Effects of Fertilizer Application Intensity on Carbon Accumulation and Greenhouse Gas Emissions in Moso Bamboo Forest–Polygonatum cyrtonema Hua Agroforestry Systems
Abstract
:1. Introduction
2. Results
2.1. Effects of Compound Fertilizer Intervention Intensity on Soil Environmental Factors and Unstable Carbon and Nitrogen Pools
2.2. Effect of Compound Fertilizer Intervention Intensity on Greenhouse Gas Emissions from Agroforestry Management Systems
2.3. Environmental Soil Factors’ Impact on Soil Greenhouse Gas Emissions
2.4. Effects of the Intensity of Compound Fertilizer Intervention on the Carbon Concentrations of Polygonatum Cyrtonema Hua
2.5. Effects of Compound Fertilizer Intervention Intensity on Vegetation and Soil Carbon Sequestration
3. Discussion
3.1. Soil GHG Emissions Respond to the Intensity of Compound Fertilizer Intervention
3.2. The Annual Carbon Sequestration of Vegetation, Soil, and Ecosystem Carbon Accumulation Respond to the Intensity of Compound Fertilizer Intervention
4. Materials and Methods
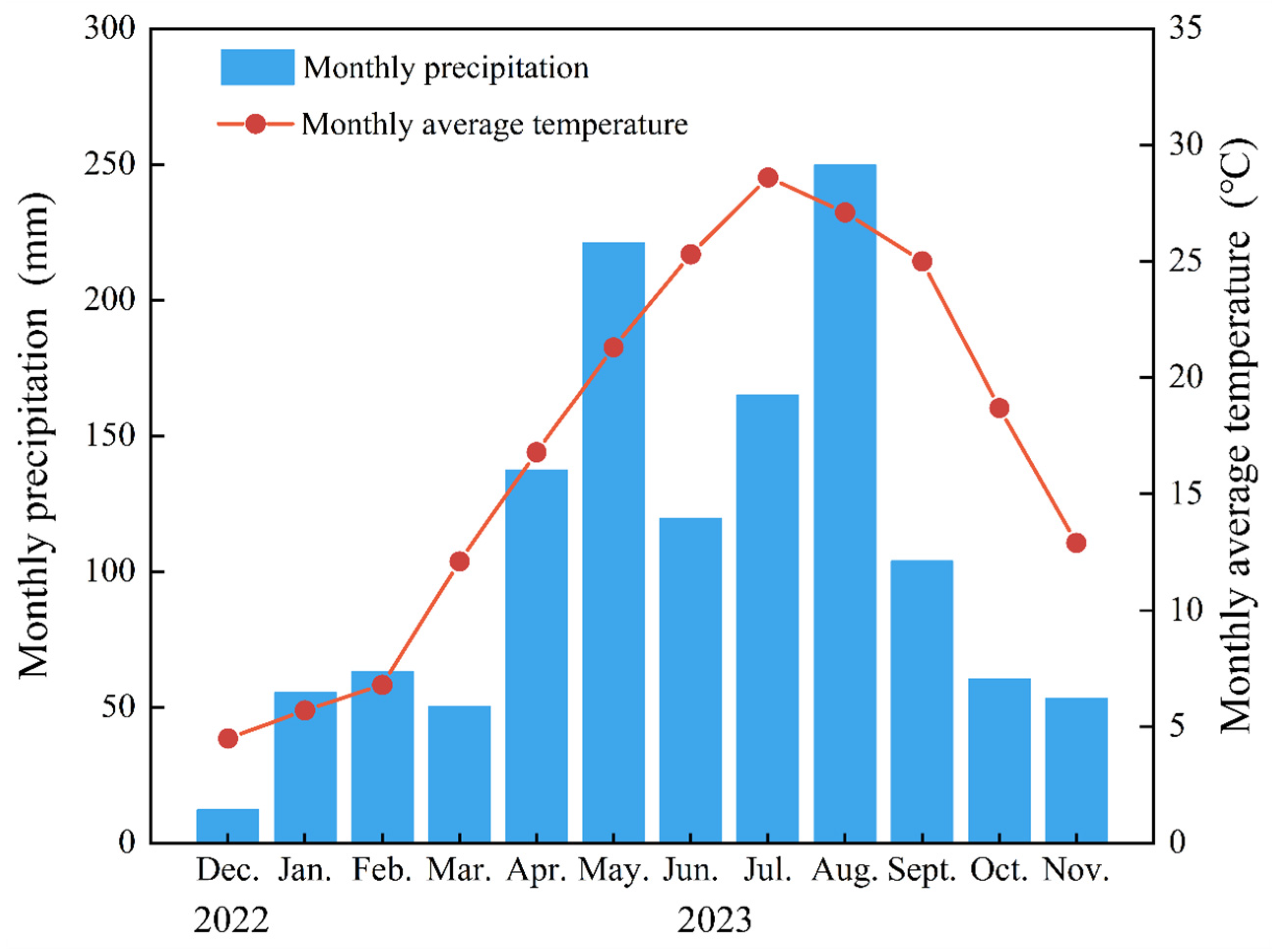
4.1. Experimental Region
4.2. Experimental Design
4.3. Measurements of Soil GHG Emissions
4.4. Soil Sampling Collection and Physico-Chemical Property Analyses
4.5. Determinations of Vegetation Carbon Storage
4.6. Determination of Carbon Concentrations of Polygonatum Cyrtonema Hua
4.7. Determination of the Ecosystem Carbon Sequestration
4.8. Statistics and Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calvin, K.; Dasgupta, D.; Krinner, G.; Mukherji, A.; Thorne, P.W.; Trisos, C.; Romero, J.; Aldunce, P.; Barrett, K.; Blanco, G.; et al. IPCC, 2023: Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change [Core Writing Team, H. Lee and J. Romero (Eds.)]; Arias, P., Bustamante, M., Elgizouli, I., Flato, G., Howden, M., Méndez-Vallejo, C., Pereira, J.J., Pichs-Madruga, R., Rose, S.K., Saheb, Y., et al., Eds.; IPCC: Geneva, Switzerland, 2023.
