Nutrient and Bioactive Fraction Content of Olea europaea L. Leaves: Assessing the Impact of Drying Methods in a Comprehensive Study of Prominent Cultivars in Morocco
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Effect of ‘Variety’ and ‘Drying Method’ on the Nutritional and Bioactive Content of Olive Leaves
2.2. Effect of the Drying Process on the Total Nitrogen and Crude Protein Contents of Olive Leaves
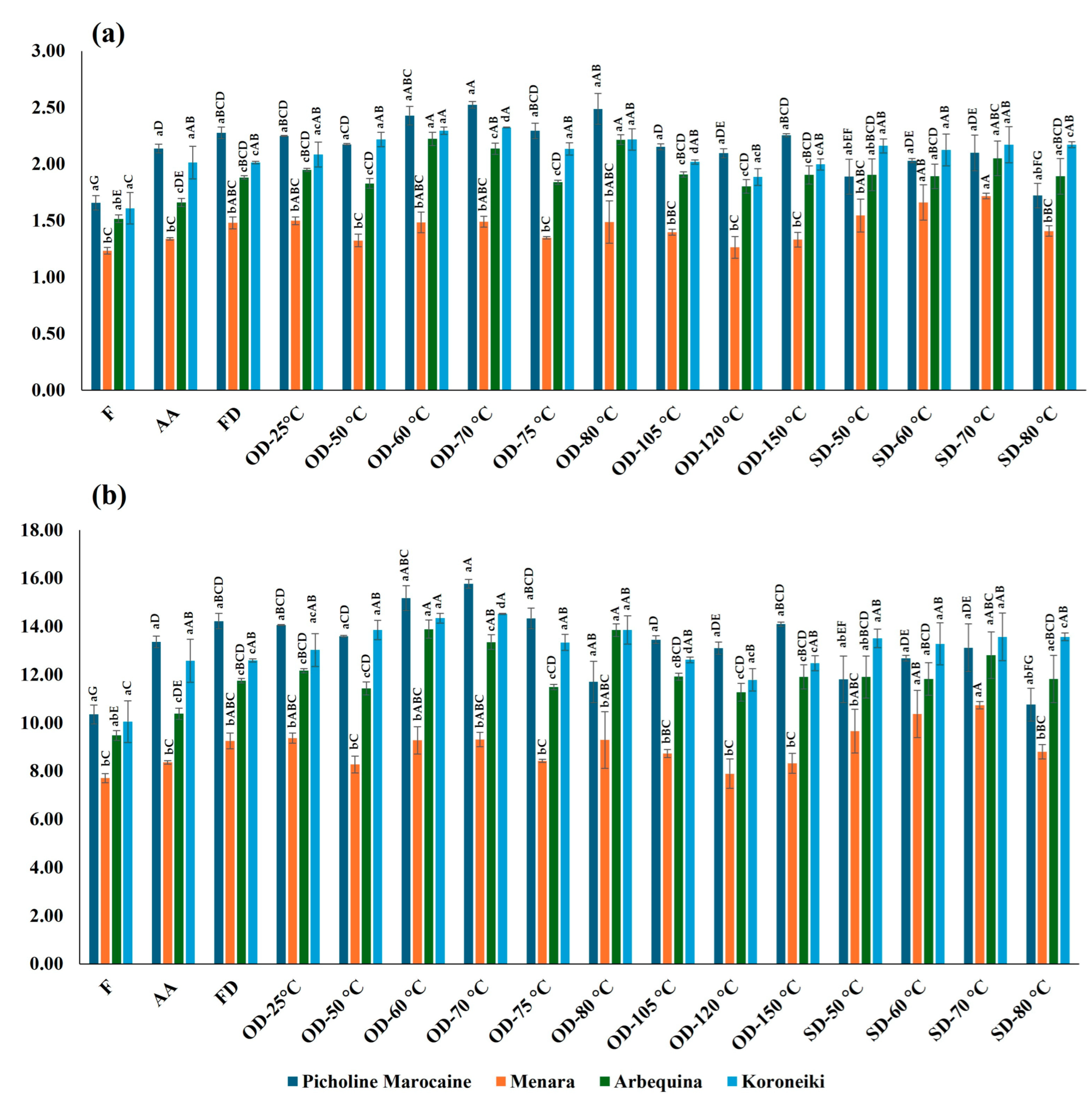
2.3. Effect of the Drying Process on Total Phenolic Content
2.4. Effect of the Drying Process on Total Flavonoid Content
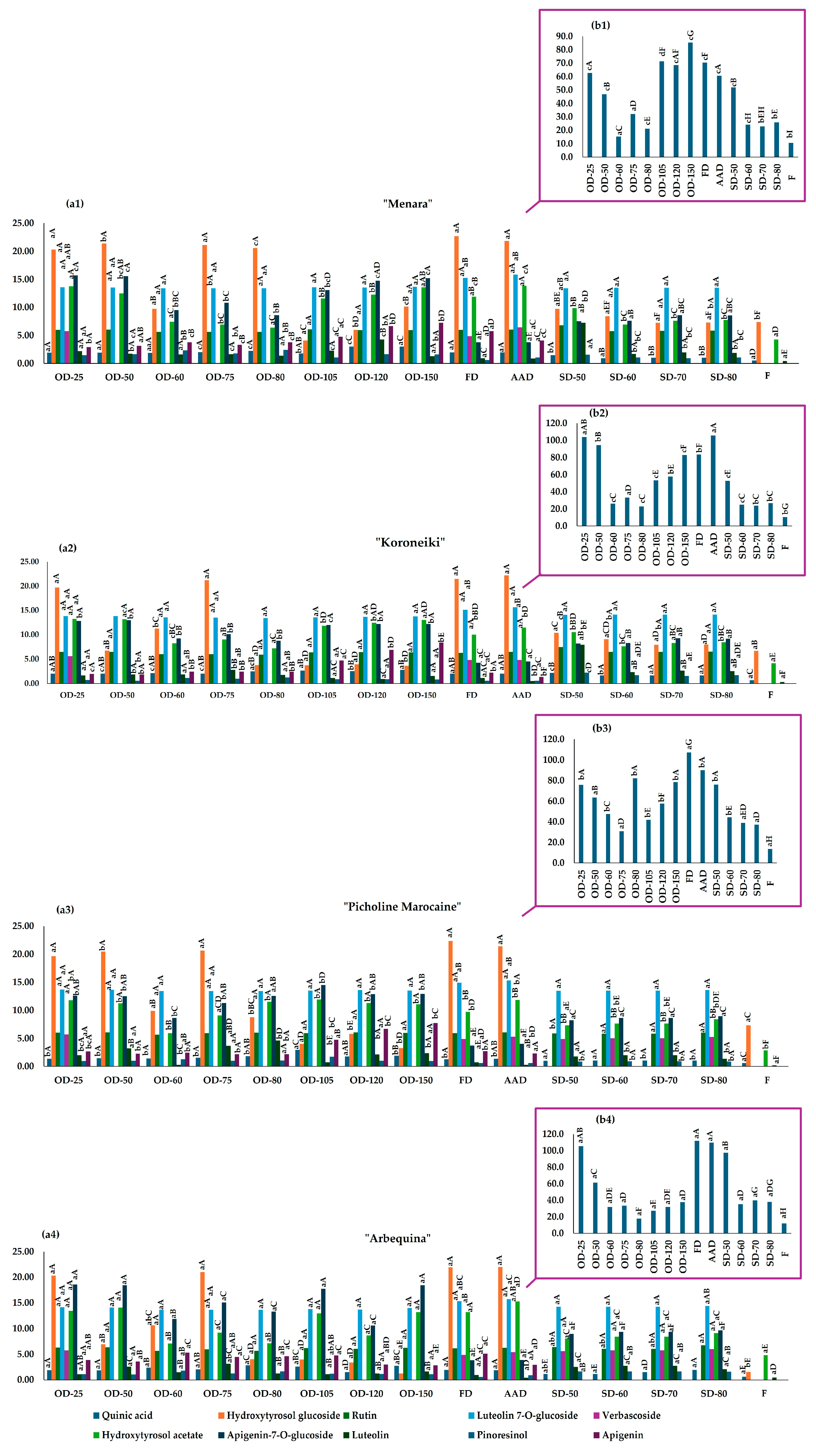
2.5. Effect of Drying on Olive Leaves’ Bioactive Fraction
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Sampling
3.3. Drying Methods
3.3.1. Air-Drying
3.3.2. Oven-Drying
3.3.3. Freeze-Drying
3.3.4. Solar-Drying
3.4. Grinding, Sieving and Storing Samples
3.5. Nutritional and Bioactive Fraction Analysis
3.5.1. Total Nitrogen and Crude Protein Content
3.5.2. Total Phenolic Content
3.5.3. Total Flavonoid Content Determination
3.5.4. Liquid Chromatographic Analysis of Bioactive Fraction
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tsimidou, M.Z.; Papoti, V.T. Olives and Olive Oil in Health and Disease Prevention, 1st ed.; Preedy, V.R., Watson, R.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2010; pp. 349–356. [Google Scholar]
- Markhali, F.S.; Teixeira, J.A.; Rocha, C.M.R. Olive Tree Leaves—A Source of Valuable Active Compounds. Processes 2020, 8, 1177. [Google Scholar] [CrossRef]
- Özcan, M.M.; Matthäus, B. A Review: Benefit and Bioactive Properties of Olive (Olea europaea L.) Leaves. Eur. Food Res. Technol. 2017, 243, 89–99. [Google Scholar] [CrossRef]
- Hadrich, F.; Chamkha, M.; Sayadi, S. Protective Effect of Olive Leaves Phenolic Compounds against Neurodegenerative Disorders: Promising Alternative for Alzheimer and Parkinson Diseases Modulation. Food Chem. Toxicol. 2022, 159, 112752. [Google Scholar] [CrossRef] [PubMed]
- Romero-Márquez, J.M.; Forbes-Hernández, T.Y.; Navarro-Hortal, M.D.; Quirantes-Piné, R.; Grosso, G.; Giampieri, F.; Lipari, V.; Sánchez-González, C.; Battino, M.; Quiles, J.L. Molecular Mechanisms of the Protective Effects of Olive Leaf Polyphenols against Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 4353. [Google Scholar] [CrossRef]
- Talhaoui, N.; Taamalli, A.; Gómez-Caravaca, A.M.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Phenolic Compounds in Olive Leaves: Analytical Determination, Biotic and Abiotic Influence, and Health Benefits. Food Res. Int. 2015, 77, 92–108. [Google Scholar] [CrossRef]
