Hyperspectral Indices Developed from Fractional-Order Derivative Spectra Improved Estimation of Leaf Chlorophyll Fluorescence Parameters
Abstract
1. Introduction
2. Materials and Methods
2.1. Measurements and Data Preparation
2.2. Data Processing and Developing New Indices
2.3. Statistical Analysis
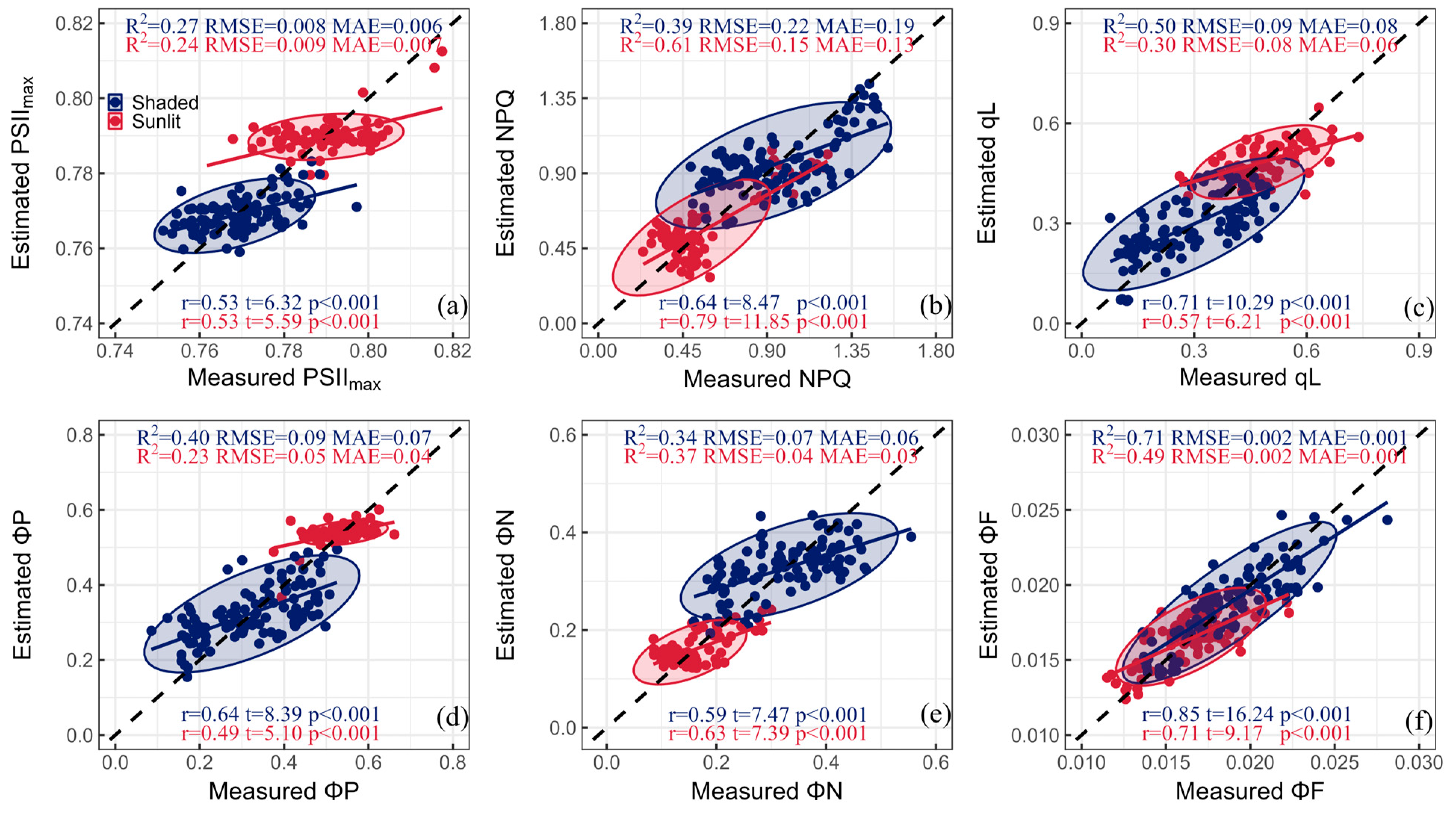
3. Results
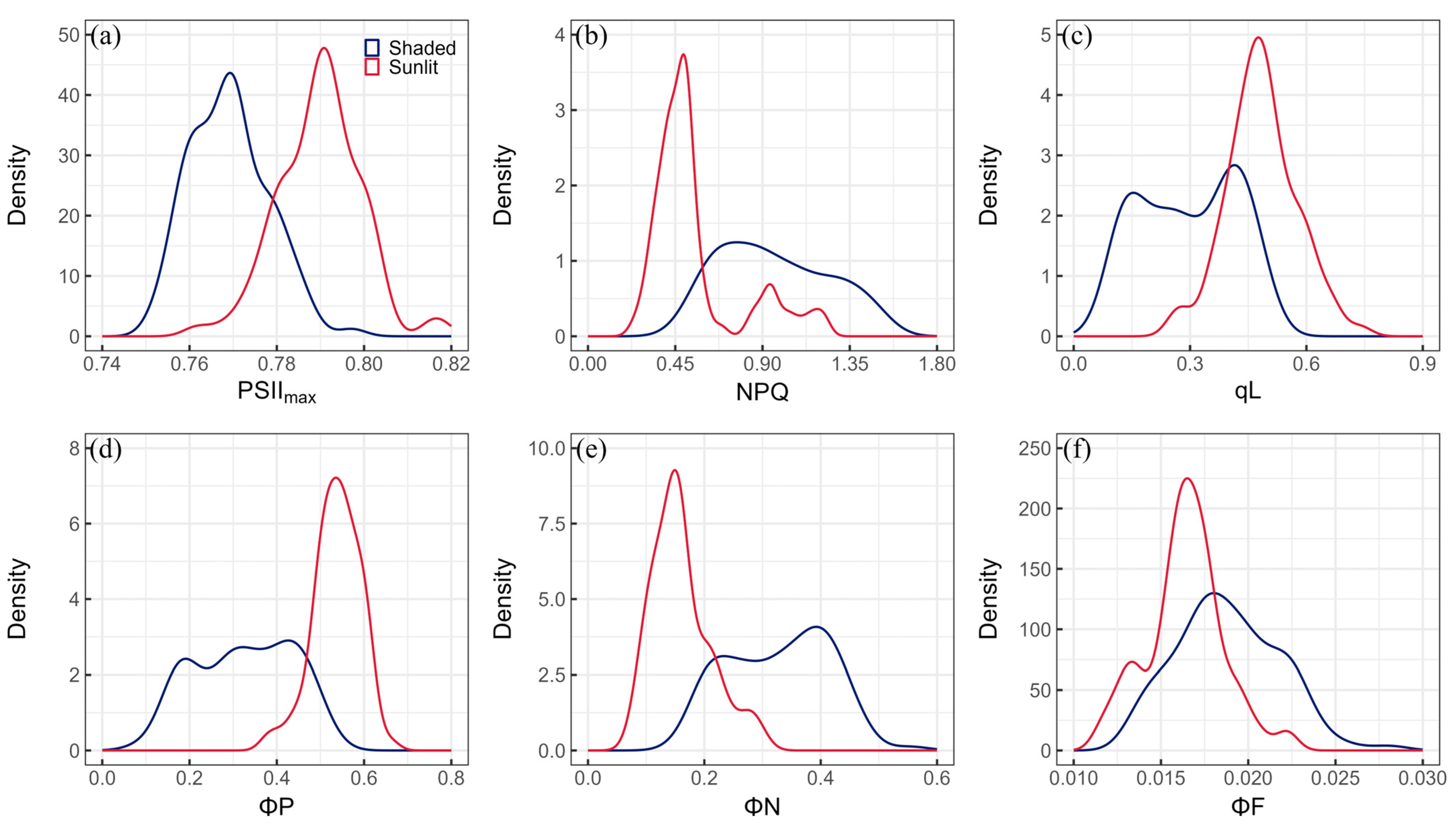
3.1. Statistical Descriptions of ChlF Parameters
3.2. Performance of Published Spectral Indices
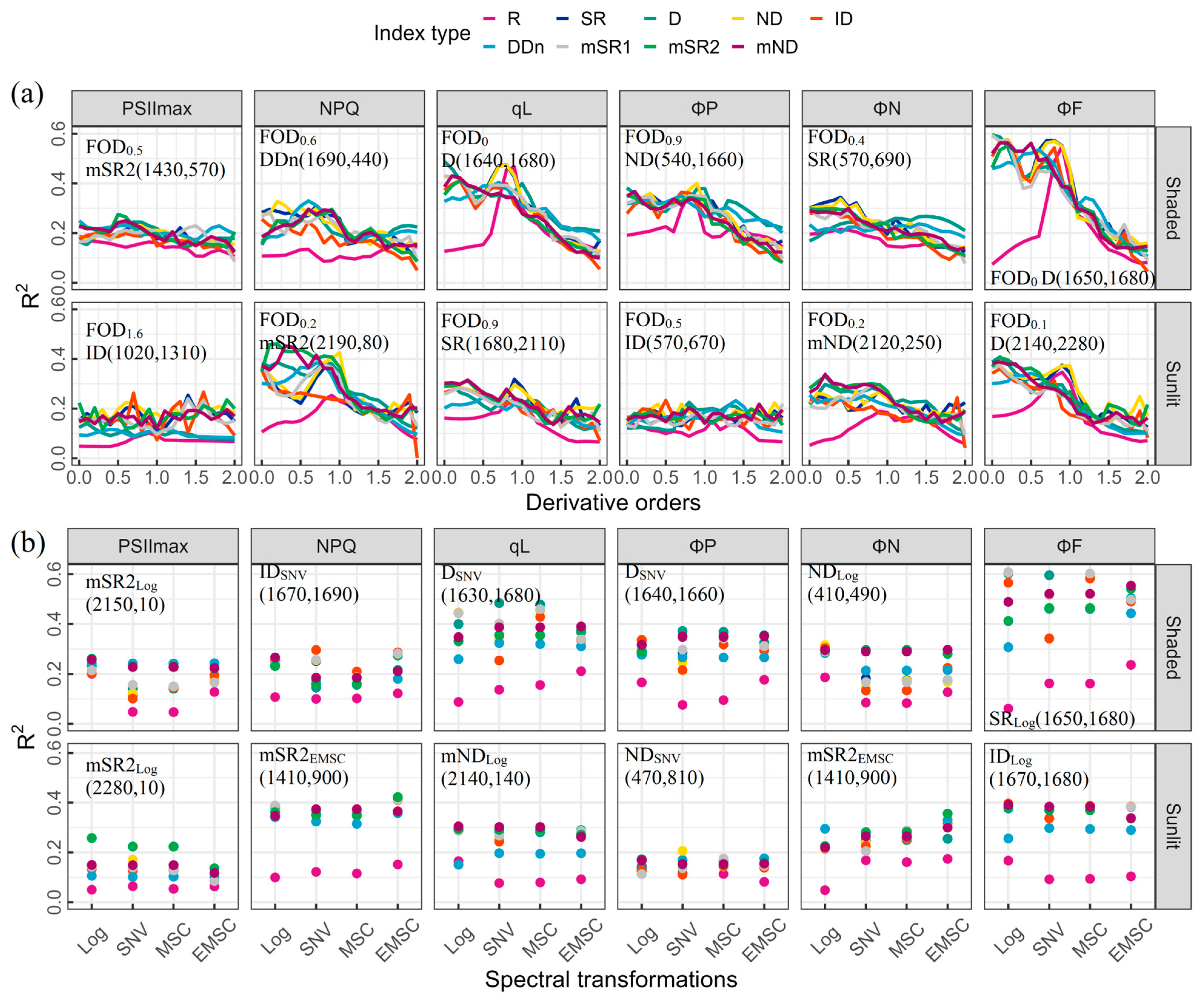
3.3. Performance of Spectral Indices Derived from Different FOD Spectra and Spectral Transformations
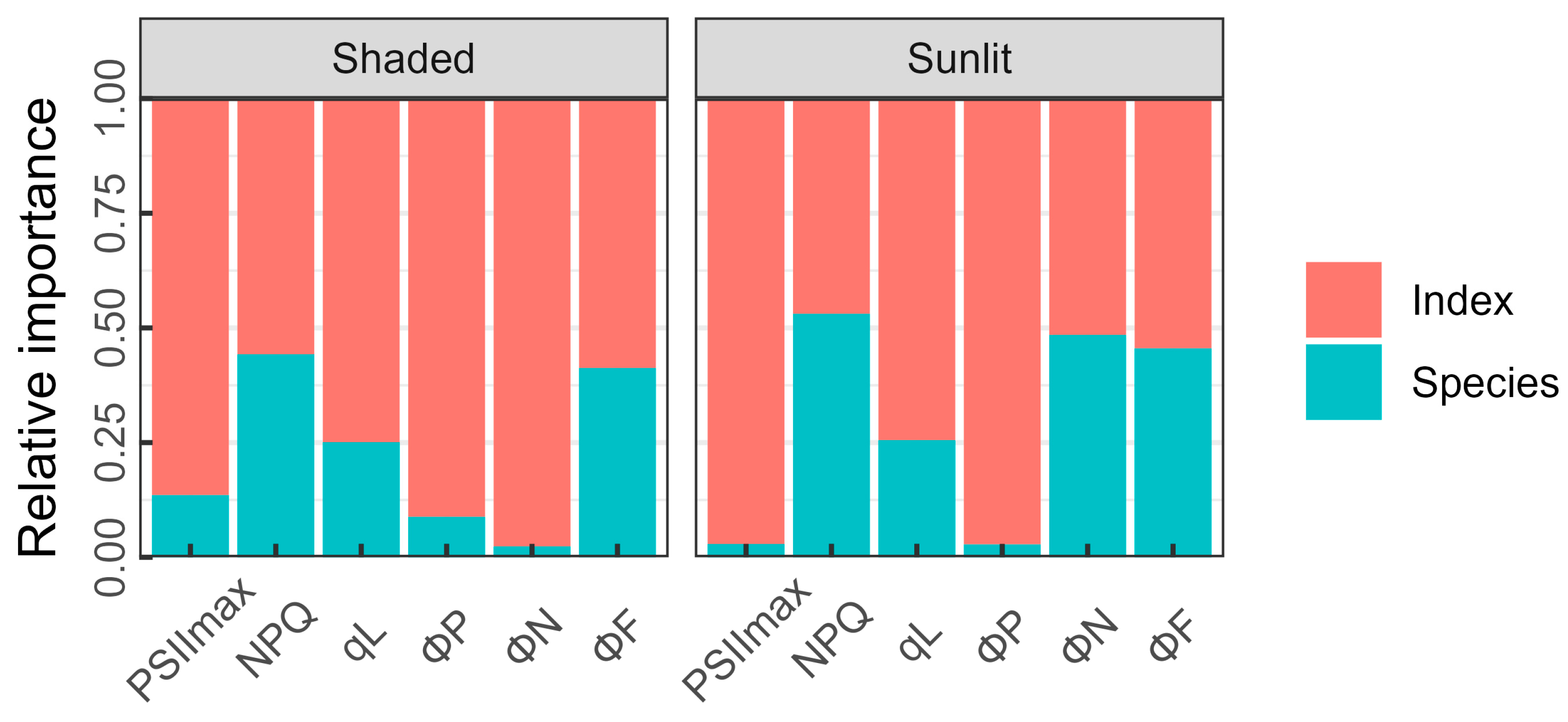
3.4. Considering the Effect of Specific Species on ChlF Parameters’ Estimation
4. Discussion
4.1. FOD Spectra and Spectral Transformations Optimize the Performance of Spectral Indices in Estimating ChlF Parameters
4.2. Effect of Species Specificity on the Estimation of ChlF Parameters
4.3. Uncertainty and Prospects
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef] [PubMed]
- Porcar-Castell, A.; Tyystjärvi, E.; Atherton, J.; van der Tol, C.; Flexas, J.; Pfündel, E.E.; Moreno, J.; Frankenberg, C.; Berry, J.A. Linking chlorophyll a fluorescence to photosynthesis for remote sensing applications: Mechanisms and challenges. J. Exp. Bot. 2014, 65, 4065–4095. [Google Scholar] [CrossRef] [PubMed]
- Krause, G.H.; Weis, E. Chlorophyll fluorescence as a tool in plant physiology. Photosynth. Res. 1984, 5, 139–157. [Google Scholar] [CrossRef] [PubMed]
- Butler, W.L. Energy Distribution in the Photochemical Apparatus of Photosynthesis. Annu. Rev. Plant Physiol. 1978, 29, 345–378. [Google Scholar] [CrossRef]
- Oxborough, K.; Baker, N.R. Resolving chlorophyll a fluorescence images of photosynthetic efficiency into photochemical and non-photochemical components—Calculation of qP and Fv-/Fm-; without measuring Fo. Photosynth. Res. 1997, 54, 135–142. [Google Scholar] [CrossRef]
- Miyake, C.; Amako, K.; Shiraishi, N.; Sugimoto, T. Acclimation of tobacco leaves to high light intensity drives the plastoquinone oxidation system—Relationship among the fraction of open PSII centers, non-photochemical quenching of Chl fluorescence and the maximum quantum yield of PSII in the dark. Plant Cell Physiol. 2009, 50, 730–743. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, M.; Butler, W.L. Quenching of chlorophyll fluorescence and primary photochemistry in chloroplasts by dibromothymoquinone. Biochim. Biophys. Acta (BBA) Bioenerg. 1975, 376, 105–115. [Google Scholar] [CrossRef]
