Understanding Ameliorating Effects of Boron on Adaptation to Salt Stress in Arabidopsis
Abstract
1. Introduction
2. Results
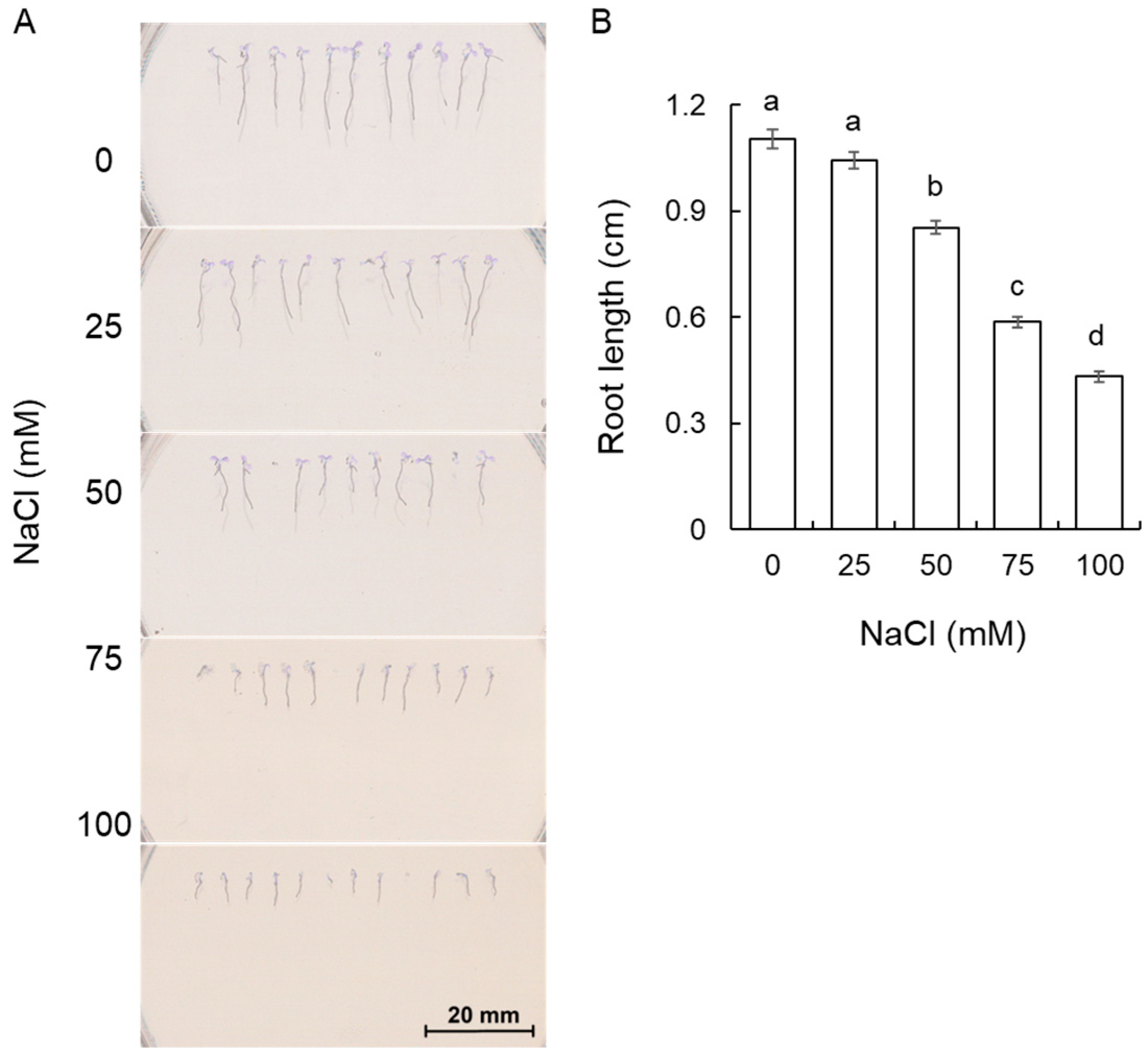
2.1. Effect of Salt on the Growth of A. thaliana
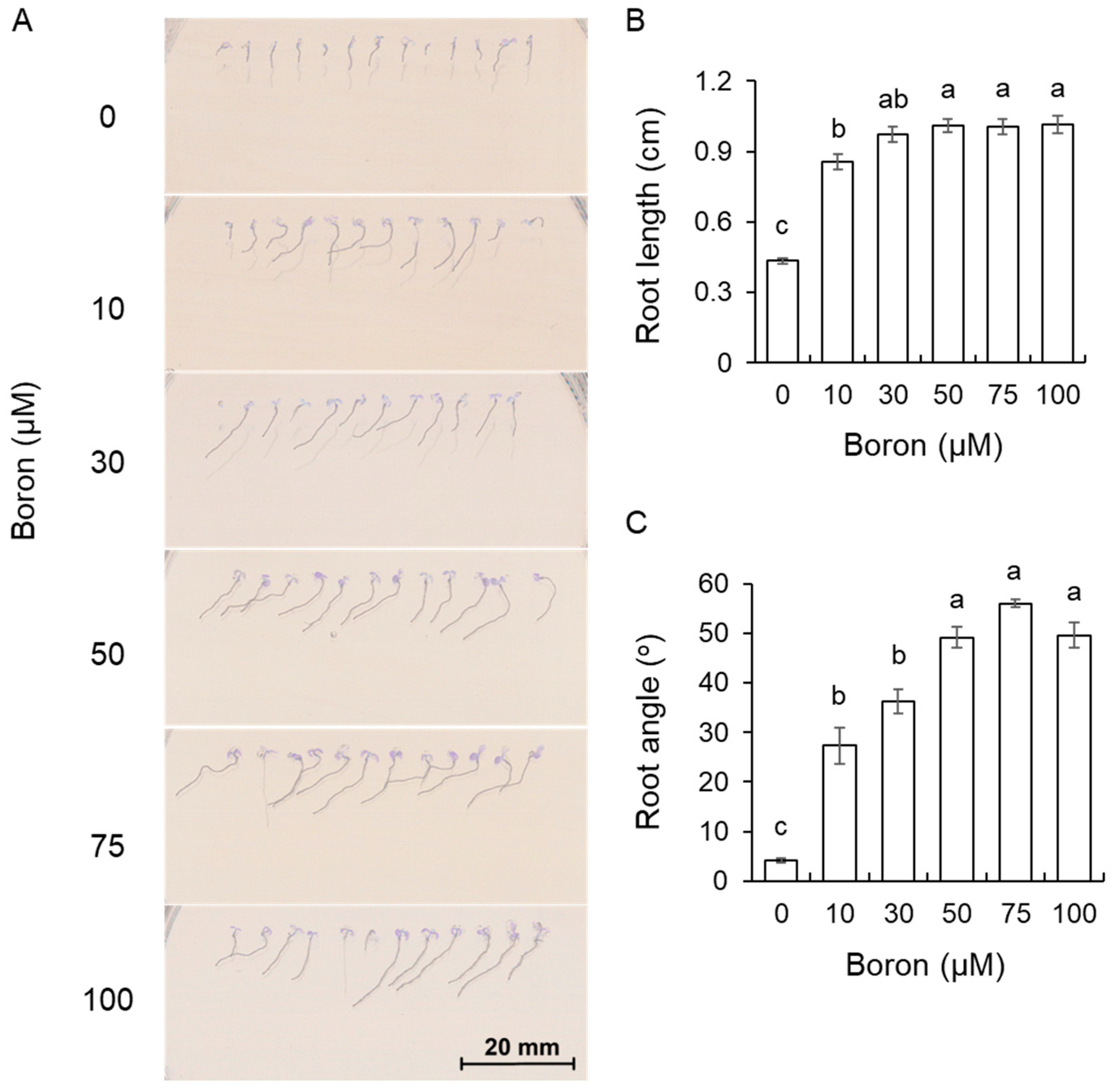
2.2. The Role of Boron in the Growth of A. thaliana in the Presence of Salt
