Conservation Potential Trough In Vitro Regeneration of Two Threatened Medicinal Plants Ungernia sewertzowii and U. victoris
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Methods
2.2.1. Sterilization of Explant
2.2.2. Explant Sources
2.2.3. Nutrient Media
2.2.4. Phytohormones and Compositions
2.2.5. Extraction
2.2.6. DPPH Free Radical Scavenging Assay
2.2.7. ABTS Free Radical Scavenging Assay
3. Result and Discussion
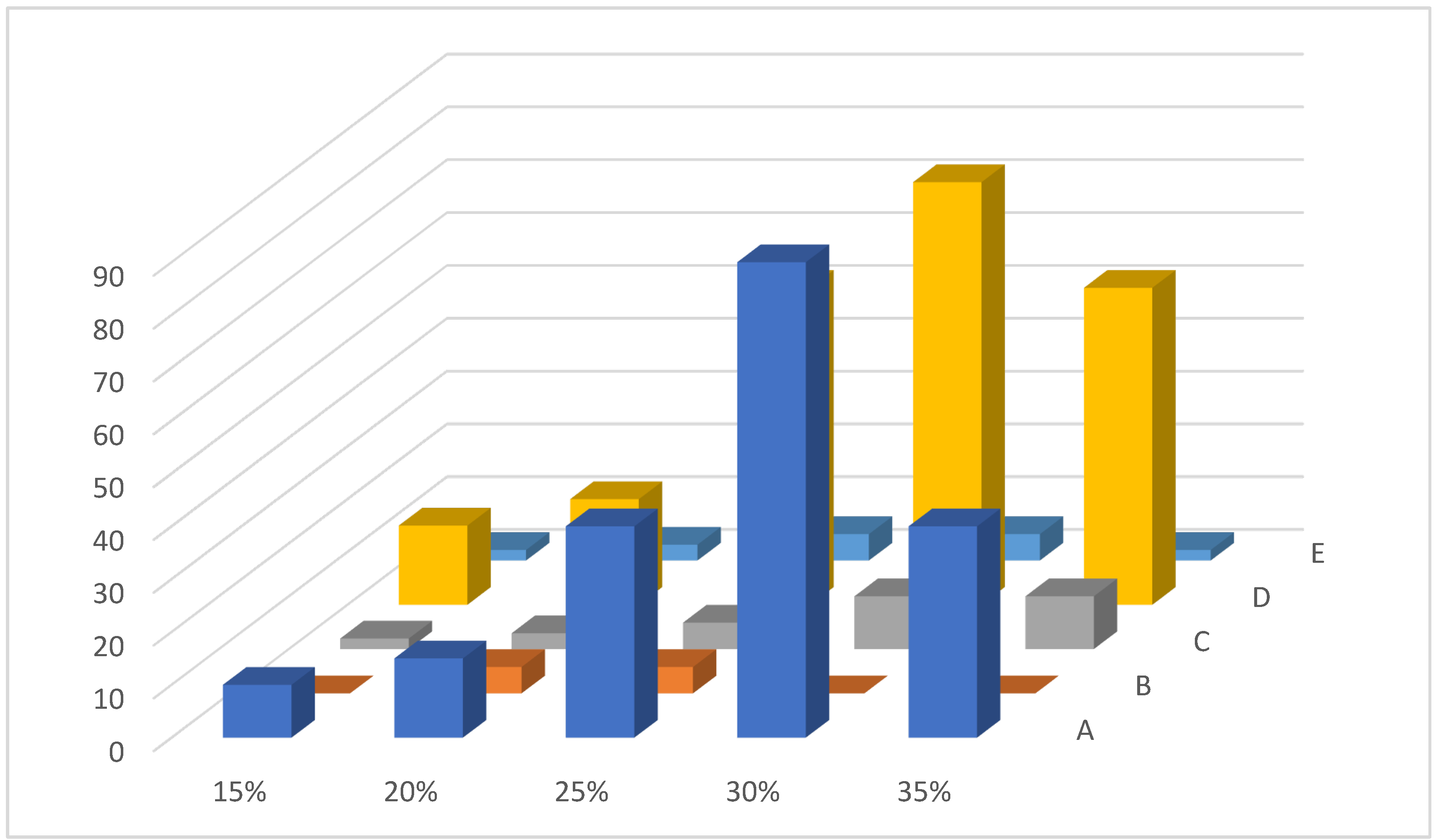
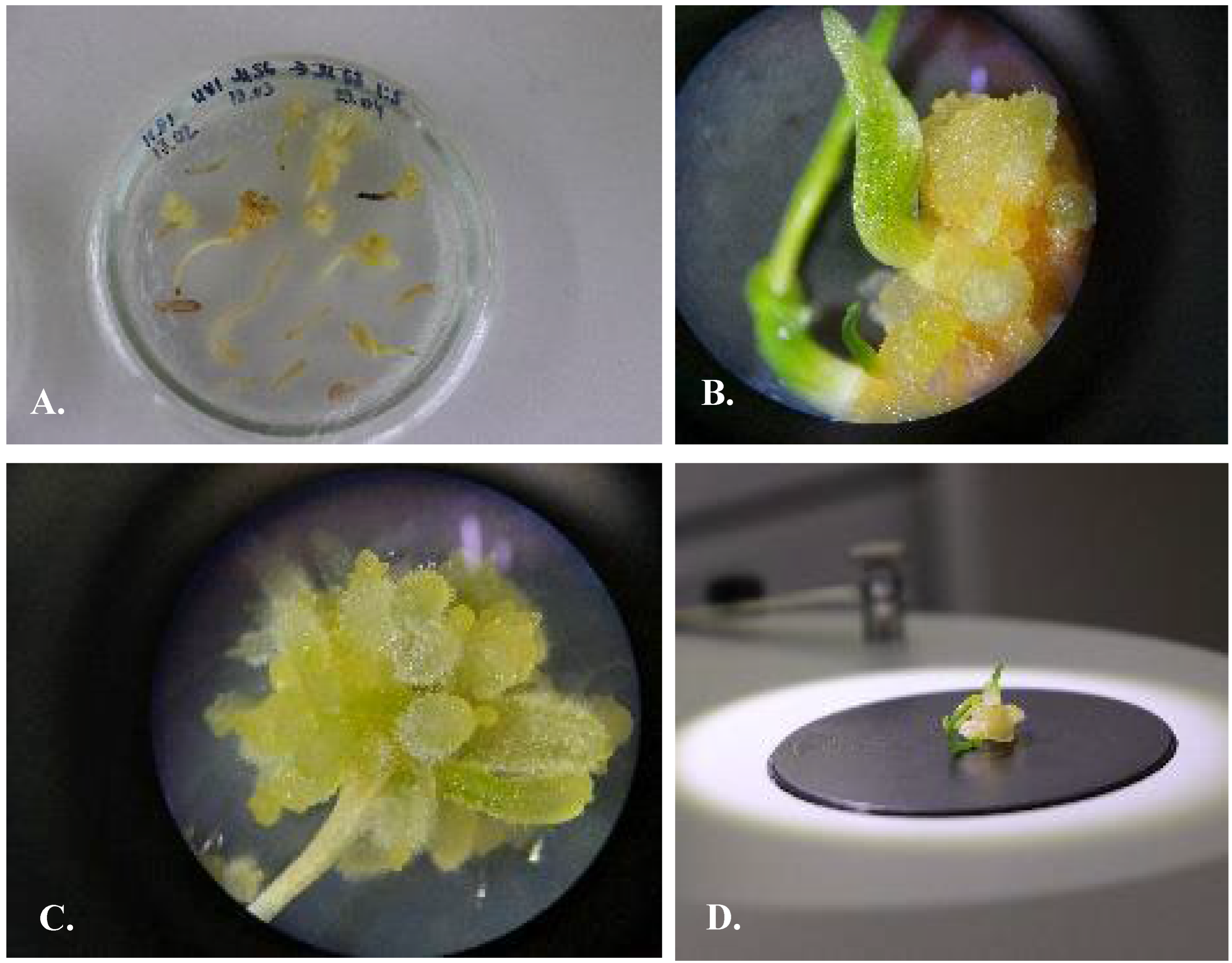
3.1. Use of Scales and Bottom of the Bulbs as the Source of Explants
Selection of Nutrient Medium
- Callusogenesis on the bulb scales of US on MS with phytohormones M39 NAA 2.0 mg/L, M55 2.4D 0.5 mg/L, M60 2.4D 1.0 mg/L + Kin 0.5 mg/L, and M56 2.4D 0.5 mg/L + Kin 0.5 mg/L and weak callus formation or its absence for UV with phytohormone 2.4D 2.0 mg/L and combination of phytohormones 2.4D 2.0–4.0 mg/L + BAP 0.5–4.0 mg/L.
- Direct somatic embryogenesis on the bulb scales of US on MS with phytohormones M40 NAA 2.0 mg/L + BAP 0.5 mg/L and M56 2.4D 0.5 mg/L + Kin 0.5 mg/L.
