Imidacloprid Uptake and Accumulation in Lettuce Plant (Lactuca sativa L. var. longipolia) and Its Effects on Abundance of Microbial Communities in Cultivated and Non-Cultivated Arid Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Soil Collection and Preparation
Soil Analysis
2.3. Planting, Growth Conditions, and Harvesting
2.4. Extraction, Purification, and Analysis
2.5. Chromatographic Conditions
2.6. Soil Microbial Characterization
2.7. Quality Assurance and Quality Control
2.8. Concentration Factors for Uptake
2.8.1. Root and Translocation Factors
2.8.2. Temporal Shift in IMI in Soil
2.9. Statistical Analysis
3. Results
3.1. Soil Physical and Chemical Parameters
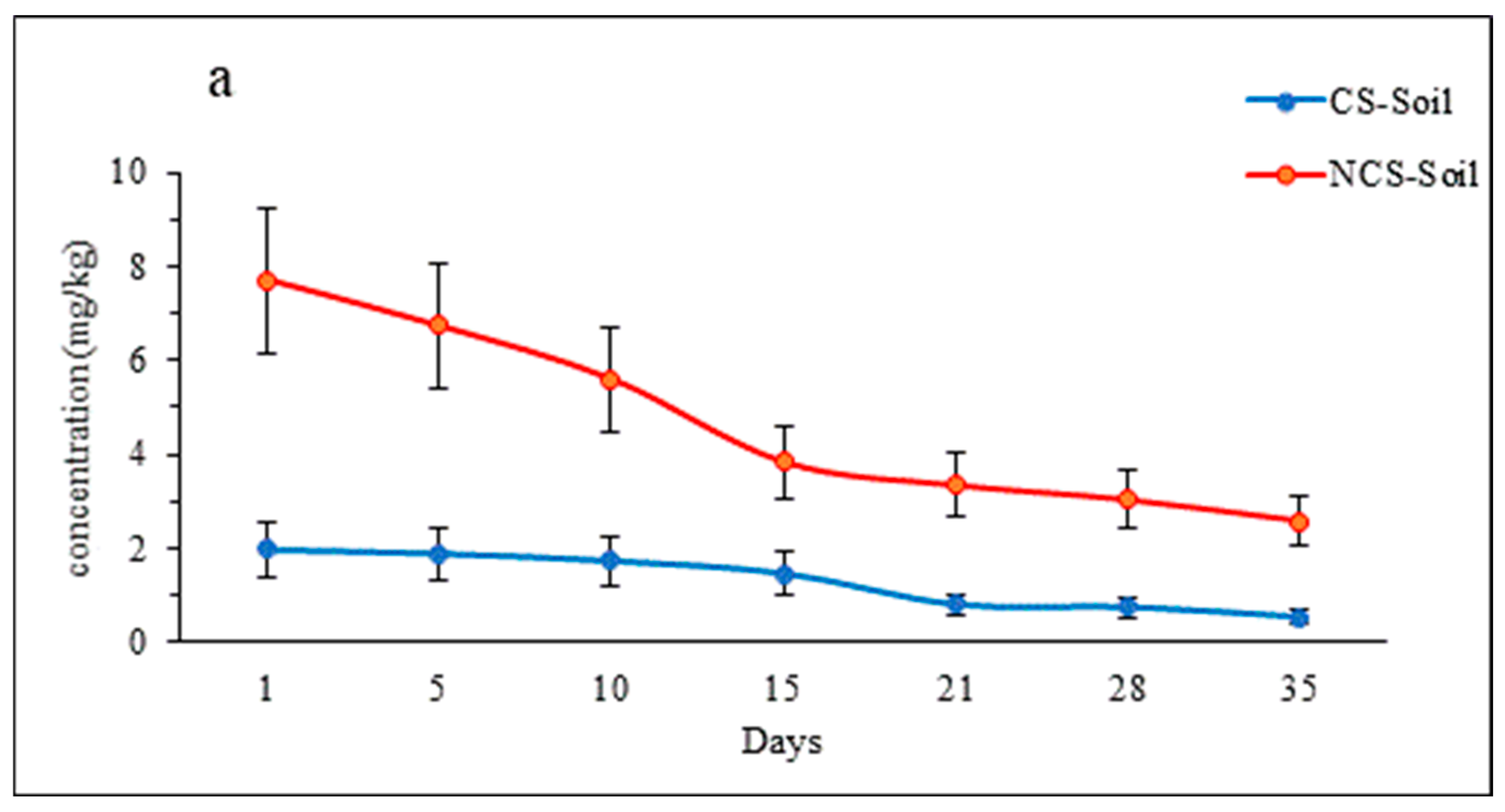
3.2. Imidacloprid (IMI) Concentration in Soil
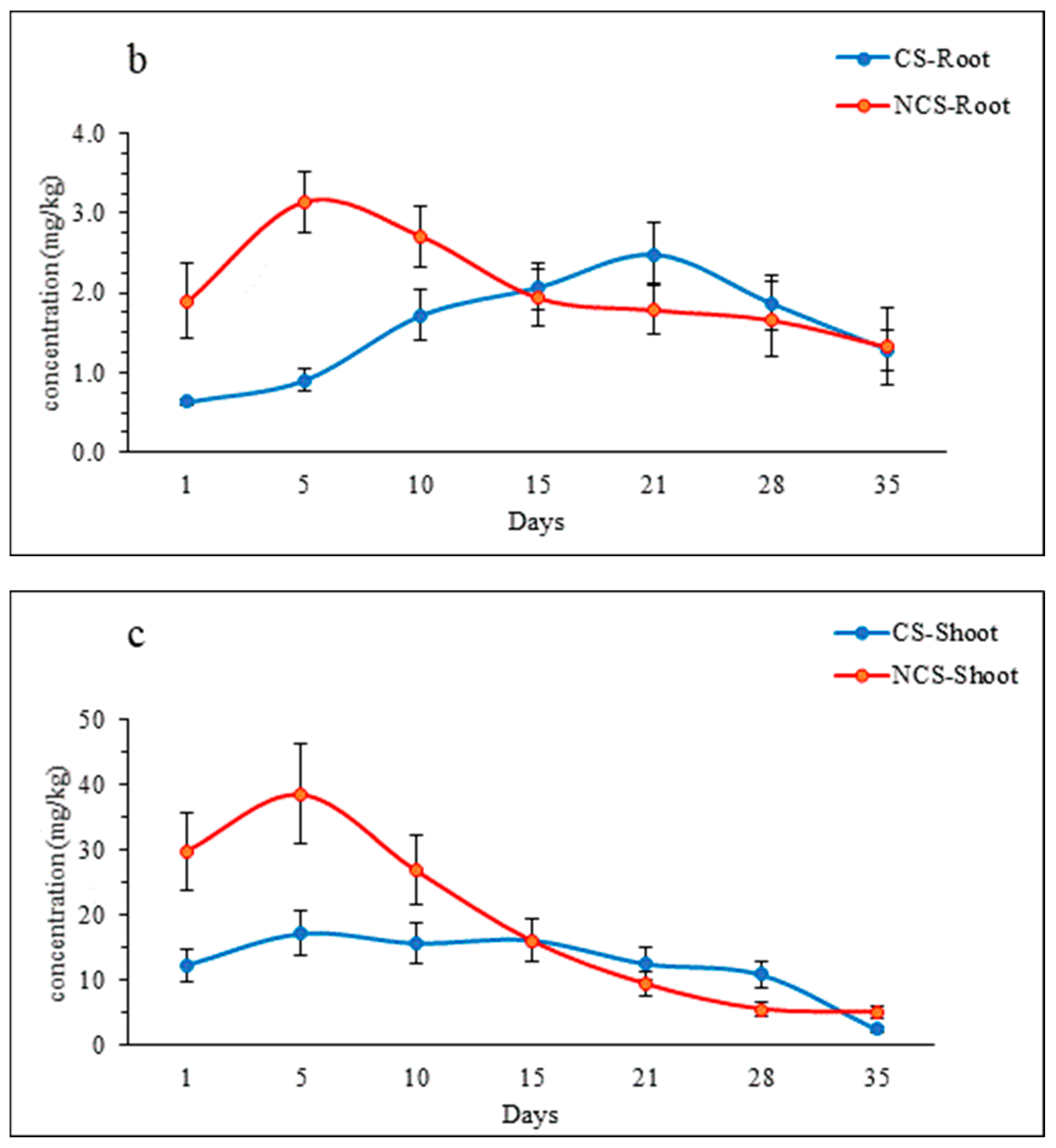
3.3. IMI Uptake by Lettuce
3.4. Root Concentration Factor (RCF) and Translocation Factor (TF)
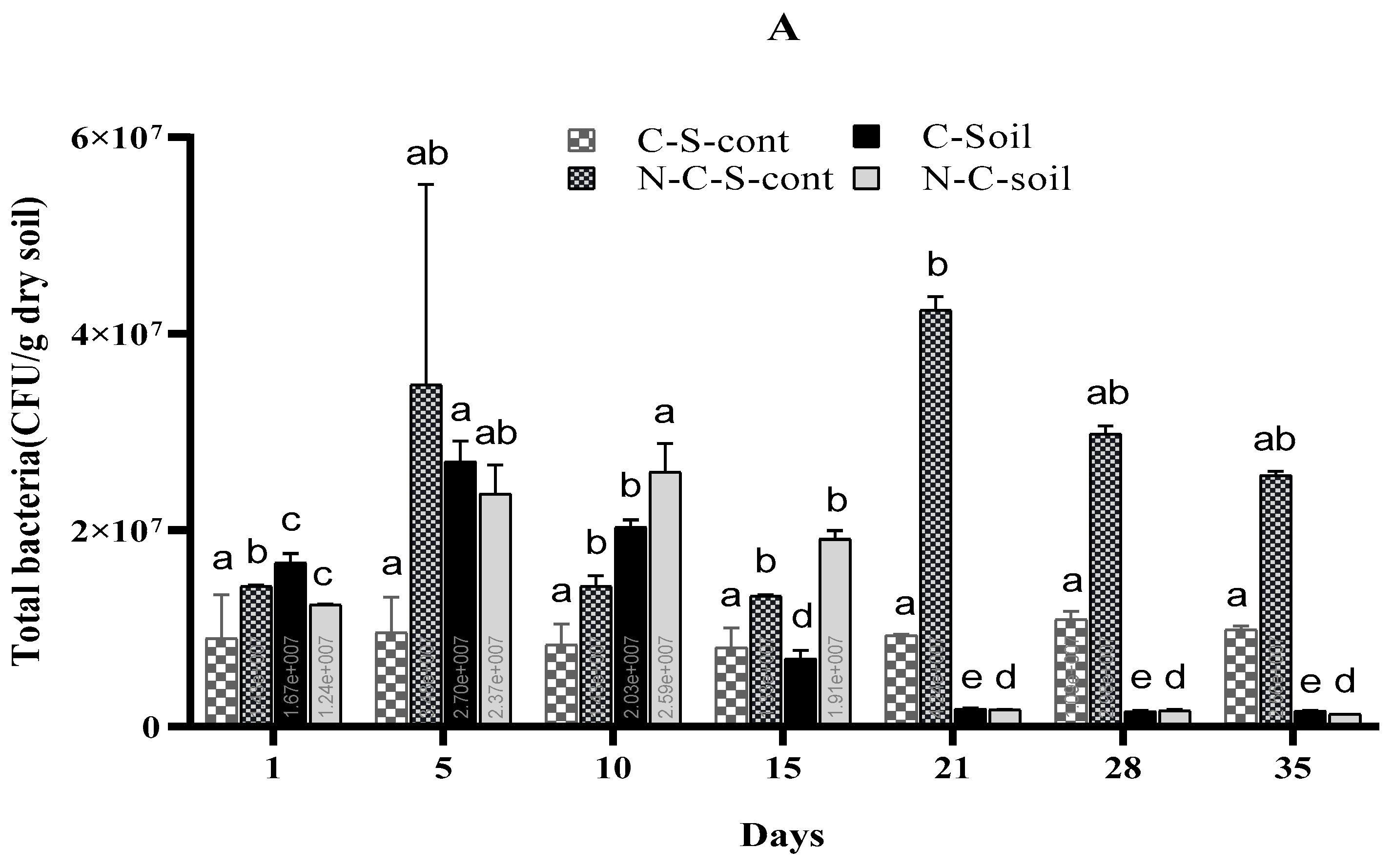
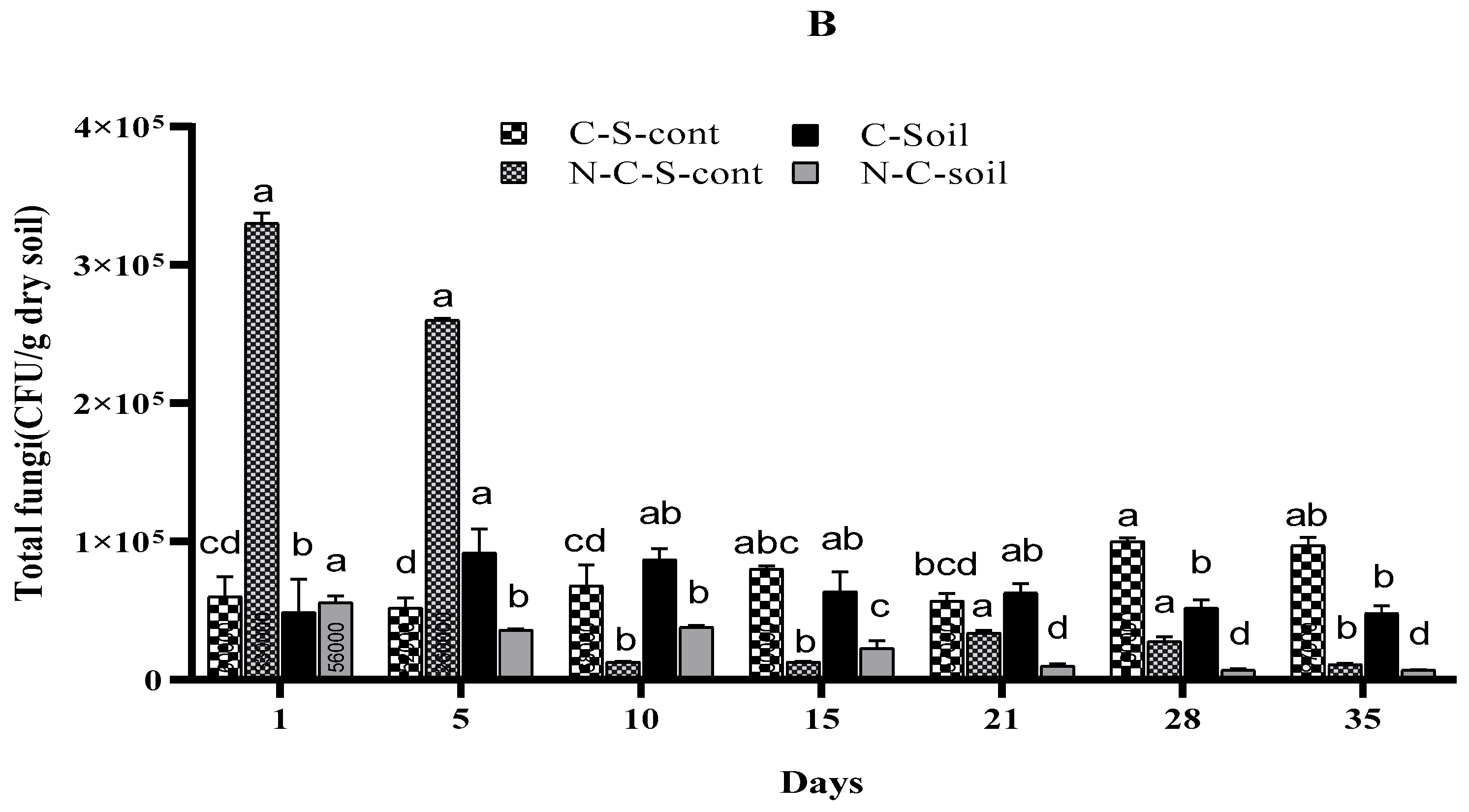
3.5. Effects of Imidacloprid on Soil Microbial Communities
4. Discussion
4.1. Study Soil Characteristics
4.2. Imidacloprid (IMI) Concentration in Soil
4.3. IMI Uptake by Lettuce
4.4. Root Concentration Factor (RCF) and Translocation Factor (TF)
4.5. Effects of Imidacloprid on Soil Microbial Communities
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bass, C.; Denholm, I.; Williamson, M.S.; Nauen, R. The global status of insect resistance to neonicotinoid insecticides. Pestic. Biochem. Physiol. 2015, 121, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Bonmatin, J.-M.; Giorio, C.; Girolami, V.; Goulson, D.; Kreutzweiser, D.; Krupke, C.; Liess, M.; Long, E.; Marzaro, M.; Mitchell, E. Environmental fate and exposure; neonicotinoids and fipronil. Environ. Sci. Pollut. Res. 2015, 22, 35–67. [Google Scholar] [CrossRef] [PubMed]
- Ford, K.A.; Casida, J.E. Comparative metabolism and pharmacokinetics of seven neonicotinoid insecticides in spinach. J. Agric. Food Chem. 2008, 56, 10168–10175. [Google Scholar] [CrossRef] [PubMed]
