Morphological and Anatomical Differentiation of Potamogeton gramineus in Relation to the Presence of Invasive Species Elodea nuttallii: A Case Study from Vlasina Lake, Serbia
Abstract
:1. Introduction
2. Results
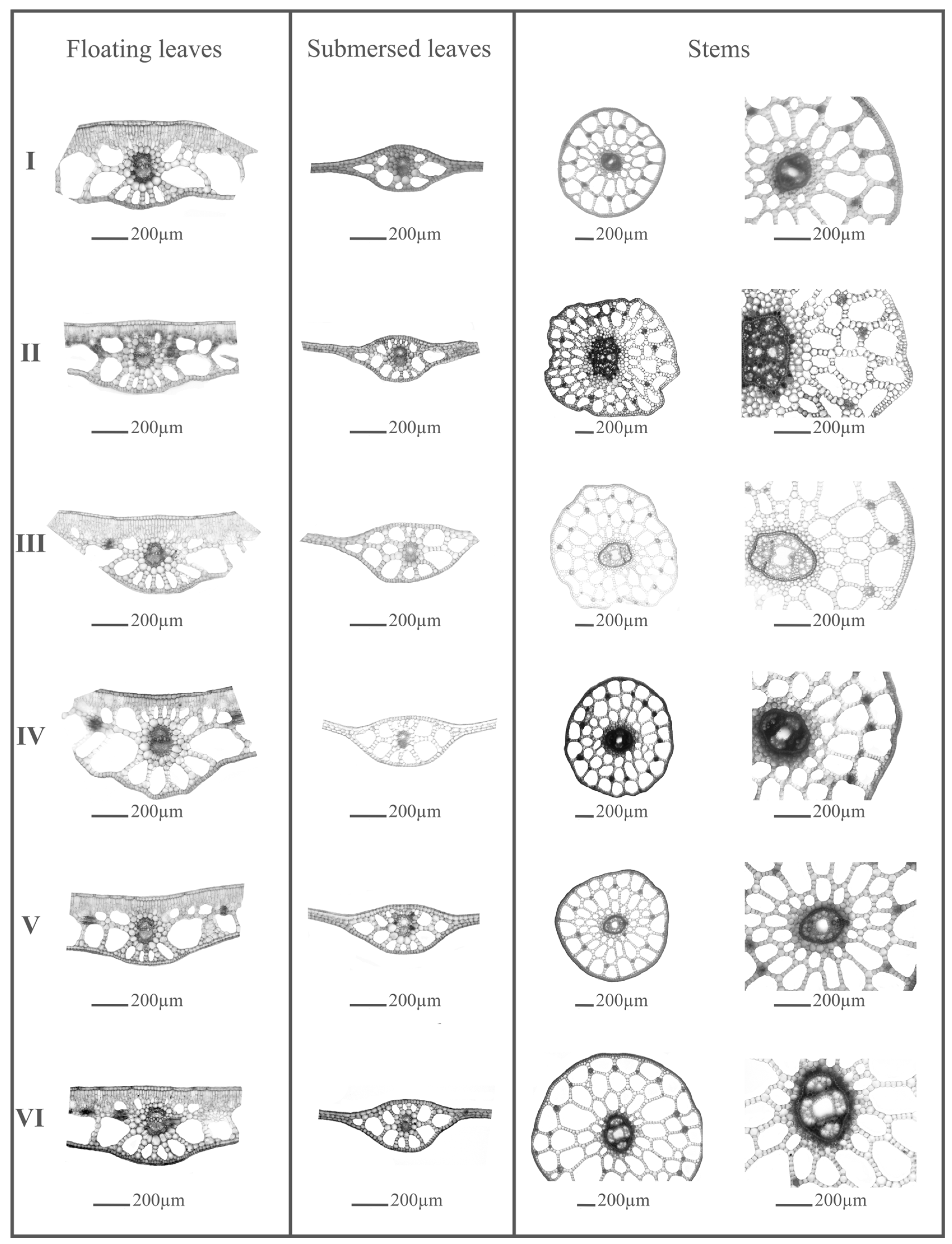
2.1. The Morphological and Anatomical Variability of Stem, Floating, and Submersed Leaves of Potamogeton gramineus
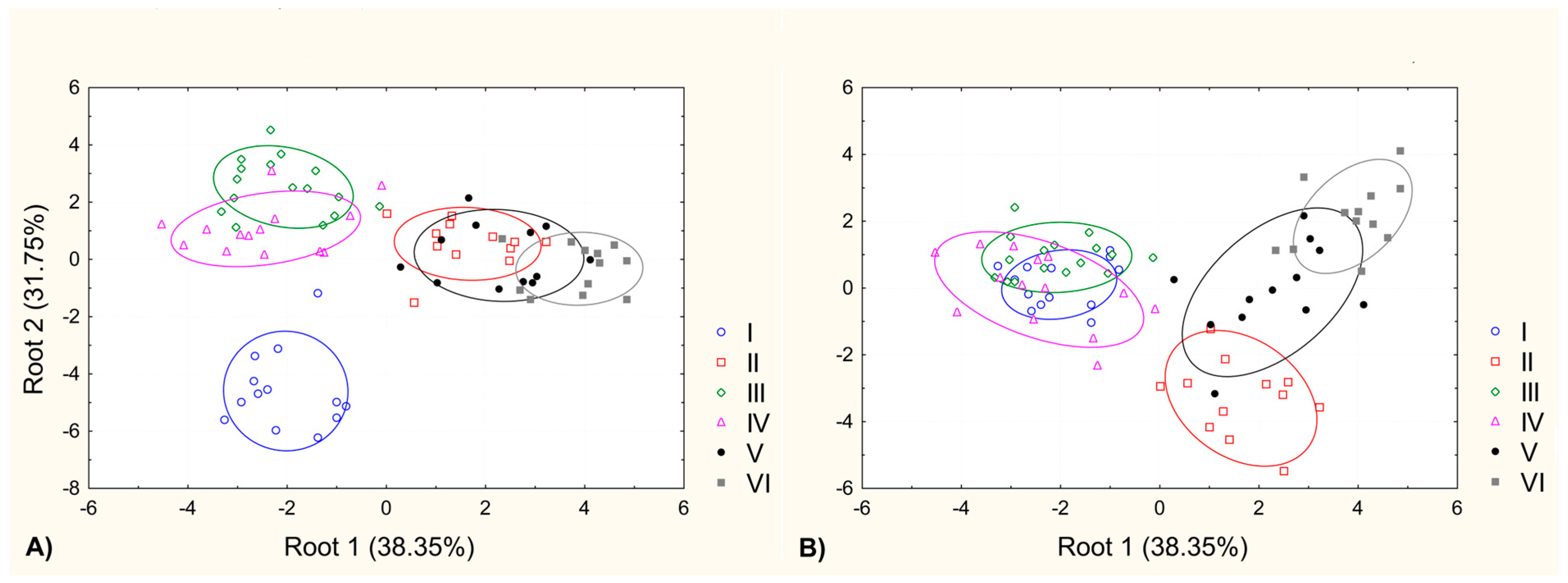
2.2. The Morphological and Anatomical Differentiation within the Analyzed Samples of Potamogeton gramineus Population
3. Discussion
4. Material and Methods
4.1. Study Area
4.2. Environmental Variables
4.3. Plant Material
4.4. Morphological and Anatomical Analysis
4.5. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodriguez, L.F. Can invasive species facilitate native species? Evidence of how, when, and why these impacts occur. Biol. Invasions 2006, 8, 927–939. [Google Scholar] [CrossRef]
- D’Antonio, C.M.; Meyerson, L.A. Exotic plant species as problems and solutions in ecological restoration: A synthesis. Restor. Ecol. 2002, 10, 703–713. [Google Scholar] [CrossRef]
- Sax, D.F.; Stachowicz, J.J.; Brown, J.H.; Bruno, J.F.; Dawson, M.N.; Gaines, S.D.; Grosberg, R.K.; Hastings, A.; Holt, R.D.; Mayfield, M.M.; et al. Ecological and evolutionary insights from species invasions. Trends Ecol. Evol. 2007, 22, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Strauss, S.Y.; Lau, J.A.; Carroll, S.P. Evolutionary responses of natives to introduced species: What do introductions tell us about natural communities? Ecol. Lett. 2006, 9, 357–374. [Google Scholar] [CrossRef]
- Freeman, A.S.; Byers, J.E. Divergent induced responses to an invasive predator in marine mussel populations. Science 2006, 313, 831–833. [Google Scholar] [CrossRef]
- Phillips, B.L.; Shine, R. An invasive species induces rapid adaptive change in a native predator: Cane toads and blacksnakes in Australia. Proc. R. Soc. B. Biol. Sci. 2006, 273, 1545–1550. [Google Scholar] [CrossRef]
- Langkilde, T. Invasive fire ants alter behaviour and morphology of native lizards. Ecology 2009, 90, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.T.; Gribben, P.E.; Byers, J.E.; Monro, K. Invasive ecosystem engineer selects for different phenotypes of an associated native species. Ecology 2012, 93, 1262–1268. [Google Scholar] [CrossRef]
