Nitrate Inhibits Nodule Nitrogen Fixation by Accumulating Ureide in Soybean Plants
Abstract
:1. Introduction
2. Results
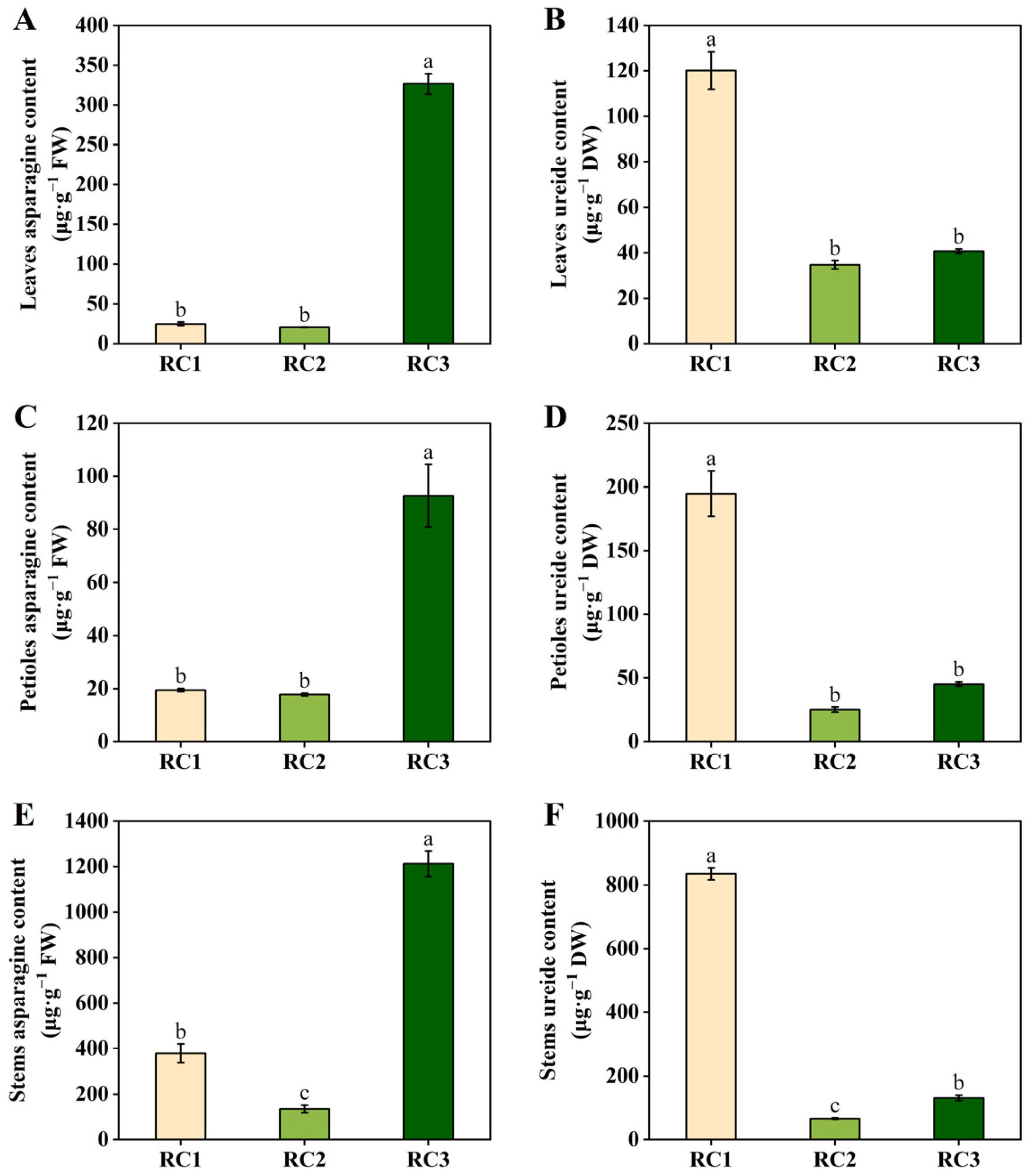
2.1. Effects of Nodule Nitrogen Fixation or Nitrate Supply on the Levels of Asparagine and Ureide in Dual-Root Soybean Shoots
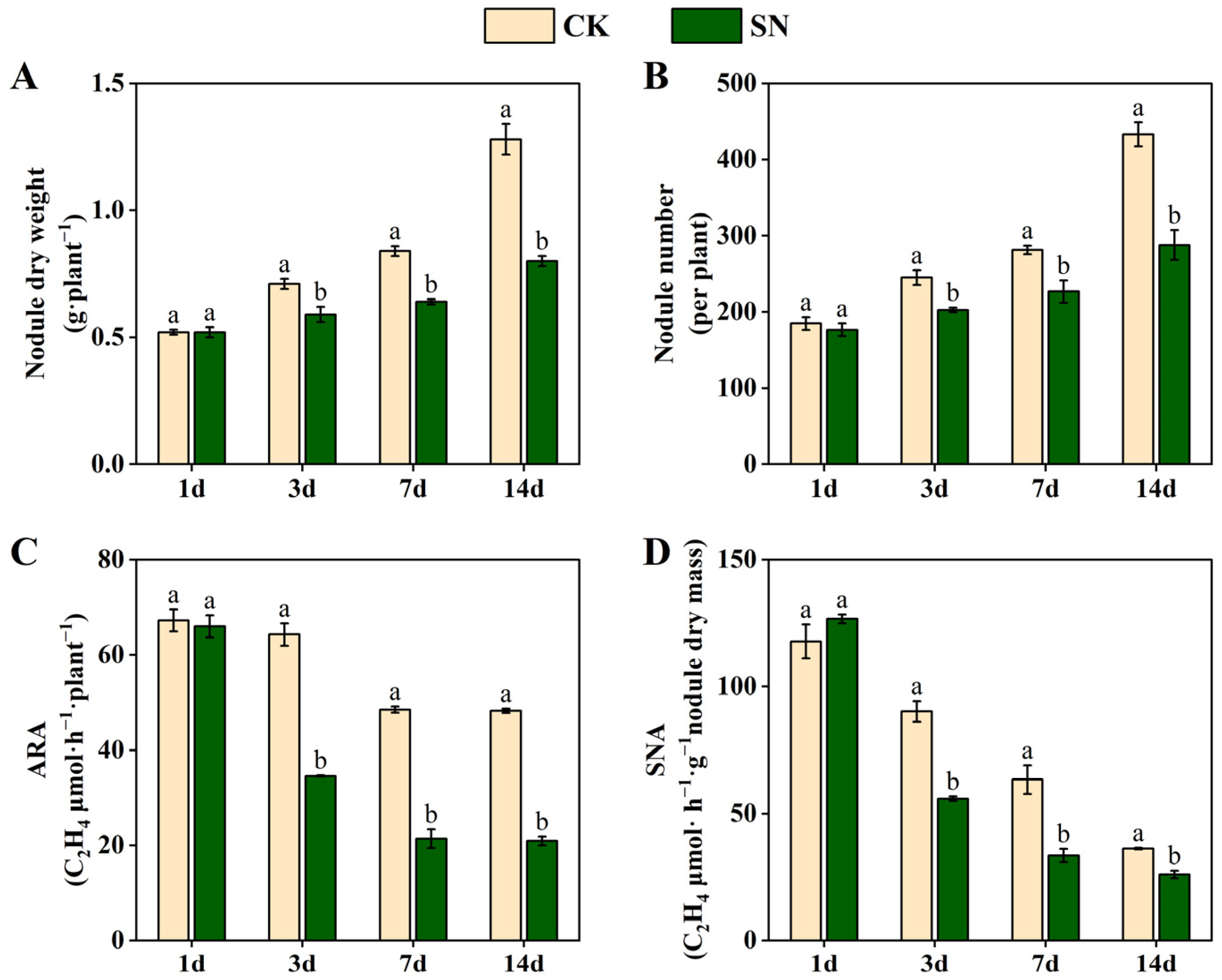
2.2. Effects of Nitrate Supply on Nodulation and Nitrogenase Activity in Dual-Root Soybeans
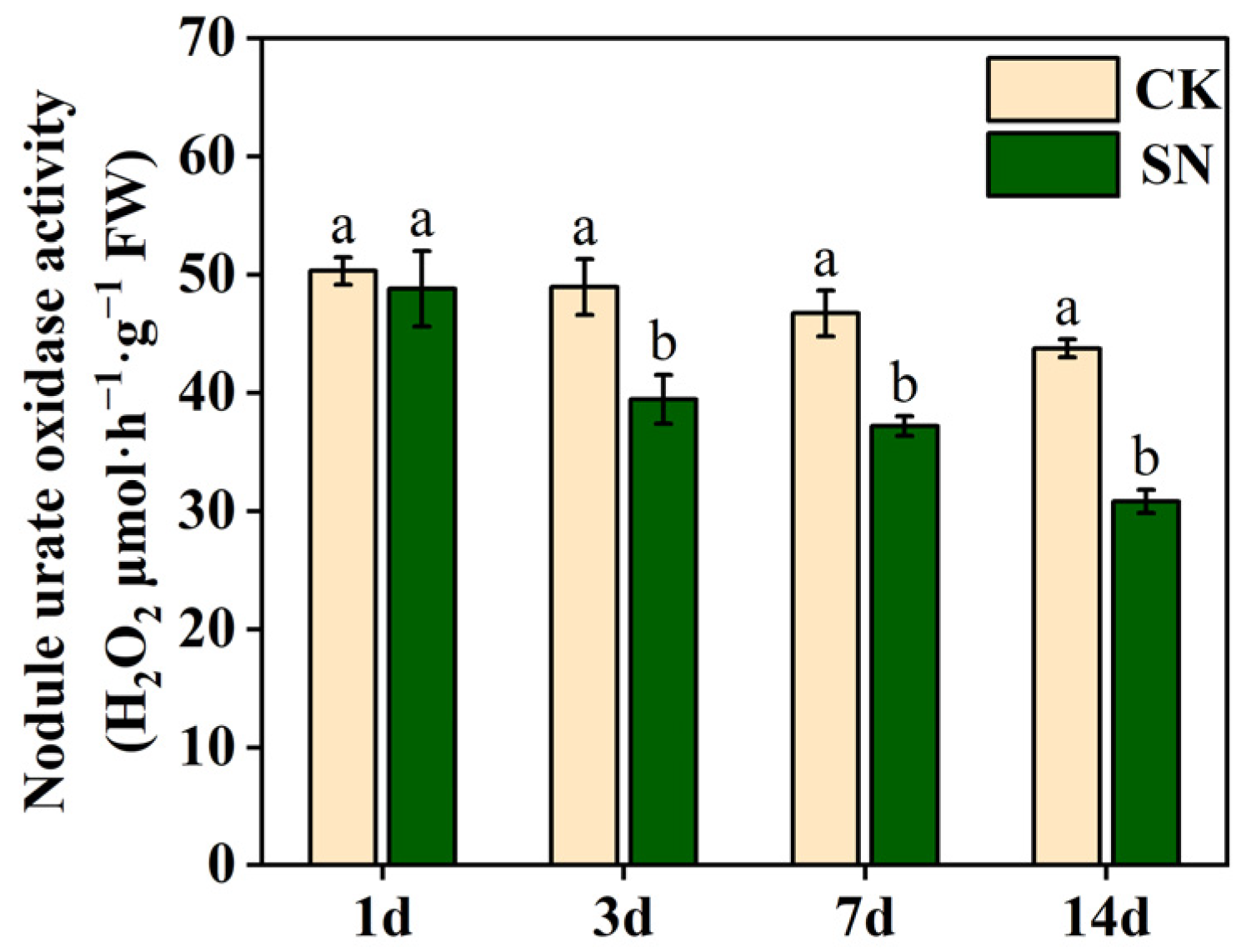
2.3. Effects of Nitrate Supply on Nodule Urate Oxidase Activity in Dual-Root Soybeans
2.4. Effects of Nitrate Supply on the Levels of Ureide in Dual-Root Soybeans
2.5. Correlations between Urate Oxidase Activity and Nitrogenase Activity in Soybean Nodules
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
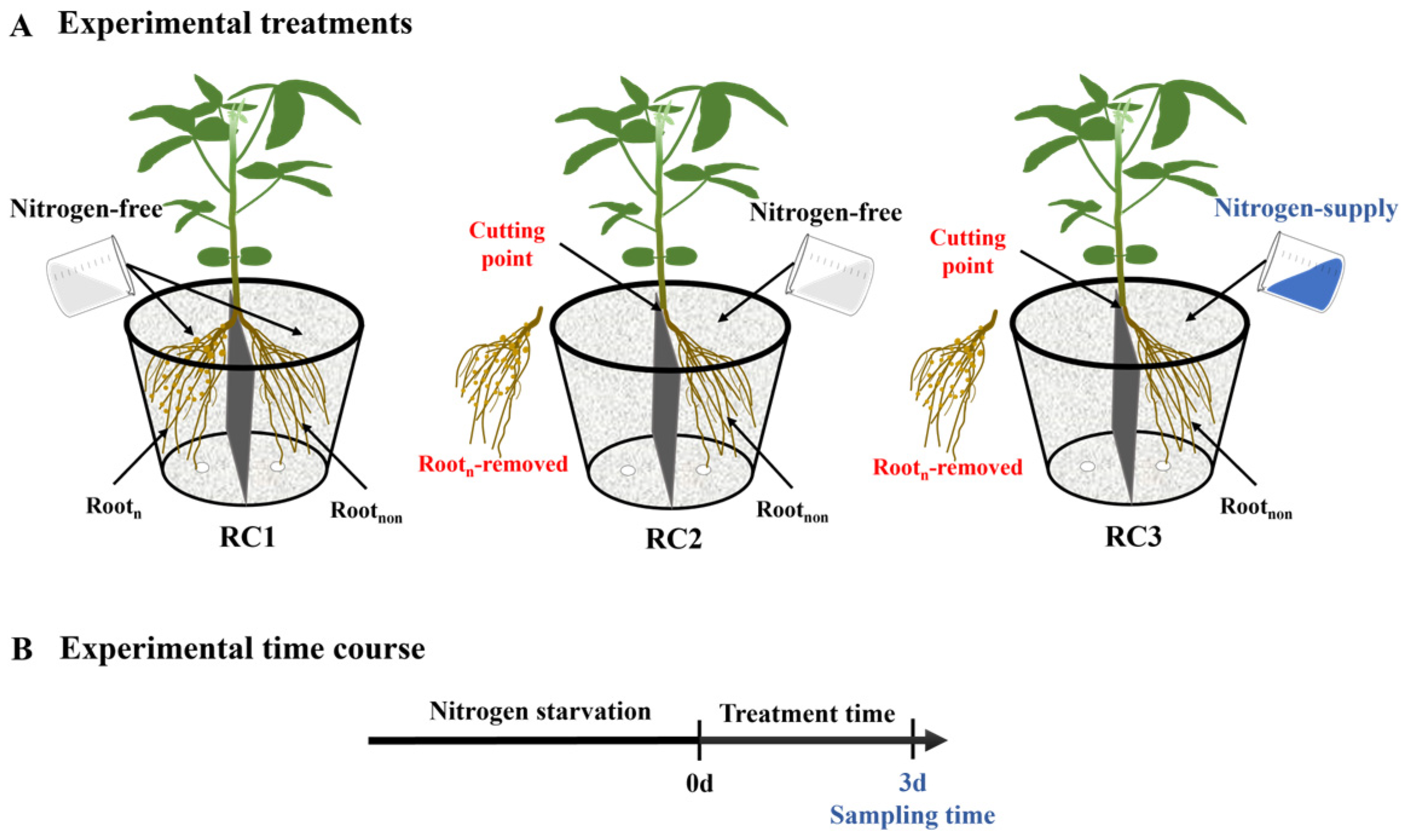
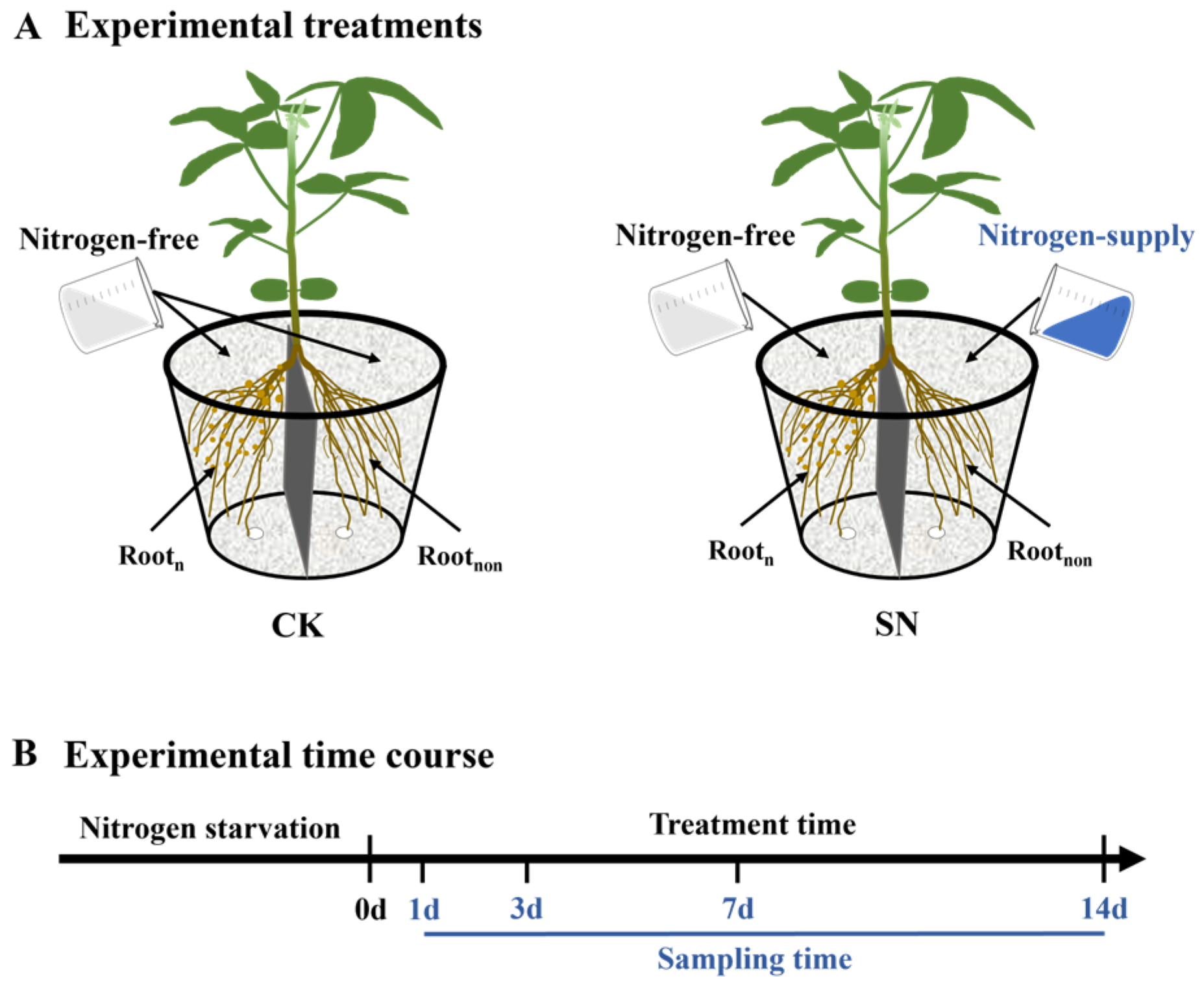
4.2. Experimental Treatments
4.3. Sampling Methods
4.4. Analysis Methods
4.5. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Saito, A.; Tanabata, S.; Tanabata, T.; Tajima, S.; Ueno, M.; Ishikawa, S.; Ohtake, N.; Sueyoshi, K.; Ohyama, T. Effect of nitrate on nodule and root growth of soybean (Glycine max (L.) Merr.). Int. J. Mol. Sci. 2014, 15, 4464–4480. [Google Scholar] [CrossRef] [PubMed]