- Höök, M.; Tang, X. Depletion of Fossil Fuels and Anthropogenic Climate Change-A Review. Energy Policy 2013, 52, 797–809. [Google Scholar] [CrossRef]
- Field, C.B.; Raupach, M.R.; Victoria, R. The Global Carbon Cycle: Integrating Humans, Climate and the Natural World; Island Press: Washington, DC, USA, 2003; pp. 1–13. [Google Scholar]
- Bonan, G.B. Forests and Climate Change: Forcings, Feedbacks, and the Climate Benefits of Forests. Science 2008, 320, 1444–1449. [Google Scholar] [CrossRef]
- Wardle, D.A.; Bardgett, R.D.; Callaway, R.M.; Van der Putten, W.H. Terrestrial Ecosystem Responses to Species Gains and Losses. Science 2011, 332, 1273–1277. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wen, J.; Wen, Y.; Zhang, Y.; Zhang, N.; Wang, Y.; Bai, L.; Su, S.; Zeng, X. Alteration of Soil-Surface Electrochemical Properties by Organic Fertilization to Reduce Dissolved Inorganic Nitrogen Leaching in Paddy Fields. Soil Tillage Res. 2021, 209, 104956. [Google Scholar] [CrossRef]
- Mao, F.; Zhou, G.; Li, P.; Du, H.; Xu, X.; Shi, Y.; Mo, L.; Zhou, Y.; Tu, G. Optimizing Selective Cutting Strategies for Maximum Carbon Stocks and Yield of Moso Bamboo Forest Using BIOME-BGC Model. J. Environ. Manag. 2017, 191, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Gratani, L.; Crescente, M.F.; Varone, L.; Fabrini, G.; Digiulio, E. Growth Pattern and Photosynthetic Activity of Different Bamboo Species Growing in the Botanical Garden of Rome. Flora Morphol. Distrib. Funct. Ecol. Plants 2008, 203, 77–84. [Google Scholar] [CrossRef]
- Xu, L.; Shi, Y.; Zhou, G.; Xu, X.; Liu, E.; Zhou, Y.; Zhang, F.; Li, C.; Fang, H.; Chen, L. Structural Development and Carbon Dynamics of Moso Bamboo Forests in Zhejiang Province, China. For. Ecol. Manag. 2018, 409, 479–488. [Google Scholar] [CrossRef]
- Feng, P.; Li, Y. China’s Bamboo Resources in 2021. World Bamboo Ratt. Newsl. 2023, 21, 100–103. (In Chinese) [Google Scholar]
- Bian, F.; Zhong, Z.; Zhang, X.; Yang, C.; Gai, X. Bamboo—An Untapped Plant Resource for the Phytoremediation of Heavy Metal Contaminated Soils. Chemosphere 2020, 246, 125750. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.; Zhou, G.; Chen, G.; Zhou, Y.; Ge, Z.; Niu, Z.; Xu, L.; Shi, Y. Effects of Different Management Practices on the Increase in Phytolith-Occluded Carbon in Moso Bamboo Forests. Front. Plant Sci. 2020, 11, 591852. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, S.; Fang, Y.; Hui, D.; Tang, C.; Van Zwieten, L.; Zhou, J.; Jiang, Z.; Cai, Y.; Yu, B.; et al. Nitrogen Deposition-Induced Stimulation of Soil Heterotrophic Respiration Is Counteracted by Biochar in a Subtropical Forest. Agric. For. Meteorol. 2024, 349, 109940. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, P.; Wang, H.; Zhou, G.; Wu, J.; Yang, F.; Qian, X. Seasonal Soil CO2 Efflux Dynamics after Land Use Change from a Natural Forest to Moso Bamboo Plantations in Subtropical China. For. Ecol. Manag. 2011, 262, 1131–1137. [Google Scholar] [CrossRef]