- Berthon, K. How do native species respond to invaders? Mechanistic and trait-based perspectives. Biol. Invasions 2015, 17, 2199–2211. [Google Scholar] [CrossRef]
- Oduor, A.M. Evolutionary responses of native plant species to invasive plants: A review. New Phytol. 2013, 200, 986–992. [Google Scholar] [CrossRef]
- Callaway, R.M.; Ridenour, W.M.; Laboski, T.; Weir, T.; Vivanco, J.M. Natural selection for resistance to the allelopathic effects of invasive plants. J. Ecol. 2005, 93, 576–583. [Google Scholar] [CrossRef]
- Rowe, C.L.J.; Leger, E.A. Competitive seedlings and inherited traits: A test of rapid evolution of Elymus multisetus (big squirrel tail) in response to cheat grass invasion. Evol. Appl. 2011, 4, 485–498. [Google Scholar] [CrossRef]
- Leger, E.A.; Espeland, E.K. Coevolution between native and invasive plant competitors: Implications for invasive species management. Evol. Appl. 2010, 3, 169–178. [Google Scholar] [CrossRef]
- Daehler, C.C. Performance Comparisons of Co-Occurring Native and Alien Invasive Plants: Implications for Conservation and Restoration. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 183–211. [Google Scholar] [CrossRef]
- Fan, J.; Harris, W. Effects of soil fertility level and cutting frequency on interference among Hieracium pilosella, H. praealtum, Rumex acetosella, and Festuca novae-zelandiae. N. Z. J. Agric. Res. 1996, 39, 1–32. [Google Scholar] [CrossRef]
- Marler, M.J.; Zabinski, C.A.; Callaway, R.M. Mycorrhizae Indirectly Enhance Competitive Effects of an Invasive Forb on a Native Bunchgrass. Ecology 1999, 80, 1180–1186. [Google Scholar] [CrossRef]
- D’Antonio, C.M.; Mahall, B.E. Root profiles and competition between the invasive, exotic perennial, Carpobrotus edulis, and two native shrub species in California coastal scrub. Am. J. Bot. 1991, 78, 885–894. [Google Scholar] [CrossRef]
- Baruch, Z.; Goldstein, G. Leaf construction cost, nutrient concentration, and net CO2 assimilation of native and invasive species in Hawaii. Oecologia 1999, 121, 183–192. [Google Scholar] [CrossRef]
- EU. Regulation (EU) No 1143/2014 of the European Parliament and of the Council on the Prevention and Management of the Introduction and Spread of Invasive Alien Species; OJEU L 317/35; EU: Luxembourg, 2014. [Google Scholar]