- Ghanbari, R.; Anwar, F.; Alkharfy, K.M.; Gilani, A.H.; Saari, N. Valuable Nutrients and Functional Bioactives in Different Parts of Olive (Olea europaea L.)—A Review. Int. J. Mol. Sci. 2012, 13, 3291–3340. [Google Scholar] [CrossRef]
- Selim, S.; Albqmi, M.; Al-Sanea, M.M.; Abdelgawad, H.; Al Jaouni, S.K.; Elkelish, A.; Hussein, S.; Warrad, M.; El-Saadony, M.T. Valorizing the Usage of Olive Leaves, Bioactive Compounds, Biological Activities, and Food Applications: A Comprehensive Review. Front. Nutr. 2022, 9, 1008349. [Google Scholar] [CrossRef]
- Gomez-Cabrera, A.; Garrido, A.; Guerrero, J.E.; Ortiz, V. Nutritive Value of the Olive Leaf: Effects of Cultivar, Season of Harvesting and System of Drying. J. Agric. Sci. 1992, 119, 205–210. [Google Scholar] [CrossRef]
- Boudhrioua, N.; Bahloul, N.; Slimen, I.B.; Kechaou, N. Comparison on the Total Phenol Contents and the Color of Fresh and Infrared Dried Olive Leaves. Ind. Crops Prod. 2009, 29, 412–419. [Google Scholar] [CrossRef]
- Olmo-García, L.; Bajoub, A.; Benlamaalam, S.; Hurtado-Fernández, E.; Bagur-González, M.G.; Chigr, M.; Mbarki, M.; Fernández-Gutiérrez, A.; Carrasco-Pancorbo, A. Establishing the Phenolic Composition of Olea europaea L. Leaves from Cultivars Grown in Morocco as a Crucial Step towards Their Subsequent Exploitation. Molecules 2018, 23, 2524. [Google Scholar] [CrossRef]
- del Mar Contreras, M.; Romero, I.; Moya, M.; Castro, E. Olive-Derived Biomass as a Renewable Source of Value-Added Products. Process Biochem. 2020, 97, 43–56. [Google Scholar] [CrossRef]
- Espeso, J.; Isaza, A.; Lee, J.Y.; Sörensen, P.M.; Jurado, P.; Avena-bustillos, R.D.J.; Olaizola, M.; Arboleya, J.C. Olive Leaf Waste Management. Front. Sustain. Food Syst. 2021, 5, 660582. [Google Scholar] [CrossRef]
- Federici, F.; Fava, F.; Kalogerakis, N.; Mantzavinos, D. Valorisation of Agro-Industrial by-Products, Effluents and Waste: Concept, Opportunities and the Case of Olive Mill Wastewaters. J. Chem. Technol. Biotechnol. 2009, 84, 895–900. [Google Scholar] [CrossRef]
- Tapia-Quirós, P.; Montenegro-Landívar, M.F.; Reig, M.; Vecino, X.; Cortina, J.L.; Saurina, J.; Granados, M. Recovery of Polyphenols from Agri-Food by-Products: The Olive Oil and Winery Industries Cases. Foods 2022, 11, 362. [Google Scholar] [CrossRef] [PubMed]
- Kamran, M.; Hamlin, A.S.; Scott, C.J.; Obied, H.K. Drying at High Temperature for a Short Time Maximizes the Recovery of Olive Leaf Biophenols. Ind. Crop. Prod. 2015, 78, 29–38. [Google Scholar] [CrossRef]
