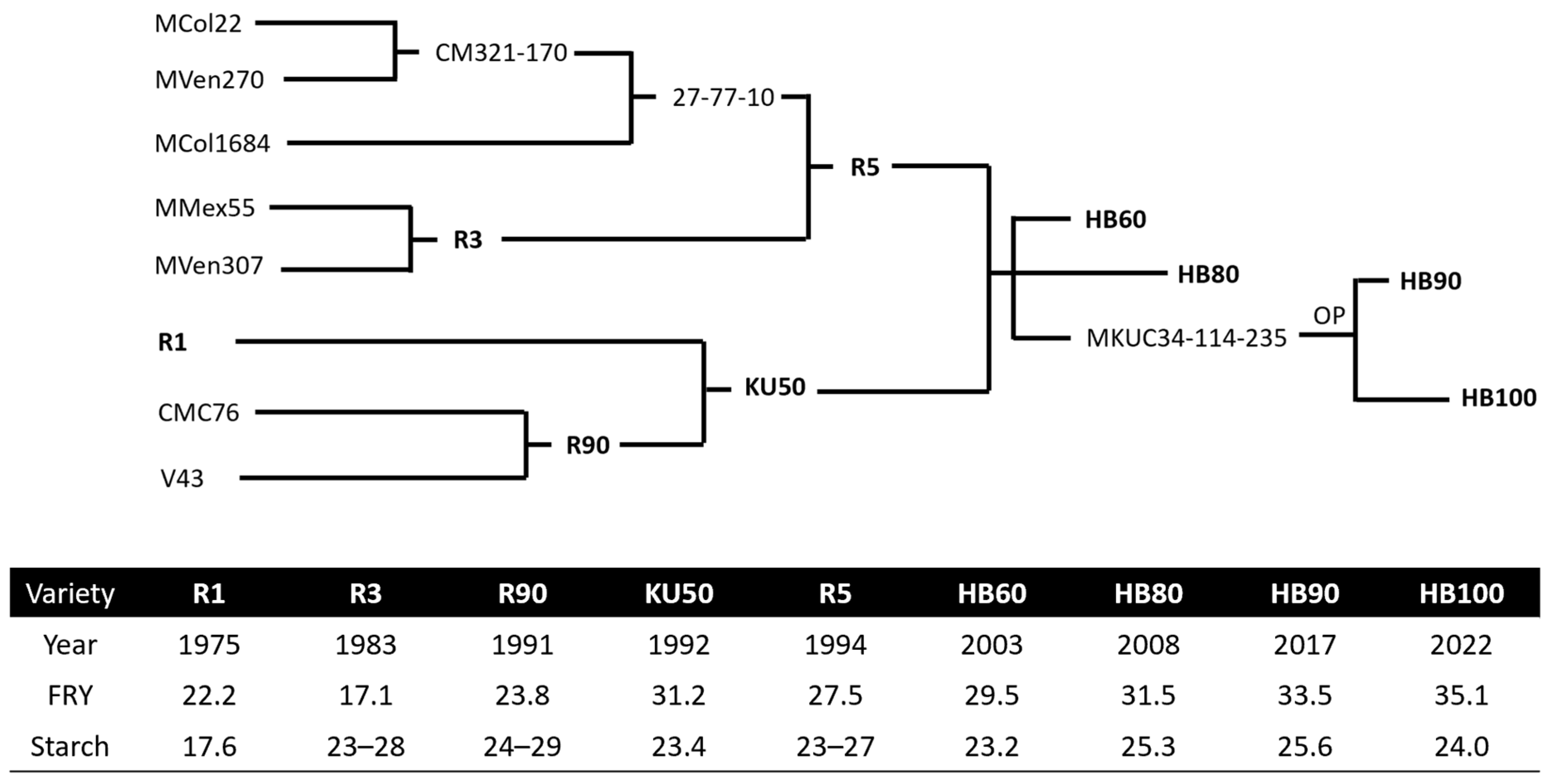
5. Released Commercial Cassava Varieties in Thailand
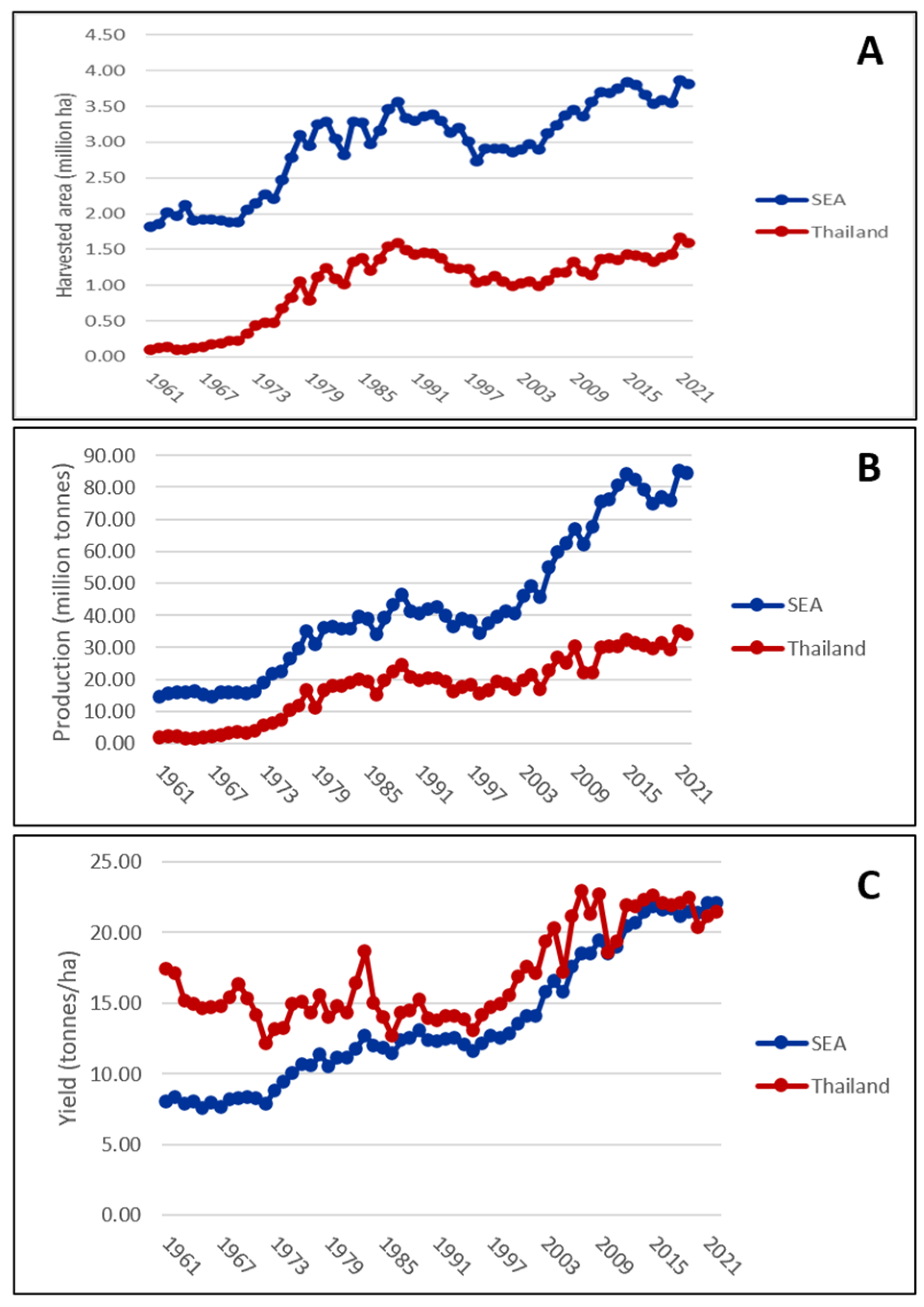
6. The Impact of KU50 in Thailand and Southeast Asia
7. Determinants of Cassava Yield and Production Quality
7.1. Yield Stability
7.2. Environmental Factors Determining Cassava Yield and Starch Production
7.3. Biotic Factors Determining Cassava Yield and Starch Production
7.4. Starch Quality Traits
8. Future of Cassava Breeding Direction
8.1. Efficient Exploitation of Heterosis and Non-Additive Genetic Variation
8.2. The Advantage of (Partially) Inbred over Non-Inbred Progenitors
8.3. Marker Assisted Breeding in Cassava
8.4. CRISPR/Cas9-Mediated Gene Editing in Cassava
8.5. The Induction of Flowering
8.6. Improved Phenotyping Tools
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rogers, D.J.; Appan, S.G. Manihot, Manihotoides (Euphorbiaceae); Flora Neotropica. Monograph No. 13; Hafner Press: New York, NY, USA, 1973. [Google Scholar]
- Lebot, V. Tropical Root and Tuber Crops: Cassava, Sweet Potato, Yams and Aroids; Crop Production Science in Horticulture. No. 17; CABI Publishing: Wallingford, UK, 2009; 413p. [Google Scholar]
- Olsen, K.M.; Schaal, B.A. Evidence on the origin of cassava: Phylogeography of Manihot esculenta. Proc. Natl. Acad. Sci. USA 1999, 96, 5586–5591. [Google Scholar] [CrossRef] [PubMed]
- Allem, A.C. The origins and taxonomy of cassava. In Cassava: Biology, Production and Utilization; Hillocks, R.J., Thresh, J.M., Bellotti, A.C., Eds.; CABI Publishing: Wallingford, UK; New York, NY, USA, 2002; pp. 1–16. [Google Scholar]
- Watling, J.; Shock, M.P.; Mongeló, G.Z.; Almeida, F.O.; Kater, T.; De Oliveira, P.E.; Neves, E.G. Direct archaeological evidence for Southwestern Amazonia as an early plant domestication and food production centre. PLoS ONE 2018, 13, e0199868. [Google Scholar] [CrossRef] [PubMed]
- Léotard, G.; Duputié, A.; Kjellberg, F.; Douzery, E.J.P.; Debain, C.; de Granville, J.-J.; McKey, D. Phylogeography and the origin of cassava: New insights from the northern rim of the Amazonian basin. Mol. Phylogenet. Evol. 2009, 53, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Ogbonna, A.C.; Braatz de Andrade, L.R.; Mueller, L.A.; de Oliveira, E.J.; Bauchet, G.J. Comprehensive genotyping of a Brazilian cassava (Manihot esculenta Crantz) germplasm bank: Insights into diversification and domestication. Theor. Appl. Genet. 2021, 134, 1343–1362. [Google Scholar] [CrossRef]
- Lyons, J.B.; Bredeson, J.V.; Mansfeld, B.N.; Bauchet, G.J.; Berry, J.; Boyher, A.; Mueller, L.A.; Rokhsar, D.S.; Bart, R.S. Current status and impending progress for cassava structural genomics. Plant Mol. Biol. 2022, 109, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Byrne, D. Breeding cassava. Plant Breed. Rev. 1984, 2, 73–134. [Google Scholar]
- Howeler, R.; NeBambi, L.; Thomas, G. Save and Grow: Cassava; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013; p. 1299. [Google Scholar]
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#data (accessed on 5 April 2024).