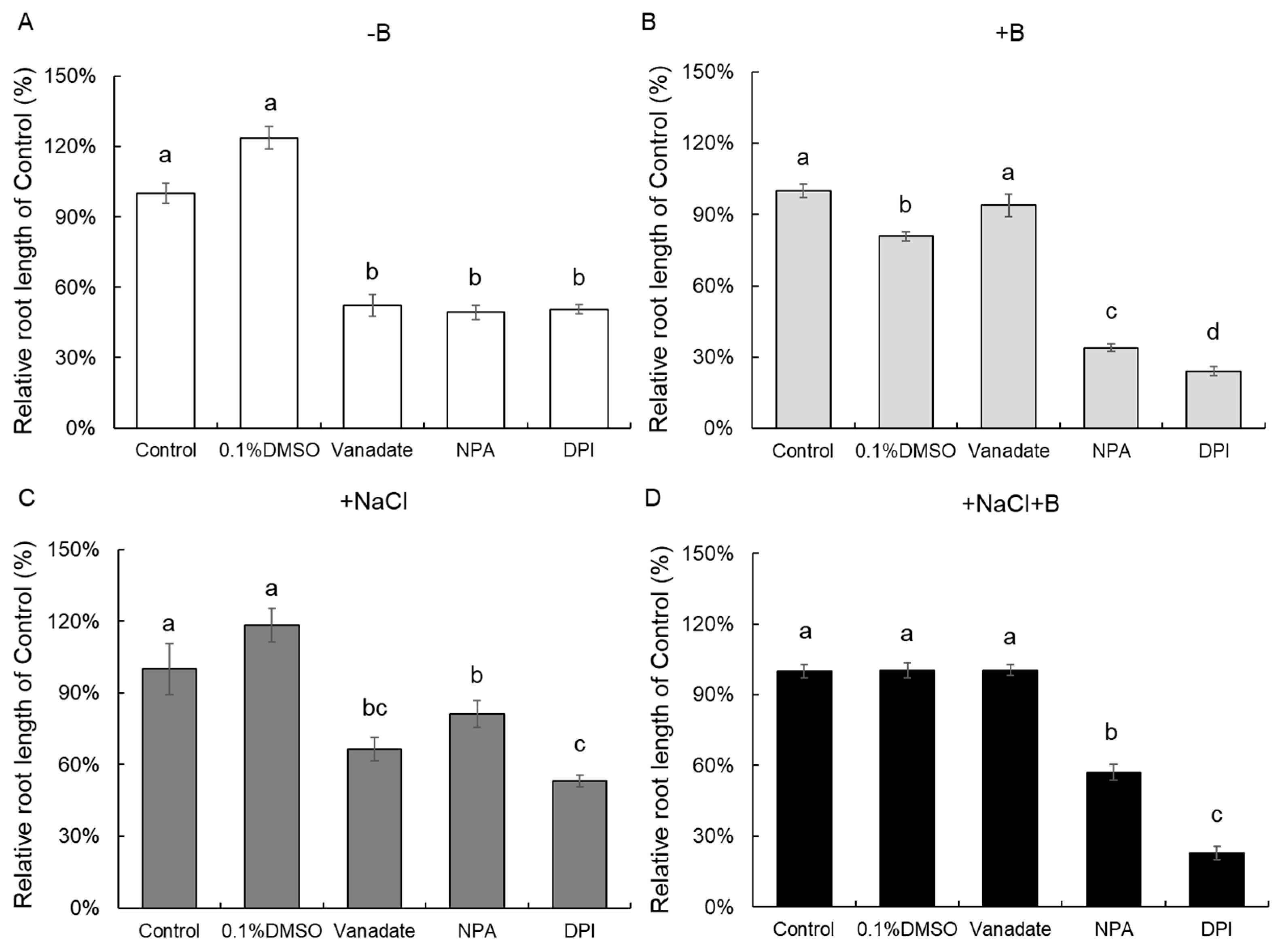
2.3. Potential Signaling Pathway Involved in Ameliorating Effects of Boron
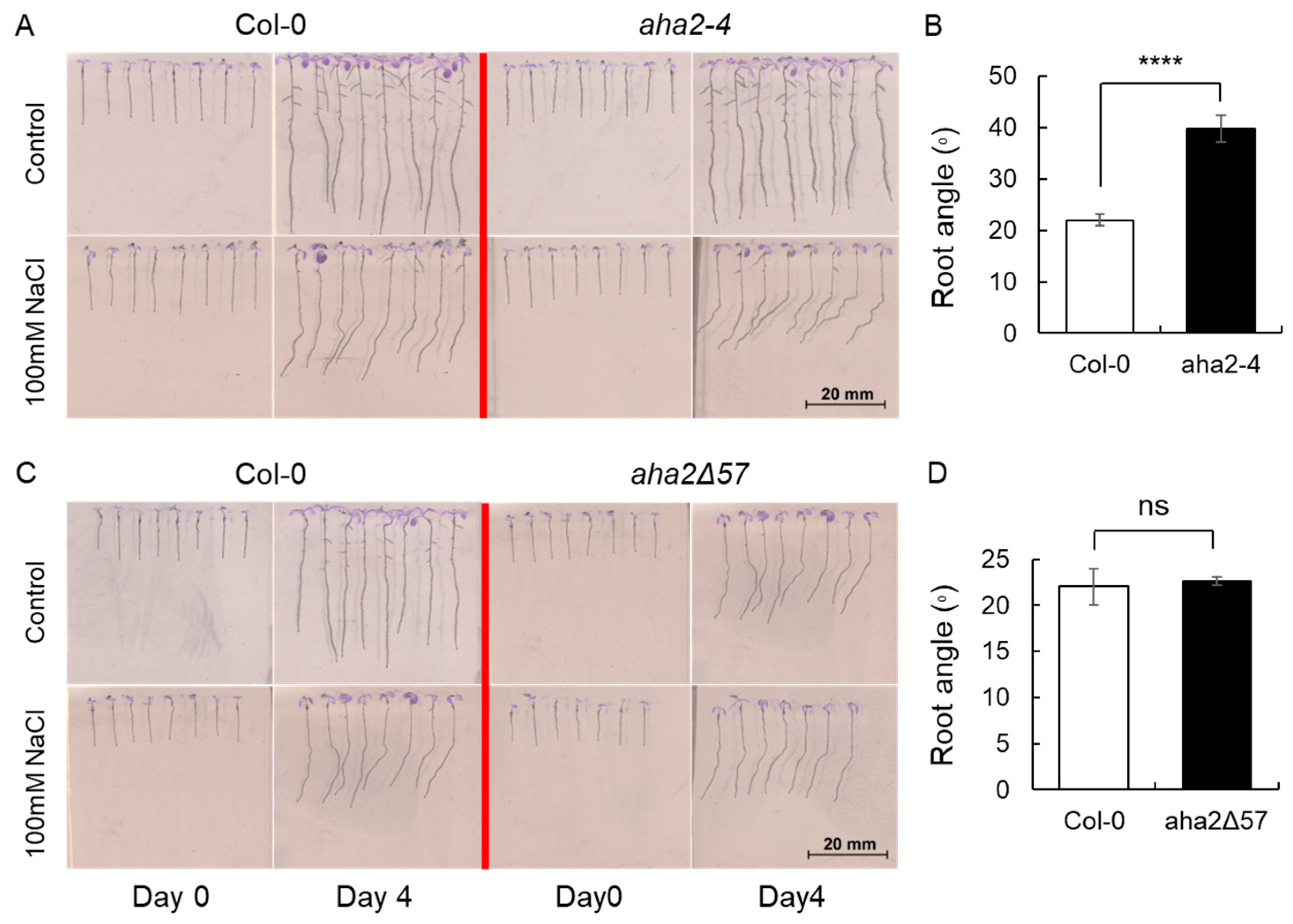
2.4. Regulation of H+-ATPase Associated with Halotropism
2.5. Dose Dependence of Boron H+ and K+ Response to NaCl
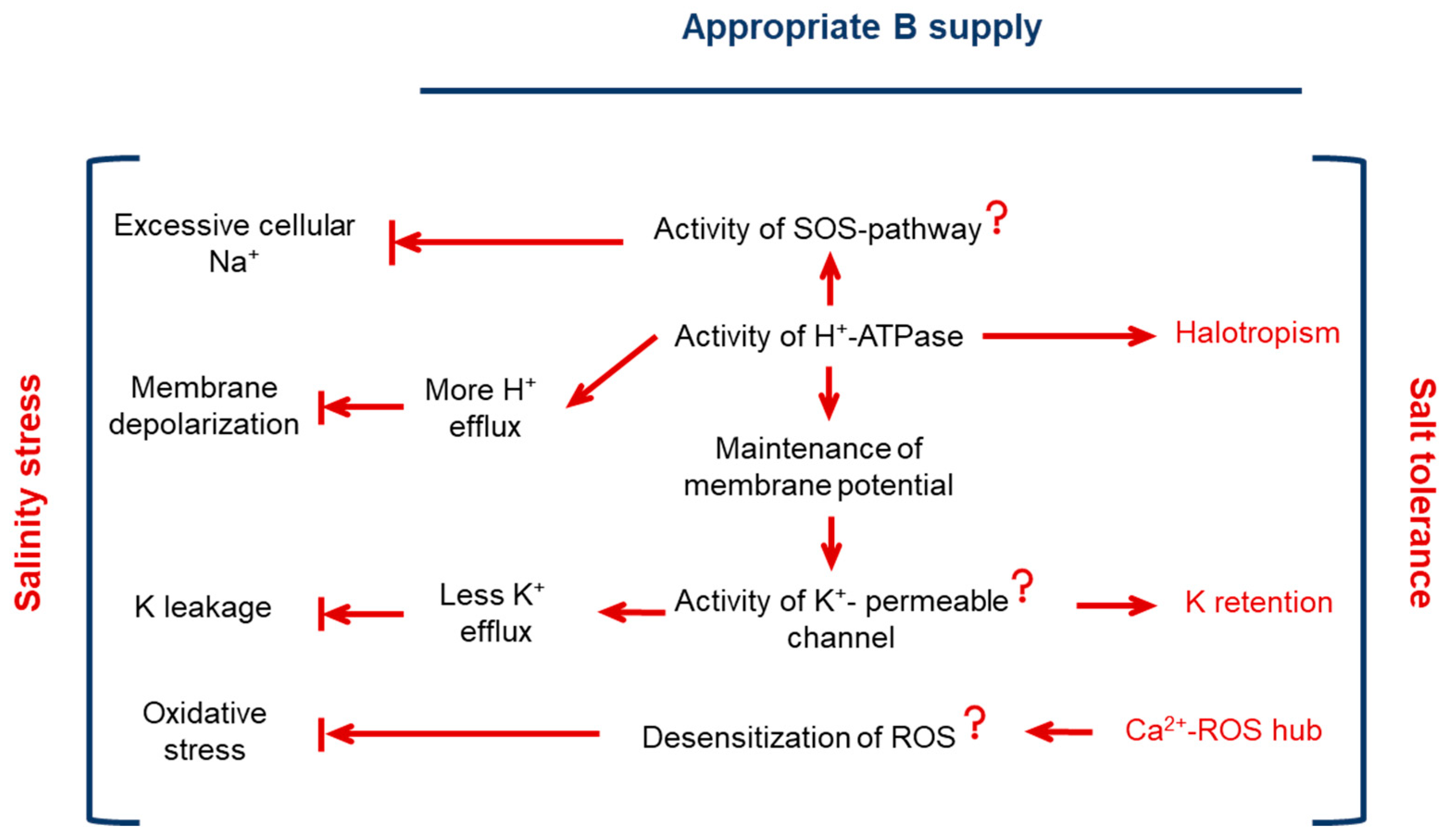
3. Discussion
4. Materials and Methods
4.1. Plant Material, Growth Conditions, and Treatment
4.2. Plant Growth Parameters
4.3. Ion Flux Measurements
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hossain, M.S.; Hasanuzzaman, M.; Sohag, M.M.H.; Bhuyan, M.B.; Fujita, M. Acetate-induced modulation of ascorbate: Glutathione cycle and restriction of sodium accumulation in shoot confer salt tolerance in Lens culinaris Medik. Physiol. Mol. Biol. Plants 2019, 25, 443–455. [Google Scholar] [CrossRef] [PubMed]
- USDA-ARS. Research Databases. Bibliography on Salt Tolerance; Salinity Lab, US Department of Agriculture, Agriculture Research Service: Riverside, CA, USA, 2008.