- Ahmad-Qasem, M.H.; Barrajón-Catalán, E.; Micol, V.; Mulet, A.; García-Pérez, J.V. Influence of Freezing and Dehydration of Olive Leaves (Var. Serrana) on Extract Composition and Antioxidant Potential. Food Res. Int. 2013, 50, 189–196. [Google Scholar] [CrossRef]
- Feng, S.; Zhang, C.; Liu, L.; Xu, Z.; Chen, T.; Zhou, L.; Yuan, M.; Li, T.; Ding, C. Comparison of Phenolic Compounds in Olive Leaves by Different Drying and Storage Methods. Separations 2021, 8, 156. [Google Scholar] [CrossRef]
- Martín-García, A.I.; Molina-Alcaide, E. Effect of Different Drying Procedures on the Nutritive Value of Olive (Olea europaea Var. Europaea) Leaves for Ruminants. Anim. Feed Sci. Technol. 2008, 142, 317–329. [Google Scholar] [CrossRef]
- Şahin, S.; Elhussein, E.; Bilgin, M.; Lorenzo, J.M.; Barba, F.J.; Roohinejad, S. Effect of Drying Method on Oleuropein, Total Phenolic Content, Flavonoid Content, and Antioxidant Activity of Olive (Olea europaea) Leaf. J. Food Process. Preserv. 2018, 42, e13604. [Google Scholar] [CrossRef]
- Markhali, F.S. Roles of Drying, Size Reduction, and Blanching in Sustainable Extraction of Phenolics from Olive Leaves. Processes 2021, 9, 1662. [Google Scholar] [CrossRef]
- Babu, A.K.; Kumaresan, G.; Aroul Raj, V.A.; Velraj, R. Review of Leaf Drying: Mechanism and Influencing Parameters, Drying Methods, Nutrient Preservation, and Mathematical Models. Renew. Sustain. Energy Rev. 2018, 90, 536–556. [Google Scholar] [CrossRef]
- Filgueira-Garro, I.; González-Ferrero, C.; Mendiola, D.; Marín-Arroyo, M.R. Effect of Cultivar and Drying Methods on Phenolic Compounds and Antioxidant Capacity in Olive (Olea europaea L.) Leaves. AIMS Agric. Food 2022, 7, 250–264. [Google Scholar] [CrossRef]
- Malik, N.S.A.; Bradford, J.M. Recovery and Stability of Oleuropein and Other Phenolic Compounds during Extraction and Processing of Olive (Olea europaea L.) Leaves. J. Food Agric. Environ. 2008, 6, 8–13. [Google Scholar]
- Taamalli, A.; Sánchez, J.L.; Jebabli, H.; Trabelsi, N.; Abaza, L.; Carretero, A.S.; Cho, J.Y.; Román, D.A. Monitoring the Bioactive Compounds Status in Olea Europaea According to Collecting Period and Drying Conditions. Energies 2019, 12, 947. [Google Scholar] [CrossRef]
- Ghelichkhani, G.; Modaresi, M.H.; Ladan, R.; Shariatifar, N.; Homapour, M.; Arabameri, M. Effect of the Spray and Freeze Dryers on the Bioactive Compounds of Olive Leaf Aqueous Extract by Chemometrics of HCA and PCA. J. Food Meas. Charact. 2019, 13, 2751–2763. [Google Scholar] [CrossRef]