- Kawano, K. Thirty years of cassava breeding for productivity-biological and social factors for success. Crop Sci. 2003, 43, 1325–1335. [Google Scholar] [CrossRef]
- Genesys. Available online: https://www.genesys-pgr.org/ (accessed on 5 April 2024).
- Hershey, C.H. A Global Conservation Strategy for Cassava (Manihot esculenta) and Wild Manihot Species. Summary of Stakeholder Deliberations and Recommendations Prepared for the Global Crop Diversity Trust. 2008. Available online: https://cgspace.cgiar.org (accessed on 5 April 2024).
- Carvajal-Yepes, M.; Ospina, J.A.; Aranzales, E.; Velez-Tobon, M.; Correa Abondano, M.; Manrique-Carpintero, N.C.; Wenzl, P. Identifying genetically redundant accessions in the world’s largest cassava collection. Front. Plant Sci. 2024, 14, 1338377. [Google Scholar] [CrossRef] [PubMed]
- Alliance Biodiversity & CIAT. Available online: https://alliancebioversityciat.org/ (accessed on 5 April 2024).
- Ceballos, H.; Iglesias, C.A.; Pérez, C.; Dixon, A.G.O. Cassava breeding: Opportunities and Challenges. Plant Mol. Biol. 2004, 56, 503–516. [Google Scholar] [CrossRef]
- Sánchez, T.; Mafla, G.; Morante, N.; Ceballos, H.; Dufour, D.; Calle, F.; Moreno, X.; Pérez, J.C.; Debouck, D. Screening of starch quality traits in cassava (Manihot esculenta Crantz). Starch/Stärke 2009, 61, 12–19. [Google Scholar] [CrossRef]
- Ceballos, H.; Hershey, C.; Becerra-López-Lavalle, L.A. New approaches to cassava breeding. Plant Breed. Rev. 2012, 36, 427–504. [Google Scholar]
- Ferguson, M.E.; Shah, T.; Kulakow, P.; Ceballos, H. A global overview of cassava genetic diversity. PLoS ONE 2019, 14, e0224763. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.I.; Kongsil, P.; Nguyễn, V.A.; Ou, W.; Sholihin, S.P.; Sheela, M.; Becerra López-Lavalle, L.A.; Utsumi, Y.; Lu, C.; Kittipadakul, P.; et al. Cassava breeding and agronomy in Asia: 50 years of history and future directions. Breed. Sci. 2020, 70, 145–166. [Google Scholar] [CrossRef] [PubMed]
- Sarakarn, S.; Limsila, A.; Watananonta, W.; Suparhan, D.; Suriyapan, P. Cassava breeding and varietal dissemination in Thailand: Major achievements during the past 25 years. In Cassava’s Potential in Asia in the 21st Century: Present Situation and Future Research and Development Needs, Proceedings of the Sixth Regional Workshop, Ho Chi Minh City, Vietnam, 21–25 February 2000; Howeler, R., Tan, S.L., Eds.; Centro Internacional de Agricultura Tropical (CIAT), Cassava Office for Asia: Cali, CO, USA, 2001; pp. 161–166. [Google Scholar]
- Kittipadakul, P.; Kongsil, P.; Phumichai, C.; Jansky, S.H. Chapter 7: Breeding cassava for higher yield. In Achieving Sustainable Cultivation of Cassava Volume 2: Genetics, Breeding, Pests, and Diseases; Hershey, C., Ed.; Burleigh Dodds Science Publishing: Cambridge, UK, 2017; pp. 139–170. [Google Scholar]
- Xiong, L.; Kaimian, L.; Yinong, T.; Jie, H.; Ruili, X. A historical account of progress made in cassava varietal improvement in China. In Cassava’s Potential in Asia in the 21st Century: Present Situation and Future Research and Development Needs, Proceedings of the Sixth Regional Workshop, Ho Chi Minh City, Vietnam, 21–25 February 2001; Howeler, R., Tan, S.L., Eds.; Centro Internacional de Agricultura Tropical (CIAT), Cassava Office for Asia: Cali, CO, USA, 2001; pp. 185–192. [Google Scholar]
- Alves, A.A.C. Cassava botany and physiology. In Cassava: Biology, Production and Utilization; Hillocks, R.J., Thresh, J.M., Bellotti, A.C., Eds.; CABI Publishing: New York, NY, USA, 2002; pp. 67–89. [Google Scholar]
- Kawano, K. Cassava. In Hybridization of Crop Plants; Fehr, W.R., Hadley, H.H., Eds.; American Society of Agronomy–Crop Science Society of America: Madison, WI, USA, 1980; pp. 225–233. [Google Scholar]
- Ramos Abril, L.N.; Pineda, L.M.; Wasek, I.; Wedzony, M.; Ceballos, H. Reproductive biology in cassava: Stigma receptivity and pollen tube growth. Commun. Integr. Biol. 2019, 12, 96–111. [Google Scholar] [CrossRef]
- Contreras-Rojas, M.; Pérez, J.C.; Ceballos, H.; Baena, D.; Morante, N.; Calle, F. Introduction of inbreeding and analysis of inbreeding depression in eight S1 cassava families. Crop Sci. 2009, 49, 543–548. [Google Scholar] [CrossRef]
- de Freitas, J.P.X.; da Silva Santos, V.; de Oliveira, E.J. Inbreeding depression in cassava for productive traits. Euphytica 2016, 209, 137–145. [Google Scholar] [CrossRef]
- Kaweesi, T.; Kyaligonza, V.; Baguma, Y.; Kawuki, R.; Ferguson, M. Inbreeding enhances field resistance to cassava brown streak viruses. J. Plant Breed. Crop Sci. 2016, 8, 138–149. [Google Scholar] [CrossRef]
- Kawuki, R.S.; Nuwamanya, E.; Labuschagne, M.T.; Herselman, L.; Ferguson, M.E. Segregation of selected agronomic traits in six S1 cassava families. J. Plant Breed. Crop Sci. 2011, 3, 154–160. [Google Scholar]
- Sheela, M.N.; Radhika, V.S.; John, K.S.; Abraham, K. Variation in crude protein, dry matter and starch in inbred and backcross lines of cassava. J. Root Crops 2008, 34, 115–119. [Google Scholar]
- Jennings, D.L.; Iglesias, C.A. Breeding for crop improvement. In Cassava: Biology, Production and Utilization; Hillocks, R.J., Thresh, J.M., Bellotti, A.C., Eds.; CABI Publishing: Wallingford, UK, 2002; pp. 149–166. [Google Scholar]
- Ceballos, H.; Kulakow, P.; Hershey, C. Cassava breeding: Current status, bottlenecks and the potential of biotechnology tools. Trop. Plant Biol. 2012, 5, 73–87. [Google Scholar] [CrossRef]
- Bandeira e Sousa, M.; de Andrade, L.R.B.; de Souza, E.H.; Alves, A.A.C.; de Oliveira, E.J. Reproductive barriers in cassava: Factors and implications for genetic improvement. PLoS ONE 2021, 16, e0260576. [Google Scholar]
- Gonçalves Fukuda, W.M.; de Oliveira, S.; Silva, S.; Iglesias, C. Cassava breeding. Crop. Breed. Appl. Biotech. 2002, 2, 617–638. [Google Scholar] [CrossRef]
- Sriroth, K.; Santisopasri, V.; Petchalanuwat, C.; Kurotjanawong, K.; Piyachomkwan, K.; Oates, C.G. Cassava starch granule structure-function properties: Influence of time and conditions at harvest on four cultivars of cassava starch. Carbohydr. Polym. 1999, 38, 161–170. [Google Scholar] [CrossRef]
- Sriroth, K.; Piyachomkwan, K.; Wanlapatit, S.; Oates, C.G. Cassava starch technology: The Thai experience. Starch/Starke 2000, 52, 439–449. [Google Scholar] [CrossRef]
- Sriroth, K.; Piyachomkwan, K.; Santisopasri, V.; Oates, C.G. Environmental conditions during root development: Drought constraint on cassava starch quality. Euphytica 2001, 120, 95–102. [Google Scholar] [CrossRef]