- Bilger, W.; Björkman, O. Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis. Photosynth. Res. 1990, 25, 173–185. [Google Scholar] [CrossRef]
- Mohammed, G.H.; Colombo, R.; Middleton, E.M.; Rascher, U.; van der Tol, C.; Nedbal, L.; Goulas, Y.; Pérez-Priego, O.; Damm, A.; Meroni, M.; et al. Remote sensing of solar-induced chlorophyll fluorescence (SIF) in vegetation: 50 years of progress. Remote Sens. Environ. 2019, 231, 111177. [Google Scholar] [CrossRef]
- Meroni, M.; Rossini, M.; Guanter, L.; Alonso, L.; Rascher, U.; Colombo, R.; Moreno, J. Remote sensing of solar-induced chlorophyll fluorescence: Review of methods and applications. Remote Sens. Environ. 2009, 113, 2037–2051. [Google Scholar] [CrossRef]
- Song, Y.; Wang, L.; Wang, J. Improved understanding of the spatially-heterogeneous relationship between satellite solar-induced chlorophyll fluorescence and ecosystem productivity. Ecol. Indic. 2021, 129, 107949. [Google Scholar] [CrossRef]
- Yang, J.C.; Magney, T.S.; Albert, L.P.; Richardson, A.D.; Frankenberg, C.; Stutz, J.; Grossmann, K.; Burns, S.P.; Seyednasrollah, B.; Blanken, P.D.; et al. Gross primary production (GPP) and red solar induced fluorescence (SIF) respond differently to light and seasonal environmental conditions in a subalpine conifer forest. Agric. For. Meteorol. 2022, 317, 108904. [Google Scholar] [CrossRef]
- Verma, M.; Schimel, D.; Evans, B.; Frankenberg, C.; Beringer, J.; Drewry, D.T.; Magney, T.; Marang, I.; Hutley, L.; Moore, C.; et al. Effect of environmental conditions on the relationship between solar-induced fluorescence and gross primary productivity at an OzFlux grassland site. J. Geophys. Res. Biogeosci. 2017, 122, 716–733. [Google Scholar] [CrossRef]
- Zhang, Y.; Migliavacca, M.; Penuelas, J.; Ju, W. Advances in hyperspectral remote sensing of vegetation traits and functions. Remote Sens. Environ. 2021, 252, 112121. [Google Scholar] [CrossRef]
- Hallik, L.; Niinemets, Ü.; Kull, O. Photosynthetic acclimation to light in woody and herbaceous species: A comparison of leaf structure, pigment content and chlorophyll fluorescence characteristics measured in the field. Plant Biol. 2012, 14, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Dorigo, W.A.; Zurita-Milla, R.; de Wit, A.J.W.; Brazile, J.; Singh, R.; Schaepman, M.E. A review on reflective remote sensing and data assimilation techniques for enhanced agroecosystem modeling. Int. J. Appl. Earth Obs. Geoinf. 2007, 9, 165–193. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, H.; Zhang, X.; Wang, K.; Song, T.; Zeng, F. Detecting Suaeda salsa L. chlorophyll fluorescence response to salinity stress by using hyperspectral reflectance. Acta Physiol. Plant. 2012, 34, 581–588. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Pushnik, J.C.; Dobrowski, S.; Ustin, S.L. Steady-state chlorophyll a fluorescence detection from canopy derivative reflectance and double-peak red-edge effects. Remote Sens. Environ. 2003, 84, 283–294. [Google Scholar] [CrossRef]
- Stratoulias, D.; Balzter, H.; Zlinszky, A.; Tóth, V.R. Assessment of ecophysiology of lake shore reed vegetation based on chlorophyll fluorescence, field spectroscopy and hyperspectral airborne imagery. Remote Sens. Environ. 2015, 157, 72–84. [Google Scholar] [CrossRef]
- Porcar-Castell, A.; Garcia-Plazaola, J.I.; Nichol, C.J.; Kolari, P.; Olascoaga, B.; Kuusinen, N.; Fernández-Marín, B.; Pulkkinen, M.; Juurola, E.; Nikinmaa, E. Physiology of the seasonal relationship between the photochemical reflectance index and photosynthetic light use efficiency. Oecologia 2012, 170, 313–323. [Google Scholar] [CrossRef]
- Sonobe, R.; Wang, Q. Assessing hyperspectral indices for tracing chlorophyll fluorescence parameters in deciduous forests. J. Environ. Manag. 2018, 227, 172–180. [Google Scholar] [CrossRef]
- Song, G.; Wang, Q.; Zhuang, J.; Jin, J. Timely estimation of leaf chlorophyll fluorescence parameters under varying light regimes by coupling light drivers to leaf traits. Physiol. Plant. 2023, 175, e14048. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Tang, W.; Zhang, C.; Zhou, L.; Feng, L.; Shen, J.; Yan, T.; Gao, P.; He, Y.; Wu, N. Spectral preprocessing combined with deep transfer learning to evaluate chlorophyll content in cotton leaves. Plant Phenomics 2022, 2022, 9813841. [Google Scholar] [CrossRef]
- Zhuang, J.; Wang, Q.; Song, G.; Jin, J. Validating and developing hyperspectral indices for tracing leaf chlorophyll fluorescence parameters under varying light conditions. Remote Sens. 2023, 15, 4890. [Google Scholar] [CrossRef]
- Zheng, K.-Y.; Zhang, X.; Tong, P.-J.; Yao, Y.; Du, Y.-P. Pretreating near infrared spectra with fractional order Savitzky–Golay differentiation (FOSGD). Chin. Chem. Lett. 2015, 26, 293–296. [Google Scholar] [CrossRef]
- Jin, J.; Wang, Q. Hyperspectral indices developed from the low order fractional derivative spectra can capture leaf dry matter content across a variety of species better. Agric. For. Meteorol. 2022, 322, 109007. [Google Scholar] [CrossRef]