- Stamm, M.D.; Heng-Moss, T.M.; Baxendale, F.P.; Siegfried, B.D.; Blankenship, E.E.; Nauen, R. Uptake and translocation of imidacloprid, clothianidin and flupyradifurone in seed-treated soybeans. Pest Manag. Sci. 2016, 72, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Simon-Delso, N.; Amaral-Rogers, V.; Belzunces, L.P.; Bonmatin, J.-M.; Chagnon, M.; Downs, C.; Furlan, L.; Gibbons, D.W.; Giorio, C.; Girolami, V. Systemic insecticides (neonicotinoids and fipronil): Trends, uses, mode of action and metabolites. Environ. Sci. Pollut. Res. 2015, 22, 5–34. [Google Scholar] [CrossRef] [PubMed]
- Tomizawa, M.; Casida, J.E. Neonicotinoid insecticide toxicology: Mechanisms of selective action. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 247–268. [Google Scholar] [CrossRef]
- Li, X.; Degain, B.A.; Harpold, V.S.; Marçon, P.G.; Nichols, R.L.; Fournier, A.J.; Naranjo, S.E.; Palumbo, J.C.; Ellsworth, P.C. Baseline susceptibilities of B-and Q-biotype Bemisia tabaci to anthranilic diamides in Arizona. Pest Manag. Sci. 2012, 68, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Bayo, F.; Hyne, R.V. Detection and analysis of neonicotinoids in river waters–development of a passive sampler for three commonly used insecticides. Chemosphere 2014, 99, 143–151. [Google Scholar] [CrossRef]
- Botías, C.; David, A.; Hill, E.M.; Goulson, D. Contamination of wild plants near neonicotinoid seed-treated crops, and implications for non-target insects. Sci. Total Environ. 2016, 566, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Byrne, F.J.; Krieger, R.I.; Doccola, J.; Morse, J.G. Seasonal timing of neonicotinoid and organophosphate trunk injections to optimize the management of avocado thrips in California avocado groves. Crop Prot. 2014, 57, 20–26. [Google Scholar] [CrossRef]
- Kraaij, H.; Connell, D. Bioconcentration and uptake kinetics of chlorobenzenes in soy-bean roots. Chemosphere 1997, 34, 2607–2620. [Google Scholar] [CrossRef]
