Impact of Methyl Jasmonate on Terpenoid Biosynthesis and Functional Analysis of Sesquiterpene Synthesis Genes in Schizonepeta tenuifolia
Abstract
1. Introduction
2. Methods and Materials
2.1. Plant Material
2.2. Effects of MeJA Treatment on Volatile Components of Schizonepeta tenuifolia
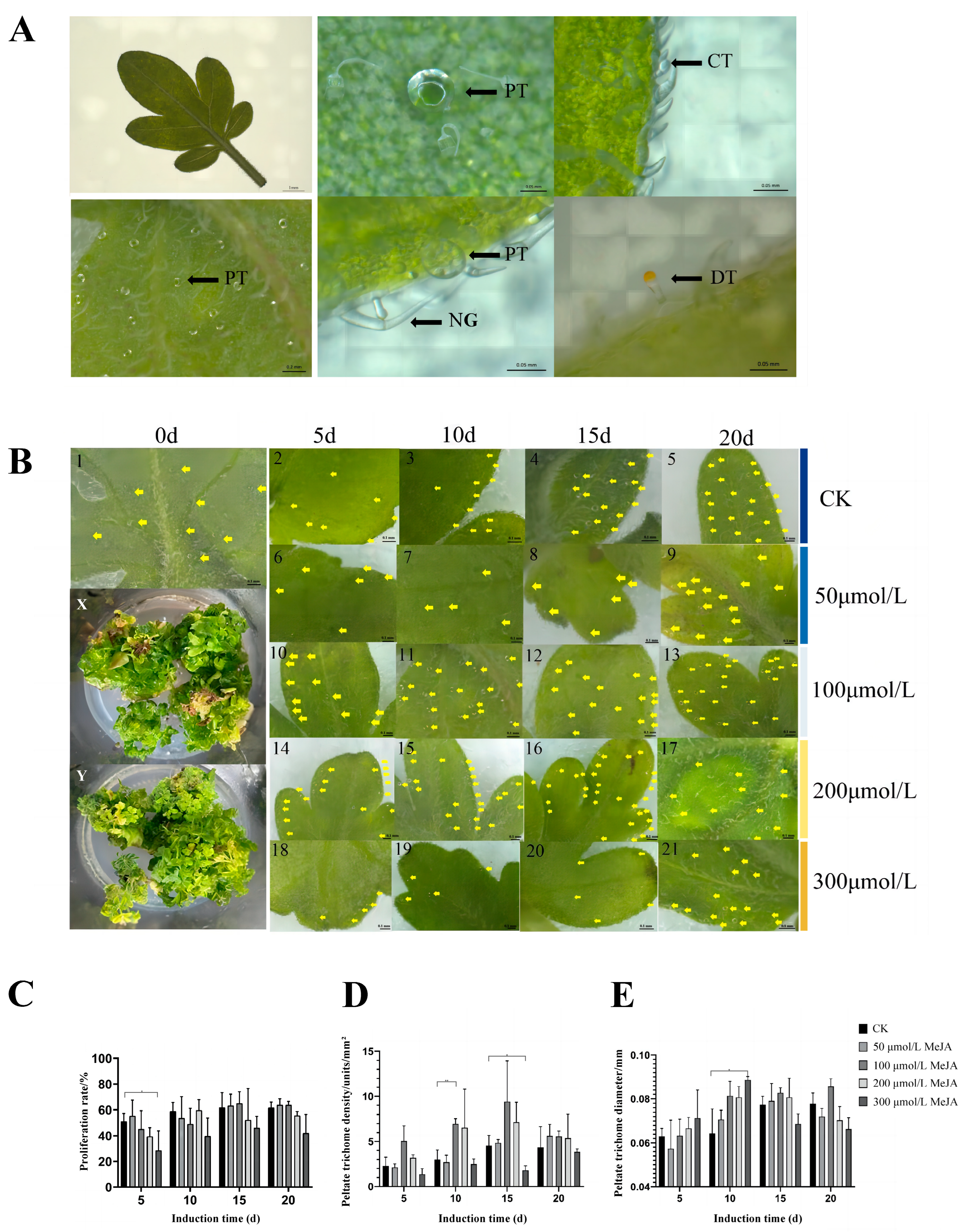
2.2.1. MeJA Induces the Formation of Schizonepeta tenuifolia
2.2.2. Effect of MeJA Treatment on the Growth of Schizonepeta tenuifolia
2.2.3. Effect of MeJA Treatment on Volatile Components of Schizonepeta tenuifolia
2.3. RNA Extraction, cDNA Library Construction and Data Assembly
2.4. Optimization and Prokaryotic Expression of Candidate Gene of Germacrene D Synthase
2.5. Functional Validation of the Protein Encoded by Candidate Gene
2.6. Analysis of the Enzyme Products of the Candidate Gene by GC-MS
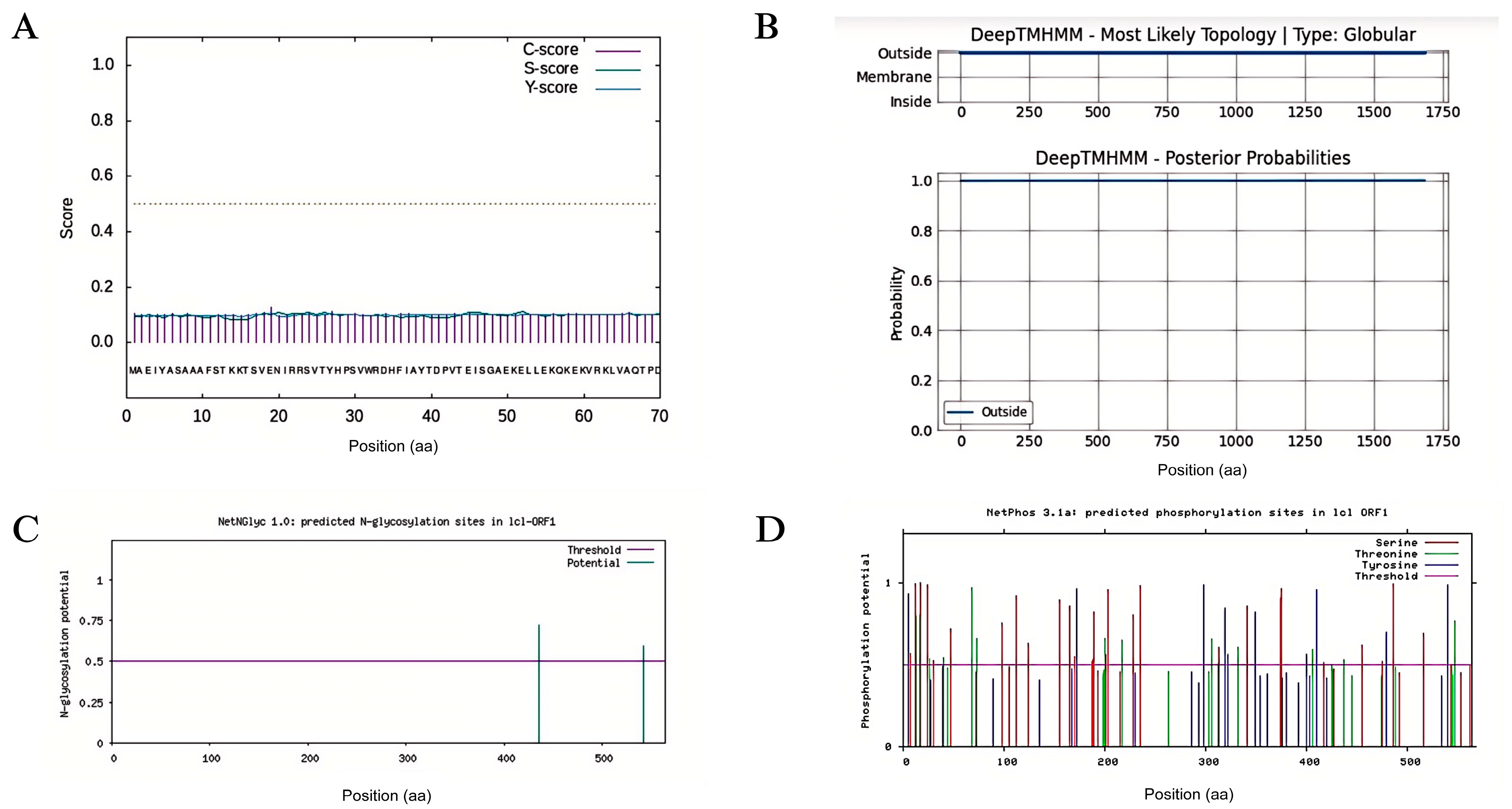
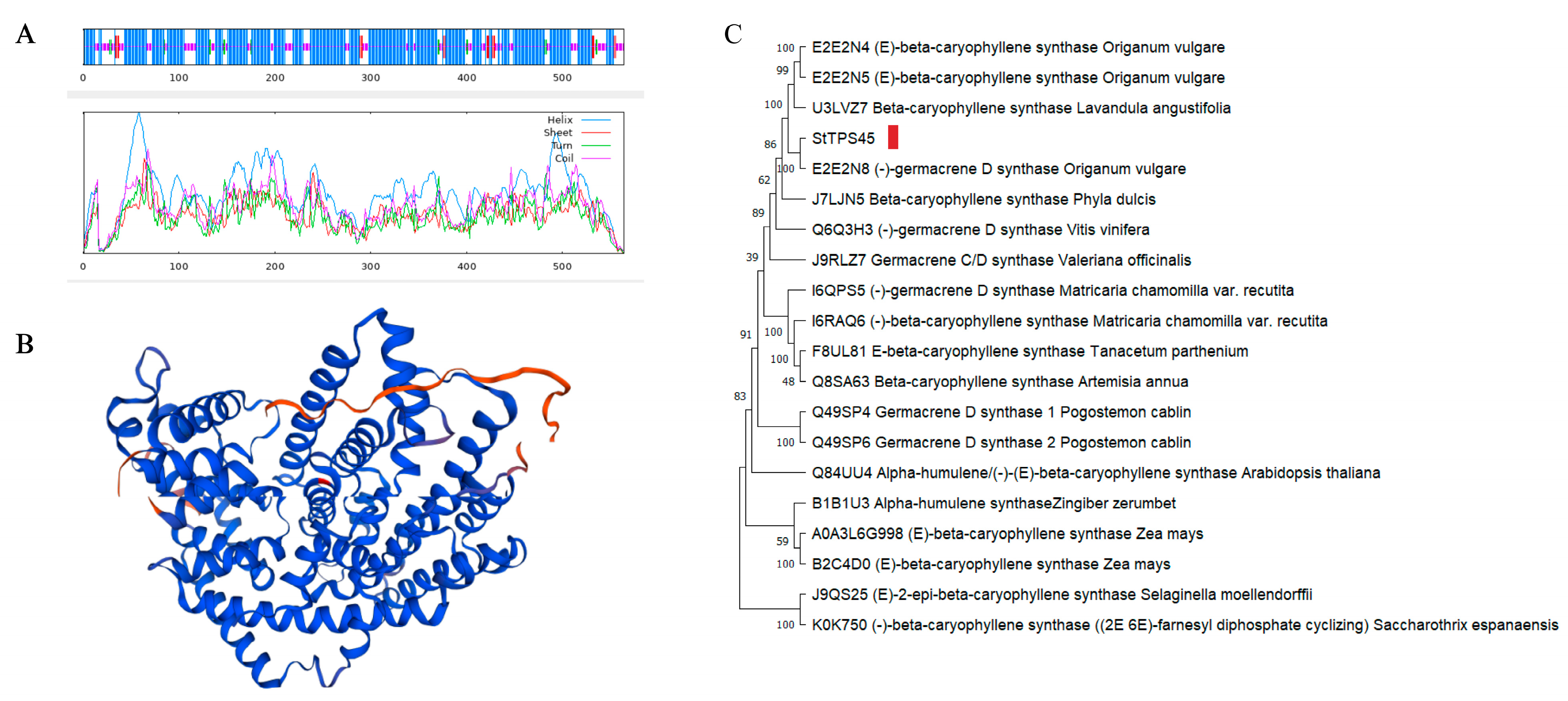
2.7. Structural Prediction and Molecular Docking of the Protein Encoded by the Candidate Gene
3. Result
3.1. Effect of MeJA Treatment on the Phenotype of Schizonepeta tenuifolia
3.2. Effects of MeJA Induction on Main Terpenoids in Volatile Oil from Schizonepeta tenuifolia
3.3. Transcriptome Analysis and Screening of Candidate Genes for Germacrene D Synthase
3.4. Overexpression and Enzyme Assay of the Protein Encoded by the Candidate Gene
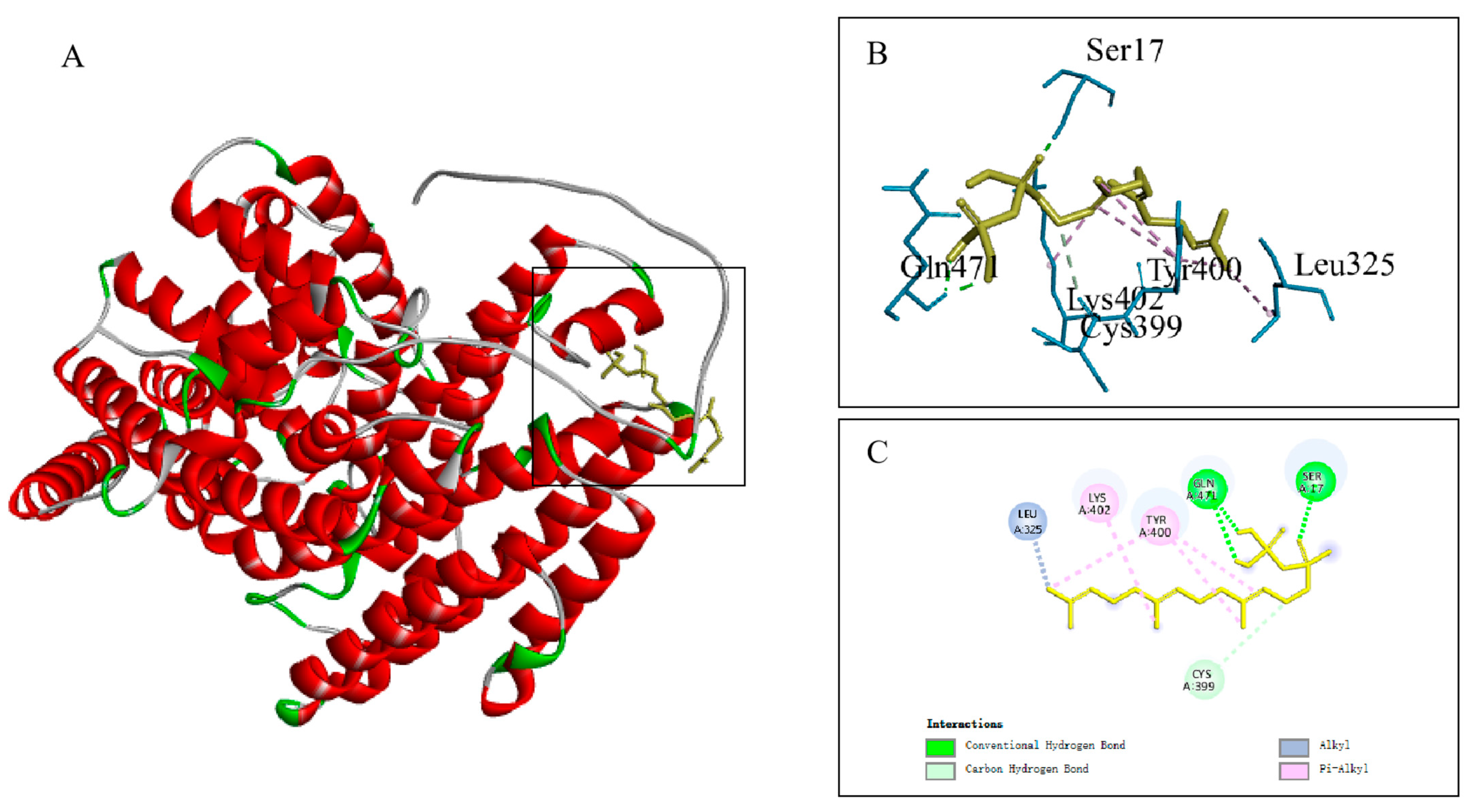
3.5. Physicochemical Property Analysis and Molecular Docking of the Protein Encoded by the Candidate Gene
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, X.; Zhou, M. Review on Chemical Constituents of Schizonepeta tenuifolia Briq. and Their Pharmacological Effects. Molecules 2022, 27, 5249. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Liu, L.; Zhang, J.; Chen, L.; Wu, T.; Aisa, H.A.; Maiwulanjiang, M. Spectrum-effect relationship between GC-QTOF-MS fingerprint and antioxidant, anti-inflammatory activities of Schizonepeta tenuifolia essential oil. Biomed. Chromatogr. 2021, 35, e5106. [Google Scholar] [CrossRef]
