Impact of Sodium Alginate-Encapsulated Iron Nanoparticles and Soil Yeasts on the Photosynthesis Performance of Lactuca sativa L. Plants
Abstract
1. Introduction
2. Results
2.1. Determination of Plant Growth-Promotion (PGP) Traits of Yeast Strains
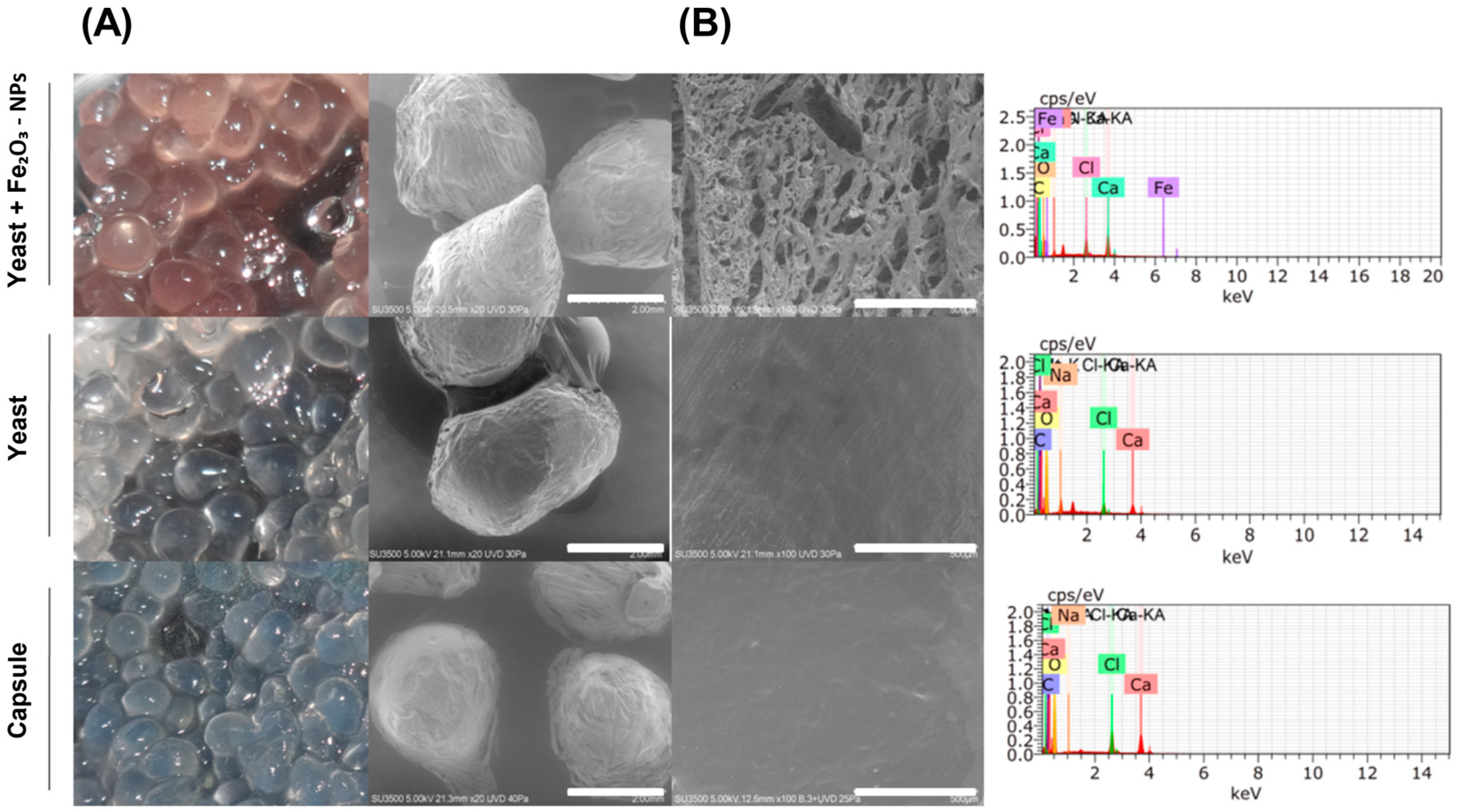
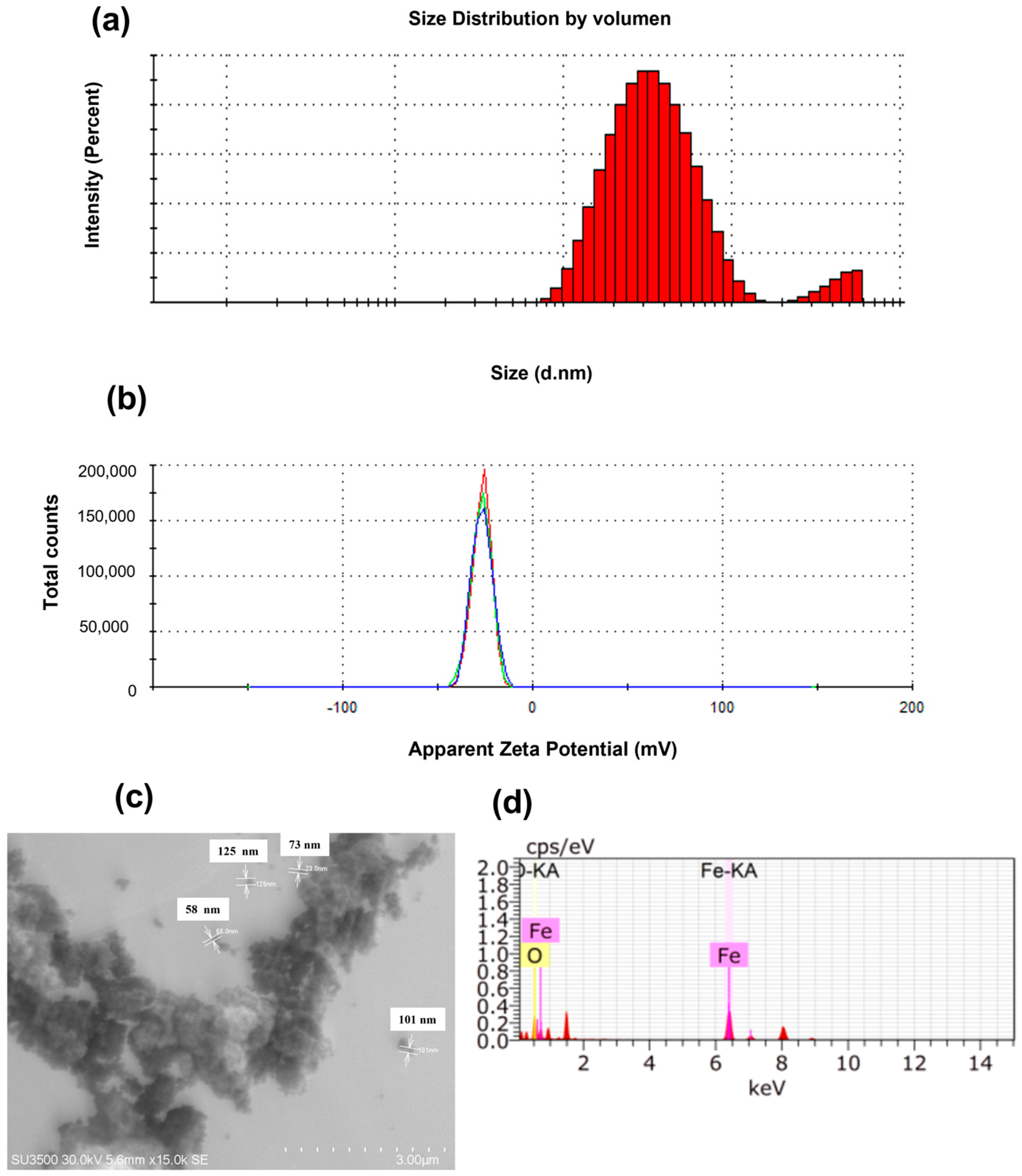
2.2. Physicochemical Characterisation of Nanocapsules and Iron Oxide Nanoparticles (α-Fe2O3-NPs)
2.3. Biomass Production of Lettuce Plants
2.4. Photosynthesis and Water Status
2.5. Photosynthetic Pigments
2.6. Multivariate Analysis
3. Discussion
4. Materials and Methods
4.1. Soil Collection and Characterisation
4.2. Determination of PGP Traits of Yeast Strains
4.3. Physicochemical Characterisation of the α-Fe2O3-NPs
4.4. In Vivo Bioassays and Plant Growth Conditions
4.5. Biomass Production
4.6. Photosynthesis Yield and Water Status
4.7. Photosynthetic Pigments
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Daszkiewicz, T. Food Production in the Context of Global Developmental Challenges. Agriculture 2022, 12, 832. [Google Scholar] [CrossRef]
- Al-Mamun, R.; Hasan, R.; Ahommed, S.; Bacchu, S.; Ali, R.; Hossain Khan, Z. Nanofertilizers towards sustainable agriculture and environment. Environ. Technol. Innov. 2021, 23, 101658. [Google Scholar] [CrossRef]
- Xu, H.; Fan, Z.; Ahmad, F.; Zhang, D. Exploring the ecological protection impacts of cultivated land transfer: Explanation based on fertilizers and pesticides. Ecol. Indic. 2024, 154, 110681. [Google Scholar] [CrossRef]
- Saroop, S.; Tamchos, S. Chapter 7—Impact of pesticide application: Positive and negative side. In Pesticides in the Environment: Impact, Assessment, and Remediation; Sharma, A., Kumar, V., Zheng, B., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 155–178. [Google Scholar]
- Shah, F.; Wu, W. Soil and Crop Management Strategies to Ensure Higher Crop Productivity within Sustainable Environments. Sustainability 2019, 11, 1485. [Google Scholar] [CrossRef]
- He, X.; Deng, H.; Hwang, H. The current application of nanotechnology in food and agriculture. J. Food Drug Anal. 2019, 27, 1–21. [Google Scholar] [CrossRef]
- Kamle, M.; Mahato, D.; Dev, S.; Tripathi, V.; Mishra, A.; Kumar, P. Nanotechnological interventions for plant health improvement and sustainable agriculture. 3 Biotech 2020, 10, 168. [Google Scholar] [CrossRef]
- Feregrino-Perez, A.; Magaña-López, E.; Guzmán, C.; Esquivel, K. A general overview of the benefits and possible negative effects of the nanotechnology in horticulture. Sci. Hortic. 2018, 238, 126–137. [Google Scholar] [CrossRef]
