The Influence of Technological Factors on the Structure and Chemical Composition of Tuberous Dahlia Roots Determined Using Vibrational Spectroscopy
Abstract
:1. Introduction
2. Results
2.1. Biochemical Composition of the Forced and Nonforced Tuberous Roots of Dahlia hybrida during an Entire Vegetative Year
2.2. FT-IR Mapping Analysis
3. Discussion
4. Materials and Methods
4.1. Experimental Site and Pedoclimatic Conditions
4.2. Plant Material
4.3. Analytical Methodology and Data Processing
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Toma, F. Floriculture and Floral Art, Species Used for Park and Garden Design; INVEL Multimedia: Bucharest, Romania, 2009; Volume 4, pp. 293–295. [Google Scholar]
- Lord, T. Flora, The Gardener’s Bible; Cassell: London, UK, 2003; Volume I (A–K), p. 466. [Google Scholar]
- Jamil, A.M.; Shagufta, G. Evaluation of Exotic Cultivars of Dahlia (Dahlia coccinea) under Rawalakot Conditions. Asian J. Plant 2002, 1, 565–566. [Google Scholar]
- Available online: https://www.gardenersworld.com/how-to/grow-plants/how-to-grow-dahlias (accessed on 10 July 2024).
- Cantor, M.; Buta, E.; Buru, T. The Culture of Ornamental Plants in a Controlled Climate; Academic Press: Cluj-Napoca, Romania, 2021; pp. 17–18. [Google Scholar]
- Traynor, J.; Mactier, R.; Geddes, C.C.; Fox, J.G. How to Measure Renal Function in Clinical Practice. BMJ 2006, 333, 733–737. [Google Scholar] [CrossRef] [PubMed]
- Zubaidah, E.; Wilda, A. Comparative Study of Inulin Extracts from Dahlia, Yam, and Gembili Tubers as Prebiotic. Food Nutr. Sci. 2013, 4, 8–12. [Google Scholar] [CrossRef]
- Available online: https://www.botanical.com/botanical/mgmh/d/dahlia02.html (accessed on 10 July 2024).
- Bernal, B.H.; Calle, J.; Duarte, Q.; Pinzó, R.; Velásquyez, M. Inulin from Tubers of Dahlia imperialis Roetz. Rev. Colomb. Cienc. Quím. Farm. 2005, 34, 122–125. [Google Scholar]