- Deng, X.; Yin, J.; Xu, L.; Shi, Y.; Zhou, G.; Li, Y.; Chen, G.; Ye, Y.; Zhang, F.; Zhou, Y.; et al. Effects of Abandonment Management on Soil C and N Pools in Moso Bamboo Forests. Sci. Total Environ. 2020, 729, 138949. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Zhao, C.; Li, X.; Gao, Q.; Huang, L.; Xiao, P.; Gao, W. The Genus Polygonatum: A Review of Ethnopharmacology, Phytochemistry and Pharmacology. J. Ethnopharmacol. 2018, 214, 274–291. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.M.; Wang, H.L.; Li, J.L.; Lu, W.J.; He, S.B.; Cheng, Z.H.; Li, Y.Y.; Luo, C.L. Study on the Quality Difference of Polygonatum in Different Growth Years and Harvest Periods in Guizhou. Spec. Wild Econ. Anim. Plant Res. 2022, 44, 75–80. (In Chinese) [Google Scholar] [CrossRef]
- Ke, S.; Yan, R.; Qiao, D.; Zhu, L. Cost-Benefit Analysis of an Agroforestry Innovation on Karst Land: Replacing Sweet Potato by Sealwort (Polygonatum cyrtonema) under Plum Trees in Chunfeng Village, Sichuan Province, China. For. Trees Livelihoods 2018, 27, 217–229. [Google Scholar] [CrossRef]
- Plieninger, T.; Muñoz-Rojas, J.; Buck, L.E.; Scherr, S.J. Agroforestry for Sustainable Landscape Management. Sustain. Sci. 2020, 15, 1255–1266. [Google Scholar] [CrossRef]
- Lorenz, K.; Lal, R. Soil Organic Carbon Sequestration in Agroforestry Systems. A Review Soil Organic Carbon Sequestration in Agroforestry Systems. A Review. Agronomy for Sustainable Development Soil Organic Carbon Sequestration in Agroforestry Systems. A Review. Agron. Sustain. Dev. 2014, 34, 443–454. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Chang, S.X.; Qin, H.; Fu, S.; Jiang, P. Understory Management and Fertilization Affected Soil Greenhouse Gas Emissions and Labile Organic Carbon Pools in a Chinese Chestnut Plantation. For. Ecol. Manag. 2015, 337, 126–134. [Google Scholar] [CrossRef]
- Xu, H.; Wang, F.; Hu, Z.; Du, Z.; Cheng, L.; Xu, X. Moso Bamboo–Polygonatum cyrtonema Agroforestry Systems: Evaluation of Soil Quality and Polygonatum Yield. Forests 2023, 14, 1426. [Google Scholar] [CrossRef]
- Mao, L.; Zha, R.; Chen, S.; Zhang, J.; Jie, L.; Zha, X. Mixture Compound Fertilizer and Super Absorbent Polymer Application Significantly Promoted Growth and Increased Nutrient Levels in Pinus massoniana Seedlings and Soil in Seriously Eroded Degradation Region of Southern China. Front. Plant Sci. 2021, 12, 763175. [Google Scholar] [CrossRef]