- Hilfiger, L.; Triaux, Z.; Marcic, C.; Héberlé, E.; Emhemmed, F.; Darbon, P.; Marchioni, E.; Petitjean, H.; Charlet, A. Anti-Hyperalgesic Properties of Menthol and Pulegone. Front. Pharmacol. 2021, 12, 753873. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Seo, S.; Lamichhane, S.; Seo, J.; Hong, J.T.; Cha, H.J.; Yun, J. Limonene has anti-anxiety activity via adenosine A2A receptor-mediated regulation of dopaminergic and GABAergic neuronal function in the striatum. Phytomedicine 2021, 83, 153474. [Google Scholar] [CrossRef]
- Araújo-Filho, H.G.D.; Dos Santos, J.F.; Carvalho, M.T.; Picot, L.; Fruitier-Arnaudin, I.; Groult, H.; Quintans-Júnior, L.J.; Quintans, J.S. Anticancer activity of limonene: A systematic review of target signaling pathways. Phytother. Res. 2021, 35, 4957–4970. [Google Scholar] [CrossRef] [PubMed]
- Chessa, M.; Sias, A.; Piana, A.; Mangano, G.S.; Petretto, G.L.; Masia, M.D.; Tirillini, B.; Pintore, G. Chemical composition and antibacterial activity of the essential oil from Mentha requienii Bentham. Nat. Prod. Res. 2013, 27, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.H.; Lin, J.Y. Menthone Inhalation Alleviates Local and Systemic Allergic Inflammation in Ovalbumin-Sensitized and Challenged Asthmatic Mice. Int. J. Mol. Sci. 2022, 23, 4011. [Google Scholar] [CrossRef]
- Huang, M.; Duan, W.; Chen, N.; Lin, G.; Wang, X. Synthesis and Antitumor Evaluation of Menthone-Derived Pyrimidine-Urea Compounds as Potential PI3K/Akt/mTOR Signaling Pathway Inhibitor. Front. Chem. 2021, 9, 815531. [Google Scholar] [CrossRef]
- Banerjee, A.; Sharkey, T.D. Methylerythritol 4-phosphate (MEP) pathway metabolic regulation. Nat. Prod. Rep. 2014, 31, 1043–1055. [Google Scholar] [CrossRef]
- Frank, A.; Groll, M. The Methylerythritol Phosphate Pathway to Isoprenoids. Chem. Rev. 2017, 117, 5675–5703. [Google Scholar] [CrossRef]
- Liu, C.; Smit, S.J.; Dang, J.; Zhou, P.; Godden, G.T.; Jiang, Z.; Liu, W.; Liu, L.; Lin, W.; Duan, J.; et al. A chromosome-level genome assembly reveals that a bipartite gene cluster formed via an inverted duplication controls monoterpenoid biosynthesis in Schizonepeta tenuifolia. Mol. Plant 2023, 16, 533–548. [Google Scholar] [CrossRef]
- Jia, C.-L.; Shu, J.; Dang, J.-J.; Wang, X.; Wu, Q.-N.; Liu, C.-C. Identification and analysis of terpene synthase (TPS) gene family in Schizonepeta tenuifolia. Zhongguo Zhong Yao Za Zhi 2023, 48, 6039–6050. [Google Scholar] [PubMed]
- Goswami, A.; Mitra, A. Light spectra manipulation stimulates growth, specialized metabolites and nutritional quality in Anethum graveolens. J. Photochem. Photobiol. B 2023, 249, 112812. [Google Scholar] [CrossRef] [PubMed]
