Loquat (Eriobotrya japonica) Is a New Natural Host of Tomato Mosaic Virus and Citrus Exocortis Viroid
Abstract
:1. Introduction
2. Results
2.1. Viruses Detected in Loquat Using High-Throughput Sequencing
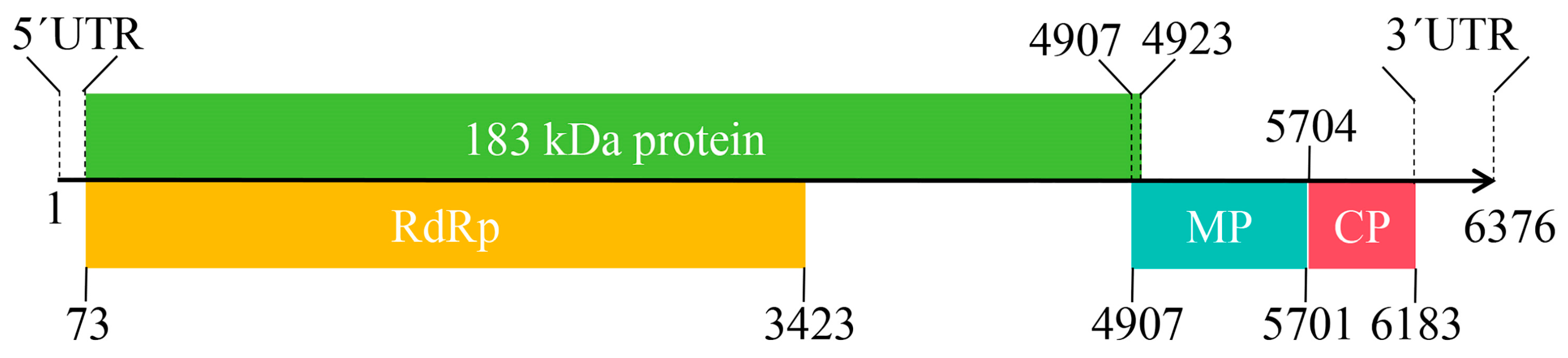
2.2. Whole Genome Sequence and Characterization of ToMV-Loquat Isolate
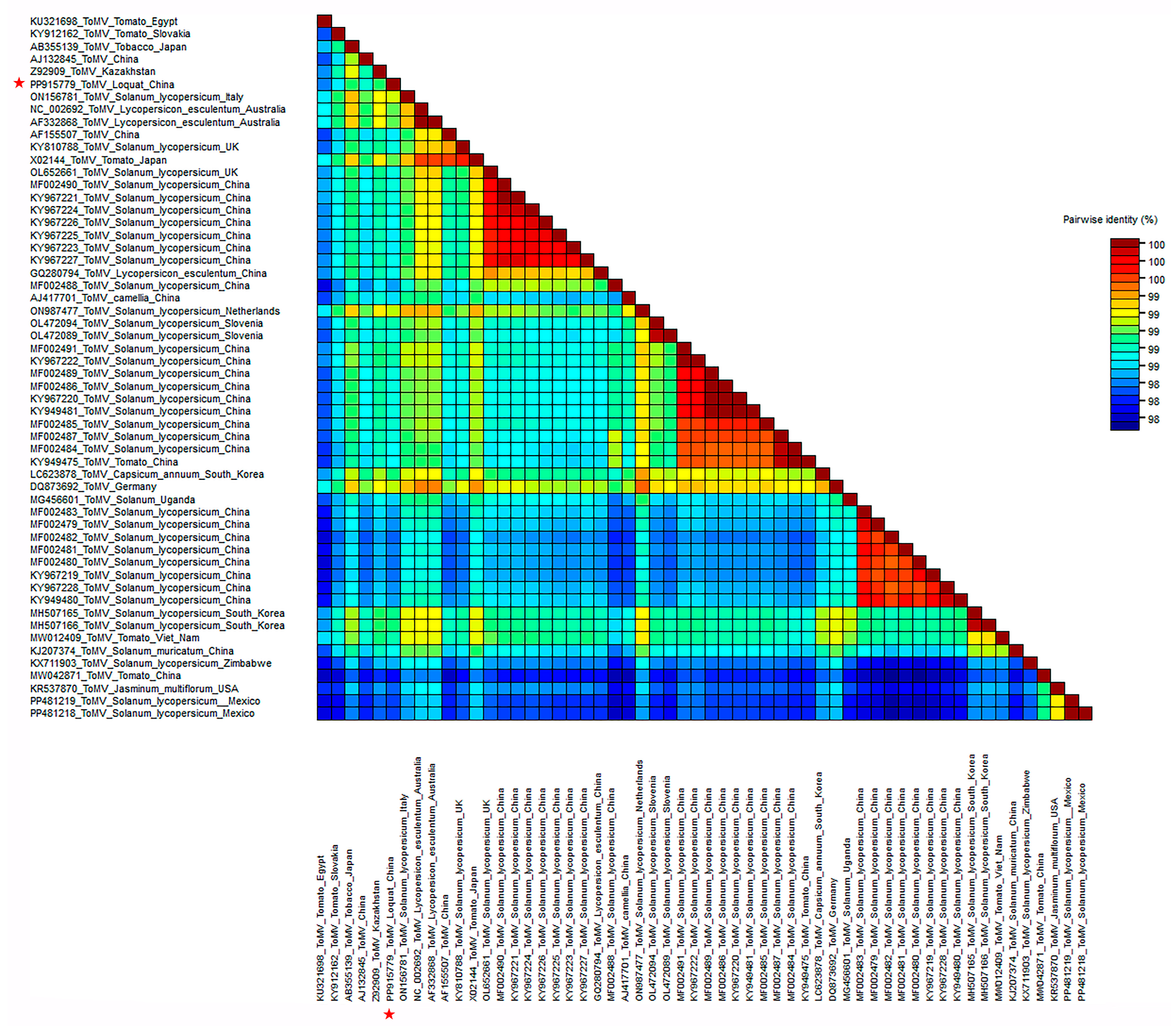
2.3. Homology and Variation between ToMV-Loquat and Other Isolates
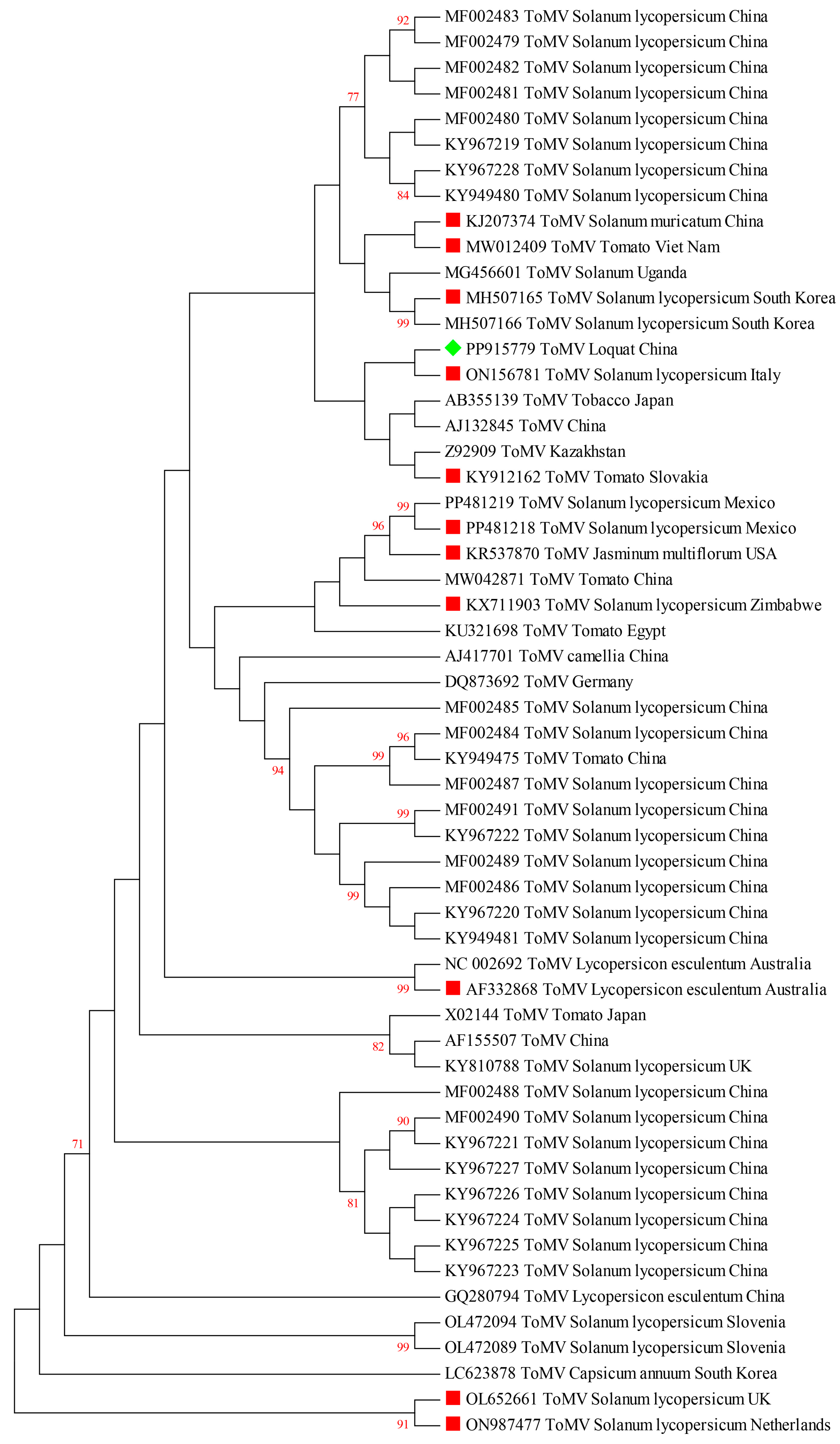
2.4. Phylogenetic Relationship between ToMV-Loquat and Other ToMV Isolates
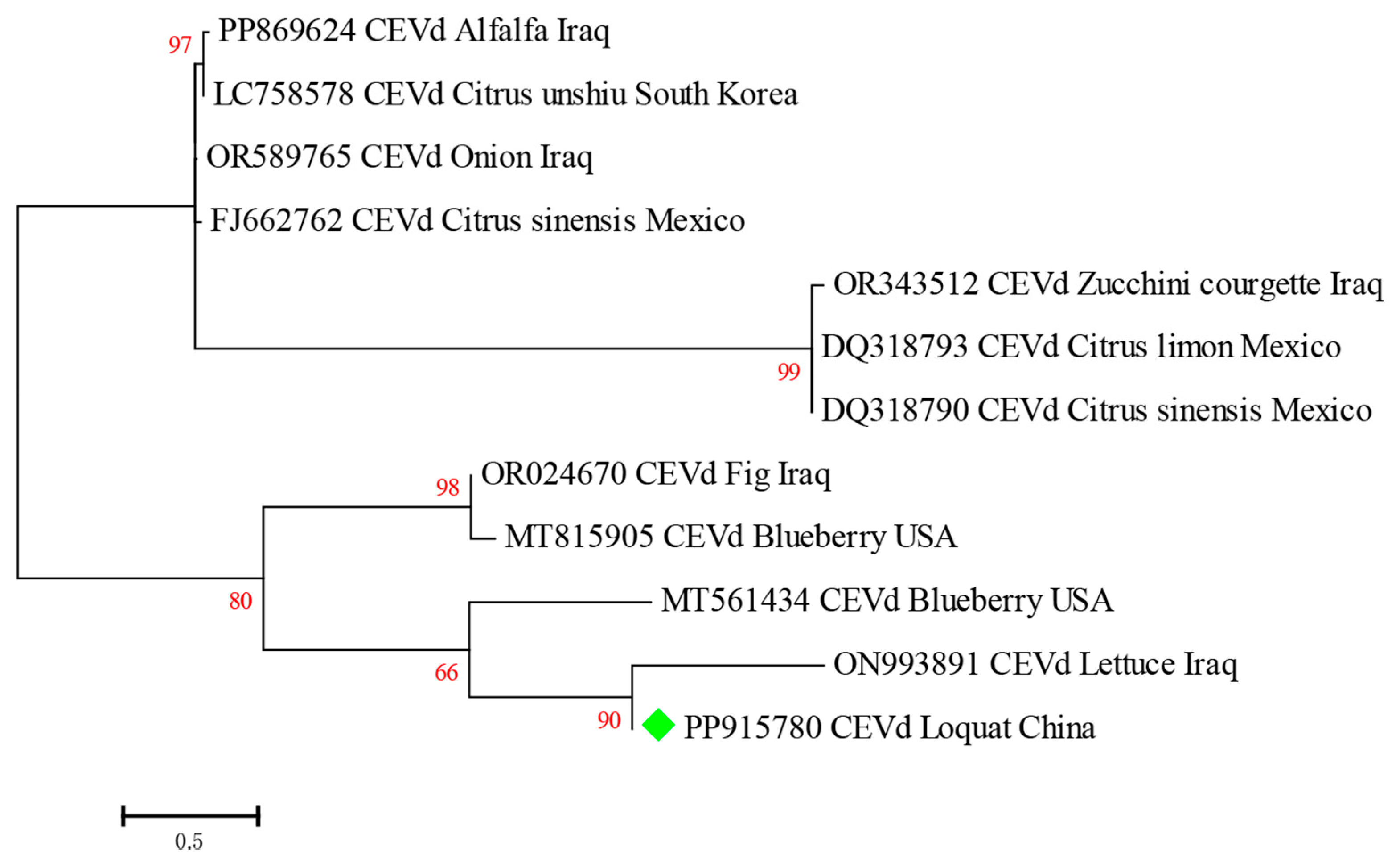
2.5. Characterization of CEVd in Loquat
3. Discussion
4. Materials and Methods
4.1. Sample Collection and RNA Isolation
4.2. cDNA Library Construction and RNA-Seq
4.3. Sequence Assembly of ToMV and CEVd
4.4. Whole Genome Sequence Amplification of ToMV
4.5. Bioinformatics Analyses
4.6. RT-PCR Amplification, Cloning, and Sequencing
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Q.; Xuan, Z.; Wu, J.; Qiu, Y.; Li, M.; Zhang, S.; Wu, D.; Li, R.; Cao, M. Loquat is a new natural host of apple stem grooving virus and apple chlorotic leaf spot virus in China. Plant Dis. 2019, 103, 3290. [Google Scholar] [CrossRef]
- Canales, C.; Morán, F.; Olmos, A.; Ruiz-García, A.B. First detection and molecular characterization of apple stem grooving virus, apple chlorotic leaf spot virus, and apple hammerhead viroid in loquat in Spain. Plants 2021, 10, 2293. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yang, L.; Xuan, Z.; Wu, J.; Qiu, Y.; Zhang, S.; Wu, D.; Zhou, C.; Cao, M. Complete nucleotide sequence of loquat virus A, a member of the family Betaflexiviridae with a novel genome organization. Arch. Virol. 2020, 165, 223–226. [Google Scholar] [CrossRef]
