Effects of Water and Nitrogen on Growth, Rhizosphere Environment, and Microbial Community of Sophora alopecuroides: Their Interrelationship
Abstract
:1. Introduction
2. Results
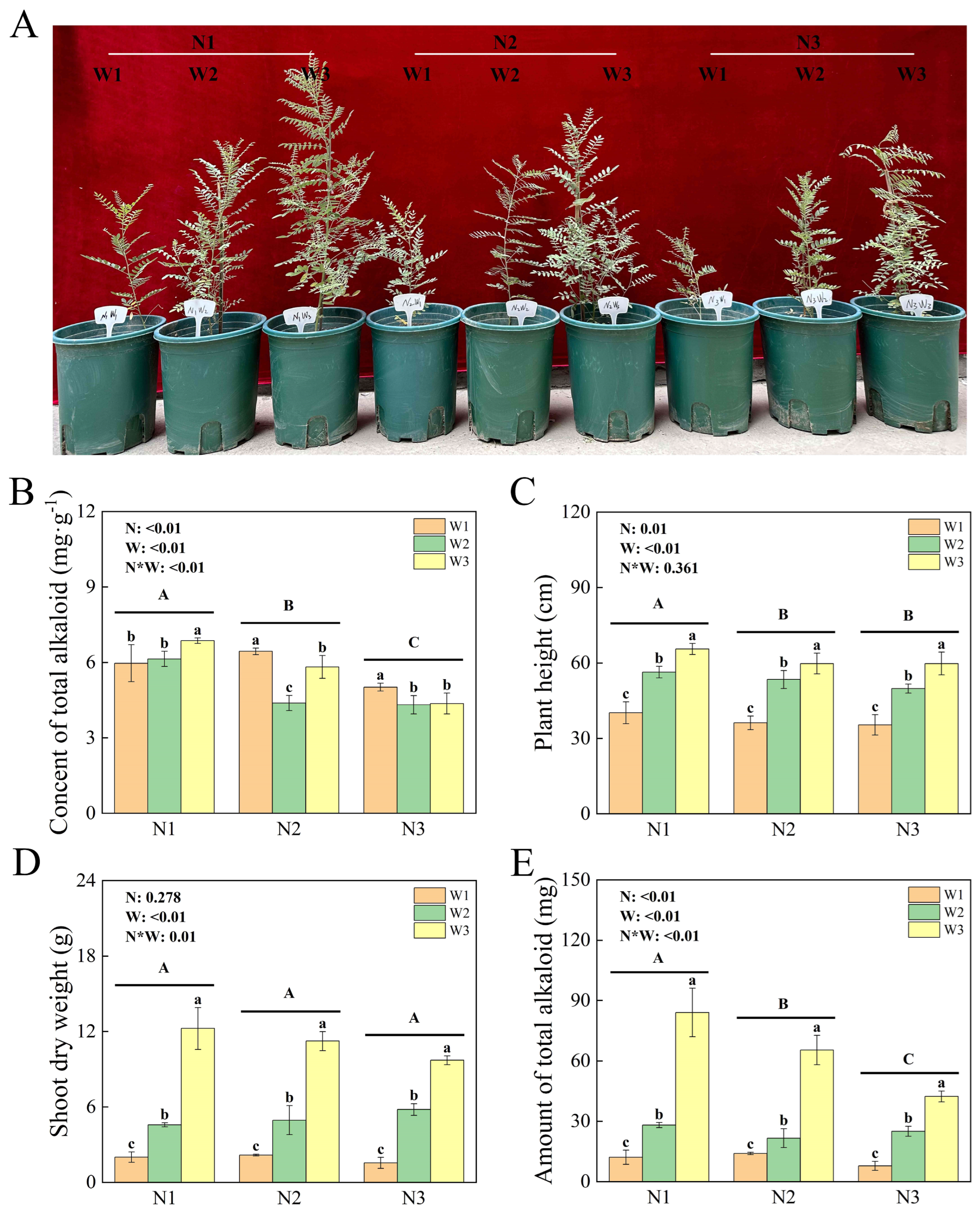
2.1. Plant Growth and Rhizospheric Soil Physicochemical Parameters
2.2. Overview of Sequencing Data
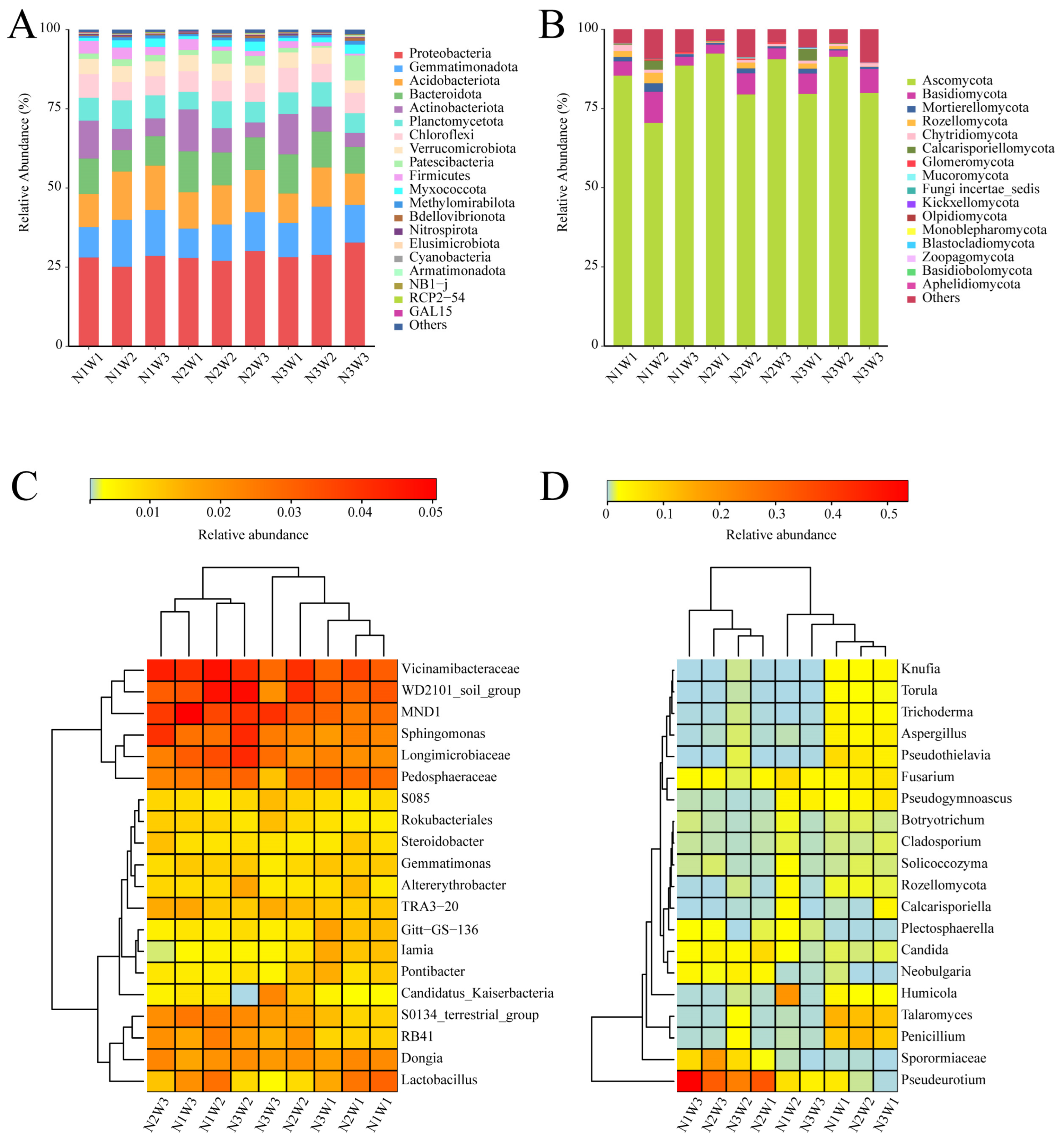
2.3. Composition of Bacterial and Fungal Communities
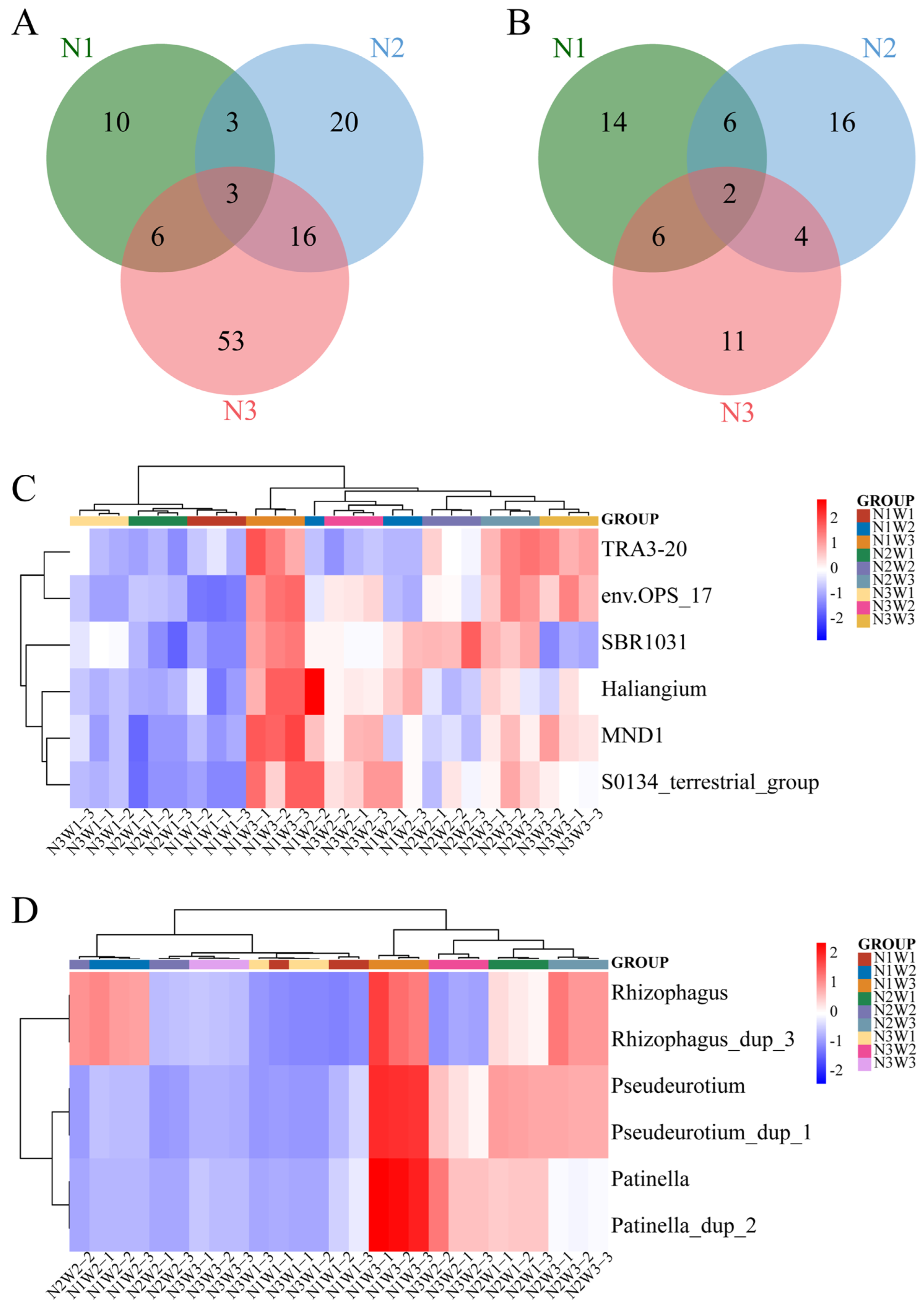
2.4. Alpha and Beta Diversity of Rhizosphere Microbiota
2.5. Analysis of Significantly Different Species
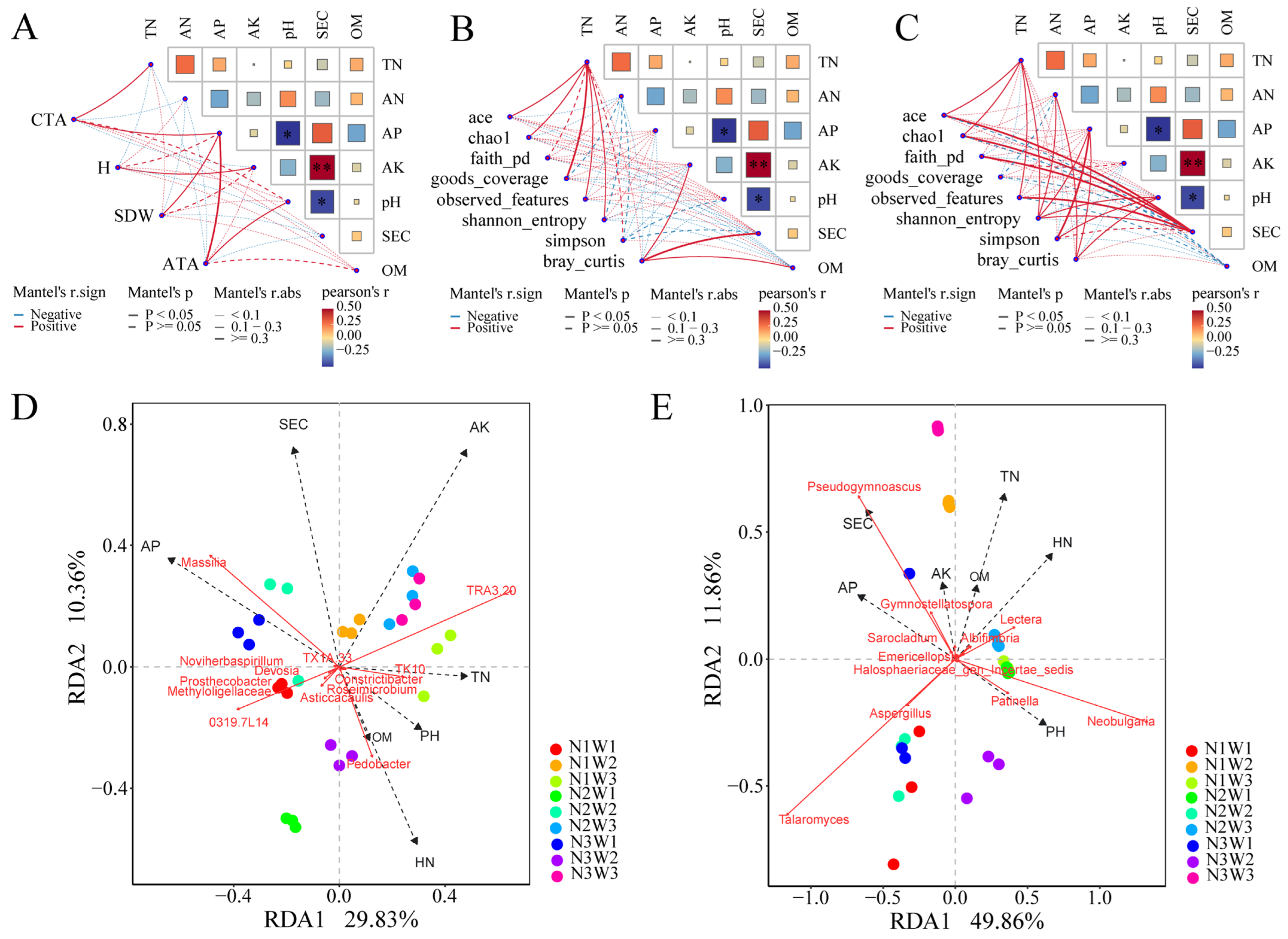
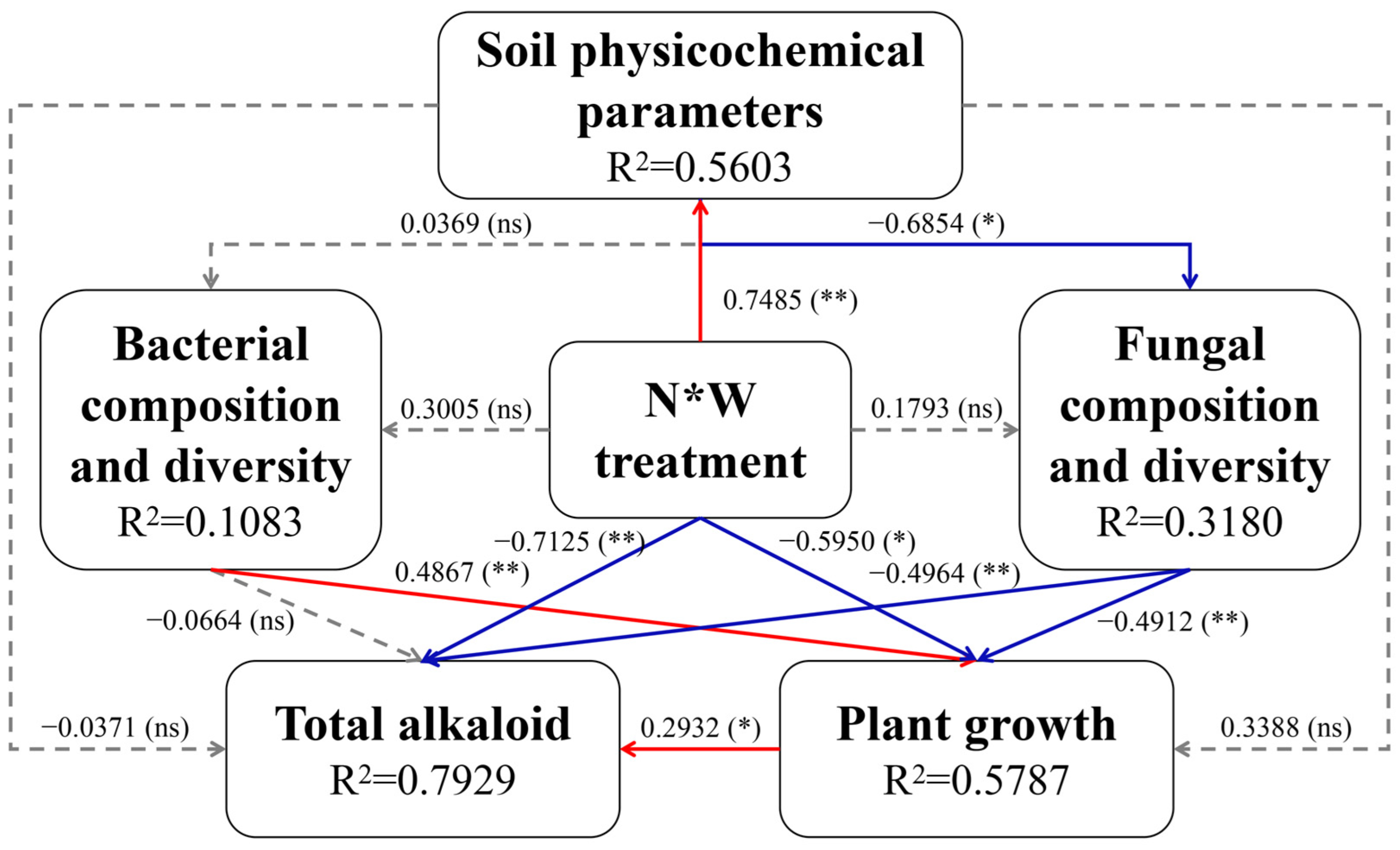
2.6. Mantel Tests and Integrated Analysis Based on PLS-PM
3. Discussion
4. Materials and Methods
4.1. Materials and Trial Design
4.2. Determination of Plant Growth Indices and Total Alkaloid Content
4.3. Collection of Rhizosphere Soil and Determination of Soil Physicochemical Parameters
4.4. Rhizosphere Microbial DNA Extraction and High-Throughput Sequencing
4.5. Alpha and Beta Diversity Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kwon, J.; Basnet, S.; Lee, J.W.; Seo, E.K.; Tsevegsuren, N.; Hwang, B.Y.; Lee, D. Chemical constituents isolated from the Mongolian medicinal plant Sophora alopecuroides L. and their inhibitory effects on LPS-induced nitric oxide production in RAW 264.7 macrophages. Bioorg. Med. Chem. Lett. 2015, 25, 3314–3318. [Google Scholar] [CrossRef] [PubMed]
- Pourahmad Jaktaji, R.; Mohammadi, P. Effect of total alkaloid extract of local Sophora alopecuroides on minimum inhibitory concentration and intracellular accumulation of ciprofloxacin, and acrA expression in highly resistant Escherichia coli clones. J. Glob. Antimicrob. Resist. 2018, 12, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Editorial Committee of Flora of China of Chinese Academy of Sciences. Flora of China; Science Press: Beijing, China, 2007. [Google Scholar]