- Malik, N.S.; Calvert, H.E.; Bauer, W.D. Nitrate induced regulation of nodule formation in soybean. Plant Physiol. 1987, 84, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Nishida, H.; Suzaki, T. Nitrate-mediated control of root nodule symbiosis. Curr. Opin. Plant Biol. 2018, 44, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, N.; Tanabata, S.; Ohtake, N.; Sueyoshi, K.; Sato, T.; Higuchi, K.; Saito, A.; Ohyama, T. Effects of different chemical forms of nitrogen on the quick and reversible inhibition of soybean nodule growth and nitrogen fixation activity. Front. Plant Sci. 2019, 10, 131. [Google Scholar] [CrossRef] [PubMed]
- Lyu, X.; Sun, C.; Zhang, J.; Wang, C.; Zhao, S.; Ma, C.; Li, S.; Li, H.; Gong, Z.; Yan, C. Integrated proteomics and metabolomics analysis of nitrogen system regulation on soybean plant nodulation and nitrogen fixation. Int. J. Mol. Sci. 2022, 23, 2545. [Google Scholar] [CrossRef] [PubMed]
- Sulieman, S.; Fischinger, S.A.; Gresshoff, P.M.; Schulze, J. Asparagine as a major factor in the N-feedback regulation of N2 fixation in Medicago truncatula. Physiol. Plant 2010, 140, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Amarante, L.d.; Lima, J.D.; Sodek, L. Growth and stress conditions cause similar changes in xylem amino acids for different legume species. Environ. Exp. Bot. 2006, 58, 123–129. [Google Scholar] [CrossRef]