- Indirect somatic embryogenesis on the bulb scales of US on MS with phytohormones M56 2.4D 0.5 mg/L + Kin 0.5 mg/L.
- No somatic embryogenesis was observed for UV on MS media.
- Callusogenesis on the bulb scales of US on Vch with phytohormones V68 IAA 0.5 mg/L + Kin 0.5 mg/L, V17 IAA 0.5 mg/L + BAP 0.5 mg/L, and V56 2.4D 0.5 mg/L + Kin 0.5 mg/L.
- Callusogenesis on the bulb scales of UV on Vch with phytohormones V56 2.4D 0.5 mg/L + Kin 0.5 mg/L, V5 2.4D 0.5 mg/L + BAP 0.5 mg/L, and V44 NAA 0.5 mg/L + Kin 0.5 mg/L.
- Direct somatic embryogenesis on the bulb scales of US on Vch with phytohormones V29 Kin 1.0 mg/L, V68 IAA 0.5 mg/L + Kin 0.5 mg/L, V56 2.4D 0.5 mg/L + Kin 0.5 mg/L, V4 2.4D 0.5 mg/L, V16 IAA 0.5 mg/L, V81 2.4D 0.5 mg/L + Kin 5.0 mg/L, V83 IAA 0.5 mg/L + BAP 5.0 mg/L, V84 2.4D 0.5 mg/L + BAP 5.0 mg/L, and V87 2.4D 0.5 mg/L + Zea 0.5 mg/L.
- Direct somatic embryogenesis on the bulb scales of UV with phytohormones V16 IAA 0.5 mg/L and V4 2.4D 0.5 mg/L.
- Indirect somatic embryogenesis was not observed for US and UV on Vch media.
