Progress on Salt Tolerance in Brassica napus
Abstract
1. Introduction
2. Molecular Mechanism of Salt Stress on Brassica napus
3. Phenotypic and Physiological Indices of Brassica napus under Salt Stress
3.1. Phenotypic Indices
3.2. Physiological Indices
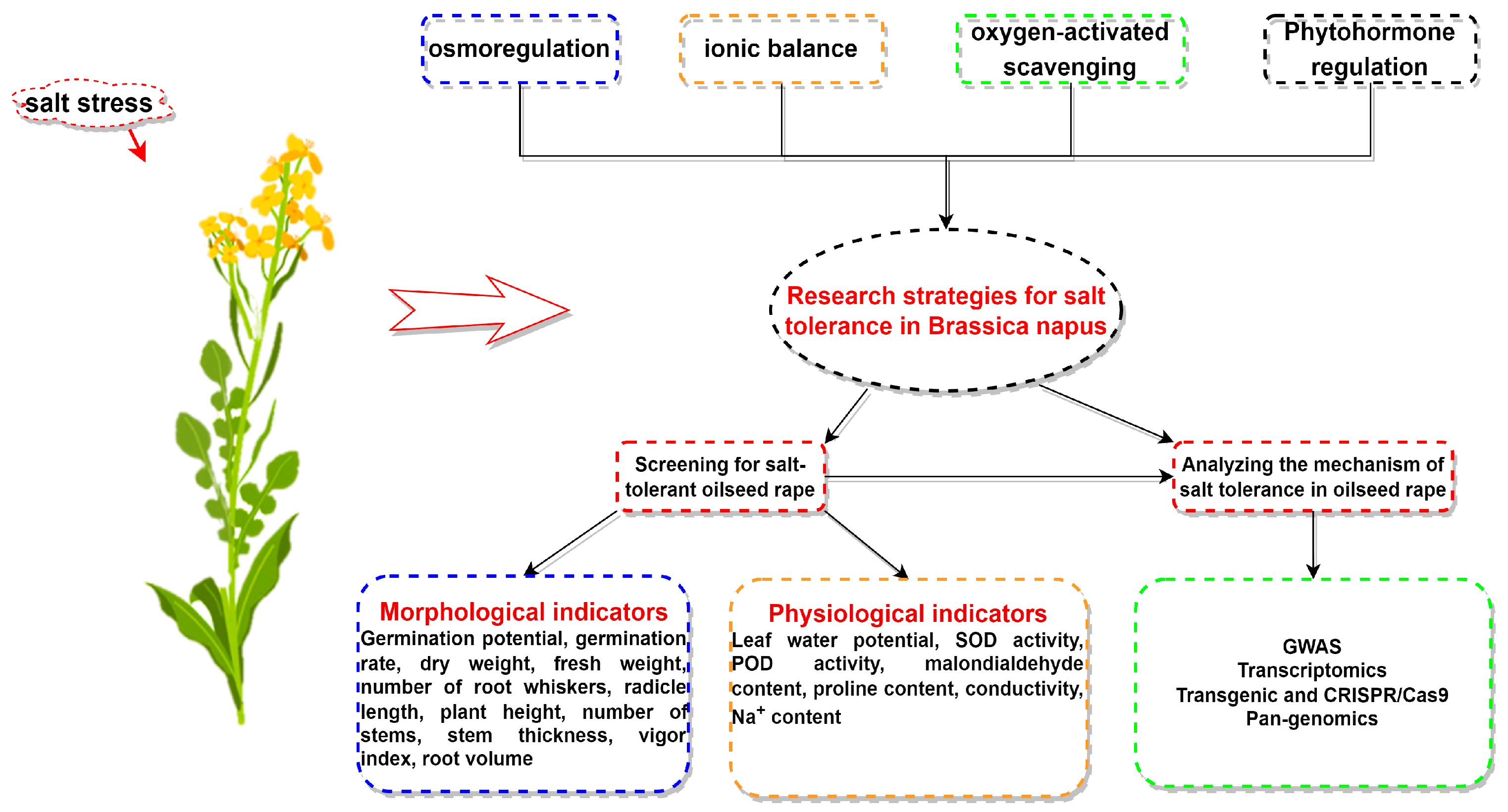
4. Research Strategy on Salt Tolerance of Brassica napus
4.1. Screening and Evaluation of Salt-Tolerant Germplasm
4.2. Mining Salt Tolerance Genes by Genetics and Genomics in Brassica napus
4.2.1. GWAS and QTL
4.2.2. Transcriptomics
4.2.3. Genetic Modification
4.2.4. Pan-Genomics
5. Exogenous Substances Promote Growth under Salt Stress in Brassica napus
6. Conclusions
7. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.M.; Horie, T. Genomics, Physiology, and molecular breeding approaches for improving salt tolerance. Annu. Rev. Plant Biol. 2017, 68, 405–434. [Google Scholar] [CrossRef] [PubMed]
- Wang, H. New-demand oriented oilseed rape industry developing strategy. Chin. J. Oil Crop Sci. 2018, 40, 613–617. [Google Scholar]
- Wang, W.; Ge, Z.; Yang, H.; Yin, F.; Huang, T.; Kuai, J.; Wang, J.; Wang, B.; Zhou, G.; Fu, T. Daptation of feed crops to saline-alkali soil Stress and effect of improving saline-alkali soil. Acta Agron. Sin. 2022, 48, 1451–1462. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Q.; Wang, H.; Tao, S.; Cao, H.; Shi, Y.; Bakirov, A.; Xu, A.; Huang, Z. Discovery of common loci and candidate genes for controlling salt-alkali tolerance and yield-related traits in Brassica napus L. Plant Cell Rep. 2023, 42, 1039–1057. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Passioura, J.B.; Colmer, T.D.; Byrt, C.S. Osmotic adjustment and energy limitations to plant growth in saline soil. New Phytol. 2020, 225, 1091–1096. [Google Scholar] [CrossRef]
- Xia, D. Research progress and breeding of plant drought resistance physiology. Mod. Hortic. 2020, 43, 221–222. [Google Scholar]
- Adem, G.D.; Roy, S.J.; Zhou, M.; Bowman, J.P.; Shabala, S. Evaluating contribution of ionic, osmotic and oxidative stress components towards salinity tolerance in barley. BMC Plant Biol. 2014, 14, 113. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Li, N.; Fan, Y.; Dong, B.; Song, Z.; Cao, H.; Du, T.; Liu, T.; Qi, M.; Niu, L. Transcriptome analysis reveals abscisic acid enhancing drought resistance by regulating genes related to flavonoid metabolism in pigeon pea. Environ. Exp. Bot. 2021, 191, 104627. [Google Scholar] [CrossRef]
- Yu, Z.; Duan, X.; Luo, L.; Dai, S.; Ding, Z.; Xia, G. How plant hormones mediate salt stress responses. Trends Plant Sci. 2020, 25, 1117–1130. [Google Scholar] [CrossRef]
- Hasan, M.M.; Liu, X.-D.; Waseem, M.; Guang-Qian, Y.; Alabdallah, N.M.; Jahan, M.S.; Fang, X.-W. ABA activated SnRK2 kinases: An emerging role in plant growth and physiology. Plant Signal. Behav. 2022, 17, 2071024. [Google Scholar] [CrossRef]
- Amjad, M.; Akhtar, J.; Anwar-ul-Haq, M.; Yang, A.; Akhtar, S.S.; Jacobsen, S.E. Integrating role of ethylene and ABA in tomato plants adaptation to salt stress. Sci. Hortic. 2014, 172, 109–116. [Google Scholar] [CrossRef]
- Luo, J.; Tang, S.; Mei, F.; Peng, X.; Li, J.; Li, X.; Yan, X.; Zeng, X.; Liu, F.; Wu, Y. BnSIP1-1, a trihelix family gene, mediates abiotic stress tolerance and ABA signaling in Brassica napus. Front. Plant Sci. 2017, 8, 44. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, Q.; Liu, M.; Zhou, H.; Ma, C.; Wang, P. Regulation of plant responses to salt stress. Int. J. Mol. Sci. 2021, 22, 4609. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Yin, M. Effects of Salt Stress on the growth and some physiological and biochemical indices of hybrid rice plantlets in Vitro. Hybrid. Rice 2008, 23, 5. [Google Scholar]