- Ciriello, M.; Cirillo, V.; Formisano, L.; De Pascale, S.; Romano, R.; Fusco, G.M.; Nicastro, R.; Carillo, P.; Kyriacou, M.C.; Soteriou, G.A.; et al. Salt-Induced Stress Impacts the Phytochemical Composition and Aromatic Profile of Three Types of Basil in a Genotype-Dependent Mode. Plants 2023, 12, 2167. [Google Scholar] [CrossRef]
- Wei, J.; Yang, Y.; Peng, Y.; Wang, S.; Zhang, J.; Liu, X.; Liu, J.; Wen, B.; Li, M. Biosynthesis and the Transcriptional Regulation of Terpenoids in Tea Plants (Camellia sinensis). Int. J. Mol. Sci. 2023, 24, 6937. [Google Scholar] [CrossRef]
- Kianersi, F.; Pour-Aboughadareh, A.; Majdi, M.; Poczai, P. Effect of Methyl Jasmonate on Thymol, Carvacrol, Phytochemical Accumulation, and Expression of Key Genes Involved in Thymol/Carvacrol Biosynthetic Pathway in Some Iranian Thyme Species. Int. J. Mol. Sci. 2021, 22, 11124. [Google Scholar] [CrossRef] [PubMed]
- Arimura, G.-I.; Garms, S.; Maffei, M.; Bossi, S.; Schulze, B.; Leitner, M.; Mithöfer, A.; Boland, W. Herbivore-induced terpenoid emission in Medicago truncatula: Concerted action of jasmonate, ethylene and calcium signaling. Planta 2008, 227, 453–464. [Google Scholar] [CrossRef]
- Ghorbel, M.; Brini, F.; Sharma, A.; Landi, M. Role of jasmonic acid in plants: The molecular point of view. Plant Cell Rep. 2021, 40, 1471–1494. [Google Scholar] [CrossRef]
- Feng, K.; Yan, Y.-J.; Sun, N.; Yang, Z.-Y.; Zhao, S.-P.; Wu, P.; Li, L.-J. Exogenous methyl jasmonate treatment induced the transcriptional responses and accumulation of volatile terpenoids in Oenanthe javanica (Blume) DC. Int. J. Biol. Macromol. 2024, 265, 131017. [Google Scholar] [CrossRef]
- Shi, J.; Wang, J.; Lv, H.; Peng, Q.; Schreiner, M.; Baldermann, S.; Lin, Z. Integrated proteomic and metabolomic analyses reveal the importance of aroma precursor accumulation and storage in methyl jasmonate-primed tea leaves. Hortic. Res. 2021, 8, 95. [Google Scholar] [CrossRef]