- Vukov, D.; Igić, R.; Boža, P.; Anačkov, G.; Janauer, G.A. Habitat and Plant Species Diversity along the River Danube in Serbia. In Proceedings 36th International Conference of IAD; Austrian Committee Danube Research, Ed.; IAD: Vienna, Austria, 2006; pp. 127–131. [Google Scholar]
- Laketić, D.L. Fitocenološka Klasifikacija Vegetacije Jezerskog Tipa u Srbiji. Ph.D. Thesis, Univerzitet u Beogradu, Beograd, Srbija, 2013. [Google Scholar]
- Anđelković, A.A.; Živković, M.M.; Cvijanović, D.L.; Novković, M.Z.; Marisavljević, D.P.; Pavlović, D.M.; Radulović, S.B. The contemporary records of aquatic plants invasion through the Danubian floodplain corridor in Serbia. Aquat. Invasions 2016, 11, 381–395. [Google Scholar] [CrossRef]
- Cvijanović, D.L.; Lakušić, D.V.; Živković, M.M.; Novković, M.Z.; Anđelković, A.A.; Pavlović, D.M.; Vukov, D.M.; Radulović, S.B. An overview of aquatic vegetation in Serbia. Tuexenia 2018, 38, 269–286. [Google Scholar]
- Howard-Williams, C. Processes of aquatic weed invasions: The New Zealand example. J. Aquat. Plant Manag. 1993, 31, 17–23. [Google Scholar]
- Mjelde, M.; Lombardo, P.; Berge, D.; Johansen, S.W. Mass invasion of non-native Elodea canadensis Michx. in a large, clear-water, species-rich Norwegian lake—Impact on macrophyte biodiversity. Ann. Limnol. Int. J. Lim. 2012, 48, 225–240. [Google Scholar] [CrossRef]
- De Winton, D.M.; Clayton, S.J. The impact of invasive submerged weed species on seed banks in lake sediments. Aquat. Bot. 1996, 53, 31–45. [Google Scholar] [CrossRef]
- O’Hare, M.T.; Gunn, D.M.I.; Chapman, S.D.; Dudley, J.B.; Purse, V.B. Impacts of space, local environment and habitat connectivity on macrophyte communities in conservation lakes. Divers. Distrib. 2012, 18, 603–614. [Google Scholar] [CrossRef]
- Barrat-Segretain, M.H. Competition between invasive and indigenous species: Impact of spatial pattern and developmental stage. Plant Ecol. 2005, 180, 153–160. [Google Scholar] [CrossRef]
- Szabo, S.; Scheffer, M.; Roijackers, R.; Waluto, B.; Braun, M.; Nagy, P.T.; Borics, G.; Zambrano, L. Strong growth limitation of a floating plant (Lemna gibba) by the submerged macrophyte (Elodea nuttallii) under laboratory conditions. Freshw. Biol. 2010, 55, 681–690. [Google Scholar] [CrossRef]