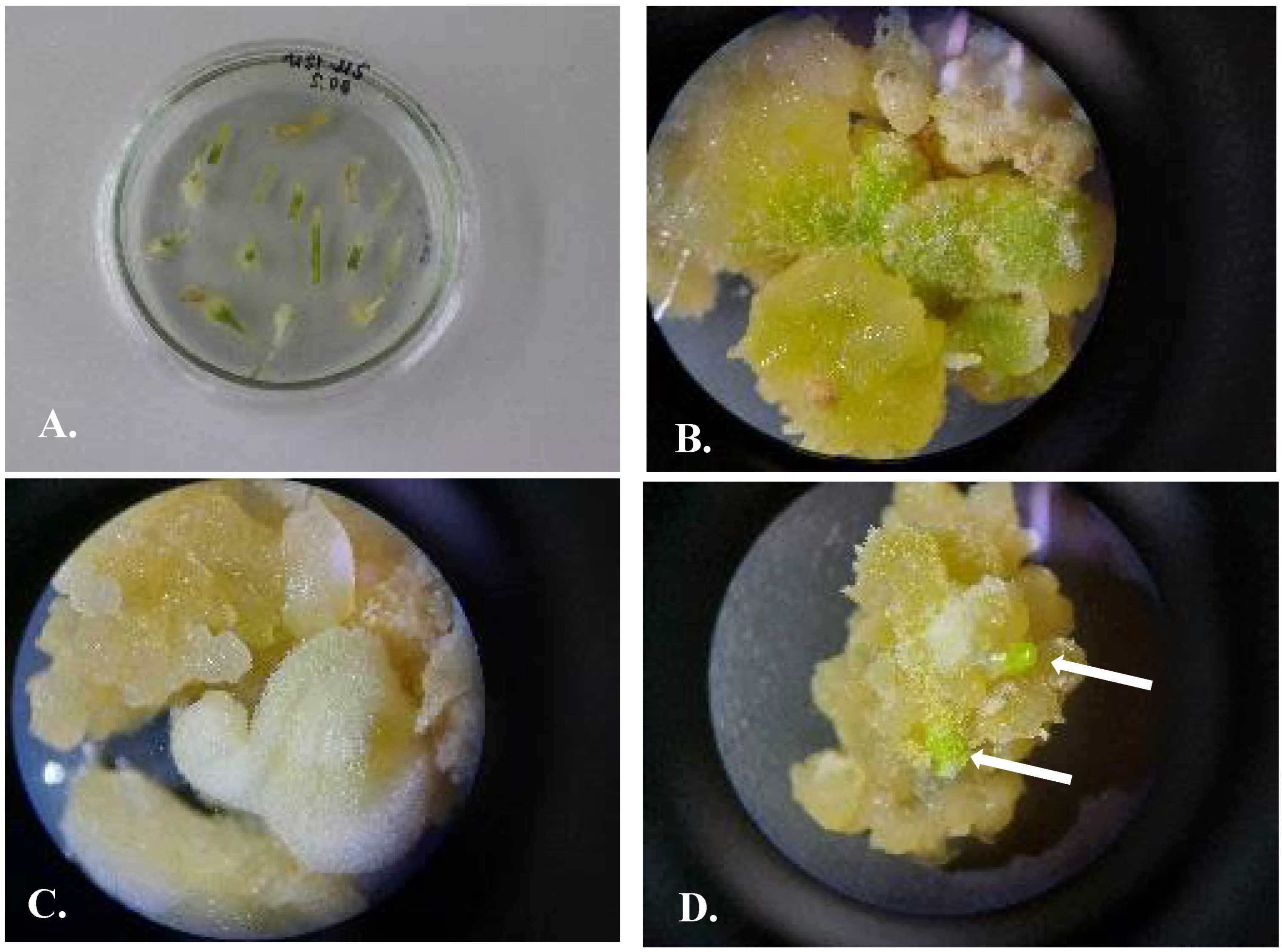
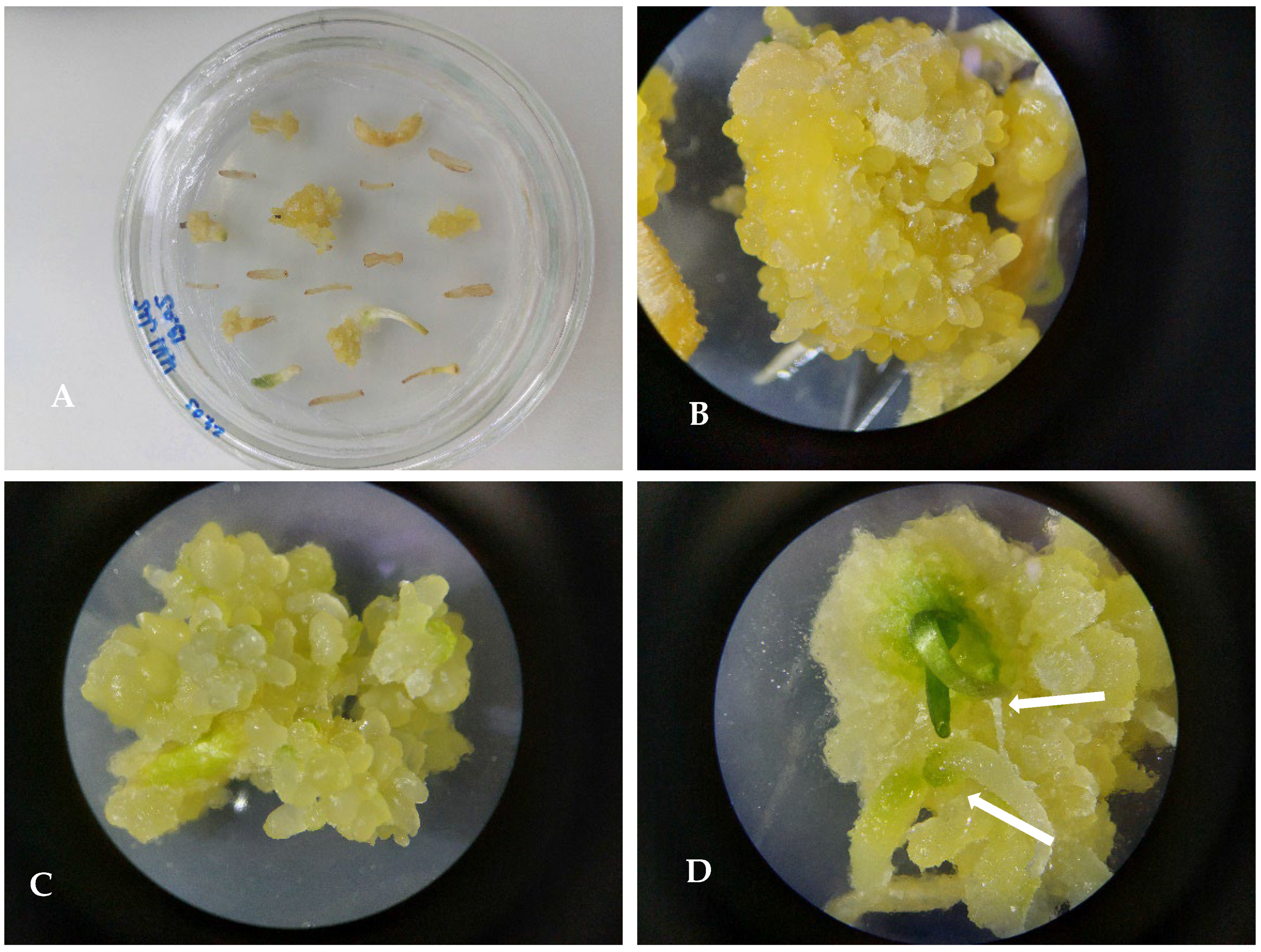
3.2. Using of Segments of Germinated Seeds as the Source of Explants
3.3. Selection of Nutrient Media
3.4. Protocol of Micropropagation of U. sewertzowii
3.4.1. Callusogenesis of U. sewertzowi
3.4.2. Direct Organogenesis
3.4.3. Indirect Organogenesis
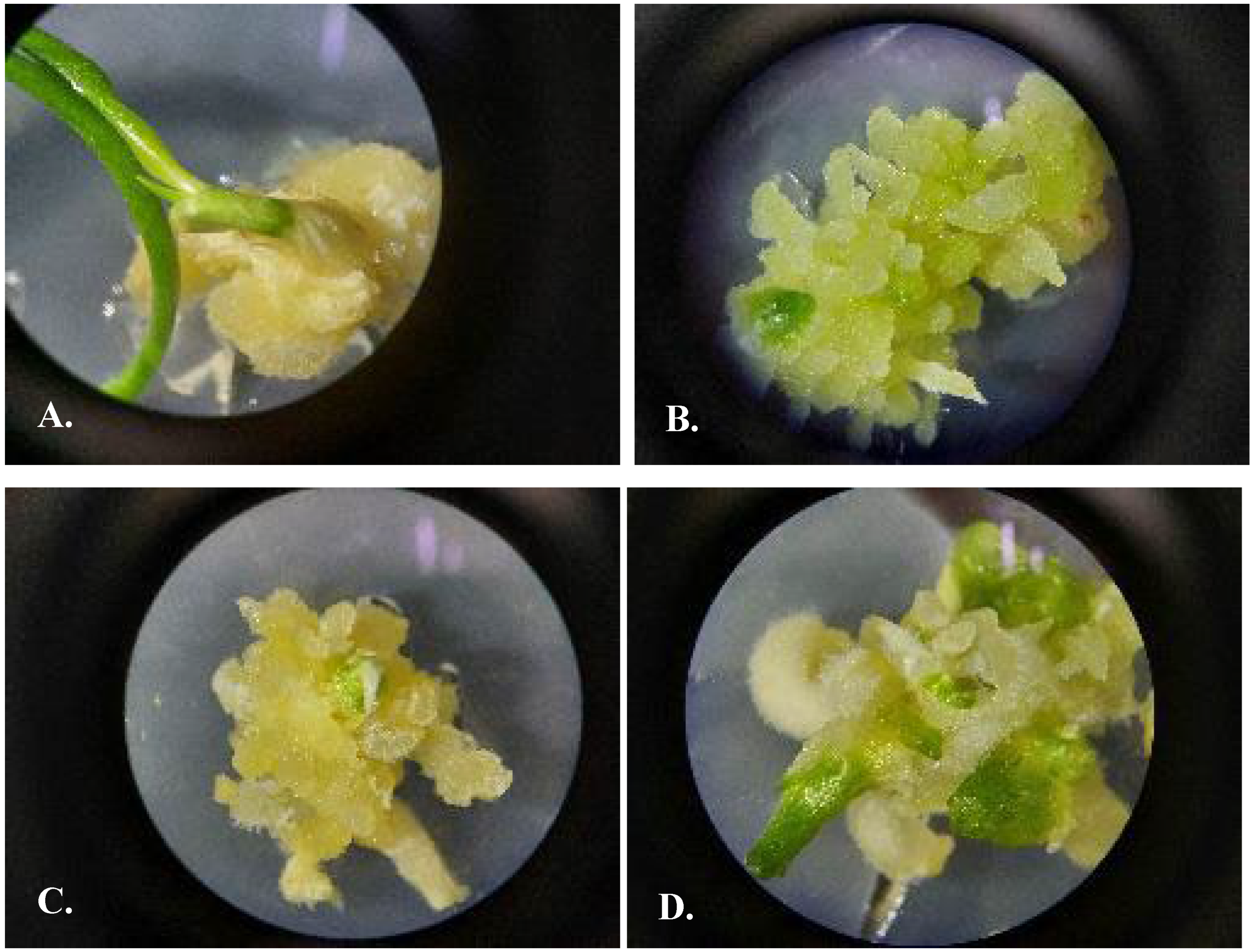
3.5. Protocol of Micropropagation of U. victoris
3.5.1. Indirect Organogenesis
3.5.2. DPPH and ABTS Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2.4D | 2,4-Dichlorophenoxyacetic acid |
| ABTS | 2,2-Azinobis (3-ethylbenzothiazoline)-6-sulphonic acid |
| atm. | Atmosphere, air pressure measurement at sea level at a temperature of 15 °C. |
| B5 | Nutrient media by Gamborg et al. (1968) [30] |
| BAC | Biologically active compounds |
| BAP | 6-Benzylaminopurine |
| DPPH | 2,2-Diphenyl-1-picrylhydryl |
| DW | Dry weight |
| IAA | Indole-3-acetic acid |
| IC50 | Half-maximal inhibitory concentration |
| Kin | Kinetin |
| l | Liter |
| M | Shortage from MS |
| masl | Meters above sea level |
| mg | Milligram |
| mM | Millimol |
| MS | Murashige and Skoog, nutrient media by Murashige and Skoog (1962) [28] |
| N6 | Nutrient media by Chu et al. (1975) [29] |
| NAA | 1-Naphthaleneacetic acid |
| nm | Nanomol |
| US | Ungernia sewertzowii |
| UV | Ungernia victoris |
| Vch | Vollosovich, nutrient media by Vollosovich (1979) [35] |
| WPM | Woody plant medium, nutrient media by Lloyd G. and McCown (1980) [31] |
| Terminology | |
| Rhizogenesis | the development of the roots. |
| Gemmogenesis | the development of the stems. |
| Gemmorhizogenesis | the development of stems and roots. |
| Callusogenesis | the formation of calli in damaged plant tissue. |
| Direct organogenesis | the production of direct buds or shoots from tissue with no intervening callus stage. |