- Rundlöf, M.; Andersson, G.K.; Bommarco, R.; Fries, I.; Hederström, V.; Herbertsson, L.; Jonsson, O.; Klatt, B.K.; Pedersen, T.R.; Yourstone, J. Seed coating with a neonicotinoid insecticide negatively affects wild bees. Nature 2015, 521, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Lu, M.; Wang, D.; Zhang, Z.; Liu, X.; Yu, X. Dissipation and distribution of chlorpyrifos in selected vegetables through foliage and root uptake. Chemosphere 2016, 144, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Girolami, K.M.; Kahng, S.; Hilker, K.A.; Girolami, P.A. Differential reinforcement of high rate behavior to increase the pace of self-feeding. Behav. Interv. Theory Pract. Resid. Community-Based Clin. Programs 2009, 24, 17–22. [Google Scholar] [CrossRef]
- Hokkanen, H.M.; Menzler-Hokkanen, I.; Keva, M. Long-term yield trends of insect-pollinated crops vary regionally and are linked to neonicotinoid use, landscape complexity, and availability of pollinators. Arthropod-Plant Interact. 2017, 11, 449–461. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, J.; Ni, Y.; Zhang, Q.; Zhao, L. Uptake by roots and translocation to shoots of polychlorinated dibenzo-p-dioxins and dibenzofurans in typical crop plants. Chemosphere 2009, 76, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Alford, A.; Krupke, C.H. Translocation of the neonicotinoid seed treatment clothianidin in maize. PLoS ONE 2017, 12, e0173836. [Google Scholar]
- Dalal, R.; Mayer, R.J. Long term trends in fertility of soils under continuous cultivation and cereal cropping in southern Queensland. I. Overall changes in soil properties and trends in winter cereal yields. Soil Res. 1986, 24, 265–279. [Google Scholar] [CrossRef]
- Paz-Gonzalez, A.; Vieira, S.; Castro, M.T.T. The effect of cultivation on the spatial variability of selected properties of an umbric horizon. Geoderma 2000, 97, 273–292. [Google Scholar] [CrossRef]
- Shepherd, T.; Saggar, S.; Newman, R.; Ross, C.; Dando, J. Tillage-induced changes to soil structure and organic carbon fractions in New Zealand soils. Soil Res. 2001, 39, 465–489. [Google Scholar] [CrossRef]
- Gleń-Karolczyk, K.; Boligłowa, E.; Antonkiewicz, J. Organic fertilization shapes the biodiversity of fungal communities associated with potato dry rot. Appl. Soil Ecol. 2018, 129, 43–51. [Google Scholar] [CrossRef]