- Yang, Y.; Guo, Y. Unraveling salt stress signaling in plants: Salt stress signaling. J. Integr. Plant Biol. 2018, 60, 796–804. [Google Scholar] [CrossRef]
- Köster, P.; Wallrad, L.; Edel, K.H.; Faisal, M.; Alatar, A.A.; Kudla, J. The battle of two ions: Ca2+ signalling against Na+ stress. Plant Biol. 2019, 21, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Ye, J.; Yang, Y.; Lin, H.; Yue, L.; Luo, J.; Long, Y.; Fu, H.; Liu, X.; Zhang, Y. The SOS2-SCaBP8 complex generates and fine-tunes an AtANN4-dependent calcium signature under salt stress. Dev. Cell 2019, 48, 697–709. [Google Scholar] [CrossRef] [PubMed]
- Yarra, R. The wheat NHX gene family: Potential role in improving salinity stress tolerance of plants. Plant Gene 2019, 18, 100178. [Google Scholar] [CrossRef]
- Yue, C.; Han, L.; Sun, S.; Chen, J.; Feng, Y.; Huang, J.; Zhou, T.; Hua, Y. Genome-wide identification of the cation/proton antiporter (CPA) gene family and functional characterization of the key member BnaA05. NHX2 in allotetraploid rapeseed. Gene 2024, 894, 148025. [Google Scholar] [CrossRef]
- Li, H.; Xu, G.; Yang, C.; Yang, L.; Liang, Z. Genome-wide identification and expression analysis of HKT transcription factor under salt stress in nine plant species. Ecotoxicol. Environ. Saf. 2019, 171, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Yue, C.-P.; Liu, Y.; Zhang, T.-Y.; Huang, J.-Y.; Hua, Y.-P. Multiomics reveal pivotal roles of sodium translocation and compartmentation in regulating salinity resistance in allotetraploid rapeseed. J. Exp. Bot. 2021, 72, 5687–5708. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Guo, Y. Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytol. 2018, 217, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Song, H.; Zhou, T.; Yue, C.; Feng, Y.; Huang, J.; Hua, Y. Identification of the core member of the HAKs family and primary analysis on their functions in allotetraploid rapeseed (Brassica napus L.). J. Plant Nutr. Fertil. 2024, 30, 515–537. [Google Scholar]
- Qi, J.; Song, C.-P.; Wang, B.; Zhou, J.; Kangasjärvi, J.; Zhu, J.K.; Gong, Z. Reactive oxygen species signaling and stomatal movement in plant responses to drought stress and pathogen attack: ROS signaling and stomatal movement. J. Integr. Plant Biol. 2018, 60, 805–826. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006, 141, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, B.; Rehman, A.; Tanveer, M.; Wang, L.; Park, S.K.; Ali, A. Salt stress in brassica: Effects, tolerance mechanisms, and management. J. Plant Growth Regul. 2022, 41, 781–795. [Google Scholar] [CrossRef]
- Shah, O.U.; Khan, L.U.; Basharat, S.; Zhou, L.; Ikram, M.; Peng, J.; Khan, W.U.; Liu, P.; Waseem, M. Genome-Wide Investigation of Class III Peroxidase Genes in Brassica napus Reveals Their Responsiveness to Abiotic Stresses. Plants 2024, 13, 942. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Su, W.; Gao, A.; Mehmood, S.S.; Hussain, M.A.; Nie, W.; Lv, Y.; Zou, X.; Zhang, X. Catalase (CAT) Gene Family in Rapeseed (Brassica napus L.): Genome-Wide Analysis, Identification, and Expression Pattern in Response to Multiple Hormones and Abiotic Stress Conditions. Int. J. Mol. Sci. 2021, 22, 4281. [Google Scholar] [CrossRef]
- Rasheed, R.; Ashraf, M.A.; Parveen, S.; Iqbal, M.; Hussain, I. Effect of salt stress on different growth and biochemical attributes in two canola (Brassica napus L.) cultivars. Commun. Soil. Sci. Plant Anal. 2014, 45, 669–679. [Google Scholar] [CrossRef]
- Guo, M.; Liu, J.; Hou, L.; Zhang, T.; Liu, H. Research progress on the production and clearance mechanism of reactive oxygen species in plants. Sci. Technol. Vis. 2021, 11, 104–106. [Google Scholar]