- Erbay, Z.; Icier, F. Optimization of Drying of Olive Leaves in a Pilot-Scale Heat Pump Dryer. Dry. Technol. 2009, 27, 416–427. [Google Scholar] [CrossRef]
- Alibas, I.; Zia, M.P.; Yilmaz, A.; Asik, B.B. Drying Kinetics and Quality Characteristics of Green Apple Peel (Mallus communis L. Var. “Granny Smith”) Used in Herbal Tea Production. J. Food Process. Preserv. 2020, 44, e14332. [Google Scholar] [CrossRef]
- Satwase, A.N.; Pandhre, G.R.; Sirsat, P.G.; Wade, Y.R. Studies on Drying Characteristic and Nutritional Composition of Drumstick Leaves by Using Sun, Shadow, Cabinet and Oven Drying Methods. Sci. Rep. 2013, 2, 584. [Google Scholar] [CrossRef]
- Mansour, S.Y.; El-moity, S.F.A.; El-shourbagy, G.A.; Siliha, H.A. Effect of Drying Methods on Chemical Composition of Moringa Leaves Powder. Zagazig J. Food Dairy Res. 2016, 43, 2099–2114. [Google Scholar]
- Bahloul, N.; Boudhrioua, N.; Kouhila, M.; Kechaou, N. Effect of Convective Solar Drying on Colour, Total Phenols and Radical Scavenging Activity of Olive Leaves (Olea europaea L.). Int. J. Food Sci. Technol. 2009, 44, 2561–2567. [Google Scholar] [CrossRef]
- Abaza, B.L.; Youssef, N.B.; Manai, H.; Haddada, F.M.; Methenni, K.; Zarrouk, M. Chétoui Olive Leaf Extracts: Influence of the Solvent Type on Phenolics and Antioxidant Activities. Grasas Aceites 2011, 62, 96–104. [Google Scholar] [CrossRef]
- Nicolì, F.; Negro, C.; Vergine, M.; Aprile, A.; Nutricati, E.; Sabella, E.; Miceli, A.; Luvisi, A.; De Bellis, L. Evaluation of Phytochemical and Antioxidant Properties of 15 Italian Olea europaea L. Cultivar Leaves. Molecules 2019, 24, 1998. [Google Scholar] [CrossRef]
- Multari, S.; Marsol-Vall, A.; Keskitalo, M.; Yang, B.; Suomela, J. Effects of Different Drying Temperatures on the Content of Phenolic Compounds and Carotenoids in Quinoa Seeds (Chenopodium quinoa) from Finland. J. Food Compos. Anal. 2018, 72, 75–82. [Google Scholar] [CrossRef]
- Fabbri, A.; Galaverna, G.; Ganino, T. Polyphenol Composition of Olive Leaves with Regard to Cultivar, Time of Collection and Shoot Type. Acta Hortic. 2008, 791, 459–464. [Google Scholar] [CrossRef]
- Ahmad-Qasem, H.M.; Ahmad-Qasem, B.H.; Barrajon-Catalan, E.; Micol, V.; Cárcel, J.A.; García-Pérez, J.V. Drying and Storage of Olive Leaf Extracts. Influence on Polyphenols Stability. Ind. Crops Prod. 2016, 79, 232–239. [Google Scholar] [CrossRef]