- Zhao, L.; Deng, Z.; Yang, W.; Cao, Y.; Wang, E.; Wei, G. Diverse rhizobia associated with Sophora alopecuroides grown in different regions of Loess Plateau in China. Syst. Appl. Microbiol. 2010, 33, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Deng, X.; Gao, Q.; Wu, X.; Han, L.; Gao, X.; Zhao, S.; Chen, W.; Zhou, R.; Li, Z.; et al. Sophora alopecuroides L.: An ethnopharmacological, phytochemical, and pharmacological review. J. Ethnopharmacol. 2020, 248, 112172. [Google Scholar] [CrossRef] [PubMed]
- Li, J.C.; Dai, W.F.; Liu, D.; Zhang, Z.J.; Jiang, M.Y.; Rao, K.R.; Li, R.T.; Li, H.M. Quinolizidine alkaloids from Sophora alopecuroides with anti-inflammatory and anti-tumor properties. Bioorg. Chem. 2021, 110, 104781. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, A.; Yang, Q.; Li, J.; Wang, L.; Liu, X.; Huang, Y.; Liu, L. Beneficial effect of alkaloids from Sophora alopecuroides L. on CUMS-induced depression model mice via modulating gut microbiota. Front. Cell. Infect. Microbiol. 2021, 11, 665159. [Google Scholar] [CrossRef] [PubMed]
- Ur Rashid, H.; Rasool, S.; Ali, Y.; Khan, K.; Martines, M.A.U. Anti-cancer potential of sophoridine and its derivatives: Recent progress and future perspectives. Bioorg. Chem. 2020, 99, 103863. [Google Scholar] [CrossRef] [PubMed]
- Hazrati, S.; Khurizadeh, S.; Sadeghi, A.R. Application of zeolite improves water and nitrogen use efficiency while increasing essential oil yield and quality of Salvia officinalis under water-deficit stress. Saudi J. Biol. Sci. 2022, 29, 1707–1716. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Effah, Z.; Yan, B.; Gao, Y.; Wu, B.; Wang, Y.; Xu, P.; Wang, H.; Zhao, B.; Wang, Y. Water and nitrogen coupling increased the water-nitrogen use efficiency of Oilseed Flax. Plants 2022, 12, 51. [Google Scholar] [CrossRef]