- Rafique, R.; Zahra, Z.; Virk, N.; Shahid, M.; Pinelli, E.; Kallerhoff, J.; Park, T.J.; Arshad, M. Data on rhizosphere pH, phosphorus uptake and wheat growth responses upon TiO2 nanoparticles application. Data Brief 2018, 17, 890–896. [Google Scholar] [CrossRef]
- Fincheira, P.; Hoffmann, N.; Tortella, G.; Ruiz, A.; Cornejo, P.; Diez, M.C.; Seabra, A.B.; Benavides-Mendoza, A.; Rubilar, O. Eco-Efficient Systems Based on Nanocarriers for the Controlled Release of Fertilizers and Pesticides: Toward Smart Agriculture. J. Nanomater. 2023, 13, 1978. [Google Scholar] [CrossRef]
- Salama, H.M.H. Effects of silver nanoparticles in some crop plants, common bean (Phaseolus vulgaris L.) and corn (Zea mays L.). Int. J. Biotechnol. 2012, 3, 190–197. [Google Scholar]
- Rui, M.; Ma, C.; Hao, Y.; Guo, J.; Rui, Y.; Tang, X.; Zhao, Q.; Fan, X.; Zhang, Z.; Hou, T.; et al. Iron oxide nanoparticles as a potential Iron fertilizer for Peanut (Arachis hypogaea). Front. Plant Sci. 2016, 7, 815. [Google Scholar] [CrossRef]
- Du, W.; Yan, J.; Peng, Q.; Liang, X.; Mao, H. Comparison study of zinc nanoparticles and zinc sulphate on wheat growth: From toxicity and zinc biofortification. Chemosphere 2019, 27, 109–116. [Google Scholar] [CrossRef]
- Iqbal, M.; Raja, N.I.; Hussain, M.; Ejaz, M.; Yasmeen, F. Effect of silver nanoparticles on growth of wheat under heat stress. J. Sci. Technol. Trans. 2019, 43, 387–395. [Google Scholar] [CrossRef]
- Wang, Z.; Hassan, M.U.; Nadeem, F.; Wu, L.; Zhang, F.; Li, X. Magnesium fertilization improves crop yield in most production systems: A meta-analysis. Front. Plant Sci. 2020, 10, 1727. [Google Scholar] [CrossRef]
- Fatima, F.; Hashim, A.; Anees, S. Efficacy of nanoparticles as nanofertilizer production: A review. Environ. Sci. Pollut. Res. Int. 2021, 28, 1292–1303. [Google Scholar] [CrossRef]
- Zörb, C.; Piepho, H.; Zikeli, S.; Horneburg, B. Heritability and Variability of Quality Parameters of Tomatoes in Outdoor Production. Sci. Res. J. 2020, 9, 6707529. [Google Scholar] [CrossRef]
- Dong, H.; Li, F.; Xuan, X.; Ahiakpa, J.; Tao, J.; Zhang, X.; Ge, P.; Wang, Y.; Gai, W.; Zhang, Y. The genetic basis and improvement of photosynthesis in tomato. Hortic. Plant J. 2024, 10, 168. [Google Scholar] [CrossRef]
- Hong, T.; Cai, Z.; Li, R.; Liu, J.; Li, J.; Wang, Z.; Zhang, Z. Effects of water and nitrogen coupling on watermelon growth, photosynthesis and yield under CO2 enrichment. Agric. Water Manag. 2022, 259, 107229. [Google Scholar] [CrossRef]
- Zhang, Y.; Peng, Y.; Liu, J.; Yan, J.; Zhu, K.; Sun, X.; Bu, X.; Wang, X.; Ahammed, G.; Liu, Y.; et al. Tetratricopeptide repeat protein SlREC2 positively regulates cold tolerance in tomato. Plant Physiol. 2023, 192, 648–665. [Google Scholar] [CrossRef]