- Morán, F.; Canales, C.; Olmos, A.; Ruiz-García, A.B. Loquat (Eriobotrya japonica) is a new natural host of apple stem pitting virus. Plants 2020, 9, 1560. [Google Scholar] [CrossRef] [PubMed]
- Boben, J.; Kramberger, P.; Petrovič, N.; Cankar, K.; Peterka, M.; Štrancar, A.; Ravnikar, M. Detection and quantification of Tomato mosaic virus in irrigation waters. Eur. J. Plant. Pathol. 2007, 118, 59–71. [Google Scholar] [CrossRef]
- Choi, G.W.; Oh, J.P.; Cho, I.S.; Ju, H.K.; Hu, W.X.; Kim, B.; Seo, E.Y.; Park, J.S.; Domier, L.L.; Hammond, J.; et al. Full-Length infectious clones of two new isolates of tomato mosaic virus induce distinct symptoms associated with two differential amino acid residues in 128-kDa Protein. Plant Pathol. J. 2019, 35, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Ge, B.B.; Liu, G.J.; Wang, H.Q. First report of tomato mosaic virus infecting pepino in China. Plant Dis. 2012, 96, 1704. [Google Scholar] [CrossRef]
- Lyu, J.; Yang, Y.; Sun, X.; Jiang, S.; Hong, H.; Zhu, X.; Liu, Y. Genetic variability and molecular evolution of tomato mosaic virus populations in three northern China Provinces. Viruses 2023, 15, 1617. [Google Scholar] [CrossRef] [PubMed]
- Strasser, M.; Pfitzner, A.J. The double-resistance-breaking Tomato mosaic virus strain ToMV1-2 contains two independent single resistance-breaking domains. Arch. Virol. 2007, 152, 903–914. [Google Scholar] [CrossRef]