- Fang, Z.; Bhandari, B. Effect of Spray Drying and Storage on the Stability of Bayberry Polyphenols. Food Chem. 2011, 129, 1139–1147. [Google Scholar] [CrossRef]
- Turkmen, F.; Karasu, S.; Karadag, A. Effects of Different Drying Methods and Temperature on the Drying Behavior and Quality Attributes of Cherry Laurel Fruit. Processes 2020, 8, 761. [Google Scholar] [CrossRef]
- Cagliari, A.; Martiny, T.R.; Nascimento, R.; Morais, M.M.; da Rosa, G.S. Effects of Different Drying Conditions on Bioactive Potential of Brazilian Olive Leaf. Braz. J. Food Technol. 2022, 25, e2021147. [Google Scholar] [CrossRef]
- Patrón-Vázquez, J.; Baas-Dzul, L.; Medina-Torres, N.; Ayora-Talavera, T.; Sánchez-Contreras, Á.; García-Cruz, U.; Pacheco, N. The Effect of Drying Temperature on the Phenolic Content and Functional Behavior of Flours Obtained from Lemon Wastes. Agronomy 2019, 9, 474. [Google Scholar] [CrossRef]
- Guo, H.-L.; Chen, Y.; Xu, W.; Xu, M.-T.; Sun, Y.; Wang, X.-C.; Wang, X.-Y.; Luo, J.; Zhang, H.; Xiong, Y.-K. Assessment of Drying Kinetics, Textural and Aroma Attributes of Mentha Haplocalyx Leaves during the Hot Air Thin-Layer Drying Process. Foods 2022, 11, 784. [Google Scholar] [CrossRef]
- Saifullah, M.; McCullum, R.; McCluskey, A.; Van Vuong, Q. Effect of Drying Techniques and Operating Conditions on the Retention of Color, Phenolics, and Antioxidant Properties in Dried Lemon Scented Tea Tree (Leptospermum petersonii) Leaves. J. Food Process. Preserv. 2021, 45, e15257. [Google Scholar] [CrossRef]
- Lorini, A.; Aranha, B.C.; da Fonseca Antunes, B.; Otero, D.M.; Jacques, A.C.; Zambiazi, R.C. Metabolic Profile of Olive Leaves of Different Cultivars and Collection Times. Food Chem. 2021, 345, 128758. [Google Scholar] [CrossRef] [PubMed]
- Karam, M.C.; Petit, J.; Zimmer, D.; Djantou, E.B.; Scher, J. Effects of Drying and Grinding in Production of Fruit and Vegetable Powders: A Review. J. Food Eng. 2016, 188, 32–49. [Google Scholar] [CrossRef]
- Chang, C.-H.; Lin, H.-Y.; Chang, C.-Y.; Liu, Y.-C. Comparisons on the Antioxidant Properties of Fresh, Freeze-Dried and Hot-Air-Dried Tomatoes. J. Food Eng. 2006, 77, 478–485. [Google Scholar] [CrossRef]
- Erbay, Z.; Icier, F. Optimization of Hot Air Drying of Olive Leaves Using Response Surface Methodology. J. Food Eng. 2009, 91, 533–541. [Google Scholar] [CrossRef]
- Arslan, D.; Özcan, M.M. Study the Effect of Sun, Oven and Microwave Drying on Quality of Onion Slices. LWT—Food Sci. Technol. 2010, 43, 1121–1127. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, J.; Xin, X.; Zhu, S.; Niu, E.; Wu, Q.; Li, T.; Liu, D. Changes in Phytochemical Profiles and Biological Activity of Olive Leaves Treated by Two Drying Methods. Front. Nutr. 2022, 9, 854680. [Google Scholar] [CrossRef]