- Wang, Y.; He, X.; Li, F.; Deng, H.; Wang, Z.; Huang, C.; Han, Y.; Ba, Y.; Lei, L.; Zhang, C. Effects of water and nitrogen coupling on the photosynthetic characteristics, yield, and quality of Isatis indigotica. Sci. Rep. 2021, 11, 17356. [Google Scholar] [CrossRef]
- Li, X.L.; Cheng, T.T.; Fang, H.X.; Wang, J.F.; Xie, Y. Effects of water and nitrogen fertilizer coupling on yield and quality of Chuzhou Chrysanthemum Morifolium. Bull. Soil Water Conserv. 2014, 34, 111–115. [Google Scholar] [CrossRef]
- Taha, H.V.; Hamid, R.V.; Reza, B.; Mohammad, J.S. Effect of irrigation and nitrogen fertilizer on grain yield and essential oil percentage of medicinal plant Ajowan. Int. J. Agron. Plant Prod. 2013, 4, 1013–1022. [Google Scholar]
- Guo, X.; Li, S.; Wang, D.; Huang, Z.; Sarwar, N.; Mubeen, K.; Shakeel, M.; Hussain, M. Effects of water and fertilizer coupling on the physiological characteristics and growth of rabbiteye blueberry. PLoS ONE 2021, 16, e0254013. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.; Garbeva, P.; Raaijmakers, J.M. The rhizosphere microbiome: Significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 2013, 37, 634–663. [Google Scholar] [CrossRef] [PubMed]
- Qu, Q.; Zhang, Z.Y.; Peijnenburg, W.J.G.M.; Liu, W.Y.; Lu, T.; Hu, B.L.; Chen, J.M.; Chen, J.; Lin, Z.F.; Qian, H.F. Rhizosphere microbiome assembly and its impact on plant growth. J. Agric. Food Chem. 2020, 68, 5024–5038. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yang, X.; Tong, B.; Wang, D.; Tian, X.; Liu, J.; Chen, J.; Xiao, X.; Wang, S. Rhizobacterial compositions and their relationships with soil properties and medicinal bioactive ingredients in Cinnamomum migao. Front. Microbiol. 2023, 14, 1078886. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zuo, J.; Zhang, H.; Zu, M.; Liu, S. The Chinese medicinal plants rhizosphere: Metabolites, microorganisms, and interaction. Rhizosphere 2022, 22, 100540. [Google Scholar] [CrossRef]