- Netten, J.J.; Arts, G.H.; Gylstra, R.; van Nes, E.H.; Scheffer, M.; Roijackers, R.M. Effect of temperature and nutrients on the competition between free-floating Salvinia natans and submerged Elodea nuttallii in mesocosms. Fund. Appl. Limnol. 2010, 177, 125–132. [Google Scholar] [CrossRef]
- Wieglet, G.; Kaplan, Z. An account of the species of Potamogeton L. (Potamogetonaceae). Folia Geobot. 1998, 33, 241–316. [Google Scholar] [CrossRef]
- Kozelková, Z.; Prausová, R.; Tomasova, Z.; Safarova, L. Differences in Potamogeton praelongus morphology and habitats in Europe. Acta Soc. Bot. Pol. 2021, 90, 1–19. [Google Scholar] [CrossRef]
- Janković, M. Rod Potamogeton L. In Flora SR Srbije; Josifović, M., Ed.; SANU: Beograd, Srbija, 1975; Volume 5, pp. 480–488. (In Serbian) [Google Scholar]
- Sala, O.E.; Chapin, F.S., III; Armesto, J.; Berlow, E.; Bloomfield, J.; Dirzo, R.; Huber-Sanwald, E.; Huenneke, L.F.; Jackson, R.B.; Kinzig, A.; et al. Global biodiversity scenarios for the year 2100. Science 2000, 287, 1770–1774. [Google Scholar] [CrossRef]
- Spencer, F.D.; Ksander, G.G. Influence of planting depth on Potamogeton gramineus L. Aquat. Bot. 1990, 36, 343–350. [Google Scholar] [CrossRef]
- Spencer, F.D.; Ksander, G.G. Influence of Temperature and Light on Early Growth of Potamogeton gramineus L. J. Freshw. Ecol. 1991, 6, 227–235. [Google Scholar] [CrossRef]
- Kaplan, Z. Phenotypic plasticity in Potamogeton (Potamogetonaceae). Folia Geobot. 2002, 37, 141–170. [Google Scholar] [CrossRef]
- Daumal, M.M.; Oosterhuis, D.; Verhofsstad, M.J.J.M.; Erkens, R.H.J.; Peters, E.T.H.M. The impact of drought duration on two Potamogeton species with different growth forms. Aquat. Sci. 2024, 86, 72–85. [Google Scholar] [CrossRef]
- Bayindir, N.; Ikinci, N. The role of environmental variables on distribution of Potamogetonaceae species. Wetlands 2020, 40, 125–133. [Google Scholar] [CrossRef]
- Søndergaard, M.; Johansson, L.S.; Lauridsen, T.L.; Jørgensen, T.B.; Liboriussen, L.; Jeppesen, E. Submerged macrophytes as indicators of the ecological quality of lakes. Freshw. Biol. 2010, 55, 893–908. [Google Scholar] [CrossRef]
- Dandy, J.E. Potamogeton L. In Flora Europaea, V; Tutin, T.G., Heywood, V.H., Burges, N.A., Moore, D.M., Valentine, D.H., Walters, S.M., Webb, D.A., Eds.; Cambridge University Press: Cambridge, UK, 1980; pp. 7–11. [Google Scholar]