- De Leonardis, A.; Macciola, V.; Cuomo, F.; Lopez, F. Evidence of Oleuropein Degradation by Olive Leaf Protein Extract. Food Chem. 2015, 175, 568–574. [Google Scholar] [CrossRef]
- Chaji, S.; Zenasni, W.; Tomao, V.; Sylvie, A.; Tixier, F.; Amine, E.; Hanine, H.; Bajoub, A. Exploring the Potential of Advanced Eco-Friendly Extraction Techniques for a Rapid Recovery of Oleuropein—Rich Extracts from “Picholine Marocaine” Olive Tree Leaves. Sustain. Chem. Pharm. 2023, 36, 101248. [Google Scholar] [CrossRef]
- Andrejč, D.C.; Butinar, B.; Knez, Ž.; Tomažič, K.; Marevci, M.K. The Effect of Drying Methods and Extraction Techniques on Oleuropein Content in Olive Leaves. Plants 2022, 11, 865. [Google Scholar] [CrossRef]
- Association Française de Normalisation. Détermination de l’azote Total—Méthode Par Distillation Après Minéralisation (Kjeldahl). In Qualité Des Sols; AFNOR: Paris, France, 1983. [Google Scholar]
- Kjeldahl, J. Neue Methode Zur Bestimmung Des Stickstoffs in Organischen Körpern. Fresenius Z. Anal. Chem. 1883, 22, 366–382. [Google Scholar] [CrossRef]
- Olmo-García, L.; Kessler, N.; Neuweger, H.; Wendt, K.; Olmo-Peinado, J.M.; Fernández-Gutiérrez, A.; Baessmann, C.; Carrasco-Pancorbo, A. Unravelling the Distribution of Secondary Metabolites in Olea europaea L.: Exhaustive Characterization of Eight Olive-Tree Derived Matrices by Complementary Platforms (LC-ESI/APCI-MS and GC-APCI-MS). Molecules 2018, 23, 2419. [Google Scholar] [CrossRef] [PubMed]
- Olmo-García, L.; Polari, J.J.; Li, X.; Bajoub, A.; Fernández-Gutiérrez, A.; Wang, S.C.; Carrasco-Pancorbo, A. Deep Insight into the Minor Fraction of Virgin Olive Oil by Using LC-MS and GC-MS Multi-Class Methodologies. Food Chem. 2018, 261, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Bajoub, A.; Carrasco-Pancorbo, A.; Ajal, E.A.; Beltrán Maza, G.; Fernández-Gutiérrez, A.; Ouazzani, N. Contribution to the Establishment of a Protected Designation of Origin for Meknès Virgin Olive Oil: A 4-Years Study of Its Typicality. Food Res. Int. 2014, 66, 332–343. [Google Scholar] [CrossRef]
| Main Factor | Cultivar | Total Nitrogen (%) | Crude Protein (%) | Number of Analyzed Extracts |
|---|---|---|---|---|
| Olive variety | “Picholine Marocaine” | 0.98 ± 0.04 a | 6.11 ± 0.23 a | 6 |
| “Menara” | 0.76 ± 0.02 b | 4.78 ± 0.21 b | 6 | |