| Indirect organogenesis | the process by which plant organs are derived from a calli mass in the explant. |
References
- Komarov, V.L. Flora of the USSR. V4: Liliiflorae and Microspermae; English 1968; Akademia Nauk SSSR: Leningrad, Russia, 1935; p. 568. [Google Scholar]
- Sennikov, A.N. (Ed.) Flora of Uzbekistan; Navruz: Tashkent, Uzbekistan, 2017; p. 173. Available online: https://www.researchgate.net/publication/329705602_Flora_of_Uzbekistan_Flora_Uzbekistana_tom_2_Izdatelstvo_Navruz_Taskent_2017 (accessed on 9 July 2024).
- Gammerman, A.F.; Kadaev, G.N.; Yacenko-Hmelevskiy, A.A. Medicinal Plants; High School: Moscow, Russia, 1990; 544p. (In Russian) [Google Scholar]
- Khojimatov, O.K.; Gafforov Yu Bussmann, R.W. (Eds.) Ethnobiology of Uzbekistan; Springer: Berlin/Heidelberg, Germany, 2023; p. 1513. [Google Scholar] [CrossRef]
- Khojimatov, O.K. Medicinal Plants of Uzbekistan. Tashkent.:Manaviyat. 2021. Available online: https://www.researchgate.net/profile/Olim-Khojimatov/publication/349703500_Lekarstvennye_rastenia_Uzbekistana/links/603da4e192851c077f0e8c14/Lekarstvennye-rastenia-Uzbekistana.pdf (accessed on 9 July 2024).
- Shah, M.; Ullah, M.A.; Drouet, S.; Younas, M.; Tungmunnithum, D.; Giglioli-Guivarc, H.N.; Hano CAbbasi, B.H. Interactive effects of light and melatonin on biosynthesis of silymarin and anti-inflammatory potential in callus cultures of Silybum marianum (L.) Gaertn. Molecules 2019, 24, 1207. [Google Scholar] [CrossRef] [PubMed]
- Sminova, L.S.; Abduazimov, X.A.; Yunusov, S.Y. About the alkaloids of Ungernia severtzovii. The structure of unsevin. Rep. ASc UzSSR 1964, 154, 171–173. (In Russian) [Google Scholar]
- Dahlgren, R.M.T.; Clifford, H.T.; Yeo, P.F. The Families of the Monocotyledons: Structure, Evolution, and Taxonomy; Springer: Berlin/Heidelberg, Germany, 1985; 520p, ISBN 3-540-13655-X. [Google Scholar]
