Unlocking the Bioactive Potential and Exploring Novel Applications for Portuguese Endemic Santolina impressa
Abstract
:1. Introduction
2. Results
2.1. Phenolic Composition of Santolina impressa Infusion
2.1.1. Phenolic Acids
2.1.2. Flavonols
2.2. Antioxidant Activity of Santolina impressa Infusion
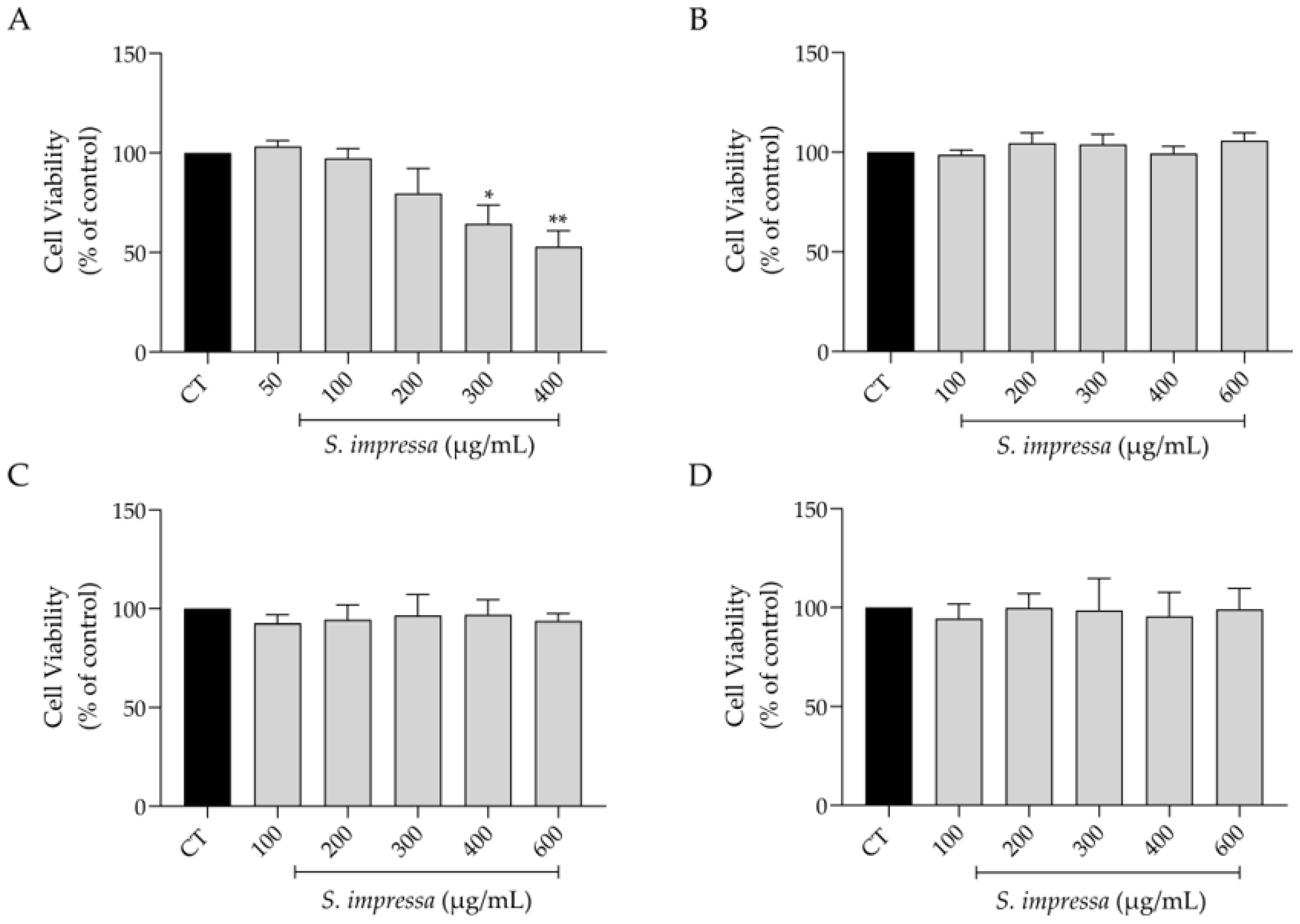
2.3. Effect on Cell Viability
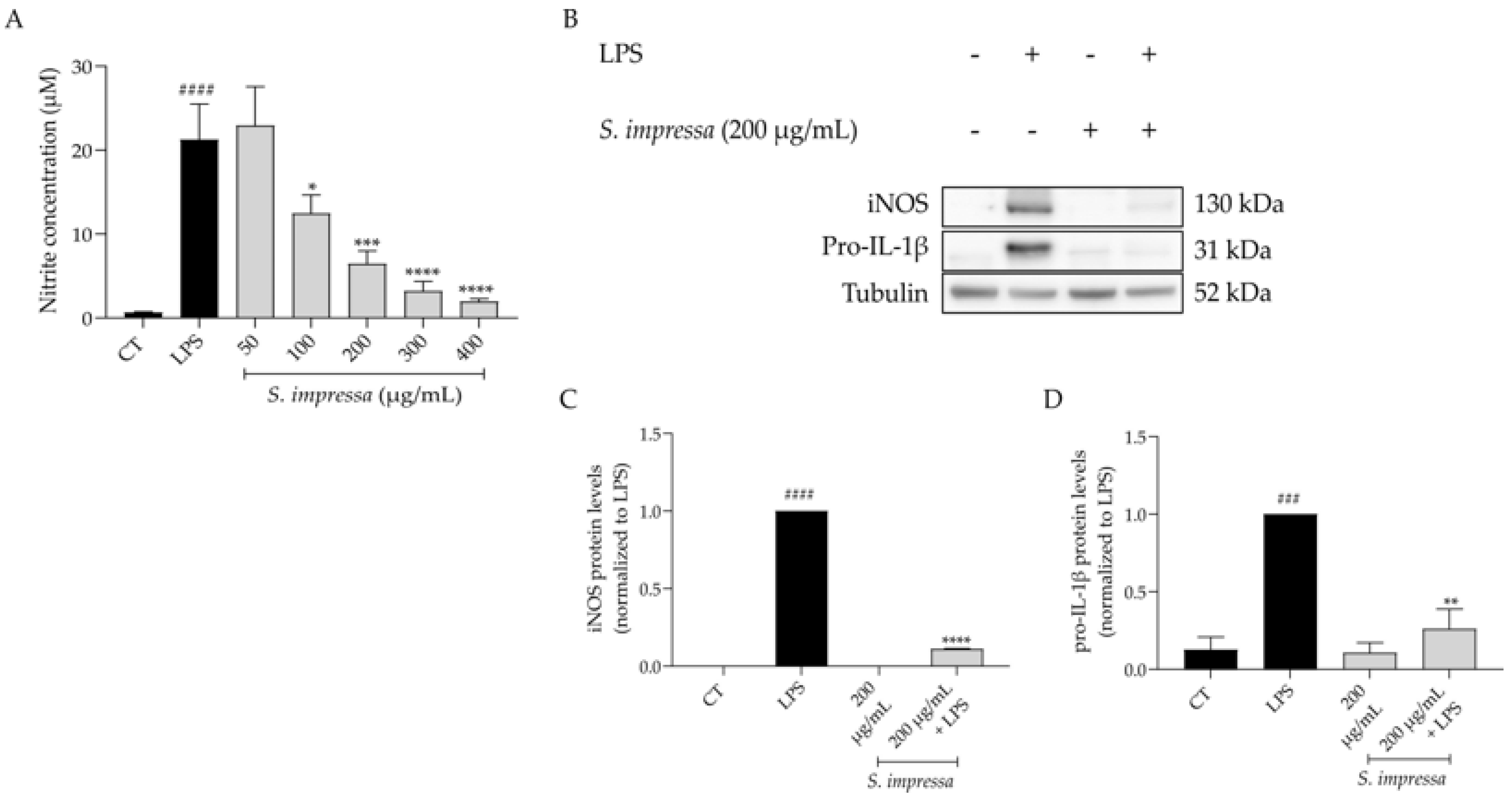
2.4. Santolina impressa Infusion Exerts Anti-Inflammatory Potential by Modulating the NF-κB Signaling Pathway