- Zhang, X.; Yang, X.; Jiao, Z. Research progress of salt tolerance evaluation in plants and tolerance evaluation strategy. J. Biol. 2018, 35, 91–94. [Google Scholar]
- Jiang, J.; Zhang, J.; Yang, L.; Zhu, J.; Wang, W.; Lei, L.; Zhou, X.; Li, Y. Effects of salt stress on germination of rapeseed seeds. Mol. Plant Breed. 2024, 1–12. [Google Scholar]
- Fang, Y.; Li, J.; Jiang, J.; Geng, Y.; Wang, J.; Wang, Y. Physiological and epigenetic analyses of Brassica napus seed germination in response to salt stress. Acta Physiol. Plant. 2017, 39, 128. [Google Scholar] [CrossRef]
- Long, W.; Pu, H.; Zhang, J.; Qi, C.; Zhang, X. Screening of Brassica napus for salinity tolerance at germination stage. Chin. J. Oil Crop Sci. 2013, 35, 271–275. [Google Scholar]
- Wang, L.; Zuo, Q.; Zheng, J.; You, J.; Yang, G.; Leng, S. Salt stress decreases seed yield and postpones growth process of canola (Brassica napus L.) by changing nitrogen and carbon characters. Sci. Rep. 2022, 12, 17884. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Huang, Z.; Zhang, X.; Lu, H.; Liu, L.; Xu, A. Physiological effects on Brassica napus seedlings under NaCl stress. Acta Bot. Boreali-Occident. Sin. 2014, 34, 2270–2276. [Google Scholar]
- Yang, Y.; Zheng, Q.; Liu, M.; Guo, S. Difference in sodium spatial distribution in the shoot of two canola cultivars under saline stress. Plant Cell Physiol. 2012, 53, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Kou, M.; Wang, Y.; Bai, Y.; Zhang, J. The effect of physiological indices in two Brassica napus leaves under salt stress. J. Northwest Norm. Univ. (Nat. Sci.) 2014, 50, 85–90. [Google Scholar]
- Keshavarzian, M.; Toorchi, M.; Shakiba, M.R. Sodium chloride salt tolerance evaluation and classification of spring rapeseed (Brassica napus L.). J. Crop Breed. 2020, 11, 145–162. [Google Scholar] [CrossRef]
- Pak, V.A.; Nabipour, M.; Meskarbashee, M. Effect of salt stress on chlorophyll content, fluorescence, Na and K ions content in rape plants (Brassica napus L.). Asian J. Agric. Res. 2009, 3, 28–37. [Google Scholar] [CrossRef]
- Long, W.; Gao, J.; Hu, M.; Chen, S.; Zhang, J.; Qi, C.; Zhang, X.; Pu, H. Cation accumulation characteristics of rapeseed under salt stress. Chin. J. Oil Crop Sci. 2016, 38, 592–597. [Google Scholar]
- Soltabayeva, A.; Ongaltay, A.; Omondi, J.O.; Srivastava, S. Morphological, physiological and molecular markers for salt-stressed plants. Plants 2021, 10, 243. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Q.; Liu, J.; Shan, J.; Zhou, J.; Wang, L.; Yang, G.; Leng, S.; Liu, H. Carbon and nitrogen assimilation and partitioning in canola (Brassica napus L.) in saline environment. Commun. Soil. Sci. Plant Anal. 2019, 50, 1700–1709. [Google Scholar] [CrossRef]
- Yadav, S.P.; Bharadwaj, R.; Nayak, H.; Mahto, R.; Singh, R.K.; Prasad, S.K. Impact of salt stress on growth, productivity and physicochemical properties of plants: A Review. Int. J. Chem. Stud. 2019, 7, 1793–1798. [Google Scholar]
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant responses to salt stress: Adaptive mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef]
- Li, P.; Yan, J.; Zhang, H.; Zhang, Y.; Tao, S.; Zhang, Q.; Aldiyar; Xu, A.; Huang, Z. Screening and evaluation of salt tolerance for 146 Brassica napus germplasms at germination Stage. Acta Agric. Boreali-Occident. Sin. 2021, 30, 848–859. [Google Scholar]
- Zhang, Y.; Gao, S.; Zhang, R.; Xie, K.; Wang, J. The effect of salt stress on the germination of different rapeseed seeds. Seed 2021, 40, 94–98. [Google Scholar]
- Wan, L.; Ni, Z.; Sun, H. Rapid Screening of salt tolerance of rapeseed by laboratory hydroponics method. J. Jinling Inst. Technol. 2019, 35, 81–84. [Google Scholar]