- D’Onofrio, C.; Matarese, F.; Cuzzola, A. Effect of methyl jasmonate on the aroma of Sangiovese grapes and wines. Food Chem. 2018, 242, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, Y.; Shi, C.; Huang, Z.; Zhang, Y.; Li, S.; Li, Y.; Ye, J.; Yu, C.; Li, Z.; et al. SOAPnuke: A MapReduce acceleration-supported software for integrated quality control and preprocessing of high-throughput sequencing data. Gigascience 2018, 7, gix120. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.-C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, H. Predicting Secretory Proteins with SignalP. Methods Mol. Biol. 2017, 1611, 59–73. [Google Scholar]
- Gupta, R.; Brunak, S. Prediction of glycosylation across the human proteome and the correlation to protein function. Pac. Symp. Biocomput. 2002, 7, 310–322. [Google Scholar]
- Blom, N.; Gammeltoft, S.; Brunak, S. Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J. Mol. Biol. 1999, 294, 1351–1362. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; De Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, w296–w303. [Google Scholar] [CrossRef] [PubMed]
- Forli, S.; Huey, R.; E Pique, M.; Sanner, M.F.; Goodsell, D.S.; Olson, A.J. Computational protein-ligand docking and virtual drug screening with the AutoDock suite. Nat. Protoc. 2016, 11, 905–919. [Google Scholar] [CrossRef] [PubMed]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Söding, J.; et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Wasternack, C.; Strnad, M. Jasmonates are signals in the biosynthesis of secondary metabolites—Pathways, transcription factors and applied aspects—A brief review. New Biotechnol. 2019, 48, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cappellari, L.D.R.; Santoro, M.V.; Schmidt, A.; Gershenzon, J.; Banchio, E. Induction of essential oil production in Mentha x piperita by plant growth promoting bacteria was correlated with an increase in jasmonate and salicylate levels and a higher density of glandular trichomes. Plant Physiol. Biochem. 2019, 141, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Majdi, M.; Malekzadeh-Mashhady, A.; Maroufi, A.; Crocoll, C. Tissue-specific gene-expression patterns of genes associated with thymol/carvacrol biosynthesis in thyme (Thymus vulgaris L.) and their differential changes upon treatment with abiotic elicitors. Plant Physiol. Biochem. 2017, 115, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Yan, T.; Shen, Q.; Lu, X.; Pan, Q.; Huang, Y.; Tang, Y.; Fu, X.; Liu, M.; Jiang, W.; et al. GLANDULAR TRICHOME-SPECIFIC WRKY 1 promotes artemisinin biosynthesis in Artemisia annua. New Phytol. 2017, 214, 304–316. [Google Scholar] [CrossRef]