- He, C.; Wang, W.; Hou, J. Plant growth and soil microbial impacts of enhancing licorice with inoculating dark septate endophytes under drought stress. Front. Microbiol. 2019, 10, 2277. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Wan, X.F.; Wang, S.; Kang, C.Z.; Zhang, W.J.; Lyu, C.G.; Sun, J.H.; Guo, L.P. Effects and mechanisms of nitrogen fertilizers on soil and tritrophic interactions in Chinese medicinal plants ecosystem. Chin. J. Chin. Mater. Med. 2021, 46, 1893–1900. [Google Scholar] [CrossRef]
- Ji, Y.; Lin, H.; Chen, Y.; Zhang, S. Nitrogen nutrition effects on character, biomass and alkaloid accumulation of Sophora alopecuroides. Acta Pratacult. Sin. 2008, 17, 40–46. [Google Scholar] [CrossRef]
- Yan, F.; Zhu, Y.; Zhao, Y.; Wang, Y.; Li, J.; Wang, Q.; Liu, Y. De novo transcriptome sequencing and analysis of salt-, alkali-, and drought-responsive genes in Sophora alopecuroides. BMC Genom. 2020, 21, 423. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Shi, C.; Wang, L.; Han, X.; Zhu, Y.; Liu, J.; Yang, X. Functional trait responses of Sophora alopecuroides L. seedlings to diverse environmental stresses in the desert steppe of Ningxia, China. Plants 2023, 13, 69. [Google Scholar] [CrossRef] [PubMed]
- Ju, X.T.; Xing, G.X.; Chen, X.P.; Zhang, S.L.; Zhang, L.J.; Liu, X.J.; Cui, Z.L.; Yin, B.; Christie, P.; Zhu, Z.L.; et al. Reducing environmental risk by improving N management in intensive Chinese agricultural systems. Proc. Natl. Acad. Sci. USA 2009, 106, 3041–3046. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.K.; Tomar, G.S.; Dwivedi, S.K. Effect of irrigation scheduling and nitrogen levels on growth, yield and water productivity of linseed (Linum usitatissimum L.) under Vertisols. J. Appl. Nat. Sci. 2017, 9, 698–705. [Google Scholar] [CrossRef]