- Toae, R.; Sriroth, K.; Rojanaridpiched, C.; Vichukit, V.; Chotineeranat, S.; Wansuksri, R.; Chatakanonda, P.; Piyachomkwan, K. Outstanding Characteristics of Thai Non-GM Bred Waxy Cassava Starches Compared with Normal Cassava Starch, Waxy Cereal Starches and Stabilized Cassava Starches. Plants 2019, 8, 447. [Google Scholar] [CrossRef] [PubMed]
- Ceballos, H.; Morante, N.; Sánchez, T.; Ortiz, D.; Aragón, I.; Chávez, A.; Pizarro, M.; Calle, F.; Dufour, D. Rapid Cycling Recurrent Selection for Increased Carotenoids Content in Cassava Roots. Crop Sci. 2013, 53, 2342–2351. [Google Scholar] [CrossRef]
- Reilly, K.; Gómez-Vásquez, R.; Buschmann, H.; Tohme, J.; Beeching, J.R. Oxidative stress responses during cassava post-harvest physiological deterioration. Plant Mol. Biol. 2003, 53, 669–685. [Google Scholar] [CrossRef]
- Reilly, K.; Bernal, D.; Cortés, D.F.; Gómez-Vásquez, R.; Tohme, J.; Beeching, J.R. Towards identifying the full set of genes expressed during cassava post-harvest physiological deterioration. Plant Mol. Biol. 2007, 64, 187–203. [Google Scholar] [CrossRef]
- An, F.; Xue, J.; Luo, X.; Chen, T.; Wei, Z.; Zhu, W.; Ou, W.; Li, K.; Cai, J.; Chen, S. MePOD12 participates the regulation to postharvest physiological deterioration by ROS scavenging and lignin accumulation in cassava tuberous roots. Postharvest. Biol. Technol. 2024, 207, 112609. [Google Scholar] [CrossRef]
- Djabou, A.S.M.; Carvalho, L.J.C.B.; Li, Q.X.; Niemenak, N.; Chen, S. Cassava postharvest physiological deterioration: A complex phenomenon involving calcium signaling, reactive oxygen species and programmed cell death. Acta Physiol. Plant. 2017, 39, 91. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Xu, J.; Feng, Y.; Wu, X.; Lu, X.; Zhang, P. Knockdown of p-Coumaroyl Shikimate/Quinate 30-Hydroxylase Delays the Occurrence of Post-Harvest Physiological Deterioration in Cassava Storage Roots. Int. J. Mol. Sci. 2022, 23, 9231. [Google Scholar] [CrossRef] [PubMed]
- Luna, J.; Dufour, D.; Tran, T.; Pizarro, M.; Calle, F.; García Domínguez, M.; Hurtado, I.M.; Sánchez, T.; Ceballos, H. Post-harvest physiological deterioration in several cassava genotypes over sequential harvests and effect of pruning prior to harvest. Int. J. Food Sci. Technol. 2020, 56, 1322–1332. [Google Scholar] [CrossRef]
- van Oirschot, Q.E.; O’Brien, G.M.; Dufour, D.; El-Sharkawy, M.A.; Mesa, E. The effect of pre-harvest pruning of cassava upon root deterioration and quality characteristics. J. Sci. Food Agric. 2000, 80, 1866–1873. [Google Scholar] [CrossRef]
- Morante, N.; Moreno, X.; Pérez, J.C.; Calle, F.; Lenis, J.I.; Ortega, E.; Jaramillo, G.; Ceballos, H. Precision of selection in early stages of cassava genetic improvement. J. Root Crops 2005, 31, 81–92. [Google Scholar]
- Du, L. The biosynthesis of cyanogenic glucosides in roots of cassava. Phytochemistry 1995, 39, 323–326. [Google Scholar] [CrossRef]
- Mkumbira, J.; Chiwona-Karltun, L.; Lagercrantz, U.; Mahungu, N.M.; Saka, J.D.K.; Mhone, A.R.; Bokanga, M.; Brimer, L.; Gullberg, U.; Rosling, H. Classification of cassava into bitter and cool in Malawi. From farmers’ perceptions to characterisation by molecular markers. Euphytica 2003, 132, 7–22. [Google Scholar] [CrossRef]
- Ospina, M.A.; Pizarro, M.; Tran, T.; Ricci, J.; Belalcazar, J.; Luna, J.L.; Londoño, L.F.; Salazar, S.; Ceballos, H.; Dufour, D.; et al. Cyanogenic, carotenoids and protein composition in leaves and roots across seven diverse population found in the world cassava germplasm collection at CIAT, Colombia. Int. J. Food Sci. Technol. 2020, 56, 1343–1353. [Google Scholar] [CrossRef]
- Ceballos, H.; Rojanaridpiched, C.; Phumichai, C.; Becerra, L.A.; Kittipadakul, P.; Iglesias, C.; Gracen, V.E. Excellence in Cassava Breeding: Perspectives for the Future. Crop Breed. Genet. Genom. 2020, 2, e200008. [Google Scholar] [CrossRef]
- Kawano, K.; Narintaraporn, K.; Narintaraporn, P.; Sarakarn, S.; Limsila, A.; Limsila, J.; Suparhan, D.; Sarawat, V.; Watananonta, W. Yield Improvement in a Multistage Breeding Program for Cassava. Crop Sci. 1998, 38, 325–332. [Google Scholar] [CrossRef]
- Ceballos, H.; Pérez, J.C.; Orlando, J.B.; Lenis, J.I.; Morante, N.; Calle, F.; Hershey, C. Cassava breeding I: The value of breeding value. Front. Plant Sci. 2016, 7, 1227. [Google Scholar] [CrossRef] [PubMed]
- Phumichai, C.; Aiemnaka, P.; Nathaisong, P.; Hunsawattanakul, S.; Fungfoo, P.; Rojanaridpiched, C.; Vichukit, V.; Kongsil, P.; Kittipadakul, P.; Wannarat, W.; et al. Genome-wide association mapping and genomic prediction of yield-related traits and starch pasting properties in cassava. Theor. Appl. Genet. 2021, 135, 145–171. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, M.D.; Chan, A.W.; Kulakow, P.; Rabbi, I.; Jannink, J.L. Genomic mating in outbred species: Predicting cross usefulness with additive and total genetic covariance matrices. Genetics 2021, 219, iyab122. [Google Scholar] [CrossRef] [PubMed]
- Aiemnaka, P.; Wongkaew, A.; Chanthaworn, J.; Nagashima, S.K.; Boonma, S.; Authapun, J.; Jenweerawat, S.; Kongsila, P.; Kittipadakul, P.; Nakasathien, S.; et al. Molecular characterization of a spontaneous waxy starch mutation in cassava (Manihot esculenta Crantz). Crop Sci. 2012, 52, 2121–2130. [Google Scholar] [CrossRef]
- Conceicão, L.V.d.; Cortes, D.F.M.; Klauser, D.; Robinson, M.; Oliveira, E.J.d. New protocol for rapid cassava multiplication in field conditions: A perspective on speed breeding. Front. Plant Sci. 2023, 14, 1258101. [Google Scholar] [CrossRef] [PubMed]
- Diniz, R.P.; Oliveira, E.J.d. Genetic parameters, path analysis and indirect selection of agronomic traits of cassava germplasm. An. Da Acad. Bras. De Ciências 2019, 91, e20180387. [Google Scholar] [CrossRef] [PubMed]
- Gracen, V.E.; Kongsil, P.; Napasintuwong, O.; Duangjit, J.; Phumichai, C. The Story of Kasetsart 50: The Most Important Cassava Variety in the World; Center for Agricultural Biotechnology, Kasetsart University, Kamphaeng Saen Campus: Nakorn Pathom, Thailand, 2018; p. 53. [Google Scholar]
- Rodjanaridpiched, C.; Limsila, A.; Supraharn, D.; Boonseng, O.; Poolsanguan, P.; Tiraporn, C.; Kawano, K. Recent progress in cassava varietal improvement in Thailand. In Proceedings of the Fourth Regional Workshop Cassava Breeding, Agronomy Research and Technology Transfer in Asia, Kerala, India, 2–6 November 1993; Howeler, R.H., Ed.; Centro Internacional de Agricultura Tropical (CIAT): Cali, CO, USA, 1995; pp. 124–134. [Google Scholar]
- Praneetvatakul, S.; Sirivirintarat, T.; Vijitsrikamol, K. Research benefits of KU50 and Huay Bong cassava breeding. Kasetsart J. Soc. Sci. 2018, 39, 684–695. [Google Scholar] [CrossRef]
- Rojanaridpiched, C.; (Kasetsart University, Bangkok, Thailand); Thai Tapioca Development Institute; (Bangkok, Thailand). Personal communication, 2022.