- Hu, Y.; Schmidhalter, U. Opportunity and challenges of phenotyping plant salt tolerance. Trends Plant Sci. 2023, 28, 552–566. [Google Scholar] [CrossRef]
- Zhang, G.; Zhou, J.; Peng, Y.; Tan, Z.; Zhang, Y.; Zhao, H.; Liu, D.; Liu, X.; Li, L.; Yu, L. High-throughput phenotyping-based QTL mapping reveals the genetic architecture of the salt stress tolerance of Brassica napus. Plant Cell Environ. 2021, 46, 549–566. [Google Scholar] [CrossRef]
- Du, W.; Ning, L.; Liu, Y.; Zhang, S.; Yang, Y.; Wang, Q.; Chao, S.; Yang, H.; Huang, F.; Cheng, H. Identification of loci and candidate gene GmSPX-RING1 responsible for phosphorus efficiency in soybean via genome-wide association analysis. BMC Genom. 2020, 21, 725. [Google Scholar] [CrossRef]
- Nordborg, M.; Atwell, S.; Huang, Y.S.; Vilhjálmsson, B.J.; Willems, G.; Horton, M.; Li, Y.; Meng, D.; Platt, A.; Tarone, A.M.; et al. Genome-wide association study of 107 phenotypes in Arabidopsis thaliana inbred lines. Nature 2010, 465, 627–631. [Google Scholar]
- Wassan, G.M.; Khanzada, H.; Zhou, Q.; Mason, A.S.; Keerio, A.A.; Khanzada, S.; Solangi, A.M.; Faheem, M.; Fu, D.; He, H. Identification of genetic variation for salt tolerance in Brassica napus using genome-wide association mapping. Mol. Genet. Genom. 2021, 296, 391–408. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhou, J.; Peng, Y.; Tan, Z.; Li, L.; Yu, L.; Jin, C.; Fang, S.; Lu, S.; Guo, L. Genome-wide association studies of salt tolerance at seed germination and seedling stages in Brassica napus. Front. Plant Sci. 2022, 12, 772708. [Google Scholar] [CrossRef] [PubMed]
- Gul, H.S.; Ulfat, M.; Zafar, Z.U.; Haider, W.; Ali, Z.; Manzoor, H.; Afzal, S.; Ashraf, M.; Athar, H.-U.-R. Photosynthesis and salt exclusion are key physiological processes contributing to salt tolerance of canola (Brassica napus L.): Evidence from physiology and transcriptome analysis. Genes 2022, 14, 3. [Google Scholar] [CrossRef]
- Ma, N.; Hu, C.; Wan, L.; Hu, Q.; Xiong, J.; Zhang, C. Strigolactones improve plant growth, photosynthesis, and alleviate oxidative stress under salinity in rapeseed (Brassica napus L.) by regulating gene expression. Front. Plant Sci. 2017, 8, 1671. [Google Scholar] [CrossRef] [PubMed]
- Baillo, E.H.; Kimotho, R.N.; Zhang, Z.; Xu, P. Transcription factors associated with abiotic and biotic stress tolerance and their potential for crops improvement. Genes 2019, 10, 771. [Google Scholar] [CrossRef]
- Shu, J.; Ma, X.; Ma, H.; Huang, Q.; Zhang, Y.; Guan, M.; Guan, C.F. Transcriptomic, proteomic, metabolomic, and functional genomic approaches of Brassica napus L. during salt stress. PLoS ONE 2022, 17, e0262587. [Google Scholar] [CrossRef]
- Ahmad, P.; Ashraf, M.; Younis, M.; Hu, X.; Kumar, A.; Akram, N.A.; Al-Qurainy, F. Role of transgenic plants in agriculture and biopharming. Biotechnol. Adv. 2012, 30, 524–540. [Google Scholar] [CrossRef]
- Zhang, H.X.; Hodson, J.N.; Williams, J.P.; Blumwald, E. Engineering salt-tolerant Brassica plants: Characterization of yield and seed oil quality in transgenic plants with increased vacuolar sodium accumulation. Proc. Natl. Acad. Sci. USA 2001, 98, 12832–12836. [Google Scholar] [CrossRef]
- Sun, X.; Feng, X.; Li, C.; Zhang, Z.; Wang, L. Study on salt tolerance with YHem1 transgenic canola (Brassica napus). Physiol. Plant. 2015, 154, 223–242. [Google Scholar] [CrossRef]
- Jha, U.C.; Bohra, A.; Nayyar, H. Advances in “omics” approaches to tackle drought stress in grain legumes. Plant Breed. 2020, 139, 1–27. [Google Scholar] [CrossRef]