- Saud, S.; Fahad, S.; Yajun, C.; Ihsan, M.Z.; Hammad, H.M.; Nasim, W.; Jr, A.; Arif, M.; Alharby, H. Effects of nitrogen supply on water stress and recovery mechanisms in Kentucky Bluegrass plants. Front. Plant Sci. 2017, 8, 983. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Bai, Y.; Zhang, Z.; Zhang, Y.; Jiang, Y.; Wang, S.; Wang, Y.; Sun, Z. Transcriptomics and metabolomics reveal the primary and secondary metabolism changes in Glycyrrhiza uralensis with different forms of nitrogen utilization. Front. Plant Sci. 2023, 14, 1229253. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gu, Y.; Zhang, W.; Yang, J. Research advances in the effects of water and nitrogen and their interaction on the grain yield, water and nitrogen use efficiencies of wheat. Crops 2023, 39, 7–15. [Google Scholar] [CrossRef]
- Marklein, A.R.; Houlton, B.Z. Nitrogen inputs accelerate phosphorus cycling rates across a wide variety of terrestrial ecosystems. New Phytol. 2021, 193, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Singh, B. Are nitrogen fertilizers deleterious to soil health? Agronomy 2018, 8, 48. [Google Scholar] [CrossRef]
- Peiffer, J.A.; Spor, A.; Koren, O.; Jin, Z.; Tringe, S.G.; Dangl, J.L.; Buckler, E.S.; Ley, R.E. Diversity and heritability of the maize rhizosphere microbiome under field conditions. Proc. Natl. Acad. Sci. USA 2013, 110, 6548–6553. [Google Scholar] [CrossRef]
- Edwards, J.; Johnson, C.; Santos-Medellín, C.; Lurie, E.; Podishetty, N.K.; Bhatnagar, S.; Eisen, J.A.; Sundaresan, V. Structure, variation, and assembly of the root-associated microbiomes of rice. Proc. Natl. Acad. Sci. USA 2015, 112, E911–E920. [Google Scholar] [CrossRef] [PubMed]
- An, Z.; Guo, F.; Chen, Y.; Bai, G.; Chen, Z. Rhizosphere bacterial and fungal communities during the growth of Angelica sinensis seedlings cultivated in an Alpine uncultivated meadow soil. PeerJ 2020, 8, e8541. [Google Scholar] [CrossRef] [PubMed]