- Joaqui, B.O.; Lenis, J.I.; Calle, F.; Morante, N.; Pérez, J.C.; Hershey, C.; Ceballos, H. Cassava breeding II: Phenotypic correlations through the different stages of selection. Front. Plant Sci. 2016, 7, 1649. [Google Scholar]
- Jennings, D.L.; Hershey, C. Cassava breeding: A decade of progress from international programmes. In Progress in Plant Breeding; Russel, G.E., Ed.; Butterworths Press: London, UK, 1985. [Google Scholar]
- Ceballos, H.; Hershey, C.; Iglesias, C.; Zhang, X. Fifty years of a public cassava breeding program: Evolution of breeding objectives, methods, and decision-making processes. Theor. Appl. Genet. 2021, 134, 2335–2353. [Google Scholar] [CrossRef]
- Eberhart, S.A.; Russell, W.A. Stability parameters for comparing varieties. Crop Sci. 1966, 6, 36–40. [Google Scholar] [CrossRef]
- Kittipadakul, P.; Rodjanaridpiched, C.; Vichukij, V.; Changlek, P.; Malumpong, C.; Keawtrakulpong, K. Cassava stability of Thai cassava. In Proceedings of the 42nd Kasetsart University Annual Conference: Plants, Agricultural Extension and Communication, Bangkok, Thailand, 3–6 February 2004; Kasetsart University: Bangkok, Thailand, 2004; pp. 191–201. (In Thai). [Google Scholar]
- Howeler, R. Chapter 3: What are the major constraints to high yields? In Sustainable Soil and Crop Management of Cassava in Asia; Howeler, R., Ed.; Centro Internacional de Agricultura Tropical (CIAT): Cali, CO, USA, 2014; 280p. [Google Scholar]
- Cobb, A.H.; Reade, J.P.H. Herbicides and Plant Physiology, 2nd ed.; Wiley-Blackwell: Chichester, UK, 2010. [Google Scholar]
- Tan, S.; Evans, R.R.; Dahmer, M.L.; Singh, B.K.; Shaner, D.L. Imidazolinone-tolerant crops: History, current status and future. Pest. Manag. Sci. 2005, 61, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Duitama, J.; Kafuri, L.; Tello, D.; Leiva, A.M.; Hofinger, B.; Datta, S.; Lentini, Z.; Aranzales, E.; Till, B.; Ceballos, H. Deep assessment of genomic diversity in cassava for herbicide tolerance and starch biosynthesis. Comput. Struct. Biotechnol. J. 2017, 15, 185–194. [Google Scholar] [CrossRef] [PubMed]
- El-Sharkawy, M.A. Drought tolerant cassava for Africa, Asia and Latin America: Breeding projects work to stabilize productivity without increasing pressures on limited natural resources. BioScience 1993, 43, 441–451. [Google Scholar] [CrossRef]
- El-Sharkawy, M.A.; de Tafur, S.M. Genotypic and within canopy variation in leaf carbon isotope discrimination and its relation to short-term leaf gas exchange characteristics in cassava grown under rain-fed conditions in the tropics. Photosynthetica 2007, 45, 515–526. [Google Scholar] [CrossRef]
- Alves, A.C.; Setter, T.L. Response of cassava to water deficit: Leaf area growth and abscisic acid. Crop Sci. 2000, 40, 131–137. [Google Scholar] [CrossRef]
- Alves, A.C.; Setter, T.L. Abscisic acid accumulation and osmotic adjustment in cassava under water deficit. Environ. Exp. Bot. 2004, 51, 259–271. [Google Scholar] [CrossRef]
- Adjebeng-Danquah, J.; Gracen, V.E.; Offei, S.K.; Asante, I.K.; Manu-Aduening, J. Genetic variability in storage root bulking of cassava genotypes under irrigation and no irrigation. Agric. Food Secur. 2016, 5, 9. [Google Scholar] [CrossRef]
- Oliveira, E.J.; Morgante, C.V.; de Tarso Aidar, S.; de Melo Chaves, A.R.; Antonio, R.P.; Cruz, J.L.; Coelho Filho, M.A. Evaluation of cassava germplasm for drought tolerance under field conditions. Euphytica 2017, 213, 188. [Google Scholar] [CrossRef]
- dos Santos Silva, P.P.; e Sousa, M.B.; de Oliveira, E.J.; Morgante, C.V.; de Oliveira, C.R.S.; Vieira, S.L.; Borel, J.C. Genome-wide association study of drought tolerance in cassava. Euphytica 2021, 217, 60. [Google Scholar] [CrossRef]
- Bolaños, J.; Edmeades, G. The importance of the anthesis-silking interval in breeding for drought tolerance in tropical maize. Field Crops Res. 1996, 48, 65–80. [Google Scholar] [CrossRef]
- El-Sharkawy, M.A. Cassava biology and physiology. Plant Mol. Biol. 2004, 56, 481–501. [Google Scholar] [CrossRef] [PubMed]
- El-Sharkawy, M.A.; Cock, J.H. Response of cassava to water stress. Plant Soil 1987, 100, 345–360. [Google Scholar] [CrossRef]
- El-Sharkawy, M.A.; Hernandez, A.D.P.; Hershey, C. Yield stability of cassava during prolonged mid-season water stress. Exp. Agric. 1992, 28, 165–174. [Google Scholar] [CrossRef]
- Okogbenin, E.; Setter, T.L.; Ferguson, M.; Mutegi, R.; Ceballos, H.; Olasanmi, B.; Fregene, M. Phenotypic approaches to drought in cassava: Review. Front. Plant Physiol. 2013, 4, 93. [Google Scholar] [CrossRef]
- Orek, C.; Gruissem, W.; Ferguson, M.; Vanderschuren, H. Morpho-physiological and molecular evaluation of drought tolerance in cassava (Manihot esculenta Crantz). Field Crops Res. 2020, 255, 107861. [Google Scholar] [CrossRef]
- Shan, Z.; Luo, X.; Wei, M.; Huang, T.; Khan, A.; Zhu, Y. Physiological and proteomic analysis on long-term drought resistance of cassava (Manihot esculenta Crantz). Sci. Rep. 2018, 8, 17982. [Google Scholar] [CrossRef] [PubMed]
- Turyagyenda, L.F.; Kizito, E.B.; Ferguson, M.; Baguma, Y.; Agaba, M.; Harvey, J.J.; Osiru, D.S. Physiological and molecular characterization of drought responses and identification of candidate tolerance genes in cassava. AoB Plants 2013, 5, plt007. [Google Scholar] [CrossRef]
- Polthanee, A.; Taboonmuang, R.; Manaonok, J. Root yield and nutrient removal of four cassava cultivars planted in early rainy season of Northeastern Thailand. Asian J. Crop Sci. 2016, 8, 24–30. [Google Scholar] [CrossRef]