- Kumar, M.; Prusty, M.R.; Pandey, M.K.; Singh, P.K.; Bohra, A.; Guo, B.; Varshney, R.K. Application of CRISPR/Cas9-mediated gene editing for abiotic stress management in crop plants. Front. Plant Sci. 2023, 14, 1157678. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, Z.; Li, J.-F. Targeted gene manipulation in plants using the CRISPR/Cas technology. J. Genet. Genom. 2016, 43, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Cabral, G.B.; Ferreira, J.L.d.P.; Souza, R.P.d.; Cunha, M.S.; Luchs, A.; Figueiredo, C.A.; Brígido, L.F.d.M. Simple protocol for population (Sanger) sequencing for Zika virus genomic regions. Memórias Inst. Oswaldo Cruz 2017, 113, 38–44. [Google Scholar] [CrossRef]
- Brookes, G.; Barfoot, P. Environmental impacts of genetically modified (GM) crop use 1996–2016: Impacts on pesticide use and carbon emissions. GM Crops Food 2018, 9, 109–139. [Google Scholar] [CrossRef]
- Bernabé-Orts, J.M.; Casas-Rodrigo, I.; Minguet, E.G.; Landolfi, V.; Garcia-Carpintero, V.; Gianoglio, S.; Vázquez-Vilar, M.; Granell, A.; Orzaez, D. Assessment of Cas12a-mediated gene editing efficiency in plants. Plant Biotechnol. J. 2019, 17, 1971–1984. [Google Scholar] [CrossRef]
- Vernikos, G.; Medini, D.; Riley, D.R.; Tettelin, H. Ten years of pan-genome analyses. Curr. Opin. Microbiol. 2015, 23, 148–154. [Google Scholar] [CrossRef]
- Wei, H.; Wang, X.; Zhang, Z.; Yang, L.; Zhang, Q.; Li, Y.; He, H.; Chen, D.; Zhang, B.; Zheng, C. Uncovering key salt-tolerant regulators through a combined eQTL and GWAS analysis using the super pan-genome in rice. Natl. Sci. Rev. 2024, 11, nwae043. [Google Scholar] [CrossRef]
- Song, J.; Guan, Z.; Hu, J.; Guo, C.; Yang, Z.; Wang, S.; Liu, D.; Wang, B.; Lu, S.; Zhou, R. Eight high-quality genomes reveal pan-genome architecture and ecotype differentiation of Brassica napus. Nat. Plants 2020, 6, 34–45. [Google Scholar] [CrossRef]
- Zhong, H.; Guo, Q.Q.; Chen, L.; Ren, F.; Wang, Q.Q.; Zheng, Y.; Li, X.B. Two Brassica napus genes encoding NAC transcription factors are involved in response to high-salinity stress. Plant Cell Rep. 2012, 31, 1991–2003. [Google Scholar] [CrossRef]
- Di, F.; Jian, H.; Wang, T.; Chen, X.; Ding, Y.; Du, H.; Lu, K.; Li, J.; Liu, L. Genome-wide analysis of the PYL gene family and identification of PYL genes that respond to abiotic stress in Brassica napus. Genes 2018, 9, 156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Song, J.; Wang, L.; Yang, Z.; Sun, D. Identification of a DEAD-box RNA helicase BnRH6 reveals its involvement in salt stress response in rapeseed (Brassica napus). Int. J. Mol. Sci. 2022, 24, 2. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, J.; Paek, K.H.; Kwon, S.Y.; Cho, H.S.; Kim, S.J.; Park, J.M. A novel WD40 protein, BnSWD1, is involved in salt stress in Brassica napus. Plant Biotechnol. Rep. 2010, 4, 165–172. [Google Scholar] [CrossRef]
- Li, Q.; Yin, M.; Li, Y.; Fan, C.; Yang, Q.; Wu, J.; Zhang, C.; Wang, H.; Zhou, Y. Expression of Brassica napus TTG2, a regulator of trichome development, increases plant sensitivity to salt stress by suppressing the expression of auxin biosynthesis genes. J. Exp. Bot. 2015, 66, 5821–5836. [Google Scholar] [CrossRef]
- Gao, Q.; Feng, D.; Liu, J.; Zhang, J.; Han, Q. Main mechanisms and classification of exogenous substances alleviating plant salt stress. J. Plant Nutr. Fertil. 2021, 27, 2030–2044. [Google Scholar]
- Shah, A.N.; Tanveer, M.; Abbas, A.; Fahad, S.; Baloch, M.S.; Ahmad, M.I.; Saud, S.; Song, Y. Targeting salt stress coping mechanisms for stress tolerance in Brassica: A research perspective. Plant Physiol. Biochem. 2021, 158, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ding, X.; Lv, Y.; Cheng, Y.; Li, C.; Yan, L.; Tian, S.; Zou, X. Exogenous serotonin improves salt tolerance in rapeseed (Brassica napus L.) seedlings. Agronomy 2021, 11, 400. [Google Scholar] [CrossRef]