- Lauber, C.L.; Strickland, M.S.; Bradford, M.A.; Fierer, N. The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biol. Biochem. 2008, 40, 2407–2415. [Google Scholar] [CrossRef]
- Zhou, J.; Ning, D. Stochastic community assembly: Does it matter in microbial ecology? Microbiol. Mol. Biol. Rev. 2017, 81, e00002-17. [Google Scholar] [CrossRef] [PubMed]
- Paungfoo-Lonhienne, C.; Visser, J.; Lonhienne, T.G.A.; Schmidt, S. Past, present and future of organic nutrients. Plant Soil 2012, 359, 1–18. [Google Scholar] [CrossRef]
- Kolb, S. The quest for atmospheric methane oxidizers in forest soils. Environ. Microbiol. Rep. 2009, 1, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Ofek, M.; Hadar, Y.; Minz, D. Ecology of root colonizing Massilia (Oxalobacteraceae). PLoS ONE 2012, 7, e40117. [Google Scholar] [CrossRef] [PubMed]
- Rivas, R.; Willems, A.; Subba-Rao, N.S.; Mateos, P.F.; Dazzo, F.B.; Kroppenstedt, R.M.; Martínez-Molina, E.; Gillis, M.; Velázquez, E. Description of Devosia neptuniae sp. nov. that nodulates and fixes nitrogen in symbiosis with Neptunia natans, an aquatic legume from India. Syst. Appl. Microbiol. 2003, 26, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Abraham, W.R.; Strömpl, C.; Meyer, H.; Lindholst, S.; Moore, E.R.; Christ, R.; Vancanneyt, M.; Tindall, B.J.; Bennasar, A.; Smit, J.; et al. Phylogeny and polyphasic taxonomy of Caulobacter species. Proposal of Maricaulis gen. nov. with Maricaulis maris (Poindexter) comb. nov. as the type species, and emended description of the genera Brevundimonas and Caulobacter. Int. J. Syst. Bacteriol. 1999, 49, 1053–1073. [Google Scholar] [CrossRef] [PubMed]
- Sundararaman, A.; Srinivasan, S.; Lee, S.S. Noviherbaspirillum humi sp. nov., isolated from soil. Anton. Leeuw. 2016, 109, 697–704. [Google Scholar] [CrossRef]