- Koruri, S.S.; Banerjee, D.; Chowdhury, R.; Bhattacharya, P. Studies on Prebiotic Food Additive (Inulin) in Indian Dietary Fibre Sources-Garlic (Allium sativum), Wheat (Triticum spp.), Oat (Avena sativa) and Dalia (Bulgur). Int. J. Pharm. Pharm. Sci. 2014, 6, 278–282. [Google Scholar]
- Herrero, A.M.; Carmona, P.; Jiménez-Colmenero, F.; Ruiz-Capillas, C. Polysaccharide Gels as Oil Bulking Agents: Technological and Structural Properties. Food Hydrocoll. 2014, 36, 374–381. [Google Scholar] [CrossRef]
- Konovalov, D.A. Polyacetylene Compounds of Plants of the Asteraceae Family (Review). Pharm. Chem. J. 2014, 48, 613–631. [Google Scholar] [CrossRef]
- Melo-De-Pinna, F.A.; Menezes, N.L. Meristematic Endodermis and Secretor Structures in Adventitious Roots of Richterago Kuntze (Mutisieae-Asteraceae). Braz. J. Bot. 2003, 26, 1–10. [Google Scholar] [CrossRef]
- Burescu, P. Botany, Plant Morphology; Universităţii: Oradea, Romania, 2002; Volume I. [Google Scholar]
- Mensink, M.A.; Frijlink, H.W.; Van der Voort Maarschalk, K.; Hinrichs, W.L.J. Inulin, a Flexible Oligosaccharide I: Review of Its Physicochemical Characteristics. Carbohydr. Polym. 2015, 130, 405–419. [Google Scholar] [CrossRef] [PubMed]
- Teferra, T.F. Possible Actions of Inulin as Prebiotic Polysaccharide: A Review. Food Front. 2021, 2, 407–416. [Google Scholar] [CrossRef]
- Caunii, A.; Butu, M.; Rodino, S.; Motoc, M.; Negrea, A.; Samfira, I.; Butnariu, M. Isolation and Separation of Inulin from Phalaris arundinacea Roots. Rev. Chim. Buchar. 2015, 66, 472–476. [Google Scholar]
- Burzo, I.; Toma, S.; Voican, V.; Amăriuţei, A.; Şelaru, E.; Popescu, V.; Crăciun, C. Physiology of Vegetables and Cultivated Plants; Întreprinderea Editorial–Poligrafică Ştiinţa: Chişinău, Moldova, 2000; Volume IV. [Google Scholar]