- Fu, X.; Peng, B.; Hassani, D.; Xie, L.; Liu, H.; Li, Y.; Chen, T.; Liu, P.; Tang, Y.; Li, L.; et al. AaWRKY9 contributes to light- and jasmonate-mediated to regulate the biosynthesis of artemisinin in Artemisia annua. New Phytol. 2021, 231, 1858–1874. [Google Scholar] [CrossRef]
- Chen, T.; Li, Y.; Xie, L.; Hao, X.; Liu, H.; Qin, W.; Wang, C.; Yan, X.; Wu-Zhang, K.; Yao, X.; et al. AaWRKY17, a positive regulator of artemisinin biosynthesis, is involved in resistance to Pseudomonas syringae in Artemisia annua. Hortic. Res. 2021, 8, 217. [Google Scholar] [CrossRef]
- Xie, L.; Yan, T.; Li, L.; Chen, M.; Ma, Y.; Hao, X.; Fu, X.; Shen, Q.; Huang, Y.; Qin, W.; et al. The WRKY transcription factor AaGSW2 promotes glandular trichome initiation in Artemisia annua. J. Exp. Bot. 2021, 72, 1691–1701. [Google Scholar] [CrossRef]
- Yuan, M.; Shu, G.; Zhou, J.; He, P.; Xiang, L.; Yang, C.; Chen, M.; Liao, Z.; Zhang, F. AabHLH113 integrates jasmonic acid and abscisic acid signaling to positively regulate artemisinin biosynthesis in Artemisia annua. New Phytol. 2023, 237, 885–899. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.X.; Li, J.X.; Yang, C.Q.; Hu, W.L.; Wang, L.J.; Chen, X.Y. The jasmonate-responsive AP2/ERF transcription factors AaERF1 and AaERF2 positively regulate artemisinin biosynthesis in Artemisia annua L. Mol. Plant 2012, 5, 353–365. [Google Scholar] [CrossRef]
- Paremskaia, A.I.; Kogan, A.A.; Murashkina, A.; Naumova, D.A.; Satish, A.; Abramov, I.S.; Feoktistova, S.G.; Mityaeva, O.N.; Deviatkin, A.A.; Volchkov, P.Y. Codon-optimization in gene therapy: Promises, prospects and challenges. Front. Bioeng. Biotechnol. 2024, 12, 1371596. [Google Scholar] [CrossRef] [PubMed]
- Loyevsky, M.; Mompoint, F.; Yikilmaz, E.; Altschul, S.F.; Madden, T.; Wootton, J.C.; Kurantsin-Mills, J.; Kassim, O.O.; Gordeuk, V.R.; Rouault, T.A. Expression of a recombinant IRP-like Plasmodium falciparum protein that specifically binds putative plasmodial IREs. Mol. Biochem. Parasitol. 2003, 126, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Burgess-Brown, N.A.; Sharma, S.; Sobott, F.; Loenarz, C.; Oppermann, U.; Gileadi, O. Codon optimization can improve expression of human genes in Escherichia coli: A multi-gene study. Protein Expr. Purif. 2008, 59, 94–102. [Google Scholar] [CrossRef]