- Therby-Vale, R.; Lacombe, B.; Rhee, S.Y.; Nussaume, L.; Rouached, H. Mineral nutrient signaling controls photosynthesis: Focus on iron deficiency-induced chlorosis. Trends Plant Sci. 2021, 27, 502–509. [Google Scholar] [CrossRef]
- Muhie, S.H. Optimization of photosynthesis for sustainable crop production. CABI Agric. Biosci. 2022, 3, 50. [Google Scholar] [CrossRef]
- Wang, C.; Liu, X.; Li, J.; Yue, L.; Yang, H.; Zou, H.; Xing, B. Copper nanoclusters promote tomato (Solanum lycopersicum L.) yield and quality through improving photosynthesis and roots growth. Environ. Pollut. 2021, 289, 117912. [Google Scholar] [CrossRef]
- Rai, S.; Singh, P.; Mankotia, S.; Swain, J.; Satbhai, S. Iron homeostasis in plants and its crosstalk with copper, zinc, and manganese. J. Plant Stress 2021, 1, 100008. [Google Scholar] [CrossRef]
- Hasan, M.; Rafique, S.; Zafar, A.; Loomba, S.; Khan, R.; Hassan, S.G.; Khan, M.W.; Zahra, S.; Zia, M.; Mustafa, G.; et al. Physiological and anti-oxidative response of biologically and chemically synthesized iron oxide: Zea mays a case study. Heliyon 2020, 6, 8. [Google Scholar] [CrossRef]
- Pariona, N.; Martinez, A.I.; Hdz-García, H.M.; Cruz, L.A.; Hernandez-Valdes, A. Effects of hematite and ferrihydrite nanoparticles on germination and growth of maize seedlings. J. Biol. Sci. 2017, 24, 1547–1554. [Google Scholar] [CrossRef]
- Abusalem, M.; Awwad, A.; Jamal, A.; Azmi, A.B. Green Synthesis of α-Fe2O3 Nanoparticles Using Pistachio Leaf Extract Influenced Seed Germination and Seedling Growth of Tomatos. J. Earth Environ. Sci. 2019, 10, 161–166. [Google Scholar]
- Plaksenkova, I.; Jermaļonoka, M.; Bankovska, l.; Gavarāne, I.; Gerbreders, V.; Sledevskis, E.; Sniķeris, J.; Kokina, I. Effects of Fe3O4 Nanoparticle Stress on the Growth and Development of Rocket Eruca sativa. J. Nanomater. 2019, 10, 2678247. [Google Scholar] [CrossRef]
- De Souza, C.; Nogueira, B.; Rostelato, M. Review of the methodologies used in the synthesis gold nanoparticles by chemical reduction. J. Alloys Compd. 2019, 798, 714–740. [Google Scholar] [CrossRef]
- Kokina, I.; Jahundoviča, I.; Mickeviča, I.; Sledevskis, E.; Ogurcovs, A.; Polyakov, B.; Jermaļonoka, M.; Strautiņš, J.; Gerbreders, V. The Impact of CdS Nanoparticles on Ploidy and DNA Damage of Rucola (Eruca sativa Mill.) Plants. J. Nanomater. 2015, 2015, 470250. [Google Scholar] [CrossRef]
- Sarabia, M.; Cazares, S.; González-Rodríguez, A.; Mora, F.; Carreón-Abud, Y.; Larsen, J. Plant growth promotion traits of rhizosphere yeasts and their response to soil characteristics and crop cycle in maize agroecosystems. Rhizosphere 2018, 6, 67–73. [Google Scholar] [CrossRef]