- Saengthongpinit, W.; Sajjaanantakul, T. Influence of Harvest Time and Storage Temperature on Characteristics of Inulin from Jerusalem Artichoke (Helianthus tuberosus L.) Tubers. Postharvest Biol. Technol. 2005, 37, 93–100. [Google Scholar] [CrossRef]
- Ohno, S.; Deguchi, A.; Hosokawa, M.; Tatsuzawa, F.; Doi, M. A Basic Helix-Loop-Helix Transcription Factor DvIVS Determines Flower Color Intensity in Cyanic Dahlia Cultivars. Planta 2013, 238, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Kosasih, W.; Pudjiraharti, S.; Ratnaningrum, D.; Priatni, S. Preparation of Inulin from Dahlia Tubers. Int. Symp. Appl. Chem. Procedia Chem. 2015, 16, 190–194. [Google Scholar] [CrossRef]
- Deguchi, A.; Ohno, S.; Hosokawa, M.; Tatsuzawa, F.; Doi, M. Endogenous Post-Transcriptional Gene Silencing of Flavone Synthase Resulting in High Accumulation of Anthocyanins in Black Dahlia Cultivars. Planta 2013, 237, 1325–1335. [Google Scholar] [CrossRef] [PubMed]
- Barclay, T.; Ginic-Markovic, M.; Cooper, P.; Petrovsky, N. Inulin—A Versatile Polysaccharide with Multiple Pharmaceutical and Food Chemical Uses. J. Excip. Food Chem. 2010, 1, 27–50. [Google Scholar]
- Hakiki, M.; Agustine, S.; Yety, M.I.; Puspa, D.L.; Desak, G.S.A. Characterization of Inulin from Local Red Dahlia (Dahlia sp. L.) Tubers by Infrared Spectroscopy. Int. Symp. Appl. Chem. 2015, 16, 78–84. [Google Scholar]
- Baranska, M.; Roman, M.; Dobrowolski, J.C.Z.; Schulz, H.; Baranski, R. Recent Advances in Raman Analysis of Plants: Alkaloids, Carotenoids and Polyacetylenes. Curr. Anal. Chem. 2013, 9, 108–127. [Google Scholar] [CrossRef]
- Serra, O.; Geldner, N. The Making of Suberin. New Phytol. 2022, 235, 848–866. [Google Scholar] [CrossRef]