- Antunes, F.; Aguilar, M.; Pineda, M.; Sodek, L. Nitrogen stress and the expression of asparagine synthetase in roots and nodules of soybean (Glycine max). Physiol. Plant 2008, 133, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lyu, X.; Wang, X.; Zhao, S.; Ma, C.; Yan, C.; Gong, Z. Assimilation of nitrate into asparagine for transport in soybeans. Agronomy 2023, 13, 2767. [Google Scholar] [CrossRef]
- Bacanamwo, M.; Harper, J.E. The feedback mechanism of nitrate inhibition of nitrogenase activity in soybean may involve asparagine and/or products of its metabolism. Physiol. Plant 1997, 100, 371–377. [Google Scholar] [CrossRef]
- Vadez, V.; Sinclair, T.R.; Serraj, R. Asparagine and ureide accumulation in nodules and shoots as feedback inhibitors of N2 fixation in soybean. Physiol. Plant 2000, 110, 215–223. [Google Scholar] [CrossRef]
- Lukaszewski, K.M.; Blevins, D.G.; Randall, D.D. Asparagine and boric acid cause allantoate accumulation in soybean leaves by inhibiting manganese-dependent allantoate amidohydrolase. Plant Physiol. 1992, 99, 1670–1676. [Google Scholar] [CrossRef] [PubMed]
- Werner, A.K.; Sparkes, I.A.; Romeis, T.; Witte, C.P. Identification, biochemical characterization, and subcellular localization of allantoate amidohydrolases from Arabidopsis and soybean. Plant Physiol. 2008, 146, 418–430. [Google Scholar] [CrossRef]