- Xu, J.; Fu, G.; Yan, L.; Yu, L.; Kuang, F.; Huang, Q.; Tang, X. Estimation of the relative chlorophyll content of Carya illinoensis leaves using fractional order derivative of leaf and canopy scale hyperspectral data. J. Soil Sci. Plant Nutr. 2024, 24, 1407–1423. [Google Scholar] [CrossRef]
- Abulaiti, Y.; Sawut, M.; Maimaitiaili, B.; Chunyue, M. A possible fractional order derivative and optimized spectral indices for assessing total nitrogen content in cotton. Comput. Electron. Agric. 2020, 171, 105275. [Google Scholar] [CrossRef]
- Song, G.; Wang, Q.; Jin, J. Fractional-order derivative spectral transformations improved partial least squares regression estimation of photosynthetic capacity from hyperspectral reflectance. IEEE Trans. Geosci. Remote Sens. 2023, 61, 5510110. [Google Scholar] [CrossRef]
- Foley, S.; Rivard, B.; Sanchez-Azofeifa, G.A.; Calvo, J. Foliar spectral properties following leaf clipping and implications for handling techniques. Remote Sens. Environ. 2006, 103, 265–275. [Google Scholar] [CrossRef]
- Song, G.; Wang, Q.; Jin, J. Estimation of leaf photosynthetic capacity parameters using spectral indices developed from fractional-order derivatives. Comput. Electron. Agric. 2023, 212, 108068. [Google Scholar] [CrossRef]
- Groemping, U. Relative importance for linear regression in R: The package relaimpo. J. Stat. Softw. 2006, 17, 1–27. [Google Scholar] [CrossRef]
- Naumann, J.C.; Young, D.R.; Anderson, J.E. Leaf chlorophyll fluorescence, reflectance, and physiological response to freshwater and saltwater flooding in the evergreen shrub, Myrica cerifera. Environ. Exp. Bot. 2008, 63, 402–409. [Google Scholar] [CrossRef]
- Zhuang, J.; Wang, Y.; Chi, Y.; Zhou, L.; Chen, J.; Zhou, W.; Song, J.; Zhao, N.; Ding, J. Drought stress strengthens the link between chlorophyll fluorescence parameters and photosynthetic traits. PeerJ 2020, 8, e10046. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Tian, J.; Feng, K.; Gong, X.; Liu, J. Application of a hyperspectral imaging system to quantify leaf-scale chlorophyll, nitrogen and chlorophyll fluorescence parameters in grapevine. Plant Physiol. Biochem. 2021, 166, 723–737. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Lu, X.; Li, Y.; Li, S.; Zhang, Y. Hyperspectral identification of chlorophyll fluorescence parameters of suaeda salsa in coastal wetlands. Remote Sens. 2021, 13, 2066. [Google Scholar] [CrossRef]