- Polthanee, A.; Janthajam, C.; Promkhambut, A. Growth, yield and starch content of cassava following rainfed lowland rice in Northeast Thailand. Int. J. Agric. Res. 2014, 9, 319–324. [Google Scholar] [CrossRef]
- Wongnoi, S.; Banterng, P.; Vorasoot, N.; Jogloy, S.; Theerakulpisut, P. Physiology, Growth and Yield of Different Cassava Genotypes Planted in Upland with Dry Environment during High Storage Root Accumulation Stage. Agronomy 2020, 10, 576. [Google Scholar] [CrossRef]
- Polthanee, A.; Srisutham, M. Supplementary irrigation for cassava planted in the late rainy season of Northeastern Thailand. Asian J. Crop Sci. 2017, 9, 100–108. [Google Scholar] [CrossRef]
- Phoncharoen, P.; Banterng, P.; Vorasoot, N.; Jogloy, S.; Theerakulpisut, P.; Hoogenboom, G. Identifying Suitable Genotypes for Different Cassava Production Environments—A Modeling Approach. Agronomy 2021, 11, 1372. [Google Scholar] [CrossRef]
- Herrera Campo, B.V.; Hyman, G.; Bellotti, A. Threats to cassava production: Known and potential geographic distribution of four key biotic constraints. Food Secur. 2011, 3, 329. [Google Scholar] [CrossRef]
- Malathi, V.; Nair, N.; Shantha, P. Cassava Mosaic Disease (Technical Bulletin Series 5); Central Tuber Crops Research Institute: Trivandrum, India, 1985; p. 18. [Google Scholar]
- Patil, B.L.; Fauquet, C.M. Cassava mosaic geminiviruses: Actual knowledge and perspectives. Mol. Plant. Pathol. 2009, 10, 685–701. [Google Scholar] [CrossRef] [PubMed]
- Minato, N.; Sok, S.; Chen, S.; Delaquis, E.; Phirun, I.; Le, V.X.; Burra, D.D.; Newby, J.C.; Wyckhuys, K.A.G.; de Haan, S. Surveillance for Sri Lankan cassava mosaic virus (SLCMV) in Cambodia and Vietnam one year after its initial detection in a single plantation in 2015. PLoS ONE 2019, 14, e0212780. [Google Scholar] [CrossRef] [PubMed]
- Saokham, K.; Hemniam, N.; Roekwan, S.; Hunsawattanakul, S.; Thawinampan, J.; Siriwan, W. Survey and molecular detection of Sri Lankan cassava mosaic virus in Thailand. PLoS ONE 2021, 16, e0252846. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Cui, X.Y.; Wang, X.W.; Liu, S.S. First report of Sri Lankan cassava mosaic virus infecting cassava in Cambodia. Plant Dis. 2016, 100, 1029. [Google Scholar] [CrossRef]
- The Current Status of SLCMV in Thailand. Available online: https://www.ippc.int/en/countries/thailand/pestreports/2019/03/the-current-status-of-slcmv-in-thailand/ (accessed on 20 January 2024).
- Attavanich, W.; (Kasetsart University, Bangkok, Thailand). Personal communication, 2022.
- Fauquet, C.; Stanley, J. Geminivirus classification and nomenclature: Progress and problems. Ann. Appl. Biol. 2005, 142, 165–189. [Google Scholar] [CrossRef]
- Uke, A.; Hoat, T.X.; Quan, M.V.; Liem, N.V.; Ugaki, M.; Natsuaki, K.T. First Report of Sri Lankan Cassava Mosaic Virus Infecting Cassava in Vietnam. Plant Dis. 2018, 102, 2669. [Google Scholar] [CrossRef]
- Chittarath, K.; Jimenez, J.; Vongphachanh, P.; Leiva, A.M.; Sengsay, S.; Lopez-Alvarez, D.; Bounvilayvong, T.; Lourido, D.; Vorlachith, V.; Cuellar, W.J. First Report of Cassava Mosaic Disease and Sri Lankan Cassava Mosaic Virus in Laos. Plant Dis. 2021, 105, 1861. [Google Scholar] [CrossRef]
- Hemniam, N.; Roekwan, S.; Vannatim, N.; Malichan, S.; Saokham, K.; Chaowongdee, S.; Siriwan, W. Natural infection of Cnidoscolus and Jatropha by Sri Lankan cassava mosaic virus in Thailand. J. Gen. Plant Pathol. 2022, 88, 386–391. [Google Scholar] [CrossRef]
- Akano, O.; Dixon, A.; Mba, C.; Barrera, E.; Fregene, M. Genetic mapping of a dominant gene conferring resistance to cassava mosaic disease. Theor. Appl. Genet. 2002, 105, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Rabbi, I.Y.; Kayondo, S.I.; Bauchet, G.; Yusuf, M.; Aghogho, C.I.; Ogunpaimo, K.; Uwugiaren, R.; Smith, I.A.; Peteti, P.; Agbona, A.; et al. Genome-wide association analysis reveals new insights into the genetic architecture of defensive, agro-morphological and quality-related traits in cassava. Plant Mol. Biol. 2022, 109, 195–213. [Google Scholar] [CrossRef] [PubMed]
- Houngue, J.A.; Zandjanakou-Tachin, M.; Ngalle, H.B.; Pita, J.S.; Cacaï, G.H.T.; Ngatat, S.E.; Bell, J.M.; Ahanhanzo, C. Evaluation of resistance to cassava mosaic disease in selected African cassava cultivars using combined molecular and greenhouse grafting tools. Physiol. Mol. Plant Pathol. 2019, 105, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Thuy, C.T.L.; Becerra Lopez-Lavalle, L.A.; Anh, V.N.; Huu, H.N.; Thi, N.P.; Ceballos, H.; Newby, J.; Ba, T.N.; Trong, H.N.; Ngoc, T.L.; et al. Identifying new resistance to cassava mosaic disease and validating markers for CMD2 locus. Agriculture 2021, 11, 829. [Google Scholar] [CrossRef]
- Alvarez, E.; Pardo, J.M.; Mejia de Los, J.F.; Bertaccini, A.; Thanh, N.D.; Hoat, T.X. Detection and identification of ‘Candidatus Phytoplasma asteris’-related phytoplasmas associated with a witches’ broom disease of cassava in Vietnam. Phytopathogenic Mollicutes 2013, 3, 77–81. [Google Scholar] [CrossRef]
- Klinkong, S. Phytoplasma Causes Witch’s Broom Disease: Emerging Threat in Cassav. Kaset Apirom 2017, 4, 29–31. [Google Scholar]
- Leiva, A.M.; Pardo, J.M.; Arinaitwe, W.; Newby, J.; Vongphachanh, P.; Chittarath, K.; Oeurn, S.; Thi Hang, L.; Gil-Ordóñez, A.; Rodriguez, R.; et al. Ceratobasidium sp. Is associated with cassava witches’ broom disease, a re-emerging threat to cassava cultivation in Southeast Asia. Sci. Rep. 2023, 13, 22500. [Google Scholar] [CrossRef]
- Ceballos, H.; (CIAT, Cali, Colombia). Personal communication, 2023.