- Yoneyama, T.; Karasuyama, M.; Kouchi, H.; Ishizuka, J. Occurrence of ureide accumulation in soybean plants. Soil. Sci. Plant Nutr. 1985, 31, 133–140. [Google Scholar] [CrossRef]
- Streeter, J.G. Nitrate inhibition of legume nodule growth and activity: II. short term studies with high nitrate supply. Plant Physiol. 1985, 77, 325–328. [Google Scholar] [CrossRef] [PubMed]
- King, C.A.; Purcell, L.C. Inhibition of N2 fixation in soybean is associated with elevated ureides and amino acids. Plant Physiol. 2005, 137, 1389–1396. [Google Scholar] [CrossRef]
- Serraj, R.; Vadez, V.V.; Denison, R.F.; Sinclair, T.R. Involvement of ureides in nitrogen fixation inhibition in soybean. Plant Physiol. 1999, 119, 289–296. [Google Scholar] [CrossRef]
- Serraj, R.; Sinclair, T.R. Evidence that carbon dioxide enrichment alleviates ureide-induced decline of nodule nitrogenase activity. Ann. Bot. 2003, 91, 85–89. [Google Scholar] [CrossRef]
- Parsons, R.; Stanforth, A.; Raven, J.A.; Sprent, J.I. Nodule growth and activity may be regulated by a feedback mechanism involving phloem nitrogen. Plant Cell Environ. 1993, 16, 125–136. [Google Scholar] [CrossRef]
- Fujihara, S.; Yamaguchi, M. Asparagine formation in soybean nodules. Plant Physiol. 1980, 66, 139–141. [Google Scholar] [CrossRef]
- Huber, T.A.; Streeter, J.G. Asparagine biosynthesis in soybean nodules. Plant Physiol. 1984, 74, 605–610. [Google Scholar] [CrossRef] [PubMed]