- Cai, Y.; Zhou, S.; Shi, M.; Feng, Q.; Zhang, Q. Effects of Fertilization on Growth and Main Metabolite Content of Polygonatum cyrtonema Hua. J. Chin. Med. Mater. 2022, 45, 2805–2811. (In Chinese) [Google Scholar] [CrossRef]
- Schlesinger, W.H.; Andrews, J.A. Soil Respiration and the Global Carbon Cycle. Biogeochemistry 2000, 48, 7–20. [Google Scholar] [CrossRef]
- Yu, Y.; Pei, J.; Liu, W.; Wang, M.; Yin, S. Effects of Adding 13C Enriched Corn Roots, Stems and Leaves on Brown Soil Soil Respiration and Its Stimulation Effect under Long-Term No Fertilization and Fertilization Treatments. J. Soil Sci. 2023, 60, 1077–1087. [Google Scholar]
- Yuan, N.; Wang, E.; Lv, S.; Tang, X.; Wang, T.; Wang, G.; Zhou, Y.; Zhou, G.; Shi, Y.; Xu, L. Degradation Reduces Greenhouse Gas Emissions While Weakening Ecosystem Carbon Sequestration of Moso Bamboo Forests. Sci. Total Environ. 2023, 877, 162915. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Gutknecht, J.L.M.; Herman, D.J.; Keck, D.C.; Firestone, M.K.; Cheng, W. Rhizosphere Priming Effects on Soil Carbon and Nitrogen Mineralization. Soil Biol. Biochem. 2014, 76, 183–192. [Google Scholar] [CrossRef]
- Wu, J.; Liu, Z.; Chen, D.; Huang, G.; Zhou, L.; Fu, S. Understory Plants Can Make Substantial Contributions to Soil Respiration: Evidence from Two Subtropical Plantations. Soil Biol. Biochem. 2011, 43, 2355–2357. [Google Scholar] [CrossRef]
- Beeckman, F.; Motte, H.; Beeckman, T. Nitrification in Agricultural Soils: Impact, Actors and Mitigation. Curr. Opin. Biotechnol. 2018, 50, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Wang, J.; Zhang, J.B.; Müller, C.; Wang, S.Q. Mechanistic Insights into the Effects of N Fertilizer Application on N2O-Emission Pathways in Acidic Soil of a Tea Plantation. Plant Soil 2015, 389, 45–57. [Google Scholar] [CrossRef]
- Coskun, D.; Britto, D.T.; Shi, W.; Kronzucker, H.J. Nitrogen Transformations in Modern Agriculture and the Role of Biological Nitrification Inhibition. Nat. Plants 2017, 3, 17074. [Google Scholar] [CrossRef] [PubMed]
- Quick, A.M.; Reeder, W.J.; Farrell, T.B.; Tonina, D.; Feris, K.P.; Benner, S.G. Nitrous Oxide from Streams and Rivers: A Review of Primary Biogeochemical Pathways and Environmental Variables. Earth Sci. Rev. 2019, 191, 224–262. [Google Scholar] [CrossRef]
- Reddy, N.; Crohn, D.M. Effects of Soil Salinity and Carbon Availability from Organic Amendments on Nitrous Oxide Emissions. Geoderma 2014, 235–236, 363–371. [Google Scholar] [CrossRef]
- Chen, C.; Liu, W.; Wu, J.; Jiang, X.; Zhu, X. Can Intercropping with the Cash Crop Help Improve the Soil Physico-Chemical Properties of Rubber Plantations? Geoderma 2019, 335, 149–160. [Google Scholar] [CrossRef]