- Simmons, L.C.; Yansura, D.G. Translational level is a critical factor for the secretion of heterologous proteins in Escherichia coli. Nat. Biotechnol. 1996, 14, 629–634. [Google Scholar] [CrossRef]
- Wu, X.; Jörnvall, H.; Berndt, K.D.; Oppermann, U. Codon optimization reveals critical factors for high level expression of two rare codon genes in Escherichia coli: RNA stability and secondary structure but not tRNA abundance. Biochem. Biophys. Res. Commun. 2004, 313, 89–96. [Google Scholar] [CrossRef]
- Grundy, D.J.; Chen, M.; González, V.; Leoni, S.; Miller, D.J.; Christianson, D.W.; Allemann, R.K. Mechanism of Germacradien-4-ol Synthase-Controlled Water Capture. Biochemistry 2016, 55, 2112–2121. [Google Scholar] [CrossRef]
- Hong, Y.J.; Irmisch, S.; Wang, S.C.; Garms, S.; Gershenzon, J.; Zu, L.; Köllner, T.G.; Tantillo, D.J. Theoretical and experimental analysis of the reaction mechanism of MrTPS2, a triquinane-forming sesquiterpene synthase from chamomile. Chemistry 2013, 19, 13590–13600. [Google Scholar] [CrossRef] [PubMed]
- Garms, S.; Köllner, T.G.; Boland, W. A multiproduct terpene synthase from Medicago truncatula generates cadalane sesquiterpenes via two different mechanisms. J. Org. Chem. 2010, 75, 5590–5600. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Chou, W.K.W.; Himmelberger, J.A.; Litwin, K.M.; Harris, G.G.; Cane, D.E.; Christianson, D.W. Reprogramming the chemodiversity of terpenoid cyclization by remolding the active site contour of epi-isozizaene synthase. Biochemistry 2014, 53, 1155–1168. [Google Scholar] [CrossRef] [PubMed]
- Garms, S.; Chen, F.; Boland, W.; Gershenzon, J.; Köllner, T.G. A single amino acid determines the site of deprotonation in the active center of sesquiterpene synthases SbTPS1 and SbTPS2 from Sorghum bicolor. Phytochemistry 2012, 75, 6–13. [Google Scholar] [CrossRef]
- Li, J.-X.; Fang, X.; Zhao, Q.; Ruan, J.-X.; Yang, C.-Q.; Wang, L.-J.; Miller, D.J.; Faraldos, J.A.; Allemann, R.K.; Chen, X.-Y.; et al. Rational engineering of plasticity residues of sesquiterpene synthases from Artemisia annua: Product specificity and catalytic efficiency. Biochem. J. 2013, 451, 417–426. [Google Scholar] [CrossRef]