- Wyckhuys, K.A.G.; Hughes, A.C.; Buamas, C.; Johnson, A.C.; Vasseur, L.; Reymondin, L.; Deguine, J.P.; Sheil, D. Biological control of an agricultural pest protects tropical forests. Commun. Biol. 2019, 2, 10. [Google Scholar] [CrossRef]
- Bellotti, A.C. Arthropod pests. In Cassava: Biology, Production and Utilization; Hillocks, R.J., Thresh, J.M., Bellotti, A.C., Eds.; CABI CABI Publishing: Wallingford, UK, 2002; pp. 209–236. [Google Scholar]
- Ceballos, H.; Sánchez, T.; Morante, N.; Fregene, M.; Dufour, D.; Smith, A.M.; Denyer, K.; Pérez, J.C.; Calle, F.; Mestres, C. Discovery of an Amylose-free Starch Mutant in Cassava (Manihot esculenta Crantz). J. Agric. Food Chem. 2007, 55, 7469–7476. [Google Scholar] [CrossRef]
- Rojanaridpiched, C.; Vichukit, V.; Ceballos, H.; Aeimnaka, P.; Pumichai, C.; Piyachomkwan, K. Development of waxy starch cassava varieties in Thailand. In Proceedings of the 9thStarch World Asia Conference (CMT), Bangkok, Thailand, 11–13 February 2020. [Google Scholar]
- Ceballos, H.; Sánchez, T.; Denyer, K.; Tofiño, A.P.; Rosero, E.A.; Dufour, D.; Smith, A.; Morante, N.; Pérez, J.C.; Fahy, B. Induction and Identification of a Small-Granule, High-Amylose Mutant in Cassava (Manihot esculenta Crantz). J. Agric. Food Chem. 2008, 56, 7215–7222. [Google Scholar] [CrossRef]
- Pingali, P.; Kelley, T. The role of international agricultural research in contributing to global food security and poverty alleviation: The case of the CGIAR. In Handbook of Agricultural Economics vol. 3; Evenson, R., Pingali, P., Eds.; Elsevier: New York, NY, USA, 2007; pp. 2382–2418. [Google Scholar]
- Miranda Filho, J.B. Inbreeding depression and heterosis. In The Genetic Exploitation of Heterosis in Crops; Coors, J.G., Pandey, S., Eds.; American Society of Agronomy: Madison, WI, USA, 1999; pp. 69–80. [Google Scholar]
- Yuanjit, P.; Vuttipongchaikij, S.; Wonnapinij, P.; Ceballos, H.; Kraichak, E.; Jompuk, C.; Kittipadakul, P. Evaluation of yield potential and combining ability in Thai elite cassava varieties for breeding selection. Agronomy 2023, 13, 1546. [Google Scholar] [CrossRef]
- Chipeta, M.M.; Bokosi, J.M.; Saka, V.W.; Benesi, I.R.M. Combining ability and mode of gene action in cassava for resistance to cassava green mite and cassava mealy bug in Malawi. J. Plant Breed. Crop Sci. 2013, 5, 195–202. [Google Scholar]
- Easwari Amma, C.S.; Sheela, N.; Thankamma Pillai, P.K. Combining ability analysis in cassava. J. Root Crops 1995, 21, 65–71. [Google Scholar]
- Kamau, J.; Melis, R.; Laing, M.; Derera, J.; Shanahan, P.; Ngugi, E. Combining the yield ability and secondary traits of selected cassava genotypes in the semi-arid areas of Eastern Kenya. J. Plant Breed. Crop Sci. 2010, 2, 181–191. [Google Scholar]
- Kulembeka, H.P.; Ferguson, M.; Herselman, L.; Kanju, E.; Mkamilo, G.; Masumba, E.; Fregene, M.; Labuschagne, M.T. Diallel analysis of field resistance to brown streak disease in cassava (Manihot esculenta Crantz) landraces from Tanzania. Euphytica 2012, 187, 277–288. [Google Scholar] [CrossRef]
- de Andrade, L.R.B.; e Sousa, M.B.; Wolfe, M.; Jannink, J.-L.; de Resende, M.D.V.; Azevedo, C.F.; de Oliveira, E.J. Increasing cassava root yield: Additive dominant genetic models for selection of parents and clones. Front. Plant Sci. 2022, 13, 1071156. [Google Scholar] [CrossRef]
- Wolfe, M.D.; Kulakow, P.; Rabbi, I.Y.; Jannink, J.-L. Marker-based estimates reveal significant nonadditive effects in clonally propagated cassava (Manihot esculenta): Implications for the prediction of total genetic value and the selection of varieties. G3 (Bethesda) 2016, 6, 3497. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, R. Essentials of Plant Breeding; Stemma Press Woodbury: Woodbury, NY, USA, 2014. [Google Scholar]
- Mikel, M.A.; Dudley, J.W. Evolution of North American dent corn from public to proprietary germplasm. Crop Sci. 2006, 46, 1193–1205. [Google Scholar] [CrossRef]
- Segelke, D.; Reinhardt, F.; Liu, Z.; Thaller, G. Prediction of expected genetic variation within groups of offspring for innovative mating schemes. Genet. Sel. Evol. 2014, 46, 42. [Google Scholar] [CrossRef]
- Kittipadakul, P.; Rojanaridpiched, C.; Vichukit, V.; Chantaworn, C. Efficiency of combining ability selection of parents in cassava breeding program. In Proceedings of the 49th Kasetsart University Annual Conference, Bangkok, Thailand, 1–4 February 2011; Kasetsart University: Bangkok, Thailand, 2011. (In Thai). [Google Scholar]
- Dirks, R.; van Dun, K.; de Snoo, C.B.; van den Berg, M.; Lelivelt, C.L.C.; Voermans, W.; Woudenberg, L.; de Wit, J.P.; Reinink, K.; Schut, J.W.; et al. Reverse breeding: A novel breeding approach based on engineered meiosis. Plant Biotechnol. J. 2009, 7, 837–845. [Google Scholar] [CrossRef]
- Pineda, M.; Morante, N.; Salazar, S.; Cuásquer, J.; Hyde, P.T.; Setter, T.L.; Ceballos, H. Induction of earlier flowering in cassava through extended photoperiod. Agronomy 2020, 10, 1273. [Google Scholar] [CrossRef]
- Pineda, M.; Yu, B.; Tian, Y.; Morante, N.; Salazar, S.; Hyde, P.; Setter, T.L.; Ceballos, H. Effect of pruning young branches on fruit and seed set in cassava. Front. Plant Sci. 2020, 11, 1107. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.D.; Bandeira, M.; Sousa, E.; Cunha Alves, A.A.; de Oliveira, E.J. Flowering induction in cassava using photoperiod extension premature pruning and plant growth regulators. PLoS ONE 2023, 18, e0292385. [Google Scholar] [CrossRef]
- Xu, Y.; Crouch, J.H. Marker-assisted selection in plant breeding: From publications to practice. Crop Sci. 2008, 48, 391–407. [Google Scholar] [CrossRef]
- Holland, J.B. Implementation of molecular markers for quantitative traits in breeding programs: Challenges and opportunities. In New Directions for a Diverse Planet: Proceedings of the 4th International Crop Science Congress; Fischer, T., Turner, N., Angus, J., McIntyre, L., Robertson, M., Borrell, A., Lloyd, D., Eds.; Crop Science Congress: Brisbane, Australia; Regional Institute: Gosford, Australia, 2004; p. 26. [Google Scholar]
- Ige, A.D.; Olasanmi, B.; Mbanjo, E.G.N.; Kayondo, I.S.; Parkes, E.Y.; Kulakow, P.; Egesi, C.; Bauchet, G.J.; Ng, E.; Becerra Lopez-Lavalle, L.A.; et al. Conversion and validation of uniplex snp markers for selection of resistance to cassava mosaic disease in cassava breeding programs. Agronomy 2021, 11, 420. [Google Scholar] [CrossRef]
- Ige, A.D.; Olasanmi, B.; Bauchet, J.; Kayondo, I.S.; Mbanjo, E.G.N.; Uwugiaren, R.; Motomura-Wages, S.; Norton, J.; Egesi, C.; Parkes, E.Y.; et al. Validation of KASP-SNP markers in cassava germplasm for marker-assisted selection of increased carotenoid content and dry matter content. Front. Plant Sci. 2022, 13, 1016170. [Google Scholar] [CrossRef]