- Pozharskiy, A.; Kostyukova, V.; Taskuzhina, A.; Nizamdinova, G.; Kisselyova, N.; Kalendar, R.; Karimov, N.; Gritsenko, D. Screening a collection of local and foreign varieties of Solanum lycopersicum L. in Kazakhstan for genetic markers of resistance against three tomato viruses. Heliyon 2022, 8, e10095. [Google Scholar] [CrossRef]
- Di Serio, F.; Flores, R.; Verhoeven, J.T.; Li, S.F.; Pallás, V.; Randles, J.W.; Sano, T.; Vidalakis, G.; Owens, R.A. Current status of viroid taxonomy. Arch. Virol. 2014, 159, 3467–3478. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Zhang, Z.; Hu, B.; Hu, G.; Wang, H.; Faure, C.; Marais, A.; Candresse, T.; Li, S. Identification of a viroid-like RNA in a lychee Transcriptome Shotgun Assembly. Virus Res. 2017, 240, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Steger, G.; Riesner, D. Viroid research and its significance for RNA technology and basic biochemistry. Nucleic Acids Res. 2018, 46, 10563–10576. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Qi, S.; Tang, N.; Zhang, X.; Chen, S.; Zhu, P.; Ma, L.; Cheng, J.; Xu, Y.; Lu, M.; et al. Discovery of replicating circular RNAs by RNA-seq and computational algorithms. PLoS Pathog. 2014, 10, e1004553. [Google Scholar] [CrossRef]
- Vernière, C.; Perrier, X.; Dubois, C.; Dubois, A.; Botella, L.; Chabrier, C.; Bové, J.M.; Vila, N.D. Citrus Viroids: Symptom expression and effect on vegetative growth and yield of clementine trees grafted on trifoliate orange. Plant Dis. 2004, 88, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Vernière, C.; Perrier, X.; Dubois, C.; Dubois, A.; Botella, L.; Chabrier, C.; Bové, J.M.; Vila, N.D. Interactions between citrus viroids affect symptom expression and field performance of clementine trees grafted on trifoliate orange. Phytopathology 2006, 96, 356–368. [Google Scholar] [CrossRef] [PubMed]