- Caravaca, F.; Masciandaro, G.; Ceccanti, B. Land use in relation to soil chemical and biochemical properties in a semiarid Mediterranean environment. Soil Tillage Res. 2002, 68, 23–30. [Google Scholar] [CrossRef]
- Saggar, S.; McIntosh, P.; Hedley, C.; Knicker, H. Changes in soil microbial biomass, metabolic quotient, and organic matter turnover under Hieracium (H. pilosella L.). Biol. Fertil. Soils 1999, 30, 232–238. [Google Scholar] [CrossRef]
- Cycoń, M.; Markowicz, A.; Borymski, S.; Wójcik, M.; Piotrowska-Seget, Z. Imidacloprid induces changes in the structure, genetic diversity and catabolic activity of soil microbial communities. J. Environ. Manag. 2013, 131, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yao, J.; Chen, H.; Yi, Z.; Choi, M.M. Influence of short-time imidacloprid and acetamiprid application on soil microbial metabolic activity and enzymatic activity. Environ. Sci. Pollut. Res. 2014, 21, 10129–10138. [Google Scholar] [CrossRef] [PubMed]
- Gee, G.W.; Bauder, J.W. Particle-size analysis. Methods of soil analysis: Part 1 Physical and mineralogical methods 1986, 5, 383–411. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. Methods Soil Anal. Part 2 Chem. Microbiol. Prop. 1996, 5, 961–1010. [Google Scholar]
- Westerman, R.L.; Boman, R.K.; Raun, W.R.; Johnson, G.V. Ammonium and nitrate nitrogen in soil profiles of long-term winter wheat fertilization experiments. Agron. J. 1994, 86, 94–99. [Google Scholar] [CrossRef]
- Neuwirthová, N.; Bílková, Z.; Vašíčková, J.; Hofman, J.; Bielská, L. Concentration/time-dependent dissipation, partitioning and plant accumulation of hazardous current-used pesticides and 2-hydroxyatrazine in sand and soil. Chemosphere 2018, 203, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Salminen, J.M.; Tuomi, P.M.; Suortti, A.