- Saake, B.; Lehnen, R. Lignin. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2000. [Google Scholar] [CrossRef]
- Lebo, S.E., Jr.; Gargulak, J.D.; McNally, T.J. Lignin. In Kirk-Othmer Encyclopedia of Chemical Technology; Wiley-Interscience: New York, NY, USA, 2001; Volume 15, pp. 1–32. [Google Scholar]
- Baia, M.; Aştilean, S.; Iliescu, T. Raman and SERS Investigations of Pharmaceuticals; Springer: Berlin, Germany, 2008; ISBN 978-3-540-78282-7. [Google Scholar]
- Gierlinger, N.; Schwanninger, M. The Potential of Raman Microscopy and Raman Imaging in Plant Research. J. Spectrosc. 2007, 21, 69–89. [Google Scholar] [CrossRef]
- Ivleva, N.P.; Niessner, R.; Panne, U. Characterization and Discrimination of Pollen by Raman Microscopy. Anal. Bioanal. Chem. 2005, 381, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, Z.; Li, T.; Chen, N.; Xu, W.; Liu, S. Surface-Enhanced Raman Scattering Spectra Revealing the Inter-Cultivar Differences for Chinese Ornamental Flos Chrysanthemum: A New Promising Method for Plant Taxonomy. Plant Methods 2017, 13, 92. [Google Scholar] [CrossRef]
- Baiz, C.R.; Błasiak, B.; Bredenbeck, J.; Cho, M.; Choi, J.H.; Corelli, S.A.; Dijkstra, A.G.; Feng, C.-J.; Garrett-Roe, S.; Ge, N.-H.; et al. Vibrational Spectroscopic Map, Vibrational Spectroscopy, and Intermolecular Interaction. Chem. Rev. 2020, 120, 7152–7218. [Google Scholar] [CrossRef]
- Zeier, J.; Ruel, K.; Ryser, U.; Schreiber, L. Chemical Analysis and Immune Localisation of Lignin and Suberin in Endodermal and Hypodermal/Rhizodermal Cell Walls of Developing Maize (Zea mays L.) Primary Roots. Planta 1999, 209, 1–12. [Google Scholar] [CrossRef]
- Gorgulu, S.T.; Dogan, M.; Severcan, F. The Characterization and Differentiation of Higher Plants by Fourier Transform Infrared Spectroscopy. Appl. Spectrosc. 2007, 61, 300–308. [Google Scholar] [CrossRef]