- Jin, J.; Wang, Q.; Song, G. Selecting informative bands for partial least squares regressions improves their goodness-of-fits to estimate leaf photosynthetic parameters from hyperspectral data. Photosynth. Res. 2022, 151, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Tsai, F.; Philpot, W. Derivative analysis of hyperspectral data. Remote Sens. Environ. 1998, 66, 41–51. [Google Scholar] [CrossRef]
- Wen, S.; Shi, N.; Lu, J.; Gao, Q.; Yang, H.; Gao, Z. Estimating chlorophyll fluorescence parameters of rice (Oryza sativa L.) based on spectrum transformation and a joint feature extraction algorithm. Agronomy 2023, 13, 337. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Kull, O. Sensitivity of photosynthetic electron transport to photoinhibition in a temperate deciduous forest canopy: Photosystem II center openness, non-radiative energy dissipation and excess irradiance under field conditions. Tree Physiol. 2001, 21, 899–914. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams Iii, W.W. Photoprotection in an ecological context: The remarkable complexity of thermal energy dissipation. New Phytol. 2006, 172, 11–21. [Google Scholar] [CrossRef]
- Peterson, R.B.; Oja, V.; Eichelmann, H.; Bichele, I.; Dall’Osto, L.; Laisk, A. Fluorescence F0 of photosystems II and I in developing C3 and C4 leaves, and implications on regulation of excitation balance. Photosynth. Res. 2014, 122, 41–56. [Google Scholar] [CrossRef]
- Cordon, G.; Lagorio, M.G.; Paruelo, J.M. Chlorophyll fluorescence, photochemical reflective index and normalized difference vegetative index during plant senescence. J. Plant Physiol. 2016, 199, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Vítek, P.; Mishra, K.B.; Mishra, A.; Veselá, B.; Findurová, H.; Svobodová, K.; Oravec, M.; Sahu, P.P.; Klem, K. Non-destructive insights into photosynthetic and photoprotective mechanisms in Arabidopsis thaliana grown under two light regimes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 281, 121531. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, Y.; Tomasetto, F.; Yan, W.; Tan, Z.; Liu, J.; Jiang, J. Non-destructive measurements of Toona sinensis chlorophyll and nitrogen content under drought stress using near infrared spectroscopy. Front. Plant Sci. 2022, 12, 809828. [Google Scholar] [CrossRef] [PubMed]
- Zarco-Tejada, P.J.; Miller, J.R.; Mohammed, G.H.; Noland, T.L. Chlorophyll Fluorescence Effects on Vegetation Apparent Reflectance: I. Leaf-Level Measurements and Model Simulation. Remote Sens. Environ. 2000, 74, 582–595. [Google Scholar] [CrossRef]