- Liu, Z.; Cai, J.; Li, J.; Lu, G.; Li, C.; Fu, G.; Zhang, X.; Liu, Q.; Zou, X.; Cheng, Y. Exogenous application of a low concentration of melatonin enhances salt tolerance in rapeseed (Brassica napus L.) seedlings. J. Integr. Agric. 2018, 17, 328–335. [Google Scholar]
- Ilyas, M.; Maqsood, M.F.; Shahbaz, M.; Zulfiqar, U.; Ahmad, K.; Naz, N.; Ali, M.F.; Ahmad, M.; Ali, Q.; Yong, J.W.H. Alleviating salinity stress in canola (Brassica napus L.) through exogenous application of salicylic acid. BMC Plant Biol. 2024, 24, 611. [Google Scholar] [CrossRef] [PubMed]
- Farouk, S.; Al-Amri, S.M. Exogenous zinc forms counteract NaCl-induced damage by regulating the antioxidant system, osmotic adjustment substances, and ions in canola (Brassica napus L. cv. Pactol) plants. J. Soil. Sci. Plant Nutr. 2019, 19, 887–899. [Google Scholar] [CrossRef]
- Xia, W.; Meng, W.; Peng, Y.; Qin, Y.; Zhang, L.; Zhu, N. Effects of Exogenous Isosteviol on the Physiological Characteristics of Brassica napus Seedlings under Salt Stress. Plants 2024, 13, 217. [Google Scholar] [CrossRef] [PubMed]
- El-Badri, A.M.; Batool, M.; Mohamed, I.A.; Wang, Z.; Wang, C.; Tabl, K.M.; Khatab, A.; Kuai, J.; Wang, J.; Wang, B. Mitigation of the salinity stress in rapeseed (Brassica napus L.) productivity by exogenous applications of bio-selenium nanoparticles during the early seedling stage. Environ. Pollut. 2022, 310, 119815. [Google Scholar] [CrossRef] [PubMed]
- Lei, P.; Xu, Z.; Liang, J.; Luo, X.; Zhang, Y.; Feng, X.; Xu, H. Poly (γ-glutamic acid) enhanced tolerance to salt stress by promoting proline accumulation in Brassica napus L. Plant Growth Regul. 2016, 78, 233–241. [Google Scholar] [CrossRef]
- Khan, A.; Nazar, S.; Lang, I.; Nawaz, H.; Hussain, M.A. Effect of ellagic acid on growth and physiology of canola (Brassica napus L.) under saline conditions. J. Plant Interact. 2017, 12, 520–525. [Google Scholar] [CrossRef]
- Efimova, M.; Savchuk, A.; Hasan, J.; Litvinovskaya, R.; Khripach, V.; Kholodova, V.; Kuznetsov, V.V. Physiological mechanisms of enhancing salt tolerance of Brassica napus plants with brassinosteroids. Russ. J. Plant Physiol. 2014, 61, 733–743. [Google Scholar] [CrossRef]
- Tian, T.; Wang, H.; Wang, J.; Zhu, Y.; Shi, X.; Li, W. Effects of nitrogen application on accumulation of organic osmotic regulating substances in forage rapeseed (Brassica napus) under salt stress. Acta Prataculturae Sin. 2021, 30, 125. [Google Scholar]
- Xiong, J.; Wang, H.; Tan, X.; Zhang, C.; Naeem, M.S. 5-aminolevulinic acid improves salt tolerance mediated by regulation of tetrapyrrole and proline metabolism in Brassica napus L. seedlings under NaCl stress. Plant Physiol. Biochem. 2018, 124, 88–99. [Google Scholar] [CrossRef]
- Naeem, M.S.; Warusawitharana, H.; Liu, H.; Liu, D.; Ahmad, R.; Waraich, E.A.; Xu, L.; Zhou, W. 5-Aminolevulinic acid alleviates the salinity-induced changes in Brassica napus as revealed by the ultrastructural study of chloroplast. Plant Physiol. Biochem. 2012, 57, 84–92. [Google Scholar] [CrossRef]
- Hashem, H.A.; Hassanein, R.A.; Bekheta, M.A.; El-Kady, F.A. Protective role of selenium in canola (Brassica napus L.) plant subjected to salt stress. Egypt. J. Exp. Biol. 2013, 9, 199–211. [Google Scholar]
- Zhao, H.-M.; Zheng, D.-F.; Feng, N.-J.; Zhou, G.-S.; Khan, A.; Lu, X.-T.; Deng, P.; Zhou, H.; Du, Y.-W. Regulatory effects of Hemin on prevention and rescue of salt stress in rapeseed (Brassica napus L.) seedlings. BMC Plant Biol. 2023, 23, 558. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, A.; Abdolzadeh, A.; Sadeghipour, H.R. Beneficial effects of silicon nutrition in alleviating salinity stress in hydroponically grown canola, Brassica napus L., plants. Soil. Sci. Plant Nutr. 2010, 56, 244–253. [Google Scholar] [CrossRef]