- Rizza, S.; Conesa, A.; Juarez, J.; Catara, A.; Navarro, L.; Duran-Vila, N.; Ancillo, G. Microarray analysis of Etrog citron (Citrus medica L.) reveals changes in chloroplast, cell wall, peroxidase and symporter activities in response to viroid infection. Mol. Plant Pathol. 2012, 13, 852–864. [Google Scholar] [CrossRef] [PubMed]
- Cottilli, P.; Belda-Palazón, B.; Adkar-Purushothama, C.R.; Perreault, J.P.; Schleiff, E.; Rodrigo, I.; Ferrando, A.; Lisón, P. Citrus exocortis viroid causes ribosomal stress in tomato plants. Nucleic Acids Res. 2019, 47, 8649–8661. [Google Scholar] [CrossRef] [PubMed]
- Villamor, D.E.V.; Ho, T.; Al Rwahnih, M.; Martin, R.R.; Tzanetakis, I.E. High throughput sequencing for plant virus detection and discovery. Phytopathology 2019, 109, 716–725. [Google Scholar] [CrossRef]
- Park, K.H.; Cha, B.J. Detection of TMV, ToMV, and CMV from tomato seeds and plants. Res. Plant Dis. 2002, 8, 101–106. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Trinity: Reconstructing a full-length transcriptome without agenome from RNA-Seq data. Nat. Biotechnol. 2013, 29, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Cao, K.; Zhang, Z.X.; Wang, L.R.; Li, S.F. Identification and characterization of a novel rhabdovirus infecting peach in China. Virus Res. 2020, 280, 197905. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Muhire, B.M.; Varsani, A.; Martin, D.P. SDT: A virus classification tool based on pairwise sequence alignment and identity calculation. PLoS ONE 2014, 9, e108277. [Google Scholar] [CrossRef] [PubMed]