- Cao, Z.; Yu, D.; Fu, S.; Zhang, G.; Pan, Y.; Bao, M.; Tu, J.; Shang, B.; Guo, P.; Yang, P.; et al. Lycorine hydrochloride selectively inhibits human ovarian cancer cell proliferation and tumor neovascularization with very low toxicity. Toxic. Lett. 2013, 18, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yuan, H.H.; Zhang, X.; Li, Y.P.; Shang, L.Q.; Yi, Z. Novel lycorine derivatives as anticancer agents: Synthesis and in vitro biological evaluation. Molecules 2014, 19, 2469–2480. [Google Scholar] [CrossRef] [PubMed]
- Vrijsen, R.; Vanden Berghe, D.A.; Vlietinck, A.J.; Boeyé, A. Lycorine: A eukaryotic termination inhibitor? J. Biol. Chem. 1986, 261, 505–507. [Google Scholar] [CrossRef] [PubMed]
- Diop, M.F.; Hehn, A.; Ptak, A.; Chrétien, F.; Doerper, S.; Gontier, E.; Bourgaud, F.; Henry, M.; Chapleur, Y.; Laurain-Mattar, D. Hairy root and tissue cultures of Leucojum aestivum L.—Relationships to galanthamine content. Phytochem. Rev. 2007, 6, 137–141. [Google Scholar] [CrossRef]
- Elisha, I.; Elgorashi, E.; Hussein, A.; Duncan, G.; Eloff, J. Acetlycholinesterase inhibitory effects of the bulb of Ammocharis coranica (Amaryllidaceae) and its active constituent lycorine. South Afr. J. Bot. 2013, 85, 44–47. [Google Scholar] [CrossRef]
- Jahn, S.; Seiwert, B.; Kretzing, S.; Abraham, G.; Regenthal, R.; Karst, U. Metabolic studies of the Amaryllidaceous alkaloids galanthamine and lycorine based on electrochemical simulation in addition to in vivo and in vitro models. Anal. Chim. Acta. 2012, 756, 60–72. [Google Scholar] [CrossRef]
- Red Book of Uzbekistan (Plants); Chinor ENK: Tashkent, Uzbekistan, 2019; p. 359, (In Russian). Available online: https://www.researchgate.net/publication/334913462_Red_Book_Uzbekistan (accessed on 9 July 2024).