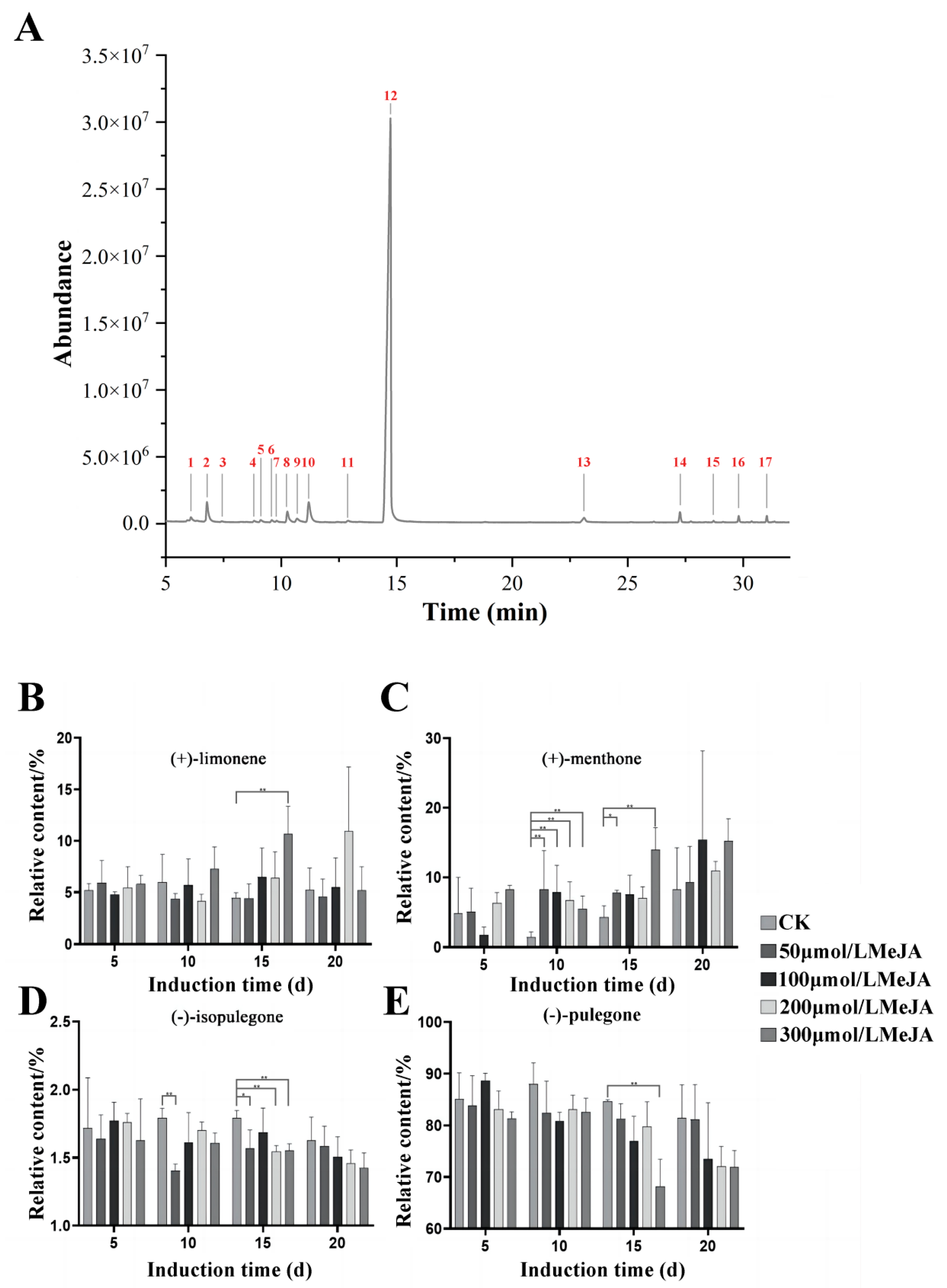


| No. | Compound | Chemical Formula | CAS | RT/min | Relative Content/% |
|---|---|---|---|---|---|
| 1 | β-Myrcene | C10H16 | 123-35-3 | 6.090 | 0.691 |
| 2 | d-Limonene | C10H16 | 89-27-5 | 6.784 | 4.327 |
| 3 | γ-Terpinene | C10H16 | 99-85-4 | 7.461 | 0.184 |
| 4 | 1-octen-3-yl-acetate | C10H18O2 | 2442-10-6 | 8.869 | 0.313 |
| 5 | Trans-p-mentha-2,8-dien-1-ol | C10H16O | 52154-82-2 | 9.190 | 0.303 |
| 6 | Cis-p-mentha-2,8-dien-1-ol | C10H16O | 22771-44-4 | 9.678 | 0.213 |
| 7 | (−)-Carveol | C10H16O | 99-48-9 | 9.854 | 0.183 |
| 8 | (+)-Menthone | C10H18O | 3391-87-5 | 10.265 | 2.268 |
| 9 | (+)-Menthofuran | C10H14O | 17957-94-7 | 10.685 | 0.842 |
| 10 | (−)-Isopulegone | C10H16O | 29606-79-9 | 11.190 | 3.737 |
| 11 | (−)-Verbenone | C10H14O | 1196-01-6 | 12.956 | 0.231 |
| 12 | (−)-Pulegone | C10H16O | 89-82-7 | 14.724 | 84.215 |
| 13 | Piperitenone | C10H14O | 491-09-8 | 23.108 | 0.557 |
| 14 | β-Caryophyllene | C15H24 | 87-44-5 | 27.264 | 1.152 |
| 15 | Germacrene D | C15H24 | 23986-74-5 | 28.802 | 0.161 |
| 16 | β-Copaene | C15H24 | 3856-25-5 | 29.806 | 0.611 |
| 17 | Cycloheptasiloxane, tetradecamethyl- | C14H42O7Si7 | 107-50-6 | 31.024 | 0.241 |
| Transcription Factor | Name | E Value | Grade | Hit Start | Hit End | Gene | CK (FPKM) | LM (FPKM) | HM (FPKM) |
|---|---|---|---|---|---|---|---|---|---|
| AtMYC2 | HiC_scaffold_6 | 4.04 × 10−38 | 41.6% | 3,066,989 | 3,067,216 | Sch000027144 | 15.22 ± 3.76 | 11.95 ± 2.46 | 13.31 ± 6.17 |
| HiC_scaffold_6 | 8.90 × 10−34 | 41.2% | 3,066,650 | 3,066,855 | Sch000027144 | 15.22 ± 3.76 | 11.95 ± 2.46 | 13.31 ± 6.17 | |
| HiC_scaffold_3 | 2.55 × 10−34 | 41.0% | 14,757,041 | 14,757,259 | Sch000015079 | 40.05 ± 4.31 | 39.08 ± 9.47 | 30.44 ± 3.88 | |
| HiC_scaffold_3 | 1.24 × 10−25 | 40.5% | 55,950,077 | 55,949,895 | Sch000013929 | 11.11 ± 3.87 | 7.92 ± 1.8 | 6.31 ± 0.21 | |
| HiC_scaffold_3 | 2.09 × 10−35 | 40.1% | 14,756,591 | 14,756,862 | Sch000015079 | 40.05 ± 4.31 | 39.08 ± 9.47 | 30.44 ± 3.88 | |
| HiC_scaffold_3 | 5.26 × 10−24 | 39.9% | 78,128,356 | 78,128,177 | Sch000013364 | 14.16 ± 1.58 | 17.29 ± 3.02 | 13.94 ± 0.43 | |
| AaWRKY1 | HiC_scaffold_508 | 1.17 × 10−35 | 55.6% | 15,781 | 15,953 | Sch000023850 | 14.11 ± 6.54 | 14.77 ± 5.81 | 20.81 ± 5.47 |