2.5. Santolina impressa Infusion Delays Cell Migration
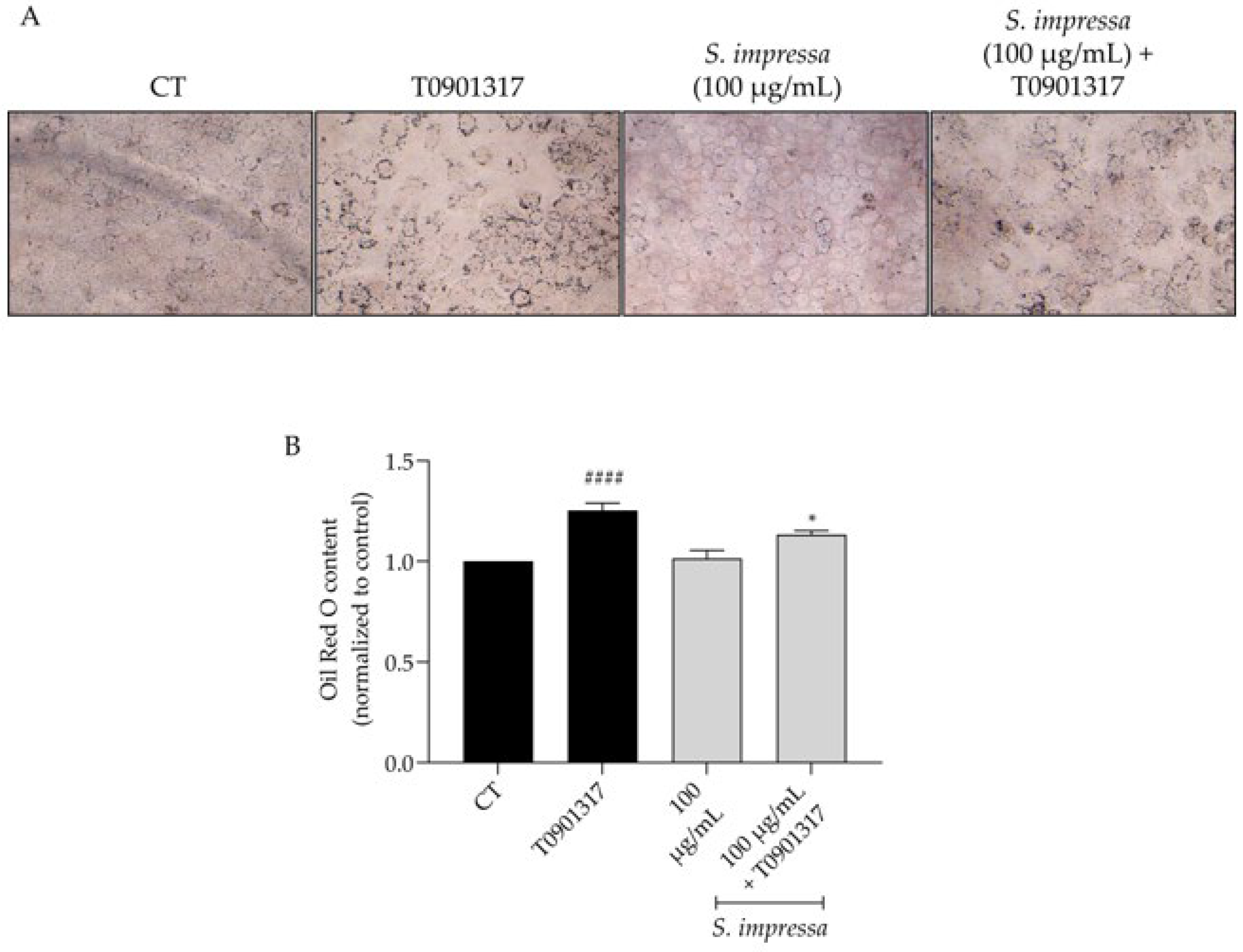
2.6. Santolina impressa Infusion Decreases Lipogenesis
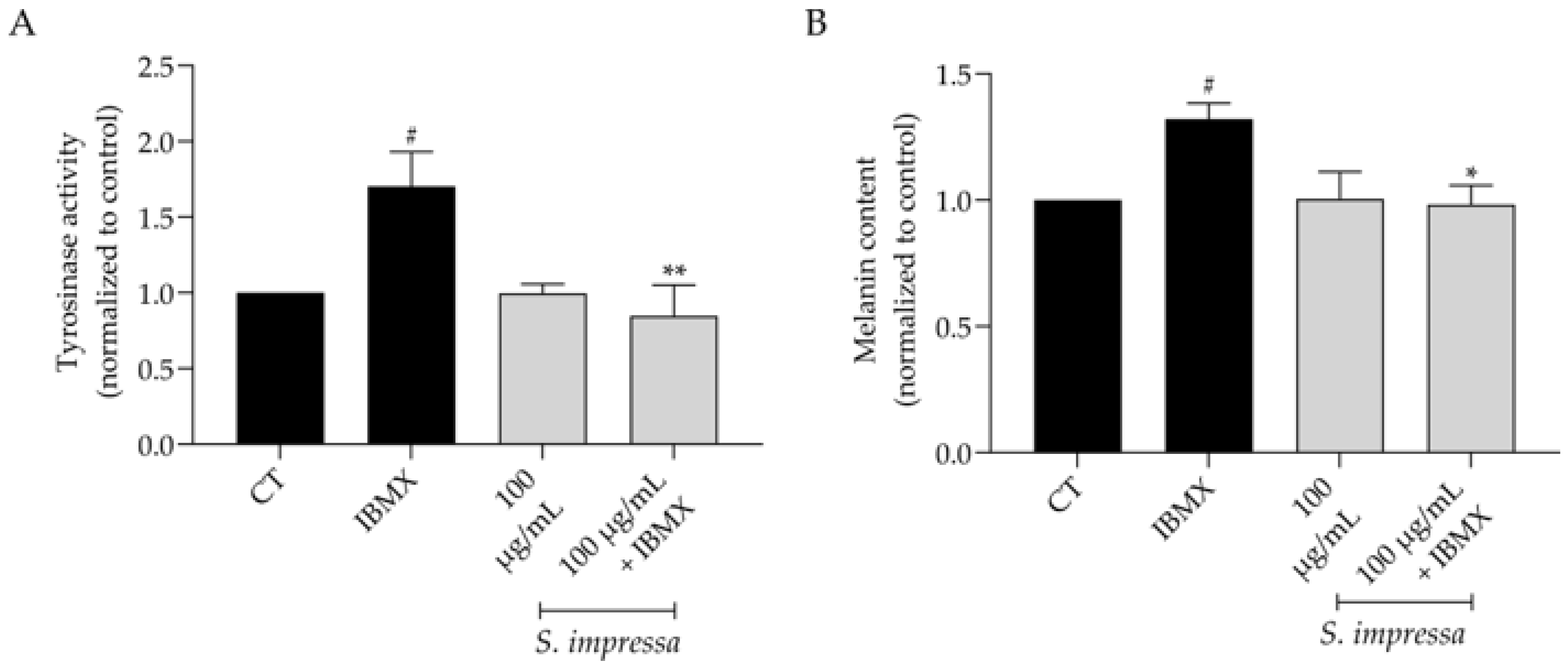
2.7. Santolina impressa Prevents Hyperpigmentation
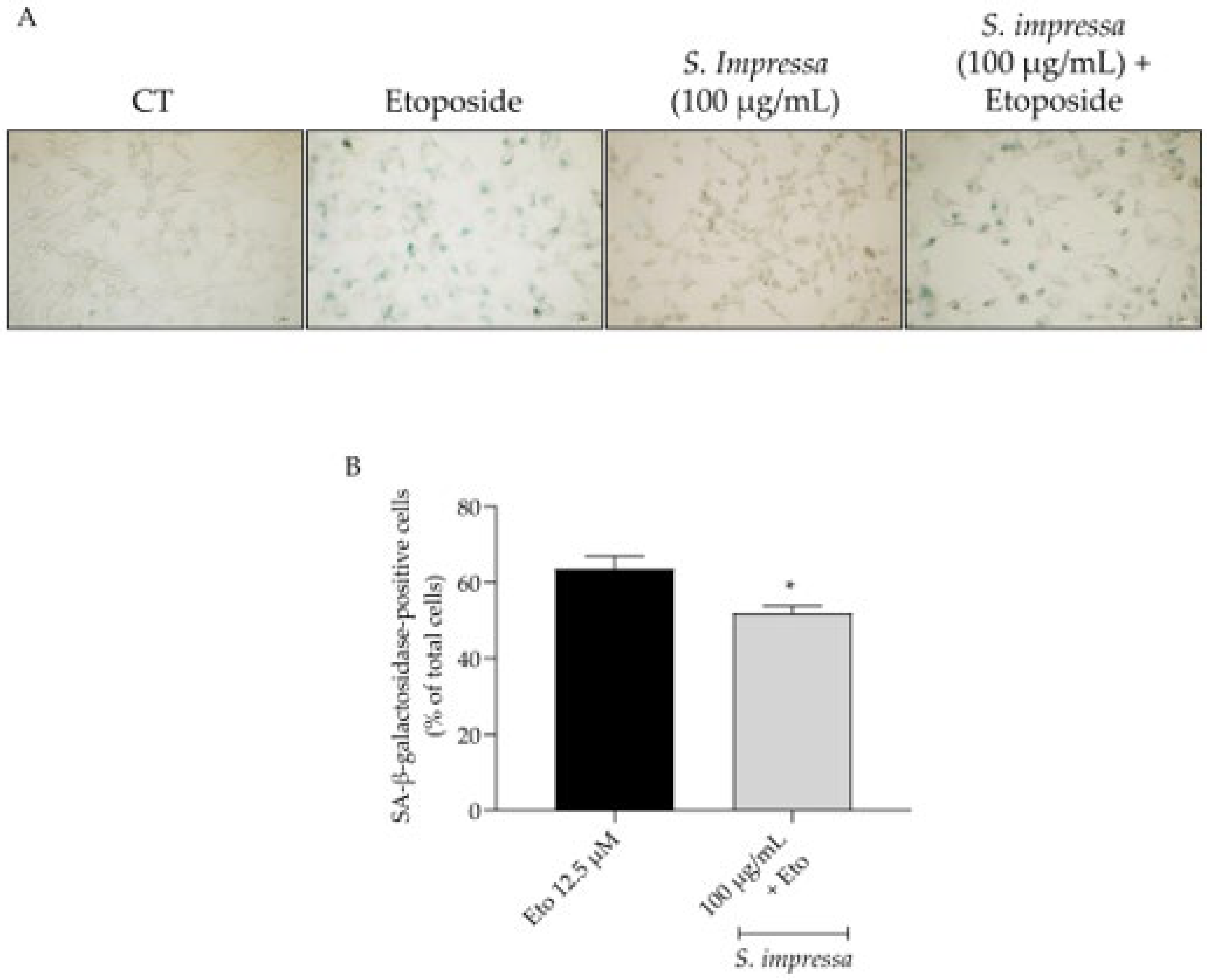
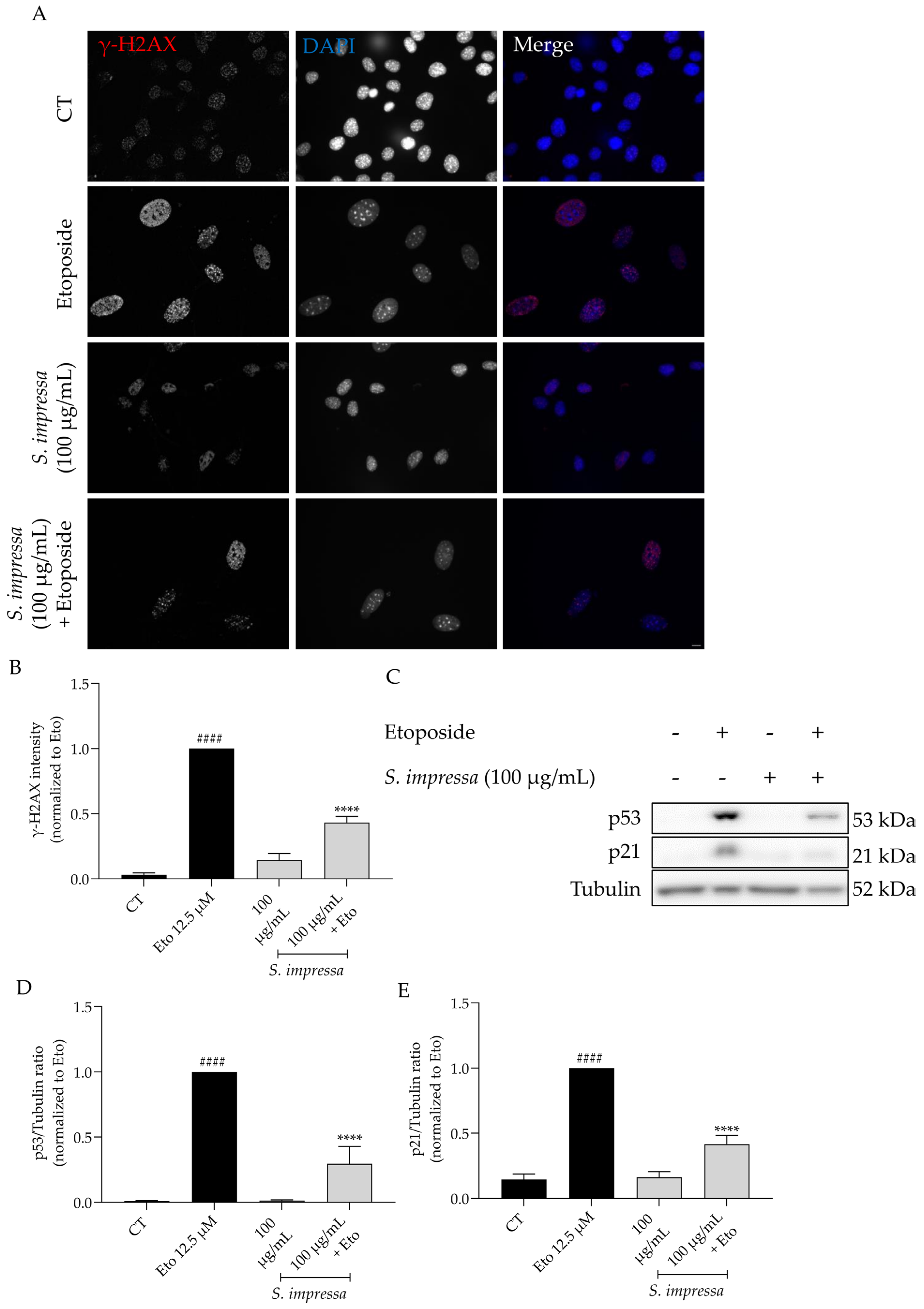
2.8. Santolina impressa Exerted Anti-Senescent Activity through Modulation of p53/p21 Pathway
3. Discussion
4. Materials and Methods
4.1. Material Collection and Santolina impressa’s Infusion Preparation
4.2. Chemical Characterization by HPLC-DAD-ESI-MSn
4.3. Antioxidant Activity
4.4. Cell Culture
4.5. Effect on Cell Viability
4.6. Anti-Inflammatory Potential
4.7. Cell Migration
4.8. Inhibition of Lipogenesis
4.9. Depigmenting Effect
4.10. Anti-Senescence Potential
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Ageing. Available online: https://www.who.int/health-topics/ageing#tab=tab_2 (accessed on 26 April 2024).