- Nigmanova, S.R.; Mutalova, D.K.; Mahmudova, B.S.; Sadikov, A.Z.; Sagdullaev, S.S. The comparative characteristics of the air segments of Ungernia victoris. Univers. Tech. Sci. Electron. Sci. J. 2023, 11. (In Russian) [Google Scholar] [CrossRef]
- Hamidhodjaev, S.A.; Korotkova, E.E. Natural Resources of Ungernia victoris. Rep. ASC UzSSR. 1966, 23, 78–89. (In Russian) [Google Scholar]
- Ding, Y.; Qu, D.; Zhang, K.-M.; Cang, X.-X.; Kou, Z.-N.; Xiao, W.; Zhu, J.-B. Phytochemical and biological investigations of Amaryllidaceae alkaloids: A review. J. Asian Nat. Prod. Res. 2016, 19, 1–48. [Google Scholar] [CrossRef] [PubMed]
- Proskurnina, N.F.; Yakovleva, A.P. About alkaloids of Galanthus woronowii. Part II. About the extraction of new alkaloids. J. Common. Chem. 1952, 22, 1899. (In Russian) [Google Scholar]
- Novikova, I.Y.; Tulaganov, A.A. Structure of chemical compounds, methods of analysis, and process control. Physicochemical methods for the analysis of galanthamine. Pharm. Chem. J. 2002, 36, 623–627. [Google Scholar] [CrossRef]
- Heinrich, M.; Teoh, H.L. Galanthamine from snowdrop—The development of a modern drug against Alzheimer’s disease from local Caucasian knowledge. J. Ethnopharm. 2004, 92, 147–162. [Google Scholar] [CrossRef]
- Laurain-Mattar, D. Galanthamine content of bulbs and in vitro cultures of Leucojum aestivum L. Nat. Prod. Commun. 2006, 1, 475–479. [Google Scholar] [CrossRef]
- Pavlov, A.; Berkov, S.; Courot, E.; Gocheva, T.; Tuneva, D.; Pandova, B.; Georgiev, V.; Yanev, S.; Burrus, M.; Ilieva, M. Galanthamine production by Leucojum aestivum in vitro systems. Proc. Biochem. 2007, 42, 734–739. [Google Scholar] [CrossRef]
- Zarotsky, V.; Sramek, J.J.; Cutler, N.R. Galanthamine hydrobromide: An agent for Alzheimer’s disease. Am. J. Health Syst. Pharm. 2003, 60, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Botyrov, R.; Azizova, M. Technological scheme for obtaining lycorine hydrochloride on the basis of new and economically effective technology. Uzb. Farm. Rep. 2017, 1, 49–55. (In Uzbek) [Google Scholar]
- Information about the Scientific Achievements of the Institute of Plants Chemical Compounds of the Academy of Sciences of the Republic of Uzbekistan. Available online: https://uzicps.uz/en/preparations/medications (accessed on 9 July 2024).