- Lopes, M.J.S.; Dias-Filho, M.B.; Gurgel, E.S.C. Successful Plant Growth-Promoting Microbes: Inoculation Methods and Abiotic Factors. Front. Sustain. Food Syst. 2021, 5, 606454. [Google Scholar] [CrossRef]
- Amprayn, K.O.; Rose, M.T.; Kecskés, M.; Pereg, L.; Nguyen, H.T.; Kennedy, I.R. Plant growth promoting characteristics of soil yeast (Candida tropicalis HY) and its effectiveness for promoting rice growth. J. Appl. Ecol. 2012, 61, 295–299. [Google Scholar] [CrossRef]
- Xu, L.; Ravnskov, S.; Larsen, J.; Nicolaisen, M. Linking fungal communities in roots, rhizosphere, and soil to the health status of Pisum sativum. Microb. Ecol. 2021, 82, 736–745. [Google Scholar] [CrossRef]
- Vidal, C.; González, F.; Santander, C.; Pérez, R.; Gallardo, V.; Santos, C.; Aponte, H.; Ruiz, A.; Cornejo, P. Management of Rhizosphere Microbiota and Plant Production under Drought Stress: A Comprehensive Review. Plants 2022, 11, 2437. [Google Scholar] [CrossRef]
- Kurtzman, C.P.; Fell, J.W.; Boekhout, T.; Robert, V. Methods for Isolation, Phenotypic Characterization and Maintenance of Yeasts. In The Yeasts: A Taxonomic Study; Elsevier: Amsterdam, The Netherlands, 2011; Volume 5, pp. 87–110. [Google Scholar]
- Silambarasan, S.; Logeswari, P.; Cornejo, P.; Kannan, V.R. Evaluation of the production of exopolysaccharide by plant growth promoting yeast Rhodotorula sp. strain CAH2 under abiotic stress conditions. Int. J. Biol. Macromol. 2019, 121, 55–62. [Google Scholar] [CrossRef]
- Fernández-San Millán, A.; Farran, I.; Larraya, L.; Ancin, M.; Arregui, L.M.; Veramendi, J. Plant growth-promoting traits of yeasts isolated from Spanish vineyards: Benefits for seedling development. Microbiol. Res. 2020, 237, 126480. [Google Scholar] [CrossRef]
- Pérez, R.; Tapia, Y.; Antilén, M.; Ruiz, A.; Pimentel, P.; Santander, C.; Aponte, H.; González, F.; Cornejo, P. Beneficial Interactive Effects Provided by an Arbuscular Mycorrhizal Fungi and Yeast on the Growth of Oenothera picensis Established on Cu Mine Tailings. Plants 2023, 12, 4012. [Google Scholar] [CrossRef]
- Balla, P.; Seelam, P.; Balaga, R.; Rajesh, R.; Perupogu, V.; Liang, T. Immobilized highly dispersed Ni nanoparticles over porous carbon as an efficient catalyst for selective hydrogenation of furfural and levulinic acid. J. Environ. Chem. Eng. 2021, 9, 106530. [Google Scholar] [CrossRef]
- López, A.; Javier, G.A.; Fenoll, J.; Hellín, P.; Flores, P. Chemical composition and antioxidant capacity of lettuce: Comparative study of regular-sized (Romaine) and baby-sized (Little Gem and Mini Romaine) types. J. Food Compos. Anal. 2014, 33, 39–48. [Google Scholar] [CrossRef]