- Zeier, J.; Schreiber, L. Comparative Investigation of Primary and Tertiary Endodermal Cell Walls Isolated from the Roots of Five Monocotyledoneous Species: Chemical Composition in Relation to Fine Structure. Planta 1998, 206, 349–361. [Google Scholar] [CrossRef]
- Chen, A.; Liu, T.; Wang, Z.; Chen, X. Plant Root Suberin: A Layer of Defence against Biotic and Abiotic Stresses. Front. Plant Sci. 2022, 13, 1056008. [Google Scholar] [CrossRef] [PubMed]
- Ciobanu, I.; Cantor, M.; Stefan, R.; Buta, E.; Magyari, K.; Baia, M. The Influence of Storage Conditions on the Biochemical Composition and Morphology of Dahlia Tuber. Not. Bot. Horti. Agrobo. 2016, 44, 459–465. [Google Scholar] [CrossRef]
- Aoba, T.; Watanabe, S.; Soma, K. Studies on the Formation of Tuberous Root in Dahlia, II Anatomical Observation of Primary Root and Tuberous Root. J. Jpn. Soc. Hortic. Sci. 1961, 30, 82–88. [Google Scholar] [CrossRef]
- Nultsch, W. General Botany; Academic Press: New York, NY, USA, 1971; pp. 171–176. [Google Scholar]
- Ingram, D.S.; Vince-Prue, D.; Gregory, P.J. Science and the Garden: The Scientific Basis of Horticultural Practice; John Wiley and Sons: Oxford, UK, 2008. [Google Scholar]
- José, G. Suberin: The Biopolyester at the Frontier of Plants. Front. Chem. 2015, 3, 62. [Google Scholar]
- Rivera-Espejel, E.A.; Cruz-Alvarez, O.; Mejiamunoz, G.; Garcia-Mateos, M.R.; Colinas-Leon, M.T.B.; Martinez-Damian, M.T. Physicochemical Quality, Antioxidant Capacity and Nutritional Value in Tuberous Roots of Some Wild Dahlia Species. Not. Bot. Horti. Agrobo. 2019, 47, 813–820. [Google Scholar] [CrossRef]
- Nsabimana, C.; Jiang, B. The Chemical Composition of Some Garden Dahlia Tubers. Br. Food J. 2011, 113, 1081–1093. [Google Scholar] [CrossRef]