- Chu, C.C. The N6 medium and its application to anther culture of cereal crops. Proc. Symp. Plant Tissue Cult. 1978, 24, 43–50. [Google Scholar]
- Murashige, I.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 159, 473–497. [Google Scholar] [CrossRef]
- Chu, C.; Wang, C.C.; Sun, C.; Hsu, C.; Yin, K.; Chu, C.C.; Fengqin, B. Establishment of an efficient medium for anther culture of rice through comparative experiments on the nitrogen sources. Sci. Sin. 1975, 18, 659–688. [Google Scholar]
- Gamborg, O.L.; Eveleigh, D.E. Culture methods and detection of glucanases in cultures of wheat and barley. Can. J. Bioch. 1968, 46, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, G.; McCown, B.H. Commercially-feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot-tip culture. Int. Plant Prop. Soc. Proc. 1980, 30, 421–427. [Google Scholar]
- Kunakh, V.A.; Mojilevska, L.P.; Muzika, V.I.; Kolonina, I.V. The Method of Microclonal Propagation of Ungernia victoris Vved. ex Artjuschenko. Patent UA (11) 85571 (13) C2, 10 February 2009. (In Ukrainian). [Google Scholar]
- Kunakh, V.A.; Mozhylevska, L.P.; Bublyk, O.M.; Kolonina, I.V.; Muzyka, V.I. Microclonal propagation of Ungernia victoris Vved. ex Artjuschenko. Biotech 2008, 1, 57–63. (In Ukrainian) [Google Scholar]
- Kunakh, V.A.; Mozhylevska, L.P.; Potapchuk, O.A.; Muzyka, V.I.; Kolonina, I.V. Obtaining Ungernia victoris tissue culture and its peculiarities upon growing in the nutrient media of different compositions. Russ. J. Biotech. 2007, 1, 14–21. (In Russian) [Google Scholar]
- Vollosovich, A.G.; Puchinina, G.M.; Nikolaeva, L.A. Optimization of macrosalt composition for tissue culture of Rauwolfia serpentina Benth. Rast. Resur. 1979, 15, 516–526. (In Russian) [Google Scholar]
- Velioglu, Y.S.; Mazza, G.; Gao, L.; Oomah, B.D. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
- Satcharoen, V.; McLean, N.J.; Kemp, S.C.; Camp, N.P.; Brown, R.C. Stereocontrolled synthesis of (-)-galanthamine. Org Lett. 2007, 9, 1867–1869. [Google Scholar] [CrossRef] [PubMed]
- Bublyk, O.M.; Andreev, I.O.; Spiridonova, K.V.; Kunakh, V.A. Genetic variability in regenerated plants of Ungernia victoris. Biol. Plant 2012, 56, 395–400. [Google Scholar] [CrossRef]
- Berkov, S.; Ivanov, I.; Georgiev, V.; Codina, C.; Pavlov, A. Galanthamine biosynthesis in plant in vitro systems. Eng. Life Sci. 2014, 14, 643–650. [Google Scholar] [CrossRef]
- Berkov, S.; Georgieva, L.; Kondakova, V.; Atanassov, A.; Viladomat, F.; Bastida, J.; Codina, C. Plant sources of galanthamine: Phytochemical and biotechnological aspects. Biotech. Biotech. Equip. 2009, 23, 1170–1176. [Google Scholar] [CrossRef]
- Berkov, S.; Pavlov, A.; Georgiev, V.; Bastida, J.; Burrus, M.; Ilieva, M.; Codina, C. Alkaloid synthesis and accumulation in Leucojum aestivum in vitro cultures. Nat. Prod. Commun. 2009, 4, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Diop, M.F.; Ptak, A.; Chretien, F.; Henry, M.; Chapleur, Y.; Laurain-Mattar, D. Production of alkaloids in plant cell and tissue cultures. Nat. Prod. Commun. 2006, 1, 165–174. [Google Scholar] [CrossRef]



| (A). Ungernia sewertzowii (three populations) | ||||
| Site of collection | Date of collection D/M/Y | Coordinates | ||
| Longitude | Latitude | Altitude, masl | ||
| Western Tien Shan, Great Chimgan mountain, Aksay and Katta Kok Say river banks | 8 April 2020 | 41.512175 | 70.050850 | 2434 |
| Western Tien Shan, Pskem range, Aksarsay river, vicinity to Nanay village | 25 June 2021 | 41.690587 | 70.234465 | 2832 |
| Tashkent region, Chatkal range, vicinity to Beldersay river | 7 June 2021 | 41.476467 | 69.975805 | 2275 |
| Gulkamsay | 7 June 2021 | 41.486467 | 69.875805 | 2375 |
| (B). Ungernia victoris (two populations) | ||||
| Site of collection | Date of collection D/M/Y | Coordinates | ||
| Longitude | Latitude | Altitude, masl | ||
| Pamir Alay, Gissar range, Saukbulak mountain, 10 km from Padang village | 29 May 2021 | 38.274326 | 67.290877 | 1838 |
| Pamir Alay, Gissar range, basin of Sangardak river, right bank, vicinity of Sangardak village | 31 May 2021 | 38.556908 | 67.502094 | 1384 |
| No. | Segment of Grown Seed | Ungernia sewertzowii | Ungernia victoris | ||
|---|---|---|---|---|---|
| The Number of Explants with a Developed Callus | The Number of Microbulbs on Each Explant | The Number of Explants with a Developed Callus | The Number of Microbulbs on Each Explant | ||
| 1 | Hypocotyl | 60–80% | 150–200 | 60–80% | 150–200 |
| 2 | Cotyledon | 50–60% | 100–120 | 50–60% | 100–120 |
| 3 | Radicle | 10–15% | 0 | 10–15% | 0 |