| “Arbequina” | 0.88 ± 0.02 a | 5.50 ± 0.12 a | 6 | |
| “Koroneiki” | 0.92 ± 0.08 a | 5.73 ± 0.50 a | 6 |
| Main Factor | Cultivar | Total Phenolic Content (mg GAE/ g FW) | Total Flavonoid Content (mg QE/g FW) | Number of Analyzed Extracts |
|---|---|---|---|---|
| Olive variety | “Picholine Marocaine” | 9.02 ± 2.05 a | 2.75 ± 0.22 a | 6 |
| “Menara” | 14.85 ± 2.66 b | 3.28 ± 0.08 a | 6 | |
| “Arbequina” | 20.18 ± 0.95 c | 3.26 ± 0.74 a | 6 | |
| “Koroneiki” | 14.15 ± 2.91 b | 3.28 ± 1.06 a | 6 |
| Drying Method | Cultivar | |||
|---|---|---|---|---|
| “Picholine Marocaine” | “Menara” | “Arbequina” | “Koroneiki” | |
| Fresh | 15.46 ± 0.24 aA | 24.11 ± 0.22 bA | 34.94 ± 0.20 cA | 24.99 ± 0.23 bA |
| Ambient-air-drying | 72.23 ± 0.21 aB | 60.95 ± 0.20 bB | 71.14 ± 0.11 cB | 64.24 ± 0.16 dB |
| Oven-drying at 25 °C | 48.16 ± 0.09 aC | 42.98 ± 0.12 b | 71.50 ± 0.20 cB | 52.84 ± 0.18 dC |
| Oven-drying at 50 °C | 45.00 ± 0.13 aD | 33.07 ± 0.21 bC | 35.23 ± 0.20 cA | 40.30 ± 0.23 dD |
| Oven-drying at 60 °C | 28.52 ± 0.12 aE | 24.82 ± 0.19 bA | 33.29 ± 0.32 cC | 28.60 ± 0.25 aE |
| Oven-drying at 70 °C | 31.61 ± 0.28 aF | 27.55 ± 0.31 bE | 26.59 ± 0.24 bD | 21.55 ± 0.21 cF |
| Oven-drying at 75 °C | 28.90 ± 0.24 aE | 25.15 ± 0.22 bD | 31.31 ± 0.30 cE | 27.68 ± 0.12 dG |
| Oven-drying at 80 °C | 19.79 ± 0.24 aG | 41.38 ± 0.37 bF | 30.27 ± 0.35 cF | 22.59 ± 0.17 dH |
| Oven-drying at 105 °C | 35.00 ± 0.20 aH | 36.60 ± 0.38 bG | 37.71 ± 0.24 bG | 31.67 ± 0.41 cI |
| Oven-drying at 120 °C | 34.39 ± 0.23 aH | 39.05 ± 0.14 bH | 47.78 ± 0.17 cH | 37.15 ± 0.26 dJ |
| Oven-drying at 150 °C | 43.03 ± 0.20 aI | 39.56 ± 0.19 bH | 41.53 ± 0.12 cI | 49.26 ± 0.23 dK |
| Freeze-drying | 39.42 ± 0.20 aJ | 45.32 ± 0.16 bI | 42.57 ± 0.16 cJ | 40.86 ± 0.20 dD |
| Solar-drying at 50 °C | 18.48 ± 0.63 aK | 21.78 ± 0.80 bJ | 20.92 ± 0.41 bK | 17.75 ± 0.41 aL |
| Solar-drying at 60 °C | 21.37 ± 0.22 aL | 25.25 ± 0.39 bD | 24.83 ± 0.61 bL | 28.87 ± 0.61 cE |
| Solar-drying at 70 °C | 35.20 ± 0.16 aM | 41.91 ± 0.65 bF | 24.83 ± 0.61 cL | 31.04 ± 0.82 dI |
| Solar-drying at 80 °C | 27.66 ± 0.53 aN | 33.12 ± 0.49 bC | 30.32 ± 0.61 cF | 27.14 ± 0.61 aG |
| Drying Method | Cultivar | |||
|---|---|---|---|---|
| “Picholine Marocaine” | “Menara” | “Arbequina” | “Koroneiki” | |
| Fresh | 4.67 ± 0.37 aA | 5.29 ± 0.13 aA | 5.61 ± 1.28 aA | 5.75 ± 1.86 aA |
| Ambient-air-drying | 20.54 ± 0.30 aB | 21.92 ± 0.22 abB | 24.01 ± 1.36 bcB | 25.45 ± 0.06 cB |