- Balan, C.; Chis, M.I.; Rachisan, A.L.; Baia, M. A Vibrational Study of Inulin by Means of Experimental and Theoretical Methods. J. Mol. Struct. 2018, 1164, 84–88. [Google Scholar] [CrossRef]
- Grube, M.; Bekers, M.; Upite, D.; Kaminska, E. Infrared Spectra of Some Fructans. Spectrosc. Lett. 2002, 16, 289–296. [Google Scholar] [CrossRef]
- Cotoz, A.P.; Dan, V.S.; Gocan, T.M.; Andreica, I.; Rózsa, S.; Cantor, M. Sedum Growth Patterns under Different Pedoclimatic Conditions. Plants 2023, 12, 2739. [Google Scholar] [CrossRef]
- Şelaru, E. The Culture of Garden Flowers; Ceres: Bucharest, Romania, 2007; pp. 337–341. [Google Scholar]
- Draghia, L. General Floriculture; Ion Ionescu de la Brad: Iași, Romania, 2011; pp. 12–17. ISBN 978-973-147-075-7. [Google Scholar]
- Hessayon, D.G. Flower Growing Expert; Alfa: Bucharest, Romania, 2007. [Google Scholar]
- Băla, M. General and Special Floriculture; Partos: Timișoara, Romania, 2012; pp. 265–268. [Google Scholar]
- Anton, D.; Nicu, C. Special Floriculture; Universitaria: Craiova, Romania, 2005; Volume I, pp. 6–8. [Google Scholar]
- Ardelean, M.; Sestraş, R.; Cordea, M. Horticultural Experimental Technique; AcademicPres: Cluj-Napoca, Romania, 2007; pp. 24–26. [Google Scholar]
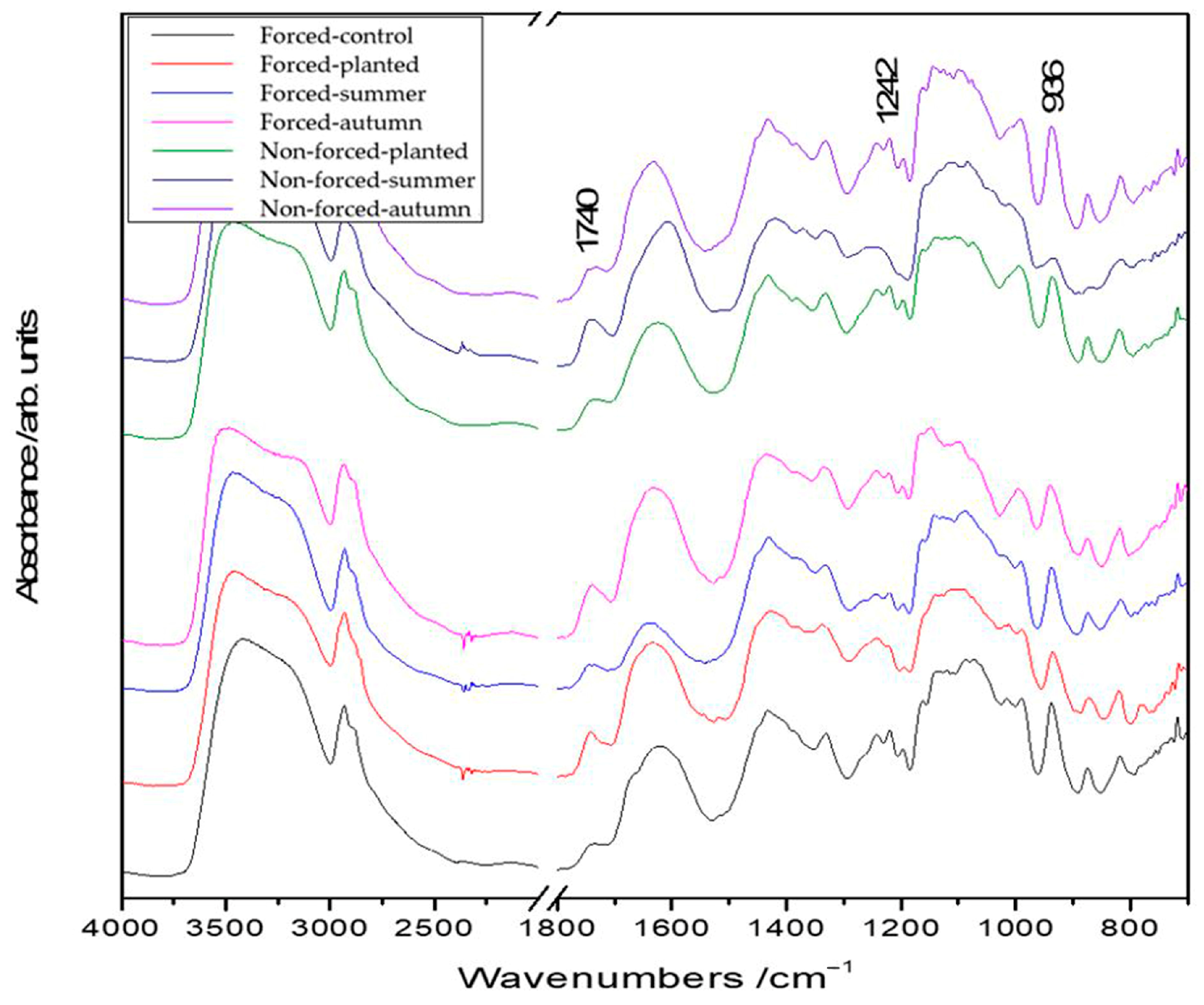


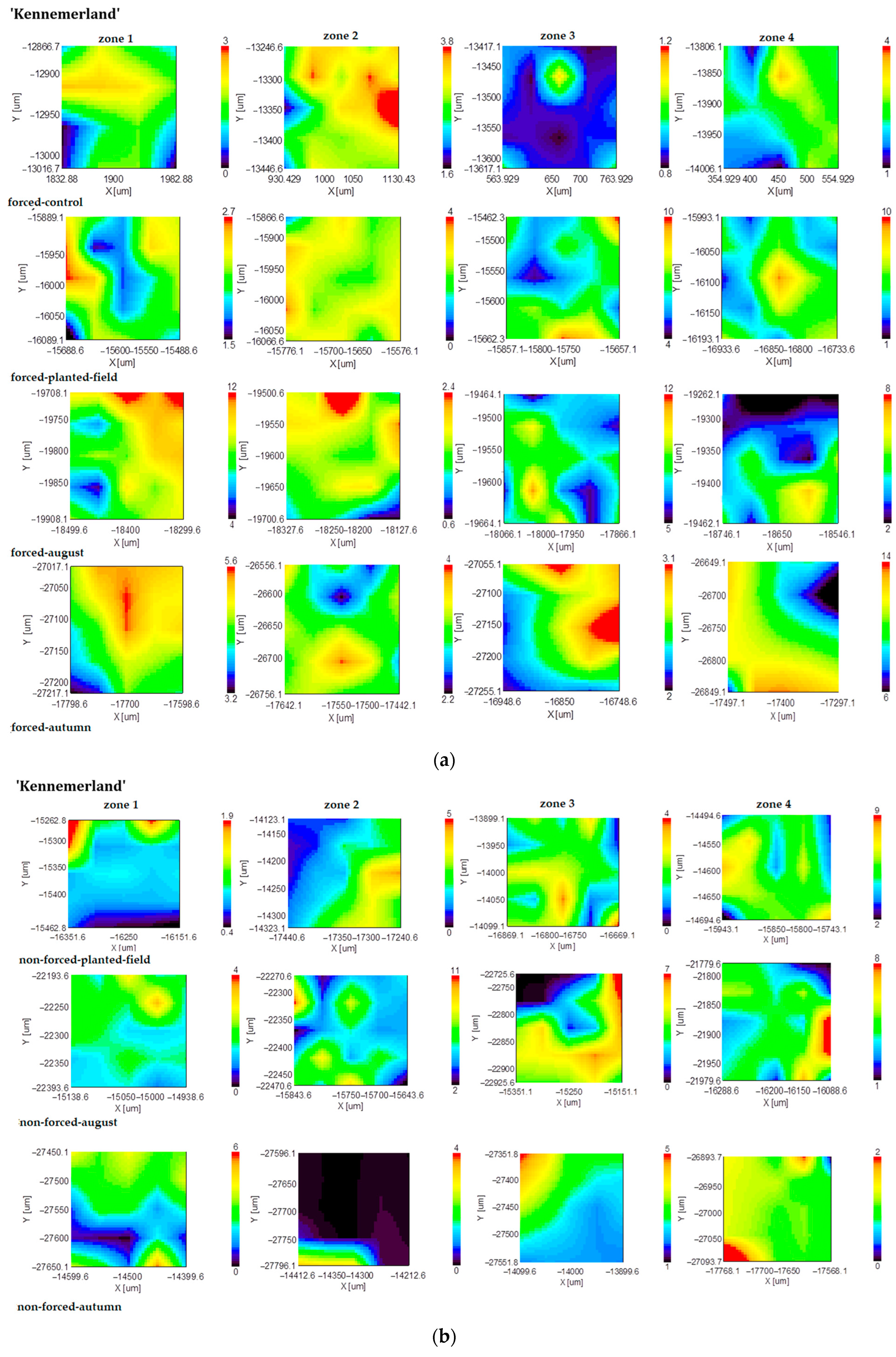


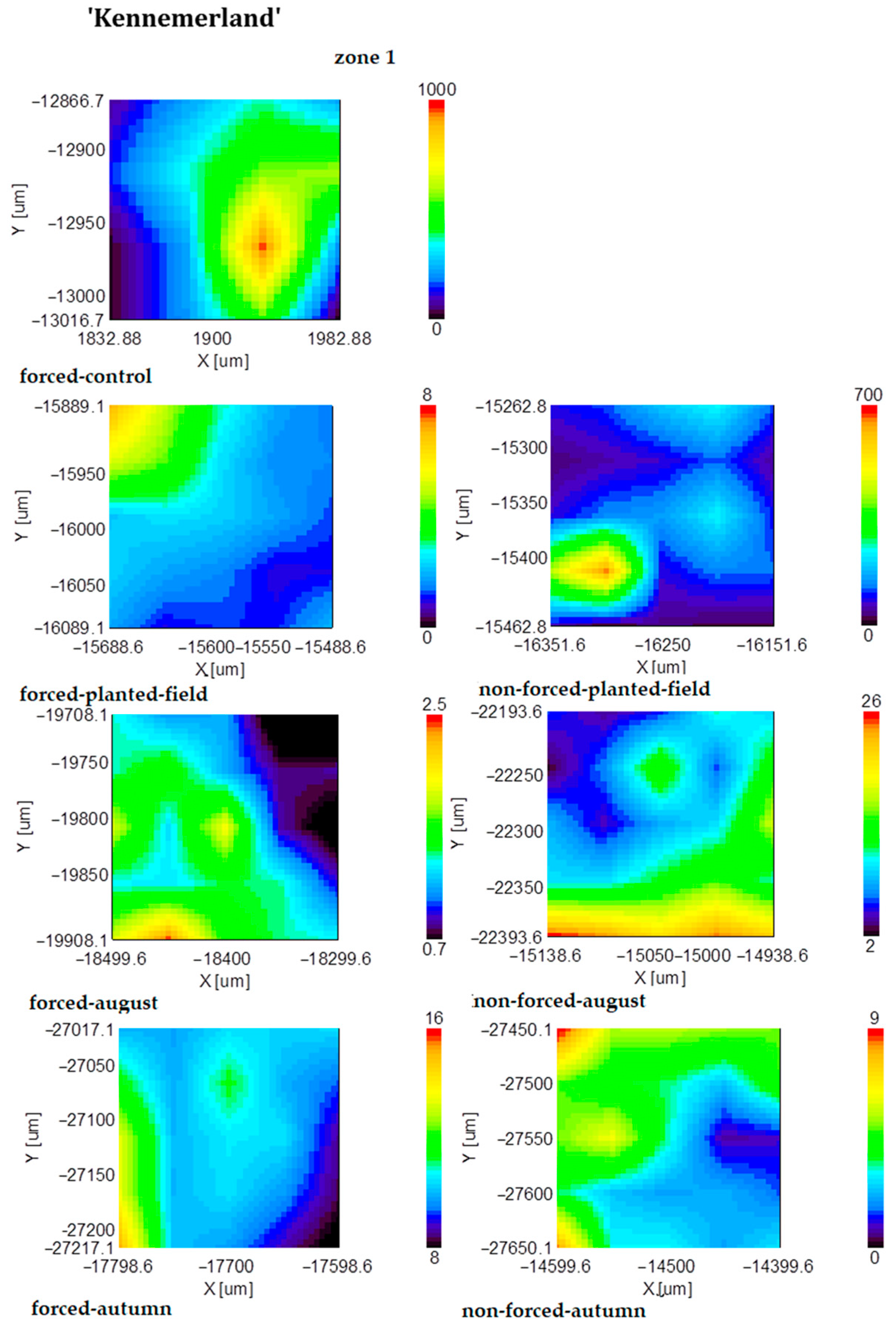
| IR Band | Vibrational Assignment |
|---|---|
| 936 m | CO stretching (inulin) |
| 1030 m | CO stretching (inulin) |
| 1060 m | COC asymmetric stretching (cellulose) |
| 1125 s | COC symmetric stretching (cellulose) |
| 1130 s | CCO stretching + OH deformation + CH deformation (inulin) |
| 1220 m/1240 m | CO stretching (lignin) |
| 1330 m | CH and CH2 groups deformation (cellulose) |
| 1430 s | CH and CH2 groups deformation (cellulose) |
| 1600 s | C=C stretching (polyphenolic compounds) |