- Shi, M.; Gu, J.; Wu, H.; Rauf, A.; Emran, T.B.; Khan, Z.; Mitra, S.; Aljohani, A.S.M.; Alhumaydhi, F.A.; Al-Awthan, Y.S.; et al. Phytochemicals, Nutrition, Metabolism, Bioavailability, and Health Benefits in Lettuce—A Comprehensive Review. Antioxidants 2022, 11, 1158. [Google Scholar] [CrossRef]
- Silambarasan, S.; Vangnai, A.S. Plant-growth promoting Candida sp. AVGB4 with capability of 4-nitroaniline biodegradation under drought stress. Environ. Toxicol. 2017, 139, 472–480. [Google Scholar] [CrossRef]
- Duca, D.R.; Glick, B.R. Indole-3-acetic acid biosynthesis and its regulation in plant-associated bacteria. Appl. Microbiol. Biotechnol. 2020, 104, 8607–8619. [Google Scholar] [CrossRef]
- Benadjila, A.; Zamoum, M.; Aouar, L.; Zitouni, A.; Goudjal, Y. Optimization of cultural conditions using response surface methodology and modeling of indole-3-acetic acid production by Saccharothrix texasensis MB15. Biocatal. Agric. Biotechnol. 2022, 39, 102271. [Google Scholar] [CrossRef]
- Vacheron, J.; Desbrosses, G.; Bouffaud, M.L.; Touraine, B.; Moënne-Loccoz, Y.; Muller, D.; Legendre, L.; Wisniewski-Dyé, F.; Prigent-Combaret, C. Plant growth-promoting rhizobacteria and root system functioning. Front. Plant Sci. 2013, 4, 356. [Google Scholar] [CrossRef]
- Gao, J.; Wang, N.; Zhang, G.; Yan, Y.; Wang, G.; Zhang, S. Influences of Saccharomyces cerevisiae on gas exchange and water-use efficiency in Vicia faba L. J. Food Agric. 2019, 31, 902–909. [Google Scholar] [CrossRef]
- Pascoli, M.; Lopes-Oliveira, P.J.; Fraceto, L.F. State of the art of polymeric nanoparticles as carrier systems with agricultural applications: A minireview. Energy Ecol. Environ. 2018, 3, 137–148. [Google Scholar] [CrossRef]
- Jiménez-Arias, D.; Morales-Sierra, S.; Borges, A.; Díaz, D. Biostimulant Nanoencapsulation: The New Keystone to Fight Hunger. J. Agric. Food Chem. 2020, 68, 7083–7085. [Google Scholar] [CrossRef]
- Yin, J.; Wang, H.; Yang, Z.; Wang, J.; Wang, Z.; Duan, L.; Li, Z.; Tan, W. Engineering lignin nanomicroparticles for the antiphotolysis and controlled release of the plant growth regulator abscisic acid. J. Agric. Food Chem. 2020, 68, 7360–7368. [Google Scholar] [CrossRef]
- Campos, S.; Pereira, A.; Aleksieienko, I.; do Carmo, G.; Gohari, G.; Santaella, C.; Fraceto, L.; Oliveira, H. Encapsulated plant growth regulators and associative microorganisms: Nature-based solutions to mitigate the effects of climate change on plants. Int. J. Plant Sci. 2023, 331, 111688. [Google Scholar] [CrossRef] [PubMed]
- Safari, M.; Motamedi, E.; Dolatabad, H.K.; Sanavy, M. Nano-carriers effects on the viability and efficiency of Pseudomonas strains as phosphate solubilizing bacteria. Heliyon 2020, 6, e05076. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Shen, D.; Liu, X.; Dong, S.; Jing, X.; Wu, W.; Tong, Y.; Gao, S.; Mao, L. Uptake of iron oxide nanoparticles inhibits the photosynthesis of the wheat after foliar exposure. Chemosphere 2020, 259, 127445. [Google Scholar] [CrossRef] [PubMed]