- McClure, P.R.; Israel, D.W. Transport of nitrogen in the xylem of soybean plants. Plant Physiol. 1979, 64, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Alamillo, J.M.; Diaz-Leal, J.L.; Sanchez-Moran, M.V.; Pineda, M. Molecular analysis of ureide accumulation under drought stress in Phaseolus vulgaris L. Plant Cell Environ. 2010, 33, 1828–1837. [Google Scholar] [CrossRef] [PubMed]
- Voß, L.; Heinemann, K.J.; Herde, M.; Medina-Escobar, N.; Witte, C.-P. Enzymes and cellular interplay required for flux of fixed nitrogen to ureides in bean nodules. Nat. Commun. 2022, 13, 5331. [Google Scholar] [CrossRef]
- Nguyen, C.X.; Dohnalkova, A.; Hancock, C.N.; Kirk, K.R.; Stacey, G.; Stacey, M.G. Critical role for uricase and xanthine dehydrogenase in soybean nitrogen fixation and nodule development. Plant Genome 2023, 16, e20171. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.W.; Lee, Y.W.; Lee, S.C.; Hwang, C.H. Nitrate inhibits soybean nodulation by regulating expression of CLE genes. Plant Sci. 2014, 229, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lyu, X.; Li, M.; Li, X.; Li, S.; Yan, C.; Ma, C.; Gong, Z. Assessing the systematic effects of the concentration of nitrogen supplied to dual-root systems of soybean plants on nodulation and nitrogen Fixation. Agronomy 2020, 10, 763. [Google Scholar] [CrossRef]