| No. | Combination of Phytohormones | Callusogenesis, % | The Number of Microbulbs on One Explant |
|---|---|---|---|
| 1 | M5 24D 0.5 mg/L + BAP 0.5 mg/L | 40 ± 0.2 | 100 |
| 2 | M56 2.4D 0.5 mg/L + Kin 0.5 mg/L | 95 ± 0.2 | 150 |
| 3 | M87 2.4D 0.5 mg/L + Zea 0.5 mg/L | 95 ± 0.2 | 150 |
| 4 | M40 IAA 2.0 mg/L + BAP 0.5 mg/L | 30 ± 0.16 | 3 |
| 5 | M44 IAA 0.5 mg/L + Kin 0.5 mg/L | 40 ± 0.1 | 4–5 |
| 6 | M68 IAA 0.5 mg/L + Kin 0.5 mg/L | 10 ± 0.1 | 3–5 |
| 7 | M32 IAA 0.5 mg/L + BAP 0.5 mg/L | 10 ± 0.1 | 4–5 |
| 8 | M17 IAA 0.5 mg/L + BAP 0.5 mg/L | 0 | 4–5 |
| Nutrient Media | Number of Microbulbs Per Explant in Direct Organogenesis | Number of Microbulbs Per Explant in Indirect Organogenesis |
|---|---|---|
| M44 NAA 0.5 mg/L + Kin 0.5 mg/L | 4–5 | 100–150 |
| M68 IAA 0.5 mg/L + Kin 0.5 mg/L | 3–5 | 100–150 |
| M32 NAA 0.5 mg/L + BAP 0.5 mg/L | 4–5 | 100–150 |
| M17 IAA 0.5 mg/L + BAP 0.5 mg/L | 4–5 | 100–150 |
| # | Ungernii sewertzowii | Ungernia victoris | Patent UA 85571 C2 (Ukraine) | |
|---|---|---|---|---|
| 1 | The source of explant | Segments of germinated seeds: cotyledon, hypocotyl, and radicle | Segments of germinated seeds: cotyledon, hypocotyl, and radicle | Scales of bulbs |
| Advantages: low degree of contamination, high degree of regeneration, year-round access to material | Advantages: low degree of contamination, high degree of regeneration, year-round access to material | Disadvantages: high degree of contamination, material availability only in spring and summer | ||
| 2 | Nutrient media | Nutrient media by Murashige and Skoog (1962) [28] | Nutrient media by Murashige and Skoog (1962) [28] | Nutrient media by Vollosovich (1979) [35] |
| 3 | Phytohormone content | Inducing in vitro culture: A. 2.4D 0.5 mg/L + BAP 0.5 mg/L B. 2.4D 0.5 mg/L +Kin 0.5 mg/L C. 2.4D 0.5 mg/L + Zea 0.5 mg/L Organogenesis (direct/indirect): M17 IAA 0.5 mg/L + BAP 0.5 mg/L M32 NAA 0.5 mg/L + BAP 0.5 mg/L M44 NAA 0.5 mg/L + Kin 0.5 mg/L, and M68 IAA 0.5 mg/L + Kin 0.5 mg/L | Inducing in vitro culture: 2,4D 0.5 mg/L + Kin 0.5 mg/L No direct organogenesis observed The indirect organogenesis were induced on MS: M17 IAA 0.5 mg/L + BAP 0.5 mg/L M32 NAA 0.5 mg/L + BAP 0.5 mg/L M44 NAA 0.5 mg/L + Kin 0.5 mg/L, and M68 IAA 0.5 mg/L + Kin 0.5 mg/L | Inducing in vitro culture: A. IAA 1.8–2.2 mg/L + Kin 0.8–1.2 mg/L Caseine hydrolysate 450–550 mg/L Mesoinosite 70–100 mg/L B. IAA 0.5 mg/L + Kin 0.02 mg/L Caseine hydrolysate 50 mg/L Mesoinosite 20 mg/L C. IAA 2.0 mg/L + Kin 0.02 mg/L Caseine hydrolysate 50 mg/L Mesoinosite 20 mg/L |
| 4 | Proliferation: The same media and phytohormones concentrations were used | Proleferation: The same media and phytohormones concentrations were used | Proliferation: IAA 2.0 mg/L + Kin 0.02 mg/L Caseine hydrolysate 40–60 mg/L Mesoinosite 10–30 mg/L | |
| 5 | Proliferation: Callusogenesis 2.4D 0.5 mg/L + Kin 0.5 mg/L Organogenesis: M17 IAA 0.5 mg/L + BAP 0.5 mg/L M32 NAA 0.5 mg/L + BAP 0.5 mg/L, and M68 IAA 0.5 mg/L + Kin 0.5 mg/L | - | Proliferation: IAA 0.2–0.5 mg/L + Kin 0.02–0.1 mg/L Caseine hydrolysate 40–60 mg/L Mesoinosite 15–25 mg/L | |
| 6 | Stimulation of rhizogenesis: NAA 2.5 mg/L + BAP 0.5 mg/L + TDZ 0.3 mg/L | Stimulation of rhizogenesis: NAA 2.5 mg/L + BAP 0.5 mg/L + TDZ 0.3 mg/L | ||
| 7 | Stimulation of rhizogenesis: NAA 0.5 mg/L | Stimulation of rhizogenesis: NAA 0.5 mg/L |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mustafina, F.U.; Juraeva, H.K.k.; Jamalova, D.N.k.; Hazratov, A.T.o.; Janabaeva, A.J.; Kim, H.J.; Na, C.S.; Lee, M.S.; Oh, Y.J.; Tojibaev, K.S.; et al. Conservation Potential Trough In Vitro Regeneration of Two Threatened Medicinal Plants Ungernia sewertzowii and U. victoris. Plants 2024, 13, 1966. https://doi.org/10.3390/plants13141966
Mustafina FU, Juraeva HKk, Jamalova DNk, Hazratov ATo, Janabaeva AJ, Kim HJ, Na CS, Lee MS, Oh YJ, Tojibaev KS, et al. Conservation Potential Trough In Vitro Regeneration of Two Threatened Medicinal Plants Ungernia sewertzowii and U. victoris. Plants. 2024; 13(14):1966. https://doi.org/10.3390/plants13141966
Chicago/Turabian StyleMustafina, Feruza Usmanovna, Hanifabonu Kobul kizi Juraeva, Dilafruz Nematilla kizi Jamalova, Abbos Tulkin ogli Hazratov, Ayimxan Jalgasbaevna Janabaeva, Hoe Jin Kim, Chae Sun Na, Min Sung Lee, Yu Jin Oh, Komiljon Sharobiddinovich Tojibaev, and et al. 2024. "Conservation Potential Trough In Vitro Regeneration of Two Threatened Medicinal Plants Ungernia sewertzowii and U. victoris" Plants 13, no. 14: 1966. https://doi.org/10.3390/plants13141966