- Ikeogu, U.N.; Akdemir, D.; Wolfe, M.D.; Okeke, U.G.; Amaefula, C.; Jannink, J.-L.; Egesi, C.N. Genetic correlation, genome-wide association and genomic prediction of portable NIRS predicted carotenoids in cassava roots. Front. Plant Sci. 2019, 10, 1570. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, M.; Rabbi, I.; Kim, D.; Gedil, M.; Lopez-Lavalle, L.A.B.; Okogbenin, E. Molecular Markers and Their Application to Cassava Breeding: Past, Present and Future. Trop. Plant Biol. 2012, 5, 95–109. [Google Scholar] [CrossRef]
- Ferguson, M.E.; Eyles, R.P.; Garcia-Oliveira, A.L.; Kapinga, F.; Masumba, E.A.; Amuge, T.; Bredeson, J.V.; Rokhsar, D.S.; Lyons, J.B.; Shah, T.; et al. Candidate genes for field resistance to cassava brown streak disease revealed through the analysis of multiple data sources. Front. Plant Sci. 2023, 14, 1270963. [Google Scholar] [CrossRef]
- Uchendu, K.; Njoku, D.N.; Paterne, A.; Rabbi, I.Y.; Dzidzienyo, D.; Tongoona, P.; Offei, S.; Egesi, C. Genome-Wide Association Study of Root Mealiness and Other Texture-Associated Traits in Cassava. Front. Plant Sci. 2021, 12, 770434. [Google Scholar] [CrossRef] [PubMed]
- Udoh, L.I.; Gedil, M.; Parkes, E.Y.; Kulakow, P.; Adesoye, A.; Nwuba, C.; Rabbi, I.Y. Candidate gene sequencing and validation of SNP markers linked to carotenoid content in cassava (Manihot esculenta Crantz). Mol. Breed. 2017, 37, 123. [Google Scholar] [CrossRef]
- Santos, C.S.D.; Sousa, M.B.; Brito, A.C.; de Oliveira, L.A.; Carvalho, C.W.P.; de Oliveira, E.J. Genome-wide association study of cassava starch paste properties. PLoS ONE 2022, 17, e0262888. [Google Scholar] [CrossRef] [PubMed]
- Mbe, J.O.; Dzidzienyo, D.K.; Abah, S.P.; Njoku, D.N.; Onyeka, J.; Tongoona, P.; Egesi, C. Novel SNP markers and other stress-related genomic regions associated with nitrogen use efficiency in cassava. Front. Plant Sci. 2024, 15, 1376520. [Google Scholar] [CrossRef] [PubMed]
- Ewa, F.; Asiwe, J.N.A.; Okogbenin, E.; Ogbonna, A.C.; Egesi, C. KASPar SNP genetic map of cassava for QTL discovery of productivity traits in moderate drought stress environment in Africa. Sci. Rep. 2021, 11, 11268. [Google Scholar] [CrossRef]
- Juma, B.S.; Mweu, C.; Piero, M.; Mbinda, W. CRISPR/Cas genome editing: A frontier for transforming precision cassava breeding. Afr. J. Biotechnol. 2021, 20, 237–250. [Google Scholar]
- Odipio, J.; Alicai, T.; Ingelbrecht, I.; Nusinow, D.A.; Bart, R.; Taylor, N.J. Efficient CRISPR/Cas9 genome editing of phytoene desaturase in cassava. Front. Plant Sci. 2017, 8, 296797. [Google Scholar] [CrossRef]
- Elegba, W.; McCallum, E.; Gruissem, W.; Vanderschuren, H. Efficient genetic transformation and regeneration of a farmer-preferred cassava cultivar from Ghana. Front. Plant Sci. 2021, 12, 668042. [Google Scholar] [CrossRef]
- Wang, Y.J.; Lu, X.H.; Zhen, X.H.; Yang, H.; Che, Y.N.; Hou, J.Y.; Geng, M.T.; Liu, J.; Hu, X.W.; Li, R.M.; et al. A transformation and genome editing system for cassava cultivar SC8. Genes 2022, 13, 1650. [Google Scholar] [CrossRef]
- Gomez, M.A.; Lin, Z.D.; Moll, T.; Chauhan, R.D.; Hayden, L.; Renninger, K.; Beyene, G.; Taylor, N.J.; Carrington, J.C.; Staskawicz, B.J.; et al. Simultaneous CRISPR/Cas9-mediated editing of cassava eIF 4E isoforms nCBP-1 and nCBP-2 reduces cassava brown streak disease symptom severity and incidence. Plant Biotechnol. J. 2019, 17, 421–434. [Google Scholar] [CrossRef]
- Mehta, D.; Stürchler, A.; Anjanappa, R.B.; Zaidi, S.S.E.A.; Hirsch-Hoffmann, M.; Gruissem, W.; Vanderschuren, H. Linking CRISPR-Cas9 interference in cassava to the evolution of editing-resistant geminiviruses. Genome Biol. 2019, 20, 80. [Google Scholar] [CrossRef] [PubMed]
- Juma, B.S.; Mukami, A.; Mweu, C.; Ngugi, M.P.; Mbinda, W. Targeted mutagenesis of the CYP79D1 gene via CRISPR/Cas9-mediated genome editing results in lower levels of cyanide in cassava. Front. Plant Sci. 2022, 13, 1009860. [Google Scholar] [CrossRef] [PubMed]
- Gomez, M.A.; Berkoff, K.C.; Gill, B.K.; Iavarone, A.T.; Lieberman, S.E.; Ma, J.M.; Schultink, A.; Karavolias, N.G.; Wyman, S.K.; Chauhan, R.D.; et al. CRISPR-Cas9-mediated knockout of CYP79D1 and CYP79D2 in cassava attenuates toxic cyanogen production. Front. Plant Sci. 2023, 13, 1079254. [Google Scholar] [CrossRef] [PubMed]
- Bull, S.E.; Seung, D.; Chanez, C.; Mehta, D.; Kuon, J.E.; Truernit, E.; Hochmuth, A.; Zurkirchen, I.; Zeeman, S.C.; Gruissem, W.; et al. Accelerated ex situ breeding of GBSS-and PTST1-edited cassava for modified starch. Sci. Adv. 2018, 4, eaat6086. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Ma, Q.; Zhong, Y.; Jing, J.; Wei, Z.; Zhou, W.; Lu, X.; Tian, Y.; Zhang, P. Editing of the starch branching enzyme gene SBE2 generates high-amylose storage roots in cassava. Plant Mol. Biol. 2022, 108, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Mukami, A.; Juma, B.S.; Mweu, C.; Oduor, R.; Mbinda, W. CRISPR-Cas9-induced targeted mutagenesis of feruloyl CoA 6′-hydroxylase gene reduces postharvest physiological deterioration in cassava roots. Postharvest Biol. Technol. 2024, 208, 112649. [Google Scholar] [CrossRef]
- Santos, A.D.; Bandeira, M.; Sousa, E.; Cunha Alves, A.A.; de Oliveira, E.J. Environmental factors influence the production of flowers and fruits of cassava. Sci. Hortic. 2024, 323, 112498. [Google Scholar] [CrossRef]
- Oluwasanya, D.N.; Gisel, A.; Stavolone, L.; Setter, T.L. Environmental responsiveness of flowering time in cassava genotypes and associated transcriptome changes. PLoS ONE 2021, 16, e0253555. [Google Scholar] [CrossRef] [PubMed]
- Oluwasanya, D.; Esan, O.; Hyde, P.T.; Kulakow, P.; Setter, T.L. Flower development in cassava is feminized by cytokinin, while proliferation is stimulated by anti-ethylene and pruning: Transcriptome responses. Front. Plant Sci. 2021, 12, 666266. [Google Scholar] [CrossRef]
- Rattanasopa, K.; Saengprachatanarug, K.; Wongpichet, S.; Posom, J.; Saikaew, K.; Ungsathittavorn, K.; Pilawut, S.; Chinapas, A.; Taira, E. UAV-Based Multispectral Imagery for Estimating Cassava Tuber Yields. Eng. Agric. Environ. Food 2022, 15, 1–12. [Google Scholar] [CrossRef]
- Wasonga, D.O.; Yaw, A.; Kleemola, J.; Alakukku, L.; Mäkelä, P.S.A. Red-Green-Blue and Multispectral Imaging as Potential Tools for Estimating Growth and Nutritional Performance of Cassava under Deficit Irrigation and Potassium Fertigation. Remote Sens. 2021, 13, 598. [Google Scholar] [CrossRef]
- Selvaraj, M.G.; Valderrama, M.; Guzman, D.; Valencia, M.; Ruiz, H.; Acharjee, A. Machine learning for high-throughput field phenotyping and image processing provides insight into the association of above and below-ground traits in cassava (Manihot esculenta Crantz). Plant Methods 2020, 16, 87. [Google Scholar] [CrossRef]
- Mbanjo, E.G.N.; Hershberger, J.; Peteti, P.; Agbona, A.; Ikpan, A.; Ogunpaimo, K.; Kayondo, S.I.; Abioye, R.S.; Nafiu, K.; Alamu, E.O.; et al. Predicting starch content in cassava fresh roots using near-infrared spectroscopy. Front. Plant Sci. 2022, 13, 990250. [Google Scholar] [CrossRef]
- Sousa, M.B.; Filho, J.S.S.; de Andrade, L.R.B.; de Oliveira, E.J. Near-infrared spectroscopy for early selection of waxy cassava clones via seed analysis. Front. Plant Sci. 2023, 14, 1089759. [Google Scholar] [CrossRef] [PubMed]