- Li, Z.; Zhang, Z.; Ren, Y.; Wang, Y.; Fang, J.; Yue, H.; Ma, S.; Guan, F. Aging and age-related diseases: From mechanisms to therapeutic strategies. Biogerontology 2021, 22, 165–187. [Google Scholar] [CrossRef]
- Anik, M.I.; Mahmud, N.; Al Masud, A.; Khan, M.I.; Islam, M.N.; Uddin, S.; Hossain, M.K. Role of reactive oxygen species in aging and age-related diseases: A review. ACS Appl. Bio Mater. 2022, 5, 4028–4054. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Hickson, L.J.; Eirin, A.; Kirkland, J.L.; Lerman, L.O. Cellular senescence: The good, the bad and the unknown. Nat. Rev. Nephrol. 2022, 18, 611–627. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, Y.; Zhao, Z.; Qiu, J. Oxidative stress in the skin: Impact and related protection. Int. J. Cosmet. Sci. 2021, 43, 495–509. [Google Scholar] [CrossRef] [PubMed]
- Solano, F. Photoprotection and skin pigmentation: Melanin-related molecules and some other new agents obtained from natural sources. Molecules 2020, 25, 1537. [Google Scholar] [CrossRef]
- Dong, Y.; Cao, J.; Wang, H.; Zhang, J.; Zhu, Z.; Bai, R.; Hao, H.; He, X.; Fan, R.; Dong, C. Nitric oxide enhances the sensitivity of alpaca melanocytes to respond to α-melanocyte-stimulating hormone by up-regulating melanocortin-1 receptor. Biochem. Biophys. Res. Commun. 2010, 396, 849–853. [Google Scholar] [CrossRef] [PubMed]
- Roméro-Graillet, C.; Aberdam, E.; Clément, M.; Ortonne, J.P.; Ballotti, R. Nitric oxide produced by ultraviolet-irradiated keratinocytes stimulates melanogenesis. J. Clin. Investig. 1997, 99, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Pedreiro, S.; Figueirinha, A.; Cavaleiro, C.; Cardoso, O.; Donato, M.M.; Salgueiro, L.; Ramos, F. Exploiting the Crithmum maritimum L. aqueous extracts and essential oil as potential preservatives in food, feed, pharmaceutical and cosmetic industries. Antioxidants 2023, 12, 252. [Google Scholar] [CrossRef] [PubMed]
- Matos, P.; Figueirinha, A.; Ferreira, I.; Cruz, M.T.; Batista, M.T. Acanthus mollis L. leaves as source of anti-inflammatory and antioxidant phytoconstituents. Nat. Prod. Res. 2019, 33, 1824–1827. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Xiao, M.; Zhao, J.; Li, Z.; Xing, B.; Li, X.; Kong, M.; Li, L.; Zhang, Q.; Liu, Y.; et al. An overview of plant phenolic compounds and their importance in human nutrition and management of type 2 diabetes. Molecules 2016, 21, 1374. [Google Scholar] [CrossRef] [PubMed]
- Alves-Silva, J.M.; Pedreiro, S.; Cavaleiro, C.; Cruz, M.T.; Figueirinha, A.; Salgueiro, L. Effect of Thymbra capitata (L.) Cav. on inflammation, senescence and cell migration. Nutrients 2023, 15, 1930. [Google Scholar] [CrossRef] [PubMed]
- Dumbuya, H.; Hafez, S.Y.; Oancea, E. Cross talk between calcium and ROS regulate the UVA-induced melanin response in human melanocytes. FASEB J. 2020, 34, 11605–11623. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-M.; Kim, M.Y.; Sohn, K.-C.; Jung, S.-Y.; Lee, H.-E.; Lim, J.W.; Kim, S.; Lee, Y.-H.; Im, M.; Seo, Y.-J.; et al. Nrf2 negatively regulates melanogenesis by modulating PI3K/Akt signaling. PLoS ONE 2014, 9, e96035. [Google Scholar] [CrossRef] [PubMed]
- Rivero-Guerra, A.O. Cytogenetics, geographical distribution, pollen stainability and fecundity of Santolina impressa (Asteraceae: Anthemideae). Folia Geobot. 2010, 45, 95–109. [Google Scholar] [CrossRef]
- Rivero-Guerra, A.O. Typification and synonymy of names in Santolina (Asteraceae: Anthemideae) published by Hoffmannsegg and Link. Nord. J. Bot. 2010, 28, 581–587. [Google Scholar] [CrossRef]
- Barra, M.I.G. Actividade Anti-Herpética de Extratos Aquosos de Plantas Aromáticas: Efeito Virucida e Inibição Da Replicação Viral; Faculdade de Ciências—Universidade de Lisboa: Lisbon, Portugal, 2014. [Google Scholar]
- Proença da Cunha, A.; Ribeiro, J.A.; Roque, O.R. Plantas Aromáticas Em Portugal—Caraterização e Utilizações; Fundação Calouste Gulbenkian: Lisboa, Portugal, 2007; ISBN 978-972-31-1170-5. [Google Scholar]
- Proença da Cunha, A.; Nogueira, M.T.; Roque, O.R. Plantas Aromáticas e Óleos Essenciais Composição e Aplicações; Fundação Calouste Gulbenkian: Lisbon, Portugal, 2012; ISBN 978-972-31-1450-8. [Google Scholar]
- Alves-Silva, J.M.; Zuzarte, M.; Gonçalves, M.J.; Cruz, M.T.; Cavaleiro, C.; Salgueiro, L. Unveiling the bioactive potential of the essential oil of a Portuguese endemism, Santolina impressa. J. Ethnopharmacol. 2019, 244, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Avello, D.; Lozano-Castellón, J.; Mardones, C.; Pérez, A.J.; Saéz, V.; Riquelme, S.; Von Baer, D.; Vallverdú-Queralt, A. Phenolic profile of grape canes: Novel compounds identified by LC-ESI-LTQ-Orbitrap-MS. Molecules 2019, 24, 3763. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, D.O.; Curto, A.F.; Guido, L.F. Determination of phenolic content in different barley varieties and corresponding malts by liquid chromatography-diode array detection-electrospray ionization tandem mass spectrometry. Antioxidants 2015, 4, 563–576. [Google Scholar] [CrossRef] [PubMed]
- Willems, J.L.; Khamis, M.M.; Mohammed Saeid, W.; Purves, R.W.; Katselis, G.; Low, N.H.; El-Aneed, A. Analysis of a series of chlorogenic acid isomers using differential ion mobility and tandem mass spectrometry. Anal. Chim. Acta 2016, 933, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yao, L.; Gong, K.; Li, K.; Sun, L.; Cai, W. Identification and quantification of chlorogenic acids from the root bark of Acanthopanax gracilistylus by UHPLC-Q-Exactive Orbitrap mass spectrometry. ACS Omega 2022, 7, 25675–25685. [Google Scholar] [CrossRef] [PubMed]
- Llorent-Martínez, E.J.; Gouveia, S.; Castilho, P.C. Analysis of phenolic compounds in leaves from endemic trees from Madeira island. A contribution to the chemotaxonomy of laurisilva forest species. Ind. Crops Prod. 2015, 64, 135–151. [Google Scholar] [CrossRef]
- Gouveia, S.; Castilho, P.C. Characterisation of phenolic acid derivatives and flavonoids from different morphological parts of Helichrysum obconicum by a RP-HPLC-DAD-(-)-ESI-MSn Method. Food Chem. 2011, 129, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Ramabulana, A.T.; Steenkamp, P.; Madala, N.; Dubery, I.A. Profiling of chlorogenic acids from Bidens pilosa and differentiation of closely related positional isomers with the aid of UHPLC-QTOF-MS/MS-based in-source collision-induced dissociation. Metabolites 2020, 10, 178. [Google Scholar] [CrossRef] [PubMed]