- ElSayed, A.I.; Mohamed, A.H.; Rafudeen, M.S.; Omar, A.A.; Awad, M.F.; Mansour, E. Polyamines mitigate the destructive impacts of salinity stress by enhancing photosynthetic capacity, antioxidant defense system and upregulation of calvin cycle-related genes in rapeseed (Brassica napus L.). Saudi J. Biol. Sci. 2022, 29, 3675–3686. [Google Scholar] [CrossRef] [PubMed]
| Gene Name | Gene ID | Arabidopsis thaliana Gene Number | Features | Function | Reference |
|---|---|---|---|---|---|
| BnCKX5 | BnaA02G0088800ZS | AT5G21482 | Encodes cytokinin dehydrogenase, which plays a role in maintaining cytokinin homeostasis and is considered a candidate gene for salt stress response. | unclear | [55] |
| BnERF3 | BnaA06G0028500ZS | AT1G50640 | Encodes an ethylene response transcription factor that plays an important role in signal transduction in many adversity stresses and is considered a candidate gene for salt stress response. | unclear | [55] |
| BnNAC2 BnNAC5 | BnaA07T0309700ZS BnaA10T0224100ZS | AT1G69490 AT5G13180 | Induced by high salt, drought, and ABA and encodes a member of the NAC transcription factor gene family. It is expressed in the floral primordium and is highly regulated by AP3 and PI. Its expression is associated with leaf senescence. | negative | [72] |
| BnPYL1-2 BnPYL7-2 | BnaA06G0421700ZS BnaA03G0276800ZS | AT5G46790 AT4G01026 | PYL is involved in the first step of ABA signaling, and some BnPYL genes are responsive to abiotic stresses such as salt and high temperature. | positive | [73] |
| BnRH6 | BnRH6 is targeted at the nucleus and cytoplasmic processing body (P-body), constitutively expressed throughout the lifespan, and induced by salt stress. | negative | [74] | ||
| BnSWD1 | BnSWD1 gene is expressed at high levels under salt stress conditions, which was upregulated after treatment with abscisic acid, salicylic acid, and methyl jasmonate. | unclear | [75] | ||
| BnaA.TTG2.a.1 | AT2G37260 | BnaA.TTG2.a.1 reduces IAA levels by repressing the expression of IAA synthesis genes, thereby making overexpressing plants salt-sensitive. | negative | [76] |
| Exogenous Substances | Application Method | Optimum Concentration | Mechanism of Exogenous Substances | Reference |
|---|---|---|---|---|
| Serotonin | Hydroponically | 200 µM | Serotonin increases the activity of catalase (CAT), superoxide dismutase (SOD), and peroxidase (POD), effectively activating the antioxidant enzyme system with the ability to scavenge reactive oxygen species, regulate osmotic pressure, and promote growth. | [79] |
| Melatonin | Hydroponically | 30 µM | Low concentrations of exogenous melatonin increase the activities of antioxidant enzymes such as POD, CAT, and APX; promote the accumulation of soluble proteins, proline, and water-soluble glucan; promote root development; and increase the biomass of Brassica napus seedlings under salt stress. | [80] |
| Salicylic acid | Foliar application | 0.02 M | SA treatment increases shoot fresh weight, root dry weight, chlorophyll a, chlorophyll b, superoxide dismutase, peroxidase, catalase, total soluble proteins, total soluble sugars, total phenolics, flavonoids, anthocyanins, endogenous ascorbic acid, glycine betaine, and total free proline. | [81] |
| ZnSO4 ZNP | Foliar application | 75 mg/L 10 mg/L | Zinc application reduces MP%, MDA, and H2O2 concentrations. Increased accumulation of proline and total soluble carbohydrates, nitrogen, potassium, and phosphorus content in plant tissues decreased sodium and chloride content. | [82] |
| Isosteviol | Seed soaking | 10−9~10−8 M | Isosteviol regulates the production of osmotic substances and ROS and reduces oxidative damage caused by salt stress in Brassica napus seedlings. Isosteviol also reduces Na+ uptake by seedling tissues, increases K+ content, and mitigates the damage caused by salt stress to plant seedlings. | [83] |