- Liu, C.; Mao, B.; Yuan, D.; Chu, C.; Duan, M. Salt tolerance in rice: Physiological responses and molecular mechanisms. Crop J. 2022, 10, 13–25. [Google Scholar] [CrossRef]
- Van Zelm, E.; Zhang, Y.; Testerink, C. Salt tolerance mechanisms of plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef] [PubMed]
- Fricke, W.; Akhiyarova, G.; Veselov, D.; Kudoyarova, G. Rapid and tissue-specific changes in ABA and in growth rate in response to salinity in barley leaves. J. Exp. Bot. 2004, 55, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Guo, J.; Passioura, J.B.; Cramer, G.R. Leaf water status controls day-time but not daily rates of leaf expansion in salt-treated barley. Funct. Plant Biol. 2000, 27, 949–957. [Google Scholar] [CrossRef]
- Demidchik, V.; Cuin, T.A.; Svistunenko, D.; Smith, S.J.; Miller, A.J.; Shabala, S.; Sokolik, A.; Yurin, V. Arabidopsis root K+-efflux conductance activated by hydroxyl radicals: Single-channel properties, genetic basis and involvement in stress-induced cell death. J. Cell Sci. 2010, 123, 1468–1479. [Google Scholar] [CrossRef] [PubMed]
- Pang, R.; Xing, K.; Yuan, L.; Liang, Z.; Chen, M.; Yue, X.; Dong, Y.; Ling, Y.; He, X.; Li, X.; et al. Peroxiredoxin alleviates the fitness costs of imidacloprid resistance in an insect pest of rice. PLoS Biol. 2021, 19, e3001190. [Google Scholar] [CrossRef] [PubMed]
- Tenhaken, R. Cell wall remodeling under abiotic stress. Front. Plant Sci. 2015, 5, 771. [Google Scholar] [CrossRef]
- Allakhverdiev, S.I.; Nishiyama, Y.; Miyairi, S.; Yamamoto, H.; Inagaki, N.; Kanesaki, Y.; Murata, N. Salt stress inhibits the repair of photodamaged photosystem II by suppressing the transcription and translation of psbA genes in Synechocystis. Plant Physiol. 2002, 130, 1443–1453. [Google Scholar] [CrossRef]
- Pospíšil, P. Production of reactive oxygen species by photosystem II. Biochim. Biophys. Acta Bioenerg. 2009, 1787, 1151–1160. [Google Scholar] [CrossRef]
- Demidchik, V. ROS-activated ion channels in plants: Biophysical characteristics, physiological functions and molecular nature. Int. J. Mol. Sci. 2018, 19, 1263. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhang, H.; Song, C.; Zhu, J.-K.; Shabala, S. Mechanisms of plant responses and adaptation to soil salinity. Innovation. 2020, 1, 100017. [Google Scholar] [CrossRef] [PubMed]
- Kohli, S.K.; Kaur, H.; Khanna, K.; Handa, N.; Bhardwaj, R.; Rinklebe, J.; Ahmad, P. Boron in plants: Uptake, deficiency and biological potential. Plant Growth Regul. 2023, 100, 267–282. [Google Scholar] [CrossRef]
- Hu, H.; Brown, P.H. Absorption of boron by plant roots. Plant Soil 1997, 193, 49–58. [Google Scholar] [CrossRef]
- Dordas, C.; Chrispeels, M.J.; Brown, P.H. Permeability and channel-mediated transport of boric acid across membrane vesicles isolated from squash roots. Plant Physiol. 2000, 124, 1349–1362. [Google Scholar] [CrossRef]
- Miwa, K.; Fujiwara, T. Boron transport in plants: Co-ordinated regulation of transporters. Ann. Bot. 2010, 105, 1103–1108. [Google Scholar] [CrossRef]
- Grieve, C.M.; Poss, J.A. Wheat response to interactive effects of boron and salinity. J. Plant Nutr. 2000, 23, 1217–1226. [Google Scholar] [CrossRef]
- Martínez-Ballesta, M.d.C.; Bastías, E.; Carvajal, M. Combined effect of boron and salinity on water transport: The role of aquaporins. Plant Signal. Behav. 2008, 3, 844–845. [Google Scholar] [CrossRef]
- Moreno, D.; Carvajal, M.; Del Carmen Mart Nez Ballesta, M. Interactive effects of boron and NaCl stress on water and nutrient transport in two broccoli cultivars. Funct. Plant Biol. 2013, 40, 739–748. [Google Scholar] [CrossRef]