- Jiang, X.J.; Liu, W.; Wu, J.; Wang, P.; Liu, C.; Yuan, Z.Q. Land Degradation Controlled and Mitigated by Rubber-Based Agroforestry Systems through Optimizing Soil Physical Conditions and Water Supply Mechanisms: A Case Study in Xishuangbanna, China. Land Degrad. Dev. 2017, 28, 2277–2289. [Google Scholar] [CrossRef]
- Lee, J.; Oh, Y.; Lee, S.T.; Seo, Y.O.; Yun, J.; Yang, Y.; Kim, J.; Zhuang, Q.; Kang, H. Soil Organic Carbon Is a Key Determinant of CH4 Sink in Global Forest Soils. Nat. Commun. 2023, 14, 3110. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Ding, B.; Pei, S.; Cao, H.; Liang, J.; Li, Z. The Impact of Organic Fertilizer Replacement on Greenhouse Gas Emissions and Its Influencing Factors. Sci. Total Environ. 2023, 905, 166917. [Google Scholar] [CrossRef] [PubMed]
- Lou, R.; Li, Y.; Liu, Y.; Jiang, Q.; Wu, Q.; He, Y.; Liu, J. Is Green Manure a Viable Substitute for Inorganic Fertilizer to Improve Grain Yields and Advance Carbon Neutrality in Paddy Agriculture? Agric. Ecosyst. Environ. 2024, 359, 108762. [Google Scholar] [CrossRef]
- Wang, L.; Qian, Y.; Brummer, J.E.; Zheng, J.; Wilhelm, S.; Parton, W.J. Simulated Biomass, Environmental Impacts and Best Management Practices for Long-Term Switchgrass Systems in a Semi-Arid Region. Biomass Bioenergy 2015, 75, 254–266. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, H.; Luo, J.; Wang, H.; Zhai, L.; Geng, Y.; Zhang, Y.; Li, J.; Lei, Q.; Bashir, M.A.; et al. Long-Term Manure Application Increased Greenhouse Gas Emissions but Had No Effect on Ammonia Volatilization in a Northern China Upland Field. Sci. Total Environ. 2018, 633, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Liu, G.; Cai, C.; Li, Y. Effects of Different Fertilization Methods on Carbon Storage and Soil Physical and Chemical Properties of Moso Bamboo Forest. J. Sichuan Agric. Univ. 2018, 36, 323–328. (In Chinese) [Google Scholar] [CrossRef]
- Homann, P.S. Convergence and Divergence of Nutrient Stoichiometry during Forest Litter Decomposition. Plant Soil 2012, 358, 251–263. [Google Scholar] [CrossRef]
- Lin, Y.; Ye, G.; Kuzyakov, Y.; Liu, D.; Fan, J.; Ding, W. Long-Term Manure Application Increases Soil Organic Matter and Aggregation, and Alters Microbial Community Structure and Keystone Taxa. Soil Biol. Biochem. 2019, 134, 187–196. [Google Scholar] [CrossRef]
- Tian, S.; Zhu, B.; Yin, R.; Wang, M.; Jiang, Y.; Zhang, C.; Li, D.; Chen, X.; Kardol, P.; Liu, M. Organic Fertilization Promotes Crop Productivity through Changes in Soil Aggregation. Soil Biol. Biochem. 2022, 165, 108533. [Google Scholar] [CrossRef]