- Buzea, C.; Pacheco, I.; Robbie, K. Nanomaterials and nanoparticles: Sources and toxicity. Biointerphases 2007, 2, MR17–MR71. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.M.; An, Y.J.; Yoon, H.; Kweon, H.S. Toxicity and bioavailability of copper nanoparticles to the terrestrial plants mung bean (Phaseolus radiatus) and wheat (Triticum aestivum): Plant agar test for water-insoluble nanoparticles. Environ. Toxicol. Chem. 2008, 27, 1915–1921. [Google Scholar] [CrossRef] [PubMed]
- Fincheira, P.; Tortella, G.; Duran, N.; Seabra, A.B.; Rubilar, O. Current applications of nanotechnology to develop plant growth inducer agents as an innovation strategy. Crit. Rev. Biotechnol. 2019, 40, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Lesuisse, E.; Blaiseau, P.L.; Dancis, A.; Camadro, J.M. Siderophore uptake and use by the yeast Saccharomyces cerevisiae. Microbiol. Res. 2001, 147, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Tapia-Vázquez, I.; Sánchez-Cruz, R.; Arroyo-Domínguez, M.; Lira-Ruan, V.; Sánchez-Reyes, A.; Sánchez-Carbente, M.; Padilla-Chacón, D.; Batista-García, D.; Folch-Mallol, J. Isolation and characterization of psychrophilic and psychrotolerant plant-growth promoting microorganisms from a high-altitude volcano crater in Mexico. Microbiol. Res. 2020, 232, 126394. [Google Scholar] [CrossRef] [PubMed]
- Lima, H.; Lima, V.; Costa, I.; Almeida, P.; Tuão, C.; Fernandes, P. Maize-associated Meyerozyma from the Brazilian semiarid region are effective plant growth-promoting yeasts. Rhizosphere 2022, 22, 100538. [Google Scholar]
- El-Maraghy, S.; Tohamy, T.; Hussein, K. Expression of SidD gene and physiological characterization of the rhizosphere plant growth-promoting yeasts. Helyon 2020, 6, e04384. [Google Scholar] [CrossRef] [PubMed]
- Lammertsma, E.I.; de Boer, H.J.; Dekker, S.C.; Dilcher, D.L.; Lotter, A.F.; Wagner-Cremer, F. Global CO2 rise leads to reduced maximum stomatal conductance in Florida vegetation. Proc. Natl. Acad. Sci. USA 2011, 108, 4035–4040. [Google Scholar] [CrossRef]
- Shah, A.; Yasin, N.; Mudassir, M.; Ramzan, M.; Hussain, I.; Siddiqui, M.; Ali, H.; Shabbir, Z.; Ali, A.; Ahmed, S.; et al. Iron oxide nanoparticles and selenium supplementation improve growth and photosynthesis by modulating antioxidant system and gene expression of chlorophyll synthase (CHLG) and protochlorophyllide oxidoreductase (POR) in arsenic-stressed Cucumis melo. Environ. Pollut. 2022, 307, 119413. [Google Scholar] [CrossRef]