- Lu, K.; Yan, L.; Riaz, M.; Babar, S.; Hou, J.; Zhang, Y.; Jiang, C. Exogenous boron alleviates salt stress in cotton by maintaining cell wall structure and ion homeostasis. Plant Physiol. Biochem. 2023, 201, 107858. [Google Scholar] [CrossRef]
- Yousefi, H.; Dalir, N.; Rahnemaie, R.; Babaei, A. The alleviation of salinity-induced stress by using boron in soilless grown rose. J. Plant Nutr. 2020, 43, 526–537. [Google Scholar] [CrossRef]
- Samet, H.; Çıkılı, Y. Response of purslane (Portulaca oleracea L.) to excess boron and salinity: Physiological approach. Russ. J. Plant Physiol. 2019, 66, 316–325. [Google Scholar] [CrossRef]
- Ekmekci, Y.; Erdal, Ş.Ç.; Nalçaiyi, A.S.B.; Cicek, N. Acquisition of boron tolerance by salt pretreatment in two sunflower cultivars. Turk. J. Bot. 2020, 44, 153–166. [Google Scholar] [CrossRef]
- Alharby, H.F.; Nahar, K.; Al-Zahrani, H.S.; Hakeem, K.R.; Hasanuzzaman, M. Enhancing salt tolerance in soybean by exogenous boron: Intrinsic study of the ascorbate-glutathione and glyoxalase pathways. Plants 2021, 10, 2085. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Rahman, K.; Sathi, K.; Alam, M.; Nahar, K.; Fujita, M.; Hasanuzzaman, M. Supplemental Selenium and Boron Mitigate Salt-Induced Oxidative Damages in Glycine max L. Plants 2021, 10, 2224. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.; Havshøi, N.W.; Huang, X.; Shabala, L.; Yu, M.; Fuglsang, A.T.; Shabala, S. Understanding the mechanistic basis of ameliorative effects of boron on salinity in barley (Hordeum vulgare). Environ. Exp. Bot. 2024, 220, 105690. [Google Scholar] [CrossRef]
- Orsini, F.; D’Urzo, M.P.; Inan, G.; Serra, S.; Oh, D.-H.; Mickelbart, M.V.; Consiglio, F.; Li, X.; Jeong, J.C.; Yun, D.J.; et al. A comparative study of salt tolerance parameters in 11 wild relatives of Arabidopsis thaliana. J. Exp. Bot. 2010, 61, 3787–3798. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant responses to salt stress: Adaptive mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef]
- Munns, R. Genes and salt tolerance: Bringing them together. New Phytol. 2005, 167, 645–663. [Google Scholar] [CrossRef]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef]
- West, G.; Inzé, D.; Beemster, G.T. Cell cycle modulation in the response of the primary root of Arabidopsis to salt stress. Plant Physiol. 2004, 135, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Bolaños, L.; Lukaszewski, K.; Bonilla, I.; Blevins, D. Why boron? Plant Physiol. Biochem. 2004, 42, 907–912. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, M.K. Role of boron in plant growth: A review. J. Agric. Res. 2009, 47, 329–338. [Google Scholar]
- Dong, X.; Sun, L.; Guo, J.; Liu, L.; Han, G.; Wang, B. Exogenous boron alleviates growth inhibition by NaCl stress by reducing Cl− uptake in sugar beet (Beta vulgaris). Plant Soil 2021, 464, 423–439. [Google Scholar] [CrossRef]
- Hager, A. Role of the plasma membrane H+-ATPase in auxin-induced elongation growth: Historical and new aspects. J. Plant Res. 2003, 116, 483–505. [Google Scholar] [CrossRef] [PubMed]
- Roldán, M.; Belver, A.; Rodríguez-Rosales, P.; Ferrol, N.; Donaire, J.P. In vivo and in vitro effects of boron on the plasma membrane proton pump of sunflower roots. Physiol. Plant. 1992, 84, 49–54. [Google Scholar] [CrossRef]
- Camacho-Cristóbal, J.J.; González-Fontes, A. Boron deficiency decreases plasmalemma H+-ATPase expression and nitrate uptake, and promotes ammonium assimilation into asparagine in tobacco roots. Planta 2007, 226, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Ishitani, M.; Kim, C.; Zhu, J.-K. The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter. Proc. Nat. Acad. Sci. USA 2000, 97, 6896–6901. [Google Scholar] [CrossRef] [PubMed]