- Yordanov, D. Potamogeton gramineus L. In Flora na NR Bulgaria, Sofia; Yordanov, D., Ed.; Publishing House Bulgarian Academy of Sciences: Sofia, Bulgaria, 1963; Volume 1, pp. 196–199. (In Bulgarian) [Google Scholar]
- Topa, E. Potamogeton L. In Flora of Socialistic Republic of Romania; Nyaradi, E., Ed.; Academiei Republicii Socialiste Romania: Bukurest, Romania, 1966; Volume 11, pp. 55–82. (In Romanian) [Google Scholar]
- Soó, R. Synopsis Systematico-Geobotanica Florae Vegetationsque Hungariae; Akadémiai Kiadó: Budapest, Hungary, 1968; Volume 3, p. 655. (In Hungarian) [Google Scholar]
- Okajima, Y.; Taneda, H.; Noguchi, K.; Terashima, I. Optimum leaf size predicted by a novel leaf energy balance model incorporating dependencies of photosynthesis on light and temperature. Ecol. Res. 2011, 27, 333–346. [Google Scholar] [CrossRef]
- Oguchi, R.; Onoda, Y.; Terashima, I.; Tholen, D. Leaf Anatomy and Function. In The Leaf: A Platform for Performing Photosynthesis. Advances in Photosynthesis and Respiration; Adams, W., III, Terashima, I., Eds.; Springer: Cham, Switzerland, 2018; Volume 44, pp. 97–139. [Google Scholar] [CrossRef]
- Lambers, H.; Chapin, F.S., III; Pons, T.L. Plant Physiological Ecology, 2nd ed.; Springer: New York, USA, 1998; p. 623. [Google Scholar]
- Nobel, P.S. Physicochemical and Environmental Plant Physiology, 5th ed.; Academic Press: London, UK, 2020; p. 654. [Google Scholar]
- Parkhurst, D.F.; Loucks, O.L. Optimal leaf size in relation to environment. J. Ecol. 1972, 60, 505–537. [Google Scholar] [CrossRef]
- Den Hartog, C.; Van Der Velde, G. Structural aspects of aquatic plant communities. In Vegetation of Inland Waters: Handbook of Vegetation Science; Symoens, J.J., Ed.; Springer: Berlin/Heidelberg, Germany, 1988; Volume 15, pp. 113–153. [Google Scholar]
- Puijalon, S.; Bouma, T.J.; Douady, C.J.; van Groenendael, J.; Anten, N.P.; Martel, E.; Bornette, G. Plant resistance to mechanical stress: Evidence of an avoidance–tolerance trade-off. New Phytol. 2011, 191, 1141–1149. [Google Scholar] [CrossRef]
- Sultan, S.E. Phenotypic plasticity in plants: A case study in ecological development. Evol. Dev. 2003, 5, 25–33. [Google Scholar] [CrossRef]