-M.; Jørgensen, K.S. Potential for aerobic and anaerobic biodegradation of petroleum hydrocarbons in boreal subsurface. Biodegradation 2004, 15, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Castle, S.J.; Byrne, F.J.; Bi, J.L.; Toscano, N.C. Spatial and temporal distribution of imidacloprid and thiamethoxam in citrus and impact on Homalodisca coagulata populations. Pest Manag. Sci. Former. Pestic. Sci. 2005, 61, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Mattina, M.J.I.; Iannucci-Berger, W.; Dykas, L. Chlordane uptake and its translocation in food crops. J. Agric. Food Chem. 2000, 48, 1909–1915. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.K.; Liu, D.C. Comparison of physical–chemical properties of type I collagen from different species. Food Chem. 2006, 99, 244–251. [Google Scholar] [CrossRef]
- Xu, H.; Song, J.; Luo, H.; Zhang, Y.; Li, Q.; Zhu, Y.; Xu, J.; Li, Y.; Song, C.; Wang, B. Analysis of the genome sequence of the medicinal plant Salvia miltiorrhiza. Mol. Plant 2016, 9, 949–952. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Satar, A.M.; Al-Khabbas, M.H.; Alahmad, W.R.; Yousef, W.M.; Alsomadi, R.H.; Iqbal, T. Quality assessment of groundwater and agricultural soil in Hail region, Saudi Arabia. Egypt. J. Aquat. Res. 2017, 43, 55–64. [Google Scholar] [CrossRef]
- Baig, S.A.; Akhter, N.A.; Ashfaq, M.; Asi, M.R.; Ashfaq, U. Imidacloprid residues in vegetables, soil and water in the southern Punjab, Pakistan. J. Agric. Technol. 2012, 8, 903–916. [Google Scholar]
- Miller, E.L.; Nason, S.L.; Karthikeyan, K.G.; Pedersen, J.A. Root Uptake of Pharmaceuticals and Personal Care Product Ingredients. Environ. Sci. Technol. 2016, 50, 525–541. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Cui, K.; Yan, H.; Li, Y.; Chai, Y.; Liu, X.; Cheng, J.; Yu, X. Uptake and translocation of imidacloprid, thiamethoxam and difenoconazole in rice plants. Environ. Pollut. 2017, 226, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Byrne, F.J.; Toscano, N.C. Uptake and persistence of imidacloprid in grapevines treated by chemigation. Crop Prot. 2006, 25, 831–834. [Google Scholar] [CrossRef]