- Spínola, V.; Llorent-Martínez, E.J.; Gouveia-Figueira, S.; Castilho, P.C. Ulex europaeus: From noxious weed to source of valuable isoflavones and flavanones. Ind. Crop. Prod. 2016, 90, 9–27. [Google Scholar] [CrossRef]
- Sinosaki, N.B.M.; Tonin, A.P.P.; Ribeiro, M.A.S.; Poliseli, C.B.; Roberto, S.B.; Da Silveira, R.; Visentainer, J.V.; Santos, O.O.; Meurer, E.C. Structural study of phenolic acids by triple quadrupole mass spectrometry with electrospray ionization in negative mode and H/D isotopic exchange. J. Braz. Chem. Soc. 2020, 31, 402–408. [Google Scholar] [CrossRef]
- Gomes, A.; Pimpão, R.C.; Fortalezas, S.; Figueira, I.; Miguel, C.; Aguiar, C.; Salgueiro, L.; Cavaleiro, C.; Gonç, M.J.; Clemente, A.; et al. Chemical characterization and bioactivity of phytochemicals from Iberian endemic Santolina semidentata and strategies for Ex situ propagation. Ind. Crops Prod. 2015, 74, 505–513. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Novikov, N.M.; Zolotaryova, S.Y.; Gautreau, A.M.; Denisov, E.V. Mutational drivers of cancer cell migration and invasion. Br. J. Cancer 2021, 124, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Łuczaj, W.; do Rosário Domingues, M.; Domingues, P.; Skrzydlewska, E. changes in lipid profile of keratinocytes from rat skin exposed to chronic UVA or UVB radiation and topical application of cannabidiol. Antioxidants 2020, 9, 1178. [Google Scholar] [CrossRef]
- Olivier, E.; Dutot, M.; Regazzetti, A.; Dargère, D.; Auzeil, N.; Laprévote, O.; Rat, P. Lipid deregulation in uv irradiated skin cells: Role of 25-hydroxycholesterol in keratinocyte differentiation during photoaging. J. Steroid Biochem. Mol. Biol. 2017, 169, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Dalmau, N.; Andrieu-Abadie, N.; Tauler, R.; Bedia, C. Phenotypic and lipidomic characterization of primary human epidermal keratinocytes exposed to simulated solar UV radiation. J. Dermatol. Sci. 2018, 92, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Hakozaki, T.; Swanson, C.L.; Bissett, D.L. Hyperpigmentation in aging skin. In Textbook of Aging Skin; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1017–1026. [Google Scholar]
- Soto-Gamez, A.; Demaria, M. Therapeutic interventions for aging: The case of cellular senescence. Drug Discov. Today 2017, 22, 786–795. [Google Scholar] [CrossRef] [PubMed]
- González-Gualda, E.; Baker, A.G.; Fruk, L.; Muñoz-Espín, D. A Guide to assessing cellular senescence in vitro and in vivo. FEBS J. 2021, 288, 56–80. [Google Scholar] [CrossRef] [PubMed]
- Kudlova, N.; De Sanctis, J.B.; Hajduch, M. Cellular senescence: Molecular targets, biomarkers, and senolytic drugs. Int. J. Mol. Sci. 2022, 23, 4168. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.M.; Vieira Falé, P.L.; Madeira, P.; Pacheco, R.; Florêncio, M.H.; Ascensão, L.; Marques Serralheiro, M.L. Phenolic profile and biological activities of decoctions from Santolina impressa, a Portuguese endemic species. J. Herb. Med. 2020, 21, 100335. [Google Scholar] [CrossRef]
- Aourach, M.; González-De-peredo, A.V.; Vázquez-Espinosa, M.; Essalmani, H.; Palma, M.; Barbero, G.F. Optimization and comparison of ultrasound and microwave-assisted extraction of phenolic compounds from cotton-lavender (Santolina chamaecyparissus L.). Agronomy 2021, 11, 84. [Google Scholar] [CrossRef]
- Boudoukha, C.; Bouriche, H.; Elmastas, M.; Aksit, H.; Kayir, O.; Genc, N.; Senator, A.; Ashraf Ahmed ElHawary, E. Antioxidant activity of polyphenolic leaf extract from Santolina chamaecyparissus L. (Asteraceae) and the isolated luteolin-7-o-glucoside. J. Pharm. Res. Int. 2018, 22, 40726. [Google Scholar] [CrossRef]
- Alcázar Magaña, A.; Kamimura, N.; Soumyanath, A.; Stevens, J.F.; Maier, C.S. Caffeoylquinic acids: Chemistry, biosynthesis, occurrence, analytical challenges, and bioactivity. Plant J. 2021, 107, 1299. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.; Dupuis, J.H.; Yada, R.Y.; Kitts, D.D. Chlorogenic acid isomers directly interact with Keap 1-Nrf2 signaling in Caco-2 Cells. Mol. Cell. Biochem. 2019, 457, 105. [Google Scholar] [CrossRef]
- Yu, S.; Li, Y.; Zhou, Y.; Follansbee, T.; Hwang, S.T. Immune mediators and therapies for pruritus in atopic dermatitis and psoriasis. J. Cutan. Immunol. Allergy 2019, 2, 4–14. [Google Scholar] [CrossRef]
- Schwingen, J.; Kaplan, M.; Kurschus, F.C. Review—Current concepts in inflammatory skin diseases evolved by transcriptome analysis: In-depth analysis of atopic dermatitis and psoriasis. Int. J. Mol. Sci. 2020, 21, 699. [Google Scholar] [CrossRef] [PubMed]
- de Sá, D.C.; Festa Neto, C. Inflammasomes and dermatology. An. Bras. Dermatol. 2016, 91, 566–578. [Google Scholar] [CrossRef]
- Song, A.; Lee, S.E.; Kim, J.H. Immunopathology and immunotherapy of inflammatory skin diseases. Immune Netw. 2022, 22, e7. [Google Scholar] [CrossRef] [PubMed]
- Goh, B.H.; Mocan, A.; Xiao, J.; Mah, S.H.; Yap, W.H. Editorial: Targeting Human Inflammatory Skin Diseases with Natural Products: Exploring Potential Mechanisms and Regulatory Pathways. Front. Pharmacol. 2021, 12, 791151. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Shin, H.S.; Satsu, H.; Totsuka, M.; Shimizu, M. 5-Caffeoylquinic acid and caffeic acid down-regulate the oxidative stress- and TNF-α-induced secretion of interleukin-8 from Caco-2 cells. J. Agric. Food Chem. 2008, 56, 3863–3868. [Google Scholar] [CrossRef] [PubMed]
- Abdel Motaal, A.; Ezzat, S.M.; Tadros, M.G.; El-Askary, H.I. In vivo anti-inflammatory activity of caffeoylquinic acid derivatives from Solidago virgaurea in rats. Pharm. Biol. 2016, 54, 2864–2870. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, M.D.; Chen, G.; Almeida, M.C.; Soares, D.M.; de Souza, G.E.P.; Lopes, N.P.; Lantz, R.C. Effects of caffeoylquinic acid derivatives and c-flavonoid from Lychnophora ericoides on in vitro inflammatory mediator production. Nat. Prod. Commun. 2010, 5, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shi, L.; Qiu, W.; Shi, Y. Ferulic acid exhibits anti-inflammatory effects by inducing autophagy and blocking NLRP3 inflammasome activation. Mol. Cell. Toxicol. 2022, 18, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.N.; Wu, W.J.; Sun, C.Z.; Liu, H.F.; Chen, W.B.; Zhan, Q.P.; Lei, Z.G.; Xin, X.; Ma, J.J.; Yao, K.; et al. Antioxidant and anti-inflammatory capacity of ferulic acid released from wheat bran by solid-state fermentation of Aspergillus niger. Biomed. Environ. Sci. 2019, 32, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Chen, X.; Qiang, S.; Su, J.; Li, J. Anti-oxidation and anti-inflammatory potency evaluation of ferulic acid derivatives obtained through virtual screening. Int. J. Mol. Sci. 2021, 22, 11305. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.O.; Yin, H.H.; Park, S.H.; Byun, E.B.; Ha, H.Y.; Jang, S. Il Anti-inflammatory activity of myricetin from Diospyros lotus through suppression of NF-ΚB and STAT1 activation and Nrf2-mediated HO-1 induction in lipopolysaccharide-stimulated RAW 264.7 macrophages. Biosci. Biotechnol. Biochem. 2016, 80, 1520–1530. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-J.; Tong, Y.; Lu, S.; Yang, R.; Liao, X.; Xu, Y.-F.; Li, X. Anti-inflammatory activity of myricetin isolated from Myrica rubra Sieb. et Zucc. leaves. Planta Med. 2010, 76, 1492–1496. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.H.; Karadeniz, F.; Lee, J.I.; Park, S.Y.; Seo, Y.; Kong, C.-S. Anticatabolic and anti-inflammatory effects of myricetin 3-O-β-D-galactopyranoside in UVA-irradiated dermal cells via repression of MAPK/AP-1 and activation of TGFβ/Smad. Molecules 2020, 25, 1331. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Lin, H.; Xie, S.; Huang, B.; Qian, Y.; Chen, K.; Niu, Y.; Shen, H.-M.; Cai, J.; Li, P.; et al. Myricetin inhibits NLRP3 inflammasome activation via reduction of ROS-dependent ubiquitination of ASC and promotion of ROS-independent NLRP3 ubiquitination. Toxicol. Appl. Pharmacol. 2019, 365, 19–29. [Google Scholar] [CrossRef]
- Lesjak, M.; Beara, I.; Simin, N.; Pintać, D.; Majkić, T.; Bekvalac, K.; Orčić, D.; Mimica-Dukić, N. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct. Foods 2018, 40, 68–75. [Google Scholar] [CrossRef]
- Saeedi-Boroujeni, A.; Mahmoudian-Sani, M.-R. Anti-inflammatory potential of quercetin in COVID-19 treatment. J. Inflamm. 2021, 18, 3. [Google Scholar] [CrossRef] [PubMed]
- Rogerio, A.P.; Kanashiro, A.; Fontanari, C.; da Silva, E.V.G.; Lucisano-Valim, Y.M.; Soares, E.G.; Faccioli, L.H. Anti-inflammatory activity of quercetin and isoquercitrin in experimental murine allergic asthma. Inflamm. Res. 2007, 56, 402–408. [Google Scholar] [CrossRef]
- Khalid, K.A.; Nawi, A.F.M.; Zulkifli, N.; Barkat, M.D.A.; Hadi, H. Aging and wound healing of the skin: A review of clinical and pathophysiological hallmarks. Life 2022, 12, 2142. [Google Scholar] [CrossRef] [PubMed]
- El-Askary, H.; Salem, H.H.; Abdel Motaal, A. Potential mechanisms involved in the protective effect of dicaffeoylquinic acids from Artemisia annua L. leaves against diabetes and its complications. Molecules 2022, 27, 857. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, S.; Ebrahimi, S.; Salehi, P.; Moridi Farimani, M.; Hamburger, M.; Jabbarzadeh, E. Wound healing potential of chlorogenic acid and myricetin-3-O-β-rhamnoside isolated from Parrotia persica. Molecules 2017, 22, 1501. [Google Scholar] [CrossRef] [PubMed]
- Ghaisas, M.M.; Kshirsagar, S.B.; Sahane, R.S. Evaluation of wound healing activity of ferulic acid in diabetic rats. Int. Wound J. 2014, 11, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Carbone, C.; Caddeo, C.; Grimaudo, M.A.; Manno, D.E.; Serra, A.; Musumeci, T. Ferulic Acid-NLC with Lavandula essential oil: A possible strategy for wound-healing? Nanomaterials 2020, 10, 898. [Google Scholar] [CrossRef] [PubMed]
- Bairagi, U.; Mittal, P.; Singh, J.; Mishra, B. Preparation, characterization, and in vivo evaluation of nano formulations of ferulic acid in diabetic wound healing. Drug Dev. Ind. Pharm. 2018, 44, 1783–1796. [Google Scholar] [CrossRef] [PubMed]
- Elloumi, W.; Mahmoudi, A.; Ortiz, S.; Boutefnouchet, S.; Chamkha, M.; Sayadi, S. Wound healing potential of quercetin-3-O-rhamnoside and myricetin-3-O-rhamnoside isolated from Pistacia lentiscus distilled leaves in rats model. Biomed. Pharmacother. 2022, 146, 112574. [Google Scholar] [CrossRef] [PubMed]
- Doersch, K.M.; Newell-Rogers, M.K. The impact of quercetin on wound healing relates to changes in AV and Β1 integrin expression. Exp. Biol. Med. 2017, 242, 1424–1431. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Huang, J.; Lin, M.; Xie, T.; You, T. Quercetin promotes diabetic wound healing via switching macrophages from M1 to M2 polarization. J. Surg. Res. 2020, 246, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Elshamy, A.I.; Ammar, N.M.; Hassan, H.A.; El-Kashak, W.A.; Al-Rejaie, S.S.; Abd-ElGawad, A.M.; Farrag, A.-R.H. Topical wound healing activity of myricetin isolated from Tecomaria capensis v. aurea. Molecules 2020, 25, 4870. [Google Scholar] [CrossRef] [PubMed]
- Buko, V.; Zavodnik, I.; Budryn, G.; Zakłos-Szyda, M.; Belonovskaya, E.; Kirko, S.; Żyżelewicz, D.; Zakrzeska, A.; Bakunovich, A.; Rusin, V.; et al. Chlorogenic acid protects against advanced alcoholic steatohepatitis in rats via modulation of redox homeostasis, inflammation, and lipogenesis. Nutrients 2021, 13, 4155. [Google Scholar] [CrossRef] [PubMed]
- Farias-Pereira, R.; Oshiro, J.; Kim, K.-H.; Park, Y. Green coffee bean extract and 5-O-caffeoylquinic acid regulate fat metabolism in Caenorhabditis elegans. J. Funct. Foods 2018, 48, 586–593. [Google Scholar] [CrossRef]
- Raineri, A.; Campagnari, R.; Dal Toso, R.; Copetti, S.; Gomez-Lira, M.; Menegazzi, M. 3,5-Dicaffeoylquinic acid lowers 3T3-L1 mitotic clonal expansion and adipocyte differentiation by enhancing Heme Oxygenase-1 expression. Molecules 2021, 26, 5027. [Google Scholar] [CrossRef] [PubMed]
- Ilavenil, S.; Kim, D.H.; Srigopalram, S.; Kuppusamy, P.; Valan Arasu, M.; Lee, K.D.; Lee, J.C.; Song, Y.H.; Jeong, Y.-I.; Choi, K.C. Ferulic acid in Lolium multiflorum inhibits adipogenesis in 3T3-L1 cells and reduced high-fat-diet-induced obesity in Swiss albino mice via regulating P38MAPK and P44/42 signal pathways. J. Funct. Foods 2017, 37, 293–302. [Google Scholar] [CrossRef]
- Little, R.; Houghton, M.J.; Carr, I.M.; Wabitsch, M.; Kerimi, A.; Williamson, G. The ability of quercetin and ferulic acid to lower stored fat is dependent on the metabolic background of human adipocytes. Mol. Nutr. Food Res. 2020, 64, e2000034. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.Y.; Ha, A.W.; Kim, W. Effects of quercetin on cell differentiation and adipogenesis in 3T3-L1 adipocytes. Nutr. Res. Pract. 2021, 15, 444. [Google Scholar] [CrossRef] [PubMed]