- Galvan-Ampudia, C.S.; Julkowska, M.M.; Darwish, E.; Gandullo, J.; Korver, R.A.; Brunoud, G.; Haring, M.A.; Munnik, T.; Vernoux, T.; Testerink, C. Halotropism is a response of plant roots to avoid a saline environment. Curr. Biol. 2013, 23, 2044–2050. [Google Scholar] [CrossRef] [PubMed]
- Han, E.H.; Petrella, D.P.; Blakeslee, J.J. ‘Bending’models of halotropism: Incorporating protein phosphatase 2A, ABCB transporters, and auxin metabolism. J. Exp. Bot. 2017, 68, 3071–3089. [Google Scholar] [CrossRef]
- Korver, R.A.; van den Berg, T.; Meyer, A.J.; Galvan-Ampudia, C.S.; Ten Tusscher, K.H.; Testerink, C. Halotropism requires phospholipase Dζ1-mediated modulation of cellular polarity of auxin transport carriers. Plant Cell Environ. 2020, 43, 143–158. [Google Scholar] [CrossRef]
- Blevins, D.G.; Lukaszewski, K.M. Boron in plant structure and function. Annu. Rev. Plant Biol. 1998, 49, 481–500. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Qin, Y.; Xie, C.; Zhao, F.; Zhao, J.; Liu, D.; Chen, S.; Fuglsang, A.F.; Palmgren, A.G.; Schumaker, K.S.; et al. The Arabidopsis chaperone J3 regulates the plasma membrane H+-ATPase through interaction with the PKS5 kinase. Plant Cell 2010, 22, 1313–1332. [Google Scholar] [CrossRef]
- Xu, W.; Jia, L.; Baluška, F.; Ding, G.; Shi, W.; Ye, N.; Zhang, J. PIN2 is required for the adaptation of Arabidopsis roots to alkaline stress by modulating proton secretion. J. Exp. Bot. 2012, 63, 6105–6114. [Google Scholar] [CrossRef] [PubMed]
- Sato, E.M.; Hijazi, H.; Bennett, M.J.; Vissenberg, K.; Swarup, R. New insights into root gravitropic signalling. J. Exp. Bot. 2015, 66, 2155–2165. [Google Scholar] [CrossRef]
- Su, S.H.; Gibbs, N.M.; Jancewicz, A.L.; Masson, P.H. Molecular mechanisms of root gravitropism. Curr. Biol. 2017, 27, R964–R972. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Xiao, X.; Huang, Q.; Zhu, H.; Feng, Y.; Li, Y.; Li, X.; Guo, Z.; Liu, J.; Wu, F.; et al. Boron supply restores aluminum-blocked auxin transport by the modulation of PIN2 trafficking in the root apical transition zone. Plant J. 2023, 114, 176–192. [Google Scholar] [CrossRef] [PubMed]
- Obermeyer, G.; Kriechbaumer, R.; Strasser, D.; Maschessnig, A.; Bentrup, F.W. Boric acid stimulates the plasma membrane H+-ATPase of ungerminated lily pollen grains. Physiol. Plant. 1996, 98, 281–290. [Google Scholar] [CrossRef]
- Zhu, J.-K. Regulation of ion homeostasis under salt stress. Curr. Opin. Cell Biol. 2003, 6, 441–445. [Google Scholar] [CrossRef]
- Jayakannan, M.; Bose, J.; Babourina, O.; Rengel, Z.; Shabala, S. Salicylic acid improves salinity tolerance in Arabidopsis by restoring membrane potential and preventing salt-induced K+ loss via a GORK channel. J. Exp. Bot. 2013, 64, 2255–2268. [Google Scholar] [CrossRef]
- Wu, H.; Shabala, L.; Zhou, M.; Shabala, S. Durum and bread wheat differ in their ability to retain potassium in leaf mesophyll: Implications for salinity stress tolerance. Plant Cell Physiol. 2014, 55, 1749–1762. [Google Scholar] [CrossRef] [PubMed]
- Ismail, H.; Maksimović, J.D.; Maksimović, V.; Shabala, L.; Živanović, B.D.; Tian, Y.; Jacobsen, S.; Shabala, S. Rutin, a flavonoid with antioxidant activity, improves plant salinity tolerance by regulating K+ retention and Na+ exclusion from leaf mesophyll in quinoa and broad beans. Funct. Plant Biol. 2015, 43, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Niu, M.; Xie, J.; Chen, C.; Cao, H.; Sun, J.; Kong, Q.; Shabala, S.; Shabala, L.; Huang, Y.; Bie, Z. An early ABA-induced stomatal closure, Na+ sequestration in leaf vein and K+ retention in mesophyll confer salt tissue tolerance in Cucurbita species. J. Exp. Bot. 2018, 69, 4945–4960. [Google Scholar] [CrossRef] [PubMed]