| Isolate | GenBank Accession No. | Host | Length (nt) | Identities (aa) % | ||||
|---|---|---|---|---|---|---|---|---|
| Origin of Country | 183 kDa Protein | RdRp | MP | CP | ||||
| GW2 | MH507166 | Solanum lycopersicum (Tomato) | South Korea | 6383 | 99.5 | 99.7 | 99.6 | 100 |
| mutoko | KX711903 | Solanum lycopersicum (Tomato) | Zimbabwe | 6383 | 99.6 | 99.9 | 99.3 | 99.4 |
| SL-1 | KY912162 | Solanum lycopersicum (Tomato) | Slovakia | 6383 | 99.5 | 99.8 | 98.9 | 98.7 |
| 99-1 | KR537870 | Jasminum multiflorum | USA | 6383 | 99.6 | 99.8 | 98.9 | 100 |
| Penghu | KJ207374 | Solanum lycopersicum (Tomato) | China | 6385 | 99.4 | 99.8 | 98.9 | 98.7 |
| Queensland | AF332868 | Solanum lycopersicum (Tomato) | Australia | 6383 | 99.7 | 99.9 | 98.9 | 100 |
| NPPO-NL 41833930 | ON987477 | Solanum lycopersicum (Tomato) | The Netherlands | 6373 | 99.6 | 99.9 | 99.3 | 98.7 |
| INIFAP JM1 | PP481218 | Solanum lycopersicum (Tomato) | Mexico | 6447 | 99.6 | 99.8 | 98.9 | 100 |
| NVWA5785660 | OL652661 | Solanum lycopersicum (Tomato) | UK | 6367 | 99.6 | 99.9 | 99.3 | 99.4 |
| DTT | MW012409 | Solanum lycopersicum (Tomato) | Vietnam | 6380 | 99.6 | 99.9 | 99.3 | 100 |
| IFA9 | ON156781 | Solanum lycopersicum (Tomato) | Italy | 6376 | 99.6 | 99.9 | 99.6 | 100 |
| Isolate | GenBank Accession No. | Origin of Country | Host | Length (nt) | Identities (nt) % |
|---|---|---|---|---|---|
| Aan-Saladin | OR589765 | Iraq | Allium cepa (Onion) | 394 | 77.8 |