| Oven-drying at 25 °C | 18.89 ± 0.54 aC | 18.43 ± 0.32 aC | 22.64 ± 0.43 bBC | 21.41 ± 0.22 bC |
| Oven-drying at 50 °C | 15.75 ± 0.22 aD | 14.22 ± 0.22 aD | 15.37 ± 3.14 aD | 20.57 ± 2.27 aC |
| Oven-drying at 60 °C | 6.17 ± 0.32 abE | 5.17 ± 0.04 aA | 6.13 ± 0.54 abA | 7.16 ± 0.65 bADE |
| Oven-drying at 70 °C | 6.30 ± 0.26 abE | 8.06 ± 0.19 bE | 5.78 ± 0.43 aA | 5.77 ± 0.93 aA |
| Oven-drying at 75 °C | 5.84 ± 0.17 aE | 8.31 ± 0.11 aE | 7.16 ± 0.65 aAE | 8.03 ± 1.10 aADE |
| Oven-drying at 80 °C | 6.06 ± 0.13 aE | 17.82 ± 1.41 bCF | 6.43 ± 0.35 aA | 6.55 ± 0.61 aAE |
| Oven-drying at 105 °C | 15.67 ± 0.76 aD | 17.28 ± 0.87 aCF | 18.35 ± 3.03 aCD | 16.97 ± 1.73 aFG |
| Oven-drying at 120 °C | 13.76 ± 0.43 aF | 16.59 ± 0.76 aF | 17.51 ± 1.19 aD | 16.13 ± 1.41 aG |
| Oven-drying at 150 °C | 19.58 ± 0.87 aC | 18.28 ± 0.11 aC | 20.34 ± 2.6 aB | 19.12 ± 0.43 aCF |
| Freeze-drying | 17.28 ± 0.65 aG | 22.64 ± 0.65 bB | 22.26 ± 2.27 abB | 21.18 ± 0.76 abC |
| Solar-drying at 50 °C | 5.48 ± 0.09 aAE | 6.64 ± 0.17 bG | 5.12 ± 0.24 aA | 5.17 ± 0.13 aA |
| Solar-drying at 60 °C | 6.09 ± 0.17 aE | 7.51 ± 0.07 bEG | 5.43 ± 0.06 cA | 9.64 ± 0.09 dDE |
| Solar-drying at 70 °C | 3.38 ± 0.06 aH | 4.10 ± 0.09 bA | 10.89 ± 0.09 cE | 8.46 ± 0.06d ADE |
| Solar-drying at 80 °C | 9.90 ± 0.07 aI | 12.08 ± 0.08 bH | 8.77 ± 0.15 cAE | 10.34 ± 0.09 dD |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaji, S.; Zenasni, W.; Ouaabou, R.; Ajal, E.A.; Lahlali, R.; Fauconnier, M.-L.; Hanine, H.; Černe, M.; Pasković, I.; Merah, O.; et al. Nutrient and Bioactive Fraction Content of Olea europaea L. Leaves: Assessing the Impact of Drying Methods in a Comprehensive Study of Prominent Cultivars in Morocco. Plants 2024, 13, 1961. https://doi.org/10.3390/plants13141961
Chaji S, Zenasni W, Ouaabou R, Ajal EA, Lahlali R, Fauconnier M-L, Hanine H, Černe M, Pasković I, Merah O, et al. Nutrient and Bioactive Fraction Content of Olea europaea L. Leaves: Assessing the Impact of Drying Methods in a Comprehensive Study of Prominent Cultivars in Morocco. Plants. 2024; 13(14):1961. https://doi.org/10.3390/plants13141961
Chicago/Turabian StyleChaji, Salah, Walid Zenasni, Rachida Ouaabou, El Amine Ajal, Rachid Lahlali, Marie-Laure Fauconnier, Hafida Hanine, Marko Černe, Igor Pasković, Othmane Merah, and et al. 2024. "Nutrient and Bioactive Fraction Content of Olea europaea L. Leaves: Assessing the Impact of Drying Methods in a Comprehensive Study of Prominent Cultivars in Morocco" Plants 13, no. 14: 1961. https://doi.org/10.3390/plants13141961