- Collier, R.; Tegeder, M. Soybean ureide transporters play a critical role in nodule development, function and nitrogen export. Plant J. 2012, 72, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Todd, C.D.; Tipton, P.A.; Blevins, D.G.; Piedras, P.; Pineda, M.; Polacco, J.C. Update on ureide degradation in legumes. J. Exp. Bot. 2006, 57, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Ladrera, R.n.; Marino, D.; Larrainzar, E.b.; González, E.M.; Arrese-Igor, C. Reduced carbon availability to bacteroids and elevated ureides in nodules, but not in shoots, are involved in the nitrogen fixation response to early drought in soybean. Plant Physiol. 2007, 145, 539–546. [Google Scholar] [CrossRef]
- Xia, X.; Ma, C.; Dong, S.; Xu, Y.; Gong, Z. Effects of nitrogen concentrations on nodulation and nitrogenase activity in dual root systems of soybean plants. Soil Sci. Plant Nutr. 2017, 63, 470–482. [Google Scholar] [CrossRef]
- Fehr, W.R.; Caviness, C.E.; Burmood, D.T.; Pennington, J.S. Stage of development descriptions for soybeans, Glycine max (L.) Merrill. Crop Sci. 1971, 11, 929–931. [Google Scholar] [CrossRef]
- Vogels, G.D.; Van der Drift, C. Differential analyses of glyoxylate derivatives. Anal. Biochem. 1970, 33, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Fazel, R.; Zarei, N.; Ghaemi, N.; Namvaran, M.M.; Enayati, S.; Mirabzadeh Ardakani, E.; Azizi, M.; Khalaj, V. Cloning and expression of Aspergillus flavus urate oxidase in Pichia pastoris. SpringerPlus 2014, 3, 395. [Google Scholar] [CrossRef]

| Traits | Urate Oxidase Activity |
|---|---|
| ARA | 0.953 ** |
| SNA | 0.829 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zhang, Y.; Lian, Z.; Lyu, X.; Yan, C.; Yan, S.; Gong, Z.; Li, S.; Ma, C. Nitrate Inhibits Nodule Nitrogen Fixation by Accumulating Ureide in Soybean Plants. Plants 2024, 13, 2045. https://doi.org/10.3390/plants13152045
Wang X, Zhang Y, Lian Z, Lyu X, Yan C, Yan S, Gong Z, Li S, Ma C. Nitrate Inhibits Nodule Nitrogen Fixation by Accumulating Ureide in Soybean Plants. Plants. 2024; 13(15):2045. https://doi.org/10.3390/plants13152045
Chicago/Turabian StyleWang, Xuelai, Yuchen Zhang, Zhaohui Lian, Xiaochen Lyu, Chao Yan, Shuangshuang Yan, Zhenping Gong, Sha Li, and Chunmei Ma. 2024. "Nitrate Inhibits Nodule Nitrogen Fixation by Accumulating Ureide in Soybean Plants" Plants 13, no. 15: 2045. https://doi.org/10.3390/plants13152045
APA StyleWang, X., Zhang, Y., Lian, Z., Lyu, X., Yan, C., Yan, S., Gong, Z., Li, S., & Ma, C. (2024). Nitrate Inhibits Nodule Nitrogen Fixation by Accumulating Ureide in Soybean Plants. Plants, 13(15), 2045. https://doi.org/10.3390/plants13152045