| HiC_scaffold_1 | 1.17 × 10−35 | 55.6% | 6,624,000 | 6,623,828 | Sch000000909 | 14.03 ± 6.35 | 14.63 ± 5.66 | 20.52 ± 5.31 | |
| HiC_scaffold_4 | 5.67 × 10−27 | 52.5% | 94,706,064 | 94,706,195 | Sch000016923 | 11.59 ± 6.83 | 10.23 ± 11.14 | 4.59 ± 4.15 | |
| HiC_scaffold_6 | 1.25 × 10−22 | 52.3% | 77,347,190 | 77,347,360 | Sch000024381 | 2.31 ± 0.81 | 0.25 ± 0.21 | 2.53 ± 2.6 | |
| HiC_scaffold_508 | 8.42 × 10−25 | 52.0% | 14,408 | 14,534 | Sch000023850 | 14.11 ± 6.54 | 14.77 ± 5.81 | 20.81 ± 5.47 | |
| HiC_scaffold_1 | 8.42 × 10−25 | 52.0% | 6,625,373 | 6,625,247 | Sch000000909 | 14.03 ± 6.35 | 14.63 ± 5.66 | 20.52 ± 5.31 | |
| HiC_scaffold_6 | 1.25 × 10−22 | 51.5% | 77,346,493 | 77,346,632 | Sch000024381 | 2.31 ± 0.81 | 0.25 ± 0.21 | 2.53 ± 2.6 | |
| AaWRKY9 | HiC_scaffold_6 | 3.59 × 10−22 | 43.4% | 86,739,994 | 86,740,109 | Sch000027590 | 3.02 ± 2.44 | 1.72 ± 1.12 | 2.36 ± 1.39 |
| AaERF1 | HiC_scaffold_6 | 7.96 × 10−27 | 51.8% | 9,096,280 | 9,096,039 | Sch000027664 | 12.24 ± 13.74 | 15.62 ± 9.39 | 9.69 ± 6.7 |
| AaERF2 | HiC_scaffold_4 | 3.29 × 10−24 | 52.1% | 41,015,067 | 41,015,255 | Sch000018564 | 234.55 ± 190.45 | 148.59 ± 49.31 | 95.03 ± 9.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Jia, C.; Lin, G.; Dang, J.; Liu, C.; Wu, Q. Impact of Methyl Jasmonate on Terpenoid Biosynthesis and Functional Analysis of Sesquiterpene Synthesis Genes in Schizonepeta tenuifolia. Plants 2024, 13, 1920. https://doi.org/10.3390/plants13141920
Li D, Jia C, Lin G, Dang J, Liu C, Wu Q. Impact of Methyl Jasmonate on Terpenoid Biosynthesis and Functional Analysis of Sesquiterpene Synthesis Genes in Schizonepeta tenuifolia. Plants. 2024; 13(14):1920. https://doi.org/10.3390/plants13141920
Chicago/Turabian StyleLi, Dishuai, Congling Jia, Guyin Lin, Jingjie Dang, Chanchan Liu, and Qinan Wu. 2024. "Impact of Methyl Jasmonate on Terpenoid Biosynthesis and Functional Analysis of Sesquiterpene Synthesis Genes in Schizonepeta tenuifolia" Plants 13, no. 14: 1920. https://doi.org/10.3390/plants13141920
APA StyleLi, D., Jia, C., Lin, G., Dang, J., Liu, C., & Wu, Q. (2024). Impact of Methyl Jasmonate on Terpenoid Biosynthesis and Functional Analysis of Sesquiterpene Synthesis Genes in Schizonepeta tenuifolia. Plants, 13(14), 1920. https://doi.org/10.3390/plants13141920