- Samson, R.A.; Visagie, C.M.; Houbraken, J.; Hong, S.B.; Hubka, V.; Klaassen, C.H.; Perrone, G.; Seifert, K.A.; Susca, A.; Tanney, J.B.; et al. Phylogeny, identification and nomenclature of the genus Aspergillus. Stud. Mycol. 2014, 78, 141–173. [Google Scholar] [CrossRef] [PubMed]
- Fierer, N.; Lauber, C.L.; Ramirez, K.S.; Zaneveld, J.; Bradford, M.A.; Knight, R. Comparative metagenomic, phylogenetic and physiological analyses of soil microbial communities across nitrogen gradients. ISME J. 2012, 6, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Y.; Yong, X.; Wu, X.; Jia, H.; Wong, J.W.C.; Wu, H.; Zhou, J. Odor emission and microbial community succession during biogas residue composting covered with a molecular membrane. Bioresour. Technol. 2020, 297, 122518. [Google Scholar] [CrossRef] [PubMed]
- Wink, M. Plant secondary metabolism: Diversity, function and its evolution. Nat. Prod. Commun. 2008, 3, 1205–1216. [Google Scholar] [CrossRef]
- Barra-Bucarei, L.; Ortiz, J.; Castro, J.F. Facultative Fungal Endophytes and Their Potential for the Development of Sustainable Agriculture; Woodhead Publishing: Cambridge, UK, 2021. [Google Scholar]
- Zhang, Y.; Han, T.; Ming, Q.; Wu, L.; Rahman, K.; Qin, L. Alkaloids produced by endophytic fungi: A review. Nat. Prod. Commun. 2012, 7, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.L.; Lu, B.Y.; Gong, Z.H.; Shah, S.N.M. Effects of drought stress on capsanthin during fruit development and ripening in pepper (Capsicum annuum L.). Agric. Water Manag. 2014, 137, 46–51. [Google Scholar] [CrossRef]
- Beckers, B.; op de Beeck, M.; Weyens, N.; Boerjan, W.; Vangronsveld, J. Structural variability and niche differentiation in the rhizosphere and endosphere bacterial microbiome of field-grown poplar trees. Microbiome 2017, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Bao, S. Soil and Agricultural Chemistry Analysis (III); China Agricultural Press: Beijing, China, 2000. [Google Scholar]