- Kaenel, B.R.; Buehrer, H.; Uehlinger, U. Effects of aquatic plant management on stream metabolism and oxygen balance in streams. Freshw. Biol. 2000, 45, 85–95. [Google Scholar] [CrossRef]
- Leandro, T.D.; Holsback, Z.D.R.; Scremin-Dias, E. The aquatic species Pontederia azurea and P. crassipes (Pontederiaceae) in the Pantanal, Brazil: Evidence of how plant structure can simultaneously reflect phylogeny and ecology. Acta Bot. Bras. 2021, 35, 79–91. [Google Scholar] [CrossRef]
- Milanović Pešić, A.; Jojić Glavonjić, T.; Denda, S.; Jakovljević, D. Sustainable Tourism Development and Ramsar Sites in Serbia: Exploring Residents’ Attitudes and Water Quality Assessment in the Vlasina Protected Area. Sustainability 2023, 15, 15391. [Google Scholar] [CrossRef]
- Stanković, S.M. Jezera Srbije: Limnološka Monografija [Lakes of Serbia: Limnological Monograph]; Center for Textbooks and Teaching Aids: Belgrade, Serbia, 2005. (In Serbian) [Google Scholar]
- Simić, V.; Simić, S.; Pešić, V. Ecological Sustainability of Fish Resources of Inland Waters of the Western Balkans. In Freshwater Fish Stocks, Sustainable Use and Conservation; Springer Nature Switzerland AG: Cham, Switzerland, 2023. [Google Scholar]
- Cattaneo, A.; Galanti, G.; Gentinetta, S.; Susana, A. Epiphytic algae and macroinvertebrates on submerged and floating-leaved macrophytes in an Italian lake. Freshw. Biol. 1998, 39, 725–740. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Gligorijević, S.; Pejčinović, D. Contribution to the methodology of anatomical sections preparation. Acta Biol. Et Med. Exp. 1983, 8, 43–45. [Google Scholar]
- Blaženčić, J. Praktikum iz Anatomije Biljaka sa Osnovama Mikroskopske Tehnike [Practicum in Plant Anatomy with the Basics of Microscopic Technique]; Naučna knjiga: Beograd, Serbia, 1979. (In Serbian) [Google Scholar]
- Ruzin, E.S. Plant Microtechnique and Microscopy. In Biological Imaging Facility; University of California: Berkeley, CA, USA; Oxford University Press: New York, NY, USA; Oxford, UK, 1999. [Google Scholar]
- StatSoft, Inc. STATISTICA (Data Analysis Software System), Version 7. 2004. Available online: www.statsoft.com (accessed on 19 January 2022).
- IBM Corp. Released IBM SPSS, Statistics for Windows, Version 19.0; IBM Corp: Armonk, NY, USA, 2010.