- Briat, J.F.; Dubos, C.; Gaymard, F. Iron nutrition, biomass production, and plant product quality. Trends Plant Sci. 2015, 20, 1360–1385. [Google Scholar] [CrossRef]
- Kohli, K.; Handa, N.; Sharma, A.; Kumar, V.; Kaur, P.; Bhardwaj, R. Synergistic effect of 24-epibrassinolide and salicylic acid on photosynthetic efficiency and gene expression in Brassica juncea L. under Pb stress. Turk. J. Biol. 2017, 41, 6. [Google Scholar] [CrossRef]
- Pereira, S.I.A.; Abreu, D.; Moreira, H.; Vega, A.; Castro, P.M.L. Plant growth-promoting rhizobacteria (PGPR) improve the growth and nutrient use efficiency in maize (Zea mays L.) under water deficit conditions. Heliyon 2020, 6, 10. [Google Scholar] [CrossRef]
- Santander, C.; González, F.; Pérez, U.; Ruiz, A.; Aroca, R.; Santos, C.; Cornejo, P.; Vidal, G. Enhancing Water Status and Nutrient Uptake in Drought-Stressed Lettuce Plants (Lactuca sativa L.) via Inoculation with Different Bacillus spp. Isolated from the Atacama Desert. Plants 2024, 13, 158. [Google Scholar] [CrossRef]
- Nephali, L.; Piater, L.A.; Dubery, I.A.; Patterson, V.; Huyser, J.; Burgess, K.; Tugizimana, F. Biostimulants for Plant Growth and Mitigation of Abiotic Stresses: A Metabolomics Perspective. Metabolites 2020, 10, 505. [Google Scholar] [CrossRef]
- González, F.; Santander, C.; Ruiz, A.; Pérez, R.; Moreira, J.; Vidal, G.; Aroca, R.; Santos, C.; Cornejo, P. Inoculation with Actinobacteria spp. Isolated from a Hyper-Arid Environment Enhances Tolerance to Salinity in Lettuce Plants (Lactuca sativa L.). Plants 2023, 12, 2018. [Google Scholar] [CrossRef] [PubMed]
- Chen, H. Metal based nanoparticles in agricultural system: Behavior, transport, and interaction with plants. Chem. Spec. Bioavailab. 2018, 30, 123–134. [Google Scholar] [CrossRef]
- Khan, R.M.; Adam, V.; Rizvi, T.F.; Zhang, B.; Ahamad, F.; Jośko, I.; Zhu, Y.; Yang, M.; Mao, C. Nanoparticle–Plant Interactions: Two-Way Traffic. Small 2019, 15, 1901794. [Google Scholar] [CrossRef] [PubMed]
- Murali, M.; Gowtham, H.G.; Brijesh Singh, S.; Shilpa, N.; Aiyaz, M.; Alomary, M.N.; Alshamrani, M.; Salawi, A.; Almoshari, Y.; Ansari, M.A.; et al. Fate, bioaccumulation and toxicity of engineered nanomaterials in plants: Current challenges and future prospects. Sci. Total Environ. 2022, 811, 152249. [Google Scholar] [CrossRef] [PubMed]
- Gowtham, H.G.; Shilpa, N.; Brijesh Singh, S.; Aiyaz, M.; Abhilash, M.R.; Nataraj, K.; Amruthesh, K.N.; Azam Ansari, M.; Alomary, M.N.; Murali, M. Toxicological effects of nanoparticles in plants: Mechanisms involved at morphological, physiological, biochemical and molecular levels. Plant Physiol. Biochem. 2024, 210, 108604. [Google Scholar] [CrossRef]