| Baghdad-1/Iraq | OR343512 | Iraq | Cucurbita pepo (Zucchini courgette) | 394 | 75.6 |
| Najaf | OR024670 | Iraq | Ficus carica (Fig) | 397 | 77.3 |
| 5/5 | DQ318793 | Mexico | Citrus limon (Lemon) | 395 | 78.1 |
| 2/3 | DQ318790 | Mexico | Citrus sinensis (Sweet orange) | 394 | 78.3 |
| CEVd | FJ662762 | Mexico | Citrus sinensis (Sweet orange) | 395 | 78.3 |
| LSS OH19 | MT561434 | USA | Vaccinium uliginosum (Blueberry) | 369 | 78.3 |
| CNU_JC | LC758578 | South Korea | Citrus unshiu (Mandarin orange) | 394 | 79.8 |
| Tikrit | ON993891 | Iraq | Lactuca sativa (Lettuce) | 370 | 77.8 |
| OH19-3 | MT815905 | USA | Vaccinium uliginosum (Blueberry) | 390 | 78.8 |
| Balad | PP869624 | Iraq | Medicago sativa (Alfalfa) | 394 | 80.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, C.; Wang, L.; Li, Y.; Zhou, K.; Zhao, K.; Chen, D.; Li, J.; Song, H.; Tu, M. Loquat (Eriobotrya japonica) Is a New Natural Host of Tomato Mosaic Virus and Citrus Exocortis Viroid. Plants 2024, 13, 1965. https://doi.org/10.3390/plants13141965
He C, Wang L, Li Y, Zhou K, Zhao K, Chen D, Li J, Song H, Tu M. Loquat (Eriobotrya japonica) Is a New Natural Host of Tomato Mosaic Virus and Citrus Exocortis Viroid. Plants. 2024; 13(14):1965. https://doi.org/10.3390/plants13141965
Chicago/Turabian StyleHe, Chengyong, Lingli Wang, Yarui Li, Kangyu Zhou, Ke Zhao, Dong Chen, Jing Li, Haiyan Song, and Meiyan Tu. 2024. "Loquat (Eriobotrya japonica) Is a New Natural Host of Tomato Mosaic Virus and Citrus Exocortis Viroid" Plants 13, no. 14: 1965. https://doi.org/10.3390/plants13141965