| Sites | Coordinates | Depth (cm) | Теmperature (°C) | pH | Oxygen Saturation (%) | Conductivity (µS/cm) | Oxygen Concentration (O2 mg/L) | Dry Biomass P. gramineus/E. nuttallii (g/m2) | Number of Individuals |
|---|---|---|---|---|---|---|---|---|---|
| I | 42°44′37″ N 22°20′13″ E | 56 | 22.2 | 6.1 | 121.5 | 84.8 | 8.8 | 242.66/0 | 13 |
| II | 42°44′09″ N 22°20′27″ E | 64 | 23.5 | 5.9 | 145.8 | 82.5 | 11.6 | 132.13/0 | 12 |
| III | 42°44′15″ N 22°20′21″ E | 34 | 26 | 5.98 | 132 | 83.3 | 8.5 | 211.6/0 | 16 |
| IV * | 42°44′31″ N 22°20′07″ E | 48 | 21.8 | 6.51 | 137.2 | 84.8 | 11 | 126.4/20 | 14 |
| V * | 42°44′12″ N 22°20′45″ E | 78 | 20.8 | 6.5 | 108.8 | 85.5 | 8.42 | 187.33/35.3 | 12 |
| VI * | 42°44′02″ N 22°20′38″ E | 57 | 20.6 | 6.6 | 117.4 | 85.8 | 8.98 | 67.2/133.06 | 12 |
| Code | Morphological and Anatomical Features | Units |
|---|---|---|
| Flo1 | Length of the floating leaf lamina | cm |
| Flo2 | Width of the floating leaf lamina | cm |
| Flo3 | Surface area of the floating leaf lamina | cm2 |
| Flo4 | Thickness of the adaxial epidermis of the floating leaf | µm |
| Flo5 | Thickness of the abaxial epidermis of the floating leaf | µm |
| Flo6 | Thickness of the mesophyll tissue of the floating leaf | µm |
| Flo7 | Thickness of the palisade tissue of the floating leaf | µm |
| Flo8 | Thickness of the aerenchyma tissue of the floating leaf | µm |
| Flo9 | Diameter of the vascular bundle of the floating leaf | µm |
| Flo10 | Number of stomata on the adaxial side of the floating leaf | / |
| Flo11 | Surface area of stomata on the adaxial side of floating leaf | µm2 |
| Flo12 | Length of stomata of the adaxial side of the floating leaf | µm |
| Flo13 | Width of stomata of the adaxial side of the floating leaf | µm |
| Flo14 | Surface area of the epidermal cells on the adaxial side of the floating leaf | µm2 |
| Flo15 | Surface area of the epidermal cells on the abaxial side of the floating leaf | µm2 |
| Flo16 | Length of the epidermal cells on the adaxial side of the floating leaf | µm |
| Flo17 | Length of the epidermal cells on the abaxial side of the floating leaf | µm |
| Sub1 | Length of the submerged leaf lamina | cm |
| Sub2 | Width of the submerged leaf lamina | cm |
| Sub3 | Surface area of the submerged leaf lamina | cm2 |
| Sub4 | Thickness of the mesophyll in the central part of the submerged leaf | µm |
| Sub5 | Thickness of the mesophyll in the lateral part of the submerged leaf | µm |
| Sub6 | Diameter of the vascular bundle of the submerged leaf | µm |
| Sub7 | Surface area of the epidermal cells of the submersed leaf | µm2 |
| Sub8 | Length of the epidermal cells of the submersed leaf | µm |
| Sub9 | Width of the epidermal cells of the submersed leaf | µm |
| Ste1 | Thickness of the stem epidermis | µm |
| Ste2 | Thickness of the stem pseudo-hypodermis | µm |
| Ste3 | Thickness of the stem aerenchyma | µm |
| Ste4 | Thickness of the stem endodermis | µm |
| Ste5 | Diameter of the stem | µm |
| Ste6 | Diameter of the stem stele | µm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolić, D.; Jenačković Gocić, D.; Raca, I.; Đorđević, M.; Savić, A.; Jušković, M. Morphological and Anatomical Differentiation of Potamogeton gramineus in Relation to the Presence of Invasive Species Elodea nuttallii: A Case Study from Vlasina Lake, Serbia. Plants 2024, 13, 1937. https://doi.org/10.3390/plants13141937
Nikolić D, Jenačković Gocić D, Raca I, Đorđević M, Savić A, Jušković M. Morphological and Anatomical Differentiation of Potamogeton gramineus in Relation to the Presence of Invasive Species Elodea nuttallii: A Case Study from Vlasina Lake, Serbia. Plants. 2024; 13(14):1937. https://doi.org/10.3390/plants13141937
Chicago/Turabian StyleNikolić, Danijela, Dragana Jenačković Gocić, Irena Raca, Miodrag Đorđević, Ana Savić, and Marina Jušković. 2024. "Morphological and Anatomical Differentiation of Potamogeton gramineus in Relation to the Presence of Invasive Species Elodea nuttallii: A Case Study from Vlasina Lake, Serbia" Plants 13, no. 14: 1937. https://doi.org/10.3390/plants13141937