- Gnoni, A.; Di Chiara Stanca, B.; Giannotti, L.; Gnoni, G.V.; Siculella, L.; Damiano, F. Quercetin reduces lipid accumulation in a cell model of NAFLD by inhibiting de novo fatty acid synthesis through the Acetyl-CoA Carboxylase 1/AMPK/PP2A Axis. Int. J. Mol. Sci. 2022, 23, 1044. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, S.; Yang, X.; You, P.; Zhang, W. Myricetin suppresses differentiation of 3T3-L1 preadipocytes and enhances lipolysis in adipocytes. Nutr. Res. 2015, 35, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Bin, H.-S.; Choi, U.-K. Myricetin inhibits adipogenesis in human adipose tissue-derived mesenchymal stem cells. Food Sci. Biotechnol. 2012, 21, 1391–1396. [Google Scholar] [CrossRef]
- Zolghadri, S.; Beygi, M.; Mohammad, T.F.; Alijanianzadeh, M.; Pillaiyar, T.; Garcia-Molina, P.; Garcia-Canovas, F.; Munoz-Munoz, J.; Saboury, A.A. Targeting tyrosinase in hyperpigmentation: Current status, limitations and future promises. Biochem. Pharmacol. 2023, 212, 115574. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.H.; Kim, J.K.; Kim, J.; Jung, S.-H.; Lee, K. Characterization of caffeoylquinic acids from Lepisorus thunbergianus and their melanogenesis inhibitory activity. ACS Omega 2020, 5, 30946–30955. [Google Scholar] [CrossRef] [PubMed]
- Akihisa, T.; Kawashima, K.; Orido, M.; Akazawa, H.; Matsumoto, M.; Yamamoto, A.; Ogihara, E.; Fukatsu, M.; Tokuda, H.; Fuji, J. Antioxidative and melanogenesis-inhibitory activities of caffeoylquinic acids and other compounds from Moxa. Chem. Biodivers. 2013, 10, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.H.; Park, S.N. Mechanism underlying inhibitory effect of six dicaffeoylquinic acid isomers on melanogenesis and the computational molecular modeling studies. Bioorg Med. Chem. 2018, 26, 4201–4208. [Google Scholar] [CrossRef] [PubMed]
- Chaiprasongsuk, A.; Onkoksoong, T.; Pluemsamran, T.; Limsaengurai, S.; Panich, U. Photoprotection by dietary phenolics against melanogenesis induced by UVA through Nrf2-dependent antioxidant responses. Redox Biol. 2016, 8, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Zduńska-Pęciak, K.; Dębowska, R.; Kołodziejczak, A.; Rotsztejn, H. Ferulic acid—A novel topical agent in reducing signs of photoaging. Dermatol. Ther. 2022, 35, e15543. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Son, Y.; Lee, S.; Jeon, Y.; Lee, J. Quercetin inhibits α-MSH-stimulated melanogenesis in B16F10 melanoma cells. Phytother. Res. 2011, 25, 1166–1173. [Google Scholar] [CrossRef] [PubMed]
- Arung, E.T.; Furuta, S.; Ishikawa, H.; Kusuma, I.W.; Shimizu, K.; Kondo, R. Anti-melanogenesis properties of quercetin- and its derivative-rich extract from Allium cepa. Food Chem. 2011, 124, 1024–1028. [Google Scholar] [CrossRef]
- Oh, J.H.; Karadeniz, F.; Seo, Y.; Kong, C.-S. Dietary flavonoid myricetin 3-O-galactoside suppresses α-Melanocyte Stimulating Hormone-induced melanogenesis in B16F10 melanoma cells by regulating PKA and ERK1/2 activation. Z. Naturforschung C 2023, 78, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Karadeniz, F.; Oh, J.H.; Seo, Y.; Yang, J.; Lee, H.; Kong, C.-S. Quercetin 3-O-galactoside isolated from Limonium tetragonum inhibits melanogenesis by regulating PKA/MITF signaling and ERK activation. Int. J. Mol. Sci. 2023, 24, 3064. [Google Scholar] [CrossRef] [PubMed]
- Takekoshi, S.; Nagata, H.; Kitatani, K. Flavonoids enhance melanogenesis in human melanoma cells. Tokai J. Exp. Clin. Med. 2014, 39, 116–121. [Google Scholar] [PubMed]
- Nagata, H.; Takekoshi, S.; Takeyama, R.; Homma, T.; Yoshiyuki Osamura, R. Quercetin enhances melanogenesis by increasing the activity and synthesis of tyrosinase in human melanoma cells and in normal human melanocytes. Pigment. Cell Res. 2004, 17, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Takekoshi, S.; Matsuzaki, K.; Kitatani, K. Quercetin stimulates melanogenesis in hair follicle melanocyte of the mouse. Tokai J. Exp. Clin. Med. 2013, 38, 129–134. [Google Scholar] [PubMed]
- Csekes, E.; Račková, L. Skin aging, cellular senescence and natural polyphenols. Int. J. Mol. Sci. 2021, 22, 12641. [Google Scholar] [CrossRef] [PubMed]
- Franco, A.C.; Aveleira, C.; Cavadas, C. Skin senescence: Mechanisms and impact on whole-body aging. Trends Mol. Med. 2022, 28, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Passos, J.F.; Nelson, G.; Wang, C.; Richter, T.; Simillion, C.; Proctor, C.J.; Miwa, S.; Olijslagers, S.; Hallinan, J.; Wipat, A.; et al. Feedback between P21 and reactive oxygen production is necessary for cell senescence. Mol. Syst. Biol. 2010, 6, 347. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.S.; François, M.; Fenech, M.F.; Leifert, W.R. Persistent γH2AX: A promising molecular marker of DNA damage and aging. Mutat. Res. Rev. Mutat. Res. 2015, 766, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Miyamae, Y.; Shigemori, H.; Isoda, H. Neuroprotective effect of 3,5-di-O-caffeoylquinic acid on SH-SY5Y Cells and senescence-accelerated-prone mice 8 through the up-regulation of Phosphoglycerate Kinase-1. Neuroscience 2010, 169, 1039–1045. [Google Scholar] [CrossRef]
- Wagle, S.; Sim, H.-J.; Bhattarai, G.; Choi, K.-C.; Kook, S.-H.; Lee, J.-C.; Jeon, Y.-M. Supplemental ferulic acid inhibits total body irradiation-mediated bone marrow damage, bone mass loss, stem cell senescence, and hematopoietic defect in mice by enhancing antioxidant defense systems. Antioxidants 2021, 10, 1209. [Google Scholar] [CrossRef] [PubMed]
- Hahn, H.J.; Kim, K.B.; Bae, S.; Choi, B.G.; An, S.; Ahn, K.J.; Kim, S.Y. Pretreatment of ferulic acid protects human dermal fibroblasts against ultraviolet A irradiation. Ann. Dermatol. 2016, 28, 740. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, X.; Zhu, X.; Wang, C.; Xu, W. Myricetin alleviated hydrogen peroxide-induced cellular senescence of nucleus pulposus cell through regulating SERPINE1. J. Orthop. Surg. Res. 2023, 18, 143. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Pan, R.; Huang, W.; Dong, S.; Wu, S.; Ye, Y. Myricetin alleviates H2O2-induced senescence and apoptosis in rat nucleus pulposus-derived mesenchymal stem cells. Folia Histochem. Cytobiol. 2023, 61, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Zoico, E.; Nori, N.; Darra, E.; Tebon, M.; Rizzatti, V.; Policastro, G.; De Caro, A.; Rossi, A.P.; Fantin, F.; Zamboni, M. Senolytic effects of quercetin in an in vitro model of pre-adipocytes and adipocytes induced senescence. Sci. Rep. 2021, 11, 23237. [Google Scholar] [CrossRef]