- Rajewicz, P.A.; Zhang, C.; Atherton, J.; Van Wittenberghe, S.; Riikonen, A.; Magney, T.; Fernandez-Marin, B.; Plazaola, J.I.G.; Porcar-Castell, A. The photosynthetic response of spectral chlorophyll fluorescence differs across species and light environments in a boreal forest ecosystem. Agric. For. Meteorol. 2023, 334, 109434. [Google Scholar] [CrossRef]
- Yi, P.; Aoli, Z.; Tinge, Z.; Shenghui, F.; Yan, G.; Yanqi, T.; Ying, Z.; Kan, L. Using remotely sensed spectral reflectance to indicate leaf photosynthetic efficiency derived from active fluorescence measurements. J. Appl. Remote Sens. 2017, 11, 026034. [Google Scholar] [CrossRef]
- Van Gaalen, K.E.; Flanagan, L.B.; Peddle, D.R. Photosynthesis, chlorophyll fluorescence and spectral reflectance in Sphagnum moss at varying water contents. Oecologia 2007, 153, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Wang, Q.; Zhuang, J.; Jin, J. Dynamics of leaf chlorophyll fluorescence parameters can well be tracked by coupling VIS-NIR-SWIR hyperspectral reflectance and light drivers in partial least-squares regression. Sci. Hortic. 2024, 325, 112651. [Google Scholar] [CrossRef]
- Mevy, J.-P.; Biryol, C.; Boiteau-Barral, M.; Miglietta, F. The optical response of a mediterranean shrubland to climate change: Hyperspectral reflectance measurements during spring. Plants 2022, 11, 505. [Google Scholar] [CrossRef] [PubMed]
- Beattie, J.R.; Esmonde-White, F.W.L. Exploration of principal component analysis: Deriving principal component analysis visually using spectra. Appl. Spectrosc. 2021, 75, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Falcioni, R.; Moriwaki, T.; Antunes, W.C.; Nanni, M.R. Rapid quantification method for yield, calorimetric energy and chlorophyll a fluorescence parameters in Nicotiana tabacum L. using Vis-NIR-SWIR hyperspectroscopy. Plants 2022, 11, 2406. [Google Scholar] [CrossRef] [PubMed]
- Falcioni, R.; Antunes, W.C.; Oliveira, R.B.; Chicati, M.L.; Demattê, J.A.M.; Nanni, M.R. Assessment of combined reflectance, transmittance, and absorbance hyperspectral sensors for prediction of chlorophyll a fluorescence parameters. Remote Sens. 2023, 15, 5067. [Google Scholar] [CrossRef]
- Bartold, M.; Kluczek, M. A machine learning approach for mapping chlorophyll fluorescence at inland wetlands. Remote Sens. 2023, 15, 2392. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, L.; Xiao, Q.; Bai, X.; Wu, B.; Wu, N.; Zhao, Y.; Wang, J.; Feng, L. End-to-end fusion of hyperspectral and chlorophyll fluorescence imaging to identify rice stresses. Plant Phenomics 2022, 2022, 9851096. [Google Scholar] [CrossRef]