- Schon, M.K.; Novacky, A.; Blevins, D.G. Boron induces hyperpolarization of sunflower root cell membranes and increases membrane permeability to K+. Plant Physiol. 1990, 93, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Shabala, L.; Zhang, J.; Pottosin, I.; Bose, J.; Zhu, M.; Fuglsang, A.T.; Velarde-Buendia, A.; Massart, A.; Hill, C.B.; Roessner, U.; et al. Cell-type-specific H+-ATPase activity in root tissues enables K+ retention and mediates acclimation of barley (Hordeum vulgare) to salinity stress. Plant Physiol. 2016, 172, 2445–2458. [Google Scholar] [CrossRef] [PubMed]
- Yokawa, K.; Fasano, R.; Kagenishi, T.; Baluška, F. Light as stress factor to plant roots–case of root halotropism. Front. Plant Sci. 2014, 5, 718. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Zheng, W.; Xing, L.; Zhu, J.-K.; Persson, S.; Zhao, Y. Root twisting drives halotropism via stress-induced microtubule reorientation. Dev. Cell 2022, 57, 2412–2425. [Google Scholar] [CrossRef] [PubMed]
- Deolu-Ajayi, A.O.; Meyer, A.J.; Haring, M.A.; Julkowska, M.M.; Testerink, C. Genetic loci associated with early salt stress responses of roots. iScience 2019, 21, 458–473. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.; Huang, X.; García-Caparrós, P.; Shabala, L.; Fuglsang, A.T.; Yu, M.; Shabala, S. Understanding the role of boron in plant adaptation to soil salinity. Physiol. Plant. 2024, 176, e14358. [Google Scholar] [CrossRef]
- Stéger, A.; Hayashi, M.; Lauritzen, E.W.; Herburger, K.; Shabala, L.; Wang, C.; Bendtsen, A.K.; Nørrevang, A.F.; Madriz-Ordeñana, K.; Ren, S.; et al. The evolution of plant proton pump regulation via the R domain may have facilitated plant terrestrialization. Commun. Biol. 2022, 5, 1312. [Google Scholar] [CrossRef]
- Shabala, S.; Cuin, T.A.; Shabala, L.; Newman, I. Quantifying kinetics of net ion fluxes from plant tissues by non-invasive microelectrode measuring MIFE technique. In Plant Salt Tolerance; Methods and Protocols; Humana Press: Totowa, NJ, USA, 2012; pp. 119–134. [Google Scholar] [CrossRef]
- Shabala, S.; Demidchik, V.; Shabala, L.; Cuin, T.A.; Smith, S.J.; Miller, A.J.; Davies, J.M.; Newman, I.A. Extracellular Ca2+ ameliorates NaCl-induced K+ loss from Arabidopsis root and leaf cells by controlling plasma membrane K+-permeable channels. Plant Physiol. 2006, 141, 1653–1665. [Google Scholar] [CrossRef] [PubMed]
- Shabala, S.N.; Newman, I.A.; Morris, J. Oscillations in H+ and Ca2+ ion fluxes around the elongation region of corn roots and effects of external pH. Plant Physiol. 1997, 113, 111–118. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, M.; Huang, X.; Shabala, L.; Fuglsang, A.T.; Yu, M.; Shabala, S. Understanding Ameliorating Effects of Boron on Adaptation to Salt Stress in Arabidopsis. Plants 2024, 13, 1960. https://doi.org/10.3390/plants13141960
Qu M, Huang X, Shabala L, Fuglsang AT, Yu M, Shabala S. Understanding Ameliorating Effects of Boron on Adaptation to Salt Stress in Arabidopsis. Plants. 2024; 13(14):1960. https://doi.org/10.3390/plants13141960
Chicago/Turabian StyleQu, Mei, Xin Huang, Lana Shabala, Anja Thoe Fuglsang, Min Yu, and Sergey Shabala. 2024. "Understanding Ameliorating Effects of Boron on Adaptation to Salt Stress in Arabidopsis" Plants 13, no. 14: 1960. https://doi.org/10.3390/plants13141960
APA StyleQu, M., Huang, X., Shabala, L., Fuglsang, A. T., Yu, M., & Shabala, S. (2024). Understanding Ameliorating Effects of Boron on Adaptation to Salt Stress in Arabidopsis. Plants, 13(14), 1960. https://doi.org/10.3390/plants13141960