- Shin, E.J.; Lee, J.S.; Hong, S.; Lim, T.G.; Byun, S. Quercetin directly targets JAK2 and PKCδ and prevents UV-induced photoaging in human skin. Int. J. Mol. Sci. 2019, 20, 5262. [Google Scholar] [CrossRef] [PubMed]
- Vicentini, F.T.M.C.; He, T.; Shao, Y.; Fonseca, M.J.V.; Verri, W.A.; Fisher, G.J.; Xu, Y. Quercetin inhibits UV irradiation-induced inflammatory cytokine production in primary human keratinocytes by suppressing NF-ΚB pathway. J. Dermatol. Sci. 2011, 61, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Alves-Silva, J.M.; Guerra, I.; Gonçalves, M.J.; Cavaleiro, C.; Cruz, M.T.; Figueirinha, A.; Salgueiro, L. Chemical composition of Crithmum maritimum L. essential oil and hydrodistillation residual water by GC-MS and HPLC-DAD-MS/MS, and their biological activities. Ind. Crops Prod. 2020, 149, 112329. [Google Scholar] [CrossRef]
- Moreira, P.; Sousa, F.J.; Matos, P.; Brites, G.S.; Gonçalves, M.J.; Cavaleiro, C.; Figueirinha, A.; Salgueiro, L.; Batista, M.T.; Branco, P.C.; et al. Chemical composition and effect against skin alterations of bioactive extracts obtained by the hydrodistillation of Eucalyptus globulus leaves. Pharmaceutics 2022, 14, 561. [Google Scholar] [CrossRef] [PubMed]
- Piras, A.; Maccioni, A.; Falconieri, D.; Porcedda, S.; Gonçalves, M.J.; Alves-Silva, J.M.; Silva, A.; Cruz, M.T.; Salgueiro, L.; Maxia, A. Chemical composition and biological activity of essential oil of Teucrium scordium L. subsp. scordioides (Schreb.) Arcang. (Lamiaceae) from Sardinia island (Italy). Nat. Prod. Res. 2022, 36, 5828–5835. [Google Scholar] [CrossRef] [PubMed]
- Cruz, M.T.; Duarte, C.B.; Gonçalo, M.; Figueiredo, A.; Carvalho, A.P.; Lopes, M.C. Granulocyte-macrophage colony-stimulating factor activates the transcription of nuclear factor kappa B and induces the expression of nitric oxide synthase in a skin dendritic cell line. Immunol. Cell Biol. 2001, 79, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Martinotti, S.; Ranzato, E. Scratch wound healing assay. In Methods in Molecular Biology; Humana Press Inc.: Totowa, NJ, USA, 2020; Volume 2109, pp. 225–229. [Google Scholar]
- Suarez-Arnedo, A.; Torres Figueroa, F.; Clavijo, C.; Arbeláez, P.; Cruz, J.C.; Muñoz-Camargo, C. An Image J plugin for the high throughput image analysis of in vitro scratch wound healing assays. PLoS ONE 2020, 15, e0232565. [Google Scholar] [CrossRef]
- Francisco, V.; Figueirinha, A.; Costa, G.; Liberal, J.; Ferreira, I.; Lopes, M.C.; García-Rodríguez, C.; Cruz, M.T.; Batista, M.T. The flavone luteolin inhibits liver X receptor activation. J. Nat. Prod. 2016, 79, 1423–1428. [Google Scholar] [CrossRef]
- Alves-Silva, J.M.; Moreira, P.; Cavaleiro, C.; Pereira, C.; Cruz, M.T.; Salgueiro, L. Effect of Ferulago lutea (Poir.) Grande essential oil on molecular hallmarks of skin aging. Plants 2023, 12, 3741. [Google Scholar] [CrossRef]
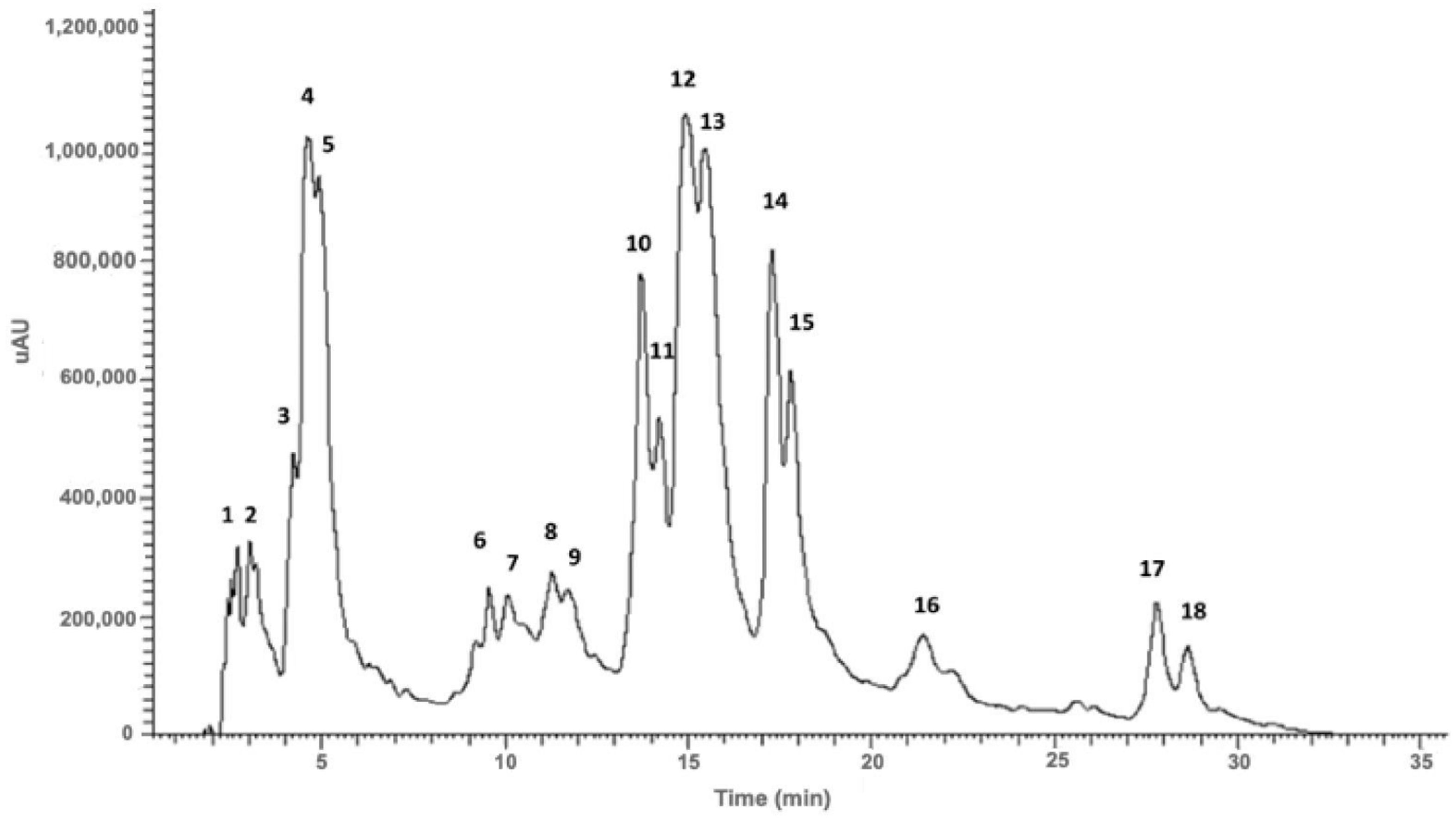

| Peak | Partial Identification | Rt (min) | λmax. (nm) | [M-H]− | MS2 | MS3 |
|---|---|---|---|---|---|---|
| 1 | Quinic acid | 2.62 | 242, 258 | 191 | [191]: 173 (30), 111 (100) | - |
| 2 | Protocatechuic acid hexoside | 3.05 | 242, 291 | 315 | [315]: 315 (100), 163 (10), 153 (80) | - |
| 3 | Unknown | 4.23 | 263, 299, 324 sh | 255 (100) | [255]: 255 (5), 237 (20), 212 (5), 211 (100) | [255 211]: 211 (100), 165 (75), 75 (25) |
| 4 | 5-O-caffeoylquinic acid | 4.62 | 259, 299, 312 sh, 329 | 707 * | [707]: 353 (100), 295 (2) | [707 353]: 191 (100), 179 (10) |
| 5 | 5-O-caffeoylquinic acid | 4.93 | 255, 299, 313, 329 | 353 | [353]: 191 (100), 179 (10), 135 (3) | [353 191]: 191 (100), 173 (25), 127 (20) |
| 6 | Ferulic acid | 9.57 | 242, 291, 319 | 193 | [193]: 149 (100) | [193 149]: 161 (50), 149 (100), 133 (75) |
| 7 | Myricetin-O-hexoside | 10.09 | 242, 281, 324 sh | 479 | [479]: 317 (100), 281 (2) | [479 317]: 317 (100), 255 (2) |
| 8 | Quercetin-O-hexoside isomer | 11.29 | 242, 282, 330 sh | 463 | [463]: 301 (100), 287 (10) | [463 301]: 301 (100), 283 (4) |
| 9 | Quercetin-O-hexoside isomer | 11.78 | 242, 282, 330 sh | 463 | [463]: 301 (100), 463 (20) | [463 301]: 301 (100), 283 (4) |
| 10 | 1,5-O-Dicaffeoylquinic acid or 3,4-O-Dicaffeoylquinic acid | 13.72 | 254, 299, 326 | 515 | [515]: 353 (100), 335 (10), 173 (10) | [515 353]: 191 (40), 179 (70), 173 (100), 135 (5) |
| 11 | 1,5-O-Dicaffeoylquinic acid or 3,4-ODicaffeoylquinic acid | 14.17 | 253, 299, 328 | 515 | [515]: 353 (100), 335 (10), 179 (5) | [515 353]: 191 (40), 179 (55), 173 (100), 135 (10) |
| 12 | 1,3-O-Dicaffeoylquinic acid or 3,5-O-Dicaffeoylquinic acid | 14.92 | 260, 299, 333 | 515 | [515]: 353 (100), 179 (2) | [515 353]: 191 (100), 179 (50), 173 (10), 135 (8) |
| 13 | 3,5-O-Dicaffeoylquinic acid or 1,3-O-Dicaffeoylquinic acid | 15.48 | 253, 299, 324 | 515 | [515]: 353 (100), 191 (2) | [515 353]: 191 (100), 179 (50), 173 (5), 135 (5) |
| 14 | 4,5-O-Dicaffeoylquinic acid or 1,4-O-Dicaffeoylquinic acid | 17.28 | 253, 299, 324 | 515 | [515]: 353 (100), 299 (15) | [515 353]: 191 (30), 179 (50), 173 (100), 135 (2) |
| 15 | 4,5-O-Dicaffeoylquinic acid or 1,4-O-Dicaffeoylquinic acid | 17.80 | 253, 300, 326 | 515 | [515]: 353 (100), 299 (15) | [515 353]: 191 (28), 179 (60), 173 (100), 135 (10) |
| 16 | Unknown | 21.23 | 220, 287, 331 sh | 725 | [725]: 707 (10), 563 (100), | [725 563]: 389 (100) |
| 17 | Unknown | 27.81 | 250, 330 | 451 | [451]: 451 (30), 407 (100), 261 (20), 246 (15), 179 (40) | - |
| 18 | Unknown | 28.70 | 251, 330 | 535 | [535]: 493 (50), 491 (75), 467 (100), 447 (65), 405 (60) | - |
| IC50 (μg/mL) a | TE (μmol/L) b | |
|---|---|---|
| DPPH | 25.29 ± 3.14 | 4.13 ± 0.22 |
| ABTS | 19.00 ± 1.26 | 3.75 ± 0.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves-Silva, J.M.; Pedreiro, S.; Zuzarte, M.; Cruz, M.T.; Figueirinha, A.; Salgueiro, L. Unlocking the Bioactive Potential and Exploring Novel Applications for Portuguese Endemic Santolina impressa. Plants 2024, 13, 1943. https://doi.org/10.3390/plants13141943
Alves-Silva JM, Pedreiro S, Zuzarte M, Cruz MT, Figueirinha A, Salgueiro L. Unlocking the Bioactive Potential and Exploring Novel Applications for Portuguese Endemic Santolina impressa. Plants. 2024; 13(14):1943. https://doi.org/10.3390/plants13141943
Chicago/Turabian StyleAlves-Silva, Jorge M., Sónia Pedreiro, Mónica Zuzarte, Maria Teresa Cruz, Artur Figueirinha, and Lígia Salgueiro. 2024. "Unlocking the Bioactive Potential and Exploring Novel Applications for Portuguese Endemic Santolina impressa" Plants 13, no. 14: 1943. https://doi.org/10.3390/plants13141943