- Qiu, J.; Chen, G.; Xu, J.; Luo, E.; Liu, Y.; Wang, F.; Zhou, H.; Liu, Y.; Zhu, F.; Ouyang, G. In vivo tracing of organochloride and organophosphorus pesticides in different organs of hydroponically grown Malabar spinach (Basella alba L.). J. Hazard. Mater. 2016, 316, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Felizeter, S.; McLachlan, M.S.; de Voogt, P. Uptake of Perfluorinated Alkyl Acids by Hydroponically Grown Lettuce (Lactuca sativa). Environ. Sci. Technol. 2012, 46, 11735–11743. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Ernst, F.; Conkle, J.L.; Gan, J. Comparative uptake and translocation of pharmaceutical and personal care products (PPCPs) by common vegetables. Environ. Int. 2013, 60, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhu, L. Plant uptake, accumulation and translocation of phenanthrene and pyrene in soils. Chemosphere 2004, 55, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Dodgen, L.; Li, J.; Parker, D.; Gan, J. Uptake and accumulation of four PPCP/EDCs in two leafy vegetables. Environ. Pollut. 2013, 182, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, B.; Adak, T.; Patil, N.K.B.; Pandi, G.G.P.; Gowda, G.B.; Jambhulkar, N.N.; Yadav, M.K.; Panneerselvam, P.; Kumar, U.; Munda, S.; et al. Imidacloprid application changes microbial dynamics and enzymes in rice soil. Ecotoxicol. Environ. Saf. 2017, 144, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, N.S.; Zakaria, M.P.; Omar, D.; Sijam, K.; Khakvar, R. Effects of imidacloprid on the biodiversity of soil microbes in selected soils of Malaysia. In Proceedings of the 2nd International Conference on Environmental Science and Development IPCBEE, Singapore, 26–28 February 2011. [Google Scholar]
- Cycoń, M.; Piotrowska-Seget, Z. Biochemical and microbial soil functioning after application of the insecticide imidacloprid. J. Environ. Sci. 2015, 27, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Cycoń, M.; Piotrowska-Seget, Z. Community structure of ammonia-oxidizing archaea and ammonia-oxidizing bacteria in soil treated with the insecticide imidacloprid. BioMed. Res. Int. 2015, 2015, 582938. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xue, C.; Wang, C. Effects of imidacloprid on soil microbial communities in different saline soils. Environ. Sci. Pollut. Res. 2015, 22, 19667–19675. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Singh, D.K. Bacterial, Azotobacter, Actinomycetes, and Fungal Population in Soil after Diazinon, Imidacloprid, and Lindane Treatments in Groundnut (Arachis hypogaea L.) Fields. J. Environ. Sci. Health Part B 2005, 40, 785–800. [Google Scholar] [CrossRef] [PubMed]
- Cela, F.; Avio, L.; Giordani, T.; Vangelisti, A.; Cavallini, A.; Turrini, A.; Sbrana, C.; Pardossi, A.; Incrocci, L. Arbuscular mycorrhizal fungi increase nutritional quality of soilless grown lettuce while overcoming low phosphorus supply. Foods 2022, 11, 3612. [Google Scholar] [CrossRef] [PubMed]
- Tu, C. Effect of some technical and formulated insecticides on microbial activities in soil. J. Environ. Sci. Health Part B 1991, 26, 557–573. [Google Scholar] [CrossRef]
- Deborah, B.V.; Madhuri, R.J. Effect of imidacloprid and triadimefon on microbial phosphatase, protease and urease enzyme activities in tomato (Lycopersicon esculentum sp.) cultivated soil. J. Appl. Nat. Sci. 2013, 5, 323–327. [Google Scholar] [CrossRef]
| Properties | NCS | CS |
|---|---|---|
| Organic matter (%) | 0.55 b | 1.79 a |
| Total nitrogen (%) | 0.05 b | 0.11 a |
| Available phosphorus (mg/kg) | 0.11 b | 0.69 a |
| Available potassium (mg/kg) | 274 a | 287 a |
| pH | 7.34 a | 7.56 a |
| EC (dS m−1) | 0.70 a | 0.69 a |
| Particle size distribution | ||
| Sand (%) | 51.1 | 39.1 |
| Clay (%) | 38.0 | 30.9 |
| Silt (%) | 10.9 | 30.0 |
| Days | Cultivated Soil (CS) | Non-Cultivated Soil (NCS) | ||
|---|---|---|---|---|
| RCF | TF | RCF | TF | |
| 1 | 0.3 ± 0.1 | 19.5 ± 0.3 | 0.3 ± 0.7 | 15.7 ± 14.9 |
| 5 | 0.5 ± 0.1 | 19.0 ± 0.2 | 0.5 ± 0.9 | 12.2 ± 19.4 |
| 10 | 1.0 ± 0.6 | 9.1 ± 0.2 | 0.5 ± 0.8 | 9.9 ± 13.0 |
| 15 | 1.4 ± 0.6 | 7.8 ± 0.2 | 0.5 ± 0.5 | 8.3 ± 6.9 |
| 21 | 3.2 ± 1.0 | 5.0 ± 0.3 | 0.5 ± 0.4 | 5.2 ± 2.4 |
| 28 | 2.5 ± 0.6 | 5.8 ± 0.3 | 0.6 ± 0.6 | 3.3 ± 1.1 |
| 35 | 2.5 ± 0.3 | 2.0 ± 0.2 | 0.5 ± 0.5 | 3.8 ± 0.9 |
| Average | 1.6 | 9.7 | 0.5 | 8.3 |