- Stanley, P.; Spertus, J.; Chiartas, J.; Stark, P.B.; Bowles, T. Valid Inferences about Soil Carbon in Heterogeneous Landscapes. Geoderma 2023, 430, 116323. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. World Reference Base for Soil Resources 2014: International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; FAO: Rome, Italy, 2014; ISBN 9789251083697. [Google Scholar]
- Xu, L.; Deng, X.; Ying, J.; Zhou, G.; Shi, Y. Silicate Fertilizer Application Reduces Soil Greenhouse Gas Emissions in a Moso Bamboo Forest. Sci. Total Environ. 2020, 747, 141380. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change. Climate Change 2013—The Physical Science Basis; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Lu, R.K. Soil Agricultural Chemical Analysis Method; China Agricultural Science and Technology Press: Beijing, China, 2000; pp. 1–315. [Google Scholar]
- McLean, E.O. Soil PH and Lime Requirement. In Methods of Soil Analysis, Part 2, Chemical and Microbiological Properties; Agronomy Monograph: Madison, WI, USA, 1982; pp. 199–224. [Google Scholar]
- Wu, J.S.; Jiang, P.K.; Chang, S.X.; Xu, Q.F.; Lin, Y. Dissolved Soil Organic Carbon and Nitrogen Were Affected by Conversion of Native Forests to Plantations in Subtropical China. Can. J. Soil Sci. 2010, 90, 27–36. [Google Scholar] [CrossRef]
- Vance, E.; Brookes, P.C.; Jenkinson, D.S. An Extraction Method for Measuring Soil Microbial Biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Buresh, R.J.; Patrick, W.H., Jr. Nitrate Reduction to Ammonium and Organic Nitrogen in an Estuarine Sediment. Soil Biol. Biochem. 1981, 13, 279–283. [Google Scholar] [CrossRef]
- Kalaiselvi, B.; Hegde, R.; Kumar, K.S.A.; Vasundhara, R.; Dharumarajan, S.; Srinivasan, R.; Lalitha, M.; Singh, S.K. Soil Organic Carbon Stocks (SOCS) in Different Land Uses of Western Ghats, Karnataka—A Case Study. J. Plant. Crops 2021, 49, 146–150. [Google Scholar] [CrossRef]
- Zhou, G.M. Carbon Storage, Fixation and Distribution in Mao Bamboo (Phyllostachys pubescens) Stands Ecosystem. Ph.D. Thesis, Zhejiang University, Hangzhou, China, 2006. [Google Scholar]
- Tao, J.X.; Du, Q.; Ji, B.Y.; Zhang, G.J.; Fu, W.J.; Xu, J.; Ge, H.L.; Yao, H.W.; Wang, W.W. The Forest Carbon Sequestration Function Monitoring in Zhejiang; China Forestry Publishing House: Beijing, China, 2014. (In Chinese) [Google Scholar]
- Xu, L.; Fang, H.; Deng, X.; Ying, J.; Lv, W.; Shi, Y.; Zhou, G.; Zhou, Y. Biochar Application Increased Ecosystem Carbon Sequestration Capacity in a Moso Bamboo Forest. For. Ecol. Manag. 2020, 475, 118447. [Google Scholar] [CrossRef]
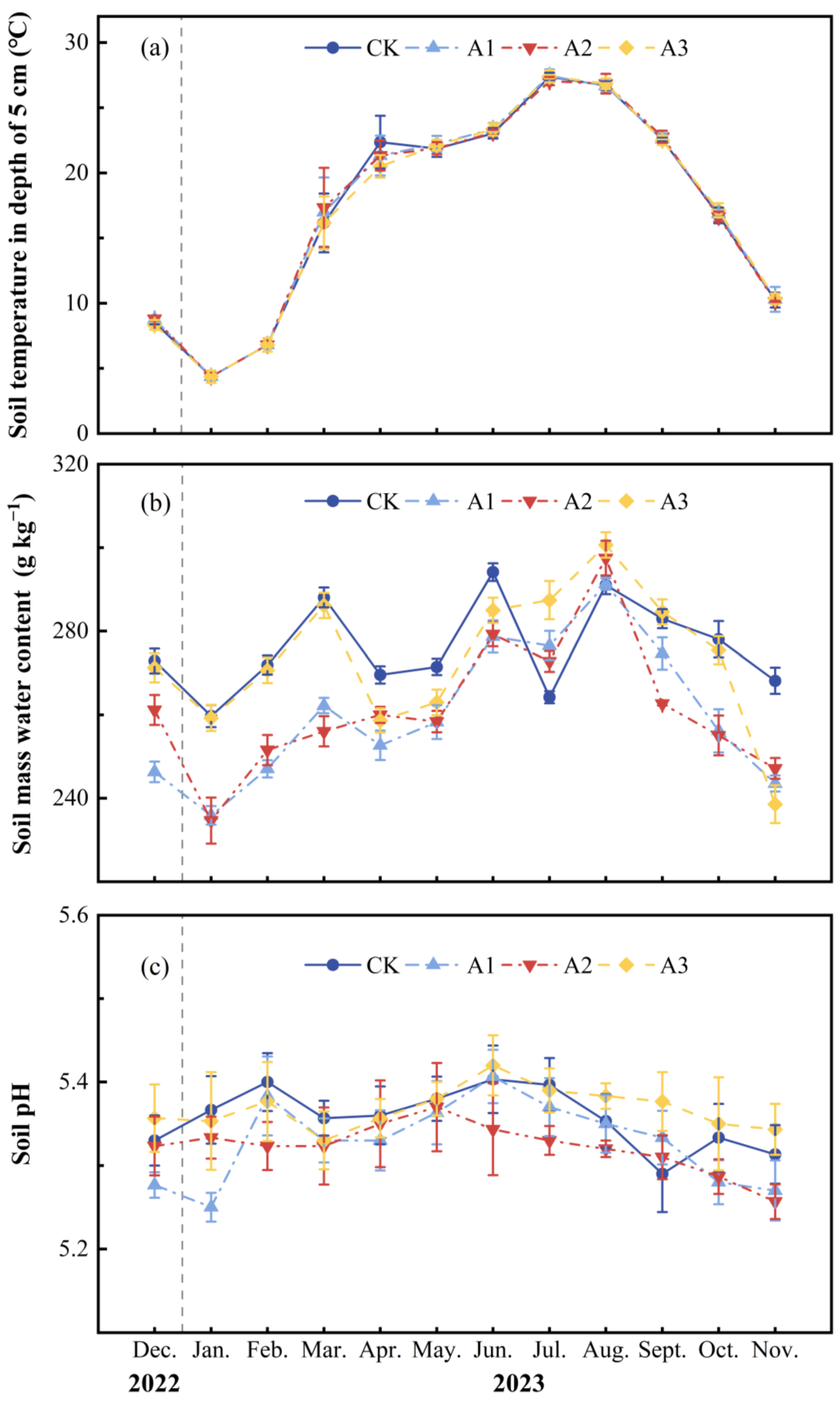

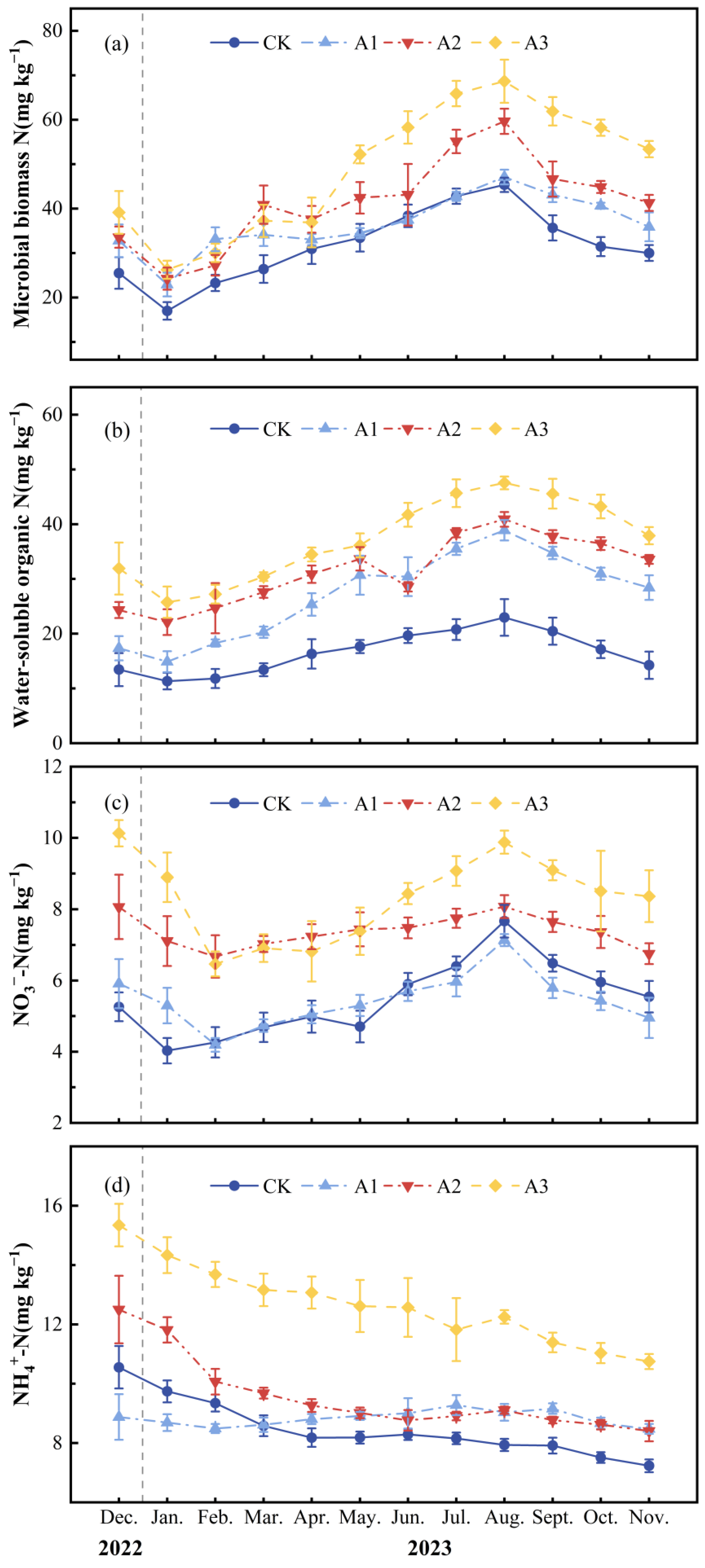
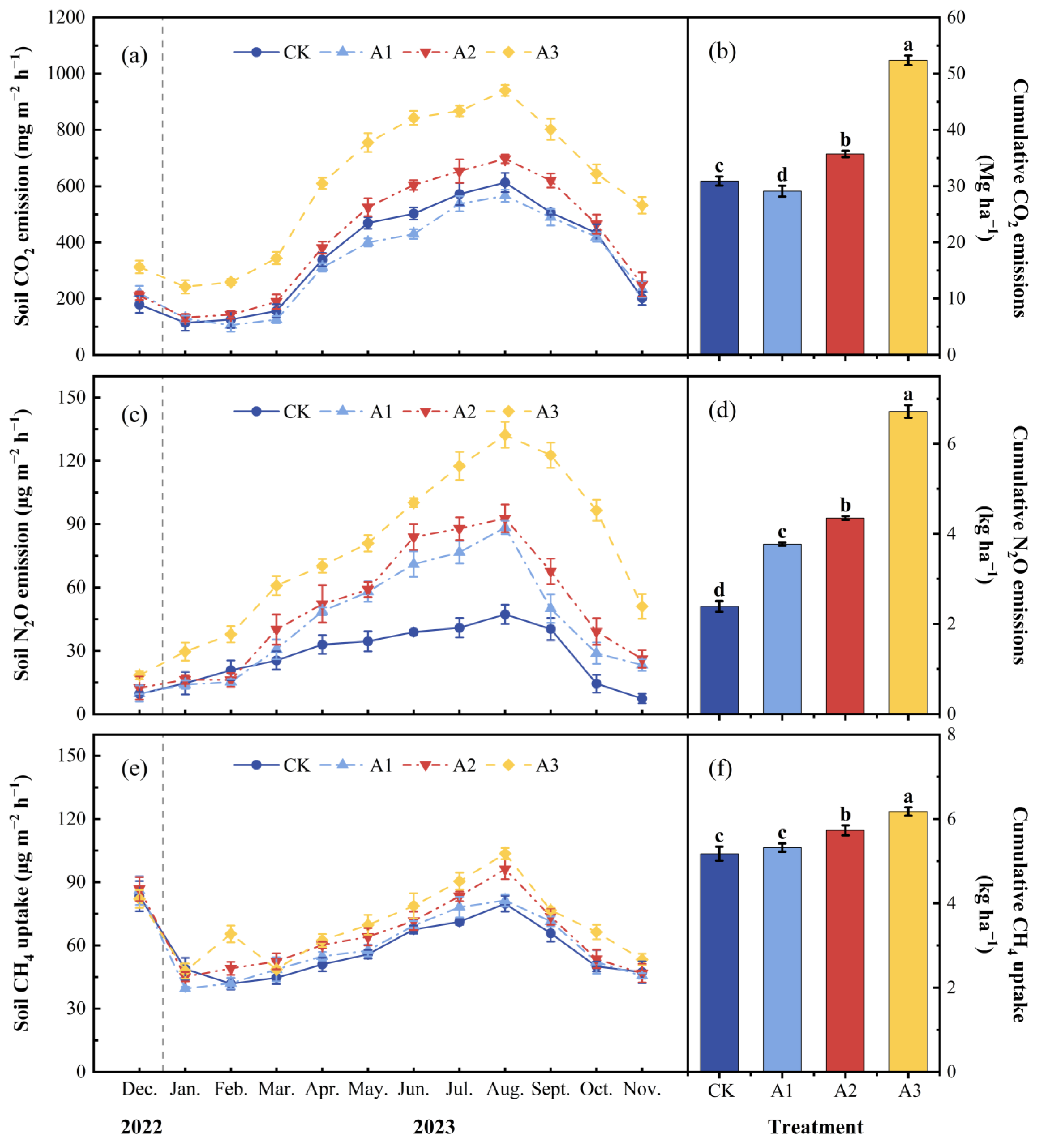

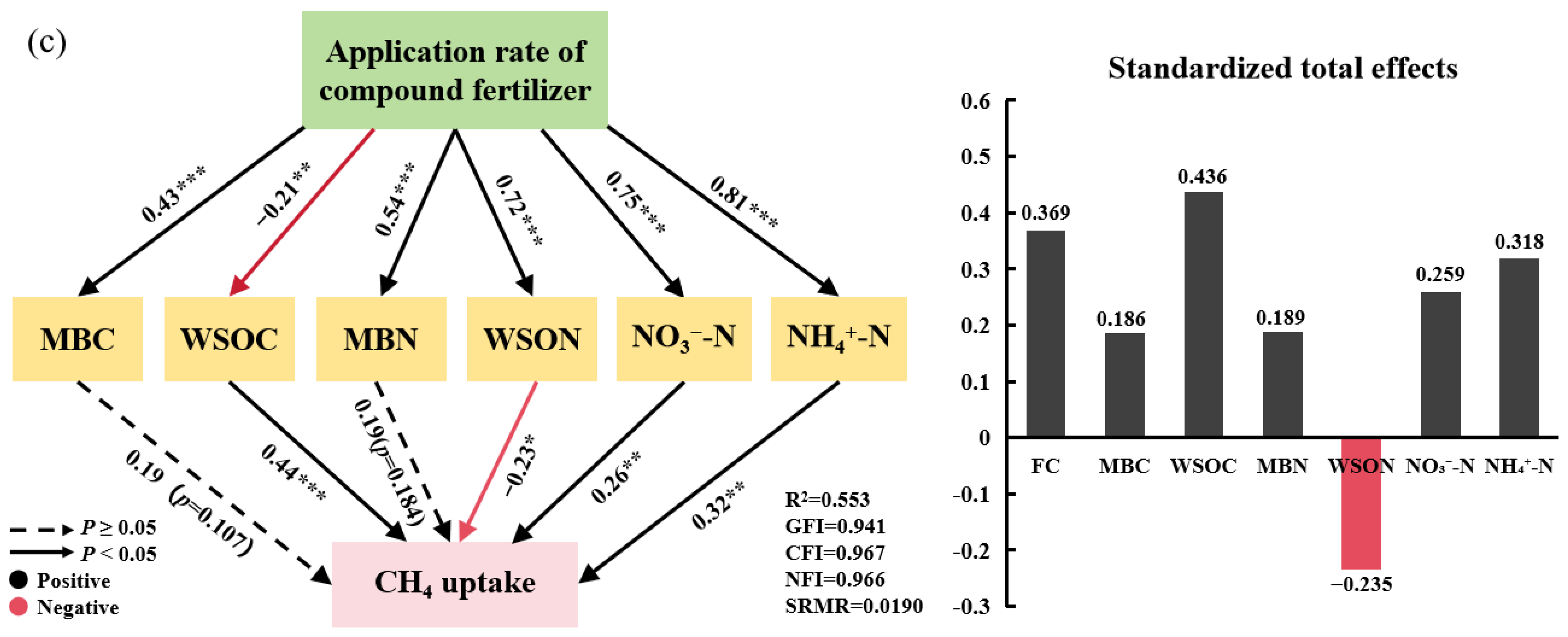