| bio-selenium nanoparticles | Pre-seed treatment | 150 μM | Biological SeNPs enhance seed vigor, improve seedling growth and physicochemical properties, regulate Na+ and K+ uptake, and improve rapeseed growth. | [84] |
| Poly (γ-glutamic acid) | Hydroponically | 20 mg/L | γ-PGA increases the resistance of Brassica napus seedlings to salt stress by activating the proline synthesis pathway and promoting proline accumulation. | [85] |
| Ellagic acid | Seed soaking | Applied based on salt concentration | Application of EA as a seed soak mitigates the effects of salinity and promotes plant growth. | [86] |
| Brassinosteroids | Hydroponically | Exogenous EBL impedes the development of NaCl-dependent lipid peroxidation and increases the osmotic potential of leaf cell contents. The protective effect of EBL under salt stress may be related to the ability of EBL to maintain intracellular ionic homeostasis regulating water status by its antioxidant action. | [87] | |
| Nitrogen | Hydroponically | Nitrogen significantly affects the contents of MDA, proline, chlorophyll, and total nitrogen in the early stage of growth, but significantly affects the contents of leaf water and soluble sugar in the late stage of growth. | [88] | |
| 5-aminolevulinic acid | Foliar application | 30 mg/L | ALA promotes increased levels of intermediates of the tetrapyrrole biosynthesis pathway, which promotes the accumulation of chlorophyll and heme, as well as enhances the accumulation of proline, thereby improving salt tolerance in Brassica napus. | [89,90] |
| Selemium | Foliar application | 5 mg/L | Se applied alone or in combination with salt treatments significantly increases plant growth, plant yield, and photosynthetic pigment content and improves the quality of Brassica napus oil. | [91] |
| Hemin | Foliar application | Hematoxylin (HS) significantly improves root length, seedling height, stem diameter and accumulates more dry matter biomass in Brassica napus seedlings under NaCl stress. It improves photosynthetic efficiency; increases the activities of antioxidant enzymes such as superoxide dismutase (SOD), peroxidase (POD), and ascorbate peroxidase (APX); reduces electrolyte leakage (EL) and malondialdehyde (MDA) content; and mitigates oxidative membrane damage. | [92] | |
| Silicon | Hydroponically | Silicon nutrition ameliorates the deleterious effects of salinity on the growth of Brassica napus plants by reducing the Na+ content of tissues and maintaining the integrity of root cell membranes. | [93] | |
| Polyamines | Foliar application | Exogenous application of Spd regulates antioxidant enzyme activities, the polyamine pathway, and Calvin cycle enzyme-related genes to alleviate salt stress injury in plants. | [94] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, R.; Zhan, N.; Geng, R.; Xu, K.; Zhou, X.; Li, L.; Yan, G.; Zhou, F.; Cai, G. Progress on Salt Tolerance in Brassica napus. Plants 2024, 13, 1990. https://doi.org/10.3390/plants13141990
Dai R, Zhan N, Geng R, Xu K, Zhou X, Li L, Yan G, Zhou F, Cai G. Progress on Salt Tolerance in Brassica napus. Plants. 2024; 13(14):1990. https://doi.org/10.3390/plants13141990
Chicago/Turabian StyleDai, Rui, Na Zhan, Rudan Geng, Kun Xu, Xiangchun Zhou, Lixia Li, Guixin Yan, Fanglin Zhou, and Guangqin Cai. 2024. "Progress on Salt Tolerance in Brassica napus" Plants 13, no. 14: 1990. https://doi.org/10.3390/plants13141990
APA StyleDai, R., Zhan, N., Geng, R., Xu, K., Zhou, X., Li, L., Yan, G., Zhou, F., & Cai, G. (2024). Progress on Salt Tolerance in Brassica napus. Plants, 13(14), 1990. https://doi.org/10.3390/plants13141990