- Alamu, E.O.; Nuwamanya, E.; Cornet, D.; Meghar, K.; Adesokan, M.; Tran, T.; Belalcazar, J.; Desfontaines, L.; Davrieux, F. Near-infrared spectroscopy applications for high-throughput phenotyping for cassava and yam: A review. Int. J. Food Sci. Technol. 2020, 56, 1491–1501. [Google Scholar] [CrossRef] [PubMed]
- Meghar, K.; Tran, T.; Delgado, L.F.; Ospina, M.A.; Moreno, J.L.; Luna, J.; Londoño, L.; Dufour, D.; Davrieux, F. Hyperspectral imaging for the determination of relevant cooking quality traits of boiled cassava. J. Sci. Food Agric. 2023, 104, 4782–4792. [Google Scholar] [CrossRef]
- Wilhelm, J.; Wojciechowski, T.; Postma, J.A.; Jollet, D.; Heinz, K.; Böckem, V.; Müller-Linow, M. Assessing the Storage Root Development of Cassava with a New Analysis Tool. Plant Phenomics 2022, 2022, 9767820. [Google Scholar] [CrossRef]
- Sunvittayakul, P.; Kittipadakul, P.; Wonnapinij, P.; Chanchay, P.; Wannitikul, P.; Sathitnaitham, S.; Phanthanong, P.; Changwitchukarn, K.; Suttangkakul, A.; Ceballos, H.; et al. Cassava root crown phenotyping using three-dimension (3D) multi-view stereo reconstruction. Sci. Rep. 2022, 12, 10030. [Google Scholar] [CrossRef]
- Sunvittayakul, P.; Wonnapinij, P.; Chanchay, P.; Wannitikul, P.; Sathitnaitham, S.; Phanthanong, P.; Changwitchukarn, K.; Suttangkakul, A.; Ceballos, H.; Gomez, L.D.; et al. Genome-Wide Association Studies of Three-Dimensional (3D) Cassava Root Crowns and Agronomic Traits Using Partially Inbred Populations. Agronomy 2024, 14, 591. [Google Scholar] [CrossRef]
- Oliveira, C.R.S.D.; Borel, J.C.; Pereira, D.A.; Carvalho, B.P.D.; Medrado, E.D.S.; Ishikawa, F.H.; Oliveira, E.J.D. Genetic parameters and path analysis for root yield of cassava under drought and early harvest. Crop Breed. Appl. Biotechnol. 2021, 21, e36162137. [Google Scholar] [CrossRef]
- Das, A.; Schneider, H.; Burridge, J.; Ascanio, A.; Wojciechowski, T.; Topp, C.N.; Lynch, J.P.; Weitz, J.S.; Bucksch, A. Digital imaging of root traits (DIRT): A high-throughput computing and collaboration platform for field-based root phenomics. Plant Methods 2015, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Kengkanna, J.; Jakaew, P.; Amawan, S.; Busener, N.; Bucksch, A.; Saengwilai, P. Phenotypic variation of cassava root traits and their responses to drought. Appl. Plant Sci. 2019, 7, e1238. [Google Scholar] [CrossRef] [PubMed]
- Gerth, S.; Claußen, J.; Eggert, A.; Wörlein, N.; Waininger, M.; Wittenberg, T.; Uhlmann, N. Semiautomated 3D Root Segmentation and Evaluation Based on X-ray CT Imagery. Plant Phenomics 2021, 2021, 8747930. [Google Scholar] [CrossRef] [PubMed]
- Claussen, J.; Wittenberg, T.; Uhlmann, N.; Gerth, S. “Chamber #8”—A holistic approach of high-throughput nondestructive assessment of plant roots. Front. Plant Sci. 2024, 14, 1269005. [Google Scholar] [CrossRef]
- Alle, J.; Gruber, R.; Wörlein, N.; Uhlmann, N.; Claußen, J.; Wittenberg, T.; Gerth, S. 3D segmentation of plant root systems using spatial pyramid pooling and locally adaptive field-of-view inference. Front. Plant Sci. 2023, 14, 1120189. [Google Scholar] [CrossRef] [PubMed]
- Delgado, A.; Novo, A.; Hays, D.B. Data Acquisition Methodologies Utilizing Ground Penetrating Radar for Cassava (Manihot esculenta Crantz) Root Architecture. Geosciences 2019, 9, 171. [Google Scholar] [CrossRef]
- Delgado, A.; Hays, D.B.; Bruton, R.K.; Ceballos, H.; Novo, A.; Boi, E.; Selvaraj, M.G. Ground penetrating radar: A case study for estimating root bulking rate in cassava (Manihot esculenta Crantz). Plant Methods 2017, 13, 65. [Google Scholar] [CrossRef] [PubMed]
- Agbona, A.; Teare, B.; Ruiz-Guzman, H.; Dobreva, I.D.; Everett, M.E.; Adams, T.; Montesinos-Lopez, O.A.; Kulakow, P.A.; Hays, D.B. Prediction of Root Biomass in Cassava Based on Ground Penetrating Radar Phenomics. Remote Sens. 2021, 13, 4908. [Google Scholar] [CrossRef]
- Agbona, A.; Montesinos-Lopez, O.A.; Everett, M.E.; Ruiz-Guzman, H.; Hays, D.B. Yield Adjustment Using GPR-Derived Spatial Covariance Structure in Cassava Field: A Preliminary Investigation. Remote Sens. 2023, 15, 1771. [Google Scholar] [CrossRef]
- Nassar, N.M.; Hashimoto, D.; Fernandes, S. Wild Manihot species: Botanical aspects, geographic distribution and economic value. Genet. Mol. Res. 2008, 7, 16–28. [Google Scholar] [CrossRef]
- Chaengsee, P.; Kongsil, P.; Siriwong, N.; Kittipadakul, P.; Piyachomkwan, K.; Petchpoung, K. Potential yield and cyanogenic glucoside content of cassava root and pasting properties of starch and flour from cassava Hanatee var. and breeding lines grown under rain-fed condition. Agric. Nat. Resour. 2020, 54, 237–244. [Google Scholar]
- Depertment of Agriculture. Plant Vareities Protection Office. Available online: https://www.doa.go.th/pvp/ (accessed on 3 May 2024).

| Cultivar | Fresh Root Yield | Dry Matter Content | Dry Root Yield | Regression |
|---|---|---|---|---|
| (t/ha) | (%) | (t/ha) | Coefficient (b) for FRY | |
| Rayong 1 | 22.4 | 30.7 | 6.9 | 0.84 |
| Rayong 3 | 19.0 | 34.2 | 6.5 | 1.02 |
| Rayong 60 | 23.6 | 31.5 | 7.4 | 1.07 |
| Rayong 90 | 23.5 | 34.7 | 8.2 | 0.98 |
| KU 50 | 24.8 | 34.0 | 8.4 | 0.96 |
| Cultivar | Fresh Root Yield | Starch Content | Dry Root Yield |
|---|---|---|---|
| (t/ha)/Regression Coefficient (b) | (%)/Regression Coefficient (b) | (t/ha)/Regression Coefficient (b) | |
| KU50 | 36.25/1.39 | 25.61/1.01 | 13.44/1.01 |
| HB60 | 36.25/0.93 | 25.55/0.89 | 13.06/0.74 |
| Rayong 5 | 35.13/1.04 | 24.70/0.74 | 12.81/1.49 |
| Rayong 72 | 31.31/0.65 | 22.88/1.36 | 11.75/0.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kongsil, P.; Ceballos, H.; Siriwan, W.; Vuttipongchaikij, S.; Kittipadakul, P.; Phumichai, C.; Wannarat, W.; Kositratana, W.; Vichukit, V.; Sarobol, E.; et al. Cassava Breeding and Cultivation Challenges in Thailand: Past, Present, and Future Perspectives. Plants 2024, 13, 1899. https://doi.org/10.3390/plants13141899
Kongsil P, Ceballos H, Siriwan W, Vuttipongchaikij S, Kittipadakul P, Phumichai C, Wannarat W, Kositratana W, Vichukit V, Sarobol E, et al. Cassava Breeding and Cultivation Challenges in Thailand: Past, Present, and Future Perspectives. Plants. 2024; 13(14):1899. https://doi.org/10.3390/plants13141899
Chicago/Turabian StyleKongsil, Pasajee, Hernan Ceballos, Wanwisa Siriwan, Supachai Vuttipongchaikij, Piya Kittipadakul, Chalermpol Phumichai, Wannasiri Wannarat, Wichai Kositratana, Vichan Vichukit, Ed Sarobol, and et al. 2024. "Cassava Breeding and Cultivation Challenges in Thailand: Past, Present, and Future Perspectives" Plants 13, no. 14: 1899. https://doi.org/10.3390/plants13141899
APA StyleKongsil, P., Ceballos, H., Siriwan, W., Vuttipongchaikij, S., Kittipadakul, P., Phumichai, C., Wannarat, W., Kositratana, W., Vichukit, V., Sarobol, E., & Rojanaridpiched, C. (2024). Cassava Breeding and Cultivation Challenges in Thailand: Past, Present, and Future Perspectives. Plants, 13(14), 1899. https://doi.org/10.3390/plants13141899