- Zhou, S.; Geng, B.; Li, M.; Li, Z.; Liu, X.; Guo, H. Comprehensive analysis of environmental factors mediated microbial community succession in nitrogen conversion and utilization of ex situ fermentation system. Sci. Total Environ. 2021, 769, 145219. [Google Scholar] [CrossRef] [PubMed]
- Masella, A.P.; Bartram, A.K.; Truszkowski, J.M.; Brown, D.G.; Neufeld, J.D. PANDAseq: Paired-end assembler for Illumina sequences. BMC Bioinform. 2012, 13, 31. [Google Scholar] [CrossRef]
- Schmieder, R.; Edwards, R. Quality control and preprocessing of metagenomic datasets. Bioinformatics 2011, 27, 863–864. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Tenenhaus, M.; Esposito Vinzi, V.; Chatelin, Y.M.; Lauro, C. PLS path modeling. Comput. Stat. Data Anal. 2005, 48, 159–205. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Niu, P.; Gao, Y.; Rong, W.; Luo, C.; Zhang, X.; Jiang, P.; Wang, M.; Chu, G. Effects of Water and Nitrogen on Growth, Rhizosphere Environment, and Microbial Community of Sophora alopecuroides: Their Interrelationship. Plants 2024, 13, 1970. https://doi.org/10.3390/plants13141970
Huang X, Niu P, Gao Y, Rong W, Luo C, Zhang X, Jiang P, Wang M, Chu G. Effects of Water and Nitrogen on Growth, Rhizosphere Environment, and Microbial Community of Sophora alopecuroides: Their Interrelationship. Plants. 2024; 13(14):1970. https://doi.org/10.3390/plants13141970
Chicago/Turabian StyleHuang, Xiang, Panxin Niu, Yude Gao, Wenwen Rong, Cunkai Luo, Xingxin Zhang, Ping Jiang, Mei Wang, and Guangming Chu. 2024. "Effects of Water and Nitrogen on Growth, Rhizosphere Environment, and Microbial Community of Sophora alopecuroides: Their Interrelationship" Plants 13, no. 14: 1970. https://doi.org/10.3390/plants13141970