| Treatment | T (°C) | M (g kg−1) | pH | CO2 Emission (mg m−2 h−1) | N2O Emission (μg m−2 h−1) | CH4 Uptake (μg m−2 h−1) | MBC (mg kg−1) | WSOC (mg kg−1) | MBN (mg kg−1) | WSON (mg kg−1) | NO3−-N (mg kg−1) | NH4+-N (mg kg−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CK | 17.23 ± 0.25 a | 276.02 ± 2.11 a | 5.36 ± 0.03 a | 351.27 ± 9.47 d | 27.28 ± 1.31 d | 58.94 ± 1.90 c | 213.02 ± 4.75 c | 288.73 ± 4.58 a | 31.68 ± 0.29 c | 16.59 ± 0.65 d | 5.49 ± 0.10 c | 8.47 ± 0.12 c |
| A1 | 17.31 ± 0.12 a | 260.28 ± 0.20 b | 5.33 ± 0.02 a | 330.74 ± 11.17 c | 42.90 ± 0.46 c | 60.59 ± 1.10 c | 209.19 ± 0.95 c | 282.48 ± 1.85 a | 36.44 ± 1.06 c | 27.14 ± 0.21 c | 5.45 ± 0.24 c | 8.83 ± 0.18 c |
| A2 | 17.25 ± 0.27 a | 261.34 ± 1.63 b | 5.32 ± 0.03 a | 406.25 ± 6.64 b | 49.47 ± 0.46 b | 65.26 ± 1.37 b | 229.98 ± 7.73 b | 272.83 ± 2.51 b | 41.38 ± 1.91 b | 31.57 ± 0.18 b | 7.39 ± 0.15 b | 9.58 ± 0.12 b |
| A3 | 17.15 ± 0.21 a | 273.38 ± 2.01 a | 5.37 ± 0.03 a | 596.18 ± 9.92 a | 76.50 ± 1.58 a | 70.46 ± 1.11 a | 276.76 ± 5.36 a | 263.42 ± 4.28 c | 48.38 ± 1.32 a | 37.29 ± 0.29 a | 8.33 ± 0.19 a | 12.67 ± 0.30 a |
| Analysis of variance between treatments | ns | *** | ns | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| GHG | Treatment | Model | df | R2 | p |
|---|---|---|---|---|---|
| CO2 | CK | Y = 0.904T | 36 | 0.812 | *** |
| Y = 0.546T + 0.429WSON | 36 | 0.866 | *** | ||
| A1 | Y = 0.912WSON | 36 | 0.826 | *** | |
| Y = 0.812WSON + 0.283NH4+-N | 36 | 0.895 | *** | ||
| Y = 0.740WSON + 0.193NH4+-N + 0.192NO3−-N | 36 | 0.913 | *** | ||
| Y = 0.589WSON + 0.141NH4+-N + 0.204NO3−-N + 0.195T | 36 | 0.921 | *** | ||
| A2 | Y = 0.945WSOC | 36 | 0.890 | *** | |
| Y = 0.888WSOC + 0.154NO3−-N | 36 | 0.909 | *** | ||
| Y = 0.892WSOC + 0.142NO3−-N + 0.120pH | 36 | 0.921 | *** | ||
| A3 | Y = 0.940MBC | 36 | 0.880 | *** | |
| Y = 0.507MBC + 0.486WSOC | 36 | 0.928 | *** | ||
| Y = 0.602MBC + 0.486WSOC−0.158M | 36 | 0.944 | *** |
| GHG | Treatment | Model | df | R2 | p |
|---|---|---|---|---|---|
| N2O | CK | Y = 0.854T | 36 | 0.721 | *** |
| Y = 0.985T + 0.232NH4+-N | 36 | 0.752 | *** | ||
| Y = 0.495T + 0.315NH4+-N + 0.585WSOC | 36 | 0.797 | *** | ||
| A1 | Y = 0.945MBC | 36 | 0.890 | *** | |
| Y = 0.627MBC + 0.353T | 36 | 0.911 | *** | ||
| A2 | Y = 0.940T | 36 | 0.880 | *** | |
| Y = 0.639T + 0.339WSON | 36 | 0.902 | *** | ||
| Y = 0.319T + 0.330WSON + 0.353MBC | 36 | 0.918 | *** | ||
| A3 | Y = 0.937MBC | 36 | 0.873 | *** | |
| Y = 0.617MBC + 0.358WSOC | 36 | 0.898 | *** | ||
| Y = 0.384MBC + 0.314WSOC + 0.212NO3−-N | 36 | 0.912 | *** |
| GHG | Treatment | Model | df | R2 | p |
|---|---|---|---|---|---|
| CH4 | CK | Y = 0.616NO3−-N | 36 | 0.361 | *** |