| 1680 sh | amide I vibrations indicating the protein |
| 1740 w | C=O groups stretching (suberin) |
| 2930 s | CH stretching |
| 3460 s broad | OH stretching |
| Sample Identification | Name Analysis | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Lab. | No. of Sample | pH | N % | Humus % | P mg L−1 | K mg L−1 | U % | Granulometric Analysis | ||||
| Coarse Sand | Sand | Dust I | Dust II | Clay | ||||||||
| 572 | Average | 6.22 | 0.18 | 2.04 | 64 | 436 | 19.2 | 17.3 | 27.8 | 7.1 | 12.5 | 35.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moldovan, I.; Cotoz, A.-P.; Rózsa, S.; Magyari, K.; Lehel, L.; Baia, M.; Cantor, M. The Influence of Technological Factors on the Structure and Chemical Composition of Tuberous Dahlia Roots Determined Using Vibrational Spectroscopy. Plants 2024, 13, 1955. https://doi.org/10.3390/plants13141955
Moldovan I, Cotoz A-P, Rózsa S, Magyari K, Lehel L, Baia M, Cantor M. The Influence of Technological Factors on the Structure and Chemical Composition of Tuberous Dahlia Roots Determined Using Vibrational Spectroscopy. Plants. 2024; 13(14):1955. https://doi.org/10.3390/plants13141955
Chicago/Turabian StyleMoldovan, Ioana, Alex-Péter Cotoz, Sándor Rózsa, Klara Magyari, Lukács Lehel, Monica Baia, and Maria Cantor. 2024. "The Influence of Technological Factors on the Structure and Chemical Composition of Tuberous Dahlia Roots Determined Using Vibrational Spectroscopy" Plants 13, no. 14: 1955. https://doi.org/10.3390/plants13141955
APA StyleMoldovan, I., Cotoz, A.-P., Rózsa, S., Magyari, K., Lehel, L., Baia, M., & Cantor, M. (2024). The Influence of Technological Factors on the Structure and Chemical Composition of Tuberous Dahlia Roots Determined Using Vibrational Spectroscopy. Plants, 13(14), 1955. https://doi.org/10.3390/plants13141955