- Zaidi, S.; Usmani, S.; Singh, B.R.; Musarrat, J. Significance of Bacillus subtilis strain SJ-101 as a bioinoculant for concurrent plant growth promotion and nickel accumulation in Brassica juncea. Chemosphere 2006, 64, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Neilands, B.B. Universal Chemical Assay for the Detection and Determination of Siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar]
- Nutaratat, P.; Srisuk, N.; Arunrattiyakorn, P.; Limtong, S. Plant growth-promoting traits of epiphytic and endophytic yeasts isolated from rice and sugar cane leaves in Thailand. Fungal Biol. 2014, 118, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Berríos, D.; Nahuelcura, J.; González, F.; Peña, F.; Cornejo, P.; Pérez-Navarro, J.; Gómez-Alonso, S.; Ruiz, A. The Biosynthesis, Accumulation of Phenolic Compounds and Antioxidant Response in Lactuca sativa L. Plants Inoculated with a Biofertilizer Based on Soil Yeast and Iron Nanoparticles. Plants 2024, 13, 388. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. In Plant Cell Membranes; Academic Press: Cambridge, MA, USA, 1987; Volume 148, pp. 350–382. ISSN 0076-6879. [Google Scholar]




| Yeast Species | Phosphate Solubilization (mg mL−1) | IAA (µg mL−1) | Siderophores | ACC Deaminase | |
|---|---|---|---|---|---|
| 24 h | Day 14 | ||||
| Candida guilliermondii | 0.17 ± 0.00 c | 1.53 ± 0.04 a | 6.46 ± 0.02 b | + | + |
| Rhodotorula mucilaginosa | 0.17 ± 0.01 c | 0.69 ± 0.02 b | 8.26 ± 0.02 a | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berríos, D.; Fincheira, P.; González, F.; Santander, C.; Cornejo, P.; Ruiz, A. Impact of Sodium Alginate-Encapsulated Iron Nanoparticles and Soil Yeasts on the Photosynthesis Performance of Lactuca sativa L. Plants. Plants 2024, 13, 2042. https://doi.org/10.3390/plants13152042
Berríos D, Fincheira P, González F, Santander C, Cornejo P, Ruiz A. Impact of Sodium Alginate-Encapsulated Iron Nanoparticles and Soil Yeasts on the Photosynthesis Performance of Lactuca sativa L. Plants. Plants. 2024; 13(15):2042. https://doi.org/10.3390/plants13152042
Chicago/Turabian StyleBerríos, Daniela, Paola Fincheira, Felipe González, Christian Santander, Pablo Cornejo, and Antonieta Ruiz. 2024. "Impact of Sodium Alginate-Encapsulated Iron Nanoparticles and Soil Yeasts on the Photosynthesis Performance of Lactuca sativa L. Plants" Plants 13, no. 15: 2042. https://doi.org/10.3390/plants13152042
APA StyleBerríos, D., Fincheira, P., González, F., Santander, C., Cornejo, P., & Ruiz, A. (2024). Impact of Sodium Alginate-Encapsulated Iron Nanoparticles and Soil Yeasts on the Photosynthesis Performance of Lactuca sativa L. Plants. Plants, 13(15), 2042. https://doi.org/10.3390/plants13152042