| Y = 0.884NO3−-N + 0.599NH4+-N | 36 | 0.646 | *** | ||
| Y = 0.619NO3−-N + 0.633NH4+-N + 0.406MBC | 36 | 0.728 | *** | ||
| A1 | Y = 0.712NO3−-N | 36 | 0.493 | *** | |
| Y = 0.508NO3−-N + 0.323T | 36 | 0.544 | *** | ||
| A2 | Y = 0.830M | 36 | 0.679 | *** | |
| Y = 0.654M + 0.357NO3−-N | 36 | 0.772 | *** | ||
| Y = 0.768M + 0.283NO3−-N + 0.244NH4+-N | 36 | 0.819 | *** | ||
| Y = 0.518M + 0.211NO3−-N + 0.501NH4+-N + 0.478WSOC | 36 | 0.855 | *** | ||
| A3 | Y = 0.683WSON | 36 | 0.451 | *** | |
| Y = 0.485WSON + 0.410M | 36 | 0.571 | *** | ||
| Y = 0.383WSON + 0.381M + 0.272NO3−-N | 36 | 0.623 | *** |
| CK | A1 | A2 | A3 | |
|---|---|---|---|---|
| Herb carbon sequestration | 0.91 ± 0.07 a | 0.70 ± 0.22 a | 0.80 ± 0.21 a | 0.96 ± 0.49 a |
| Shrub carbon sequestration | 0.58 ± 0.07 a | 0.52 ± 0.04 a | 0.64 ± 0.18 a | 0.59 ± 0.24 a |
| Moso bamboo carbon sequestration | 29.99 ± 1.52 c | 35.93 ± 1.31 b | 38.47 ± 1.01 a | 38.95 ± 1.41 a |
| Vegetation carbon sequestration | 31.48 ± 1.60 c | 37.16 ± 1.34 b | 39.90 ± 0.74 a | 40.50 ± 0.95 a |
| Cumulative soil CO2 emission | 30.90 ± 0.82 c | 29.10 ± 0.97 d | 35.72 ± 0.58 b | 52.37 ± 0.86 a |
| Cumulative soil N2O emission | 0.71 ± 0.04 d | 1.12 ± 0.01 c | 1.30 ± 0.01 b | 2.00 ± 0.04 a |
| Cumulative soil CH4 uptake | 0.13 ± 0.01 c | 0.13 ± 0.01 c | 0.14 ± 0.01 b | 0.15 ± 0.01 a |
| Total GHG emissions | 31.48 ± 0.81 c | 30.09 ± 0.96 c | 36.87 ± 0.58 b | 54.22 ± 0.89 a |
| 32.36 ± 2.31 b | 42.90 ± 5.36 a | 46.04 ± 8.61 a | 46.37 ± 3.68 a | |
| Ecosystem carbon sequestration | 32.35 ± 3.07 b | 49.96 ± 4.94 a | 49.07 ± 7.51 a | 32.65 ± 3.15 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Cheng, X.; Zhang, X.; Shi, H.; Chen, J.; Xu, R.; Chen, Y.; Ying, J.; Wu, Y.; Zhou, Y.; et al. Effects of Fertilizer Application Intensity on Carbon Accumulation and Greenhouse Gas Emissions in Moso Bamboo Forest–Polygonatum cyrtonema Hua Agroforestry Systems. Plants 2024, 13, 1941. https://doi.org/10.3390/plants13141941
Chen H, Cheng X, Zhang X, Shi H, Chen J, Xu R, Chen Y, Ying J, Wu Y, Zhou Y, et al. Effects of Fertilizer Application Intensity on Carbon Accumulation and Greenhouse Gas Emissions in Moso Bamboo Forest–Polygonatum cyrtonema Hua Agroforestry Systems. Plants. 2024; 13(14):1941. https://doi.org/10.3390/plants13141941
Chicago/Turabian StyleChen, Huiying, Xuekun Cheng, Xingfa Zhang, Haitao Shi, Jiahua Chen, Ruizhi Xu, Yangen Chen, Jianping Ying, Yixin Wu, Yufeng Zhou, and et al. 2024. "Effects of Fertilizer Application Intensity on Carbon Accumulation and Greenhouse Gas Emissions in Moso Bamboo Forest–Polygonatum cyrtonema Hua Agroforestry Systems" Plants 13, no. 14: 1941. https://doi.org/10.3390/plants13141941