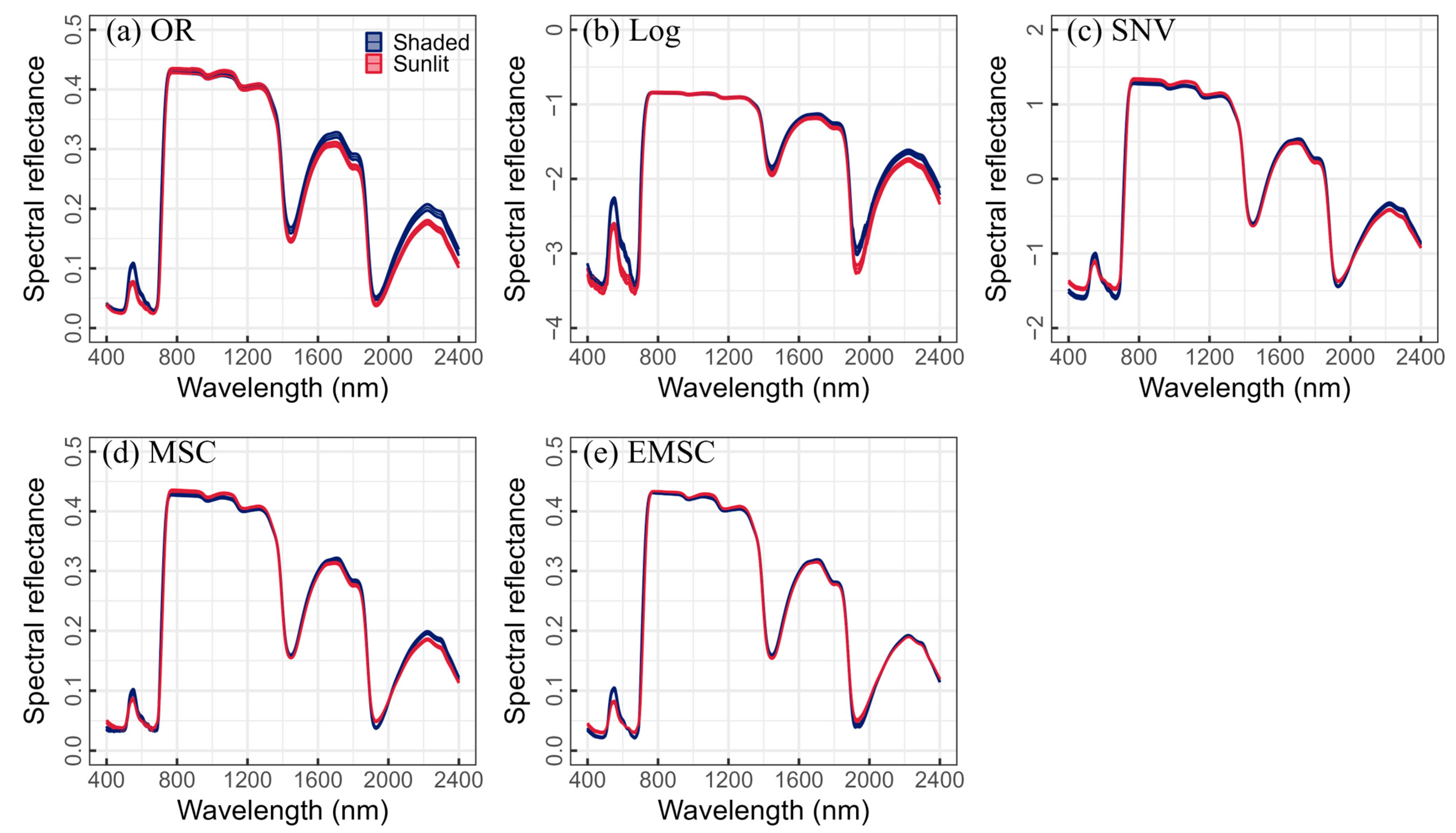
| Parameter | Calculation | Reference |
|---|---|---|
| PSIImax | Kitajima and Butler [7] | |
| NPQ | Bilger and Björkman [8] | |
| qL | Miyake, Amako, Shiraishi, and Sugimoto [6] | |
| Oxborough and Baker [5] | ||
| ΦP | Butler [4] | |
| ΦN | ||
| ΦF | ||
| Index | Wavelength | Formula |
|---|---|---|
| given wavelength (R) | λ1 | |
| simple ratio (SR) | λ1 and λ2 | |
| wavelength difference (D) | λ1 and λ2 | |
| normalized difference (ND) | λ1 and λ2 | |
| inverse differences (ID) | λ1 and λ2 | |
| double differences (DDn) | λ1 and Δλ | 2 |
| modified simple ratio 1 (mSR1) | λ1 and Δλ | ( |
| modified simple ratio 2 (mSR2) | λ1 and Δλ | ( |
| modified normalized difference (mND) | λ1 and Δλ | ( |
| Leaf Group | Parameters | Mean | Median | Minimum | Maximum | CV (%) | Skewness | Kurtosis |
|---|---|---|---|---|---|---|---|---|
| Shaded | PSIImax | 0.77 | 0.77 | 0.75 | 0.80 | 1.16 | 0.40 | −0.18 |
| NPQ | 0.96 | 0.91 | 0.49 | 1.54 | 29.13 | 0.29 | −1.06 | |
| qL | 0.30 | 0.30 | 0.08 | 0.51 | 42.08 | −0.08 | −1.39 | |
| ΦP | 0.33 | 0.33 | 0.09 | 0.53 | 34.12 | −0.13 | −1.19 | |
| ΦN | 0.33 | 0.34 | 0.16 | 0.55 | 26.54 | −0.03 | −0.99 | |
| ΦF | 0.019 | 0.019 | 0.014 | 0.028 | 15.55 | 0.32 | −0.18 | |
| Sunlit | PSIImax | 0.79 | 0.79 | 0.76 | 0.82 | 1.21 | −0.03 | 0.69 |
| NPQ | 0.55 | 0.49 | 0.24 | 1.22 | 42.54 | 1.43 | 1.01 | |
| qL | 0.48 | 0.48 | 0.26 | 0.74 | 18.65 | 0.11 | 0.22 | |
| ΦP | 0.54 | 0.54 | 0.38 | 0.66 | 9.89 | −0.51 | 0.42 | |
| ΦN | 0.16 | 0.15 | 0.09 | 0.30 | 31.23 | 0.84 | 0.21 | |
| ΦF | 0.016 | 0.016 | 0.012 | 0.022 | 13.35 | 0.08 | 0.23 |
| Leaf Group | Index | PSIImax | NPQ | qL | ΦP | ΦN | ΦF | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | RMSE | R2 | RMSE | R2 | RMSE | R2 | RMSE | R2 | RMSE | R2 | RMSE | ||
| Shaded | ARI2 | 0.018 | 0.008 | 0.042 | 0.269 | 0.054 | 0.123 | 0.087 | 0.099 | 0.114 | 0.074 | 0.005 | 0.003 |
| CRI1 | 0.001 | 0.009 | 0.017 | 0.272 | 0.008 | 0.126 | 0.002 | 0.104 | 0.008 | 0.078 | 0.020 | 0.003 | |
| CRI2 | 0.002 | 0.008 | 0.009 | 0.273 | 0.017 | 0.126 | 0.0046 | 0.104 | 0.001 | 0.078 | 0.025 | 0.003 | |
| EVI | 0.037 | 0.008 | 0.032 | 0.270 | 0.022 | 0.125 | 0.012 | 0.103 | 0.001 | 0.078 | 0.056 | 0.003 | |
| OCAR | 0.010 | 0.008 | 0.016 | 0.272 | 0.056 | 0.123 | 0.082 | 0.010 | 0.066 | 0.076 | 0.026 | 0.003 | |
| PRI | 0.052 | 0.008 | 0.003 | 0.275 | 0.103 | 0.120 | 0.118 | 0.098 | 0.040 | 0.077 | 0.113 | 0.003 | |