| Pesticides | RCFs | TFs | Plant Type | References |
| parathion-methyl | Malabar spinach | [40] | ||
| propetamphos | ||||
| endosulfan | ||||
| fenthion | ||||
| difenoconazole | 2 | Rice and lettuce | [38,41] | |
| perfluorinated alkyl acids | ||||
| imidacloprid | 7.3 | [38] | ||
| thiamethoxam | 7.2 | |||
| chlorpyrifos | 39 | 0.096 and 0.137 | pakchoi and lettuce | [13] |
| acenaphthene | 1.3 | maize | [42] | |
| fluorene | 8.9 | |||
| phenanthrene | 1.8 | |||
| perfluorinated alkyl acids | cabbage, zucchini, and tomato | |||
| di-butyl phthalate | 0.34 | lettuce | [41] | |
| di(2-ethylhexyl) phthalate | 0.77 | |||
| mono-n-butyl phthalate | 0.93 | |||
| mono (2 ethylhexyl) phthalate | 0.57 | |||
| phenanthrene | 0.006–0.12 | Same plants | [43] | |
| bisphenol A | 0.051 | collards | [44] | |
| diclofenac | 0.131 | |||
| naproxen | 0.511 | |||
| nonylphenol | 0.079 | |||
| parathion-methyl | Malabar spinach | [40] | ||
| propetamphos | ||||
| endosulfan | ||||
| fenthion |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, A.A.; Bazyad, A.; Alotaibi, F.; Alotaibi, K.D.; Codling, G.; Alharbi, H.A. Imidacloprid Uptake and Accumulation in Lettuce Plant (Lactuca sativa L. var. longipolia) and Its Effects on Abundance of Microbial Communities in Cultivated and Non-Cultivated Arid Soil. Plants 2024, 13, 2017. https://doi.org/10.3390/plants13152017
Ahmed AA, Bazyad A, Alotaibi F, Alotaibi KD, Codling G, Alharbi HA. Imidacloprid Uptake and Accumulation in Lettuce Plant (Lactuca sativa L. var. longipolia) and Its Effects on Abundance of Microbial Communities in Cultivated and Non-Cultivated Arid Soil. Plants. 2024; 13(15):2017. https://doi.org/10.3390/plants13152017
Chicago/Turabian StyleAhmed, Ahmed A., Abdulgader Bazyad, Fahad Alotaibi, Khaled D. Alotaibi, Garry Codling, and Hattan A. Alharbi. 2024. "Imidacloprid Uptake and Accumulation in Lettuce Plant (Lactuca sativa L. var. longipolia) and Its Effects on Abundance of Microbial Communities in Cultivated and Non-Cultivated Arid Soil" Plants 13, no. 15: 2017. https://doi.org/10.3390/plants13152017