| PSRI | 0.006 | 0.008 | 0.058 | 0.267 | 0.008 | 0.126 | 0.039 | 0.102 | 0.063 | 0.076 | 0.001 | 0.003 | |
| RGI | 0.018 | 0.008 | 0.060 | 0.266 | 0.011 | 0.126 | 0.042 | 0.102 | 0.086 | 0.075 | 0.008 | 0.003 | |
| RSI | 0.002 | 0.008 | 0.071 | 0.265 | 0.003 | 0.127 | 0.004 | 0.104 | 0.044 | 0.077 | 0.027 | 0.003 | |
| YCAR | 0.009 | 0.008 | 0.019 | 0.272 | 0.046 | 0.124 | 0.072 | 0.100 | 0.067 | 0.076 | 0.017 | 0.003 | |
| Sunlit | ARI2 | 0.009 | 0.009 | 0.015 | 0.246 | 0.005 | 0.091 | 0.038 | 0.053 | 0.029 | 0.051 | 0.001 | 0.002 |
| CRI1 | 0.011 | 0.009 | 0.001 | 0.247 | 0.001 | 0.091 | 0.002 | 0.054 | 0.001 | 0.052 | 0.001 | 0.002 | |
| CRI2 | 0.010 | 0.009 | 0.001 | 0.247 | 0.003 | 0.091 | 0.003 | 0.054 | 0.001 | 0.052 | 0.001 | 0.002 | |
| EVI | 0.020 | 0.009 | 0.002 | 0.247 | 0.025 | 0.090 | 0.050 | 0.053 | 0.011 | 0.052 | 0.024 | 0.002 | |
| OCAR | 0.001 | 0.009 | 0.031 | 0.244 | 0.027 | 0.090 | 0.003 | 0.054 | 0.018 | 0.052 | 0.031 | 0.002 | |
| PRI | 0.045 | 0.009 | 0.010 | 0.246 | 0.009 | 0.091 | 0.002 | 0.054 | 0.003 | 0.052 | 0.016 | 0.002 | |
| PSRI | 0.020 | 0.009 | 0.023 | 0.245 | 0.036 | 0.089 | 0.002 | 0.054 | 0.010 | 0.052 | 0.032 | 0.002 | |
| RGI | 0.039 | 0.009 | 0.070 | 0.239 | 0.011 | 0.090 | 0.020 | 0.053 | 0.066 | 0.050 | 0.018 | 0.002 | |
| RSI | 0.019 | 0.009 | 0.003 | 0.247 | 0.001 | 0.091 | 0.004 | 0.054 | 0.001 | 0.052 | 0.002 | 0.002 | |
| YCAR | 0.013 | 0.009 | 0.044 | 0.242 | 0.032 | 0.090 | 0.004 | 0.054 | 0.029 | 0.051 | 0.034 | 0.002 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuang, J.; Wang, Q. Hyperspectral Indices Developed from Fractional-Order Derivative Spectra Improved Estimation of Leaf Chlorophyll Fluorescence Parameters. Plants 2024, 13, 1923. https://doi.org/10.3390/plants13141923
Zhuang J, Wang Q. Hyperspectral Indices Developed from Fractional-Order Derivative Spectra Improved Estimation of Leaf Chlorophyll Fluorescence Parameters. Plants. 2024; 13(14):1923. https://doi.org/10.3390/plants13141923
Chicago/Turabian StyleZhuang, Jie, and Quan Wang. 2024. "Hyperspectral Indices Developed from Fractional-Order Derivative Spectra Improved Estimation of Leaf Chlorophyll Fluorescence Parameters" Plants 13, no. 14: 1923. https://doi.org/10.3390/plants13141923
APA StyleZhuang, J., & Wang, Q. (2024). Hyperspectral Indices Developed from Fractional-Order Derivative Spectra Improved Estimation of Leaf Chlorophyll Fluorescence Parameters. Plants, 13(14), 1923. https://doi.org/10.3390/plants13141923








