Targeted Screening and Quantification of Characteristic Sesquiterpene Lactones in Ambrosia artemisiifolia L. at Different Growth Stages
Abstract
1. Introduction
2. Results
2.1. HPLC/DAD Method for the Analysis of A. artemisiifolia Aerial Parts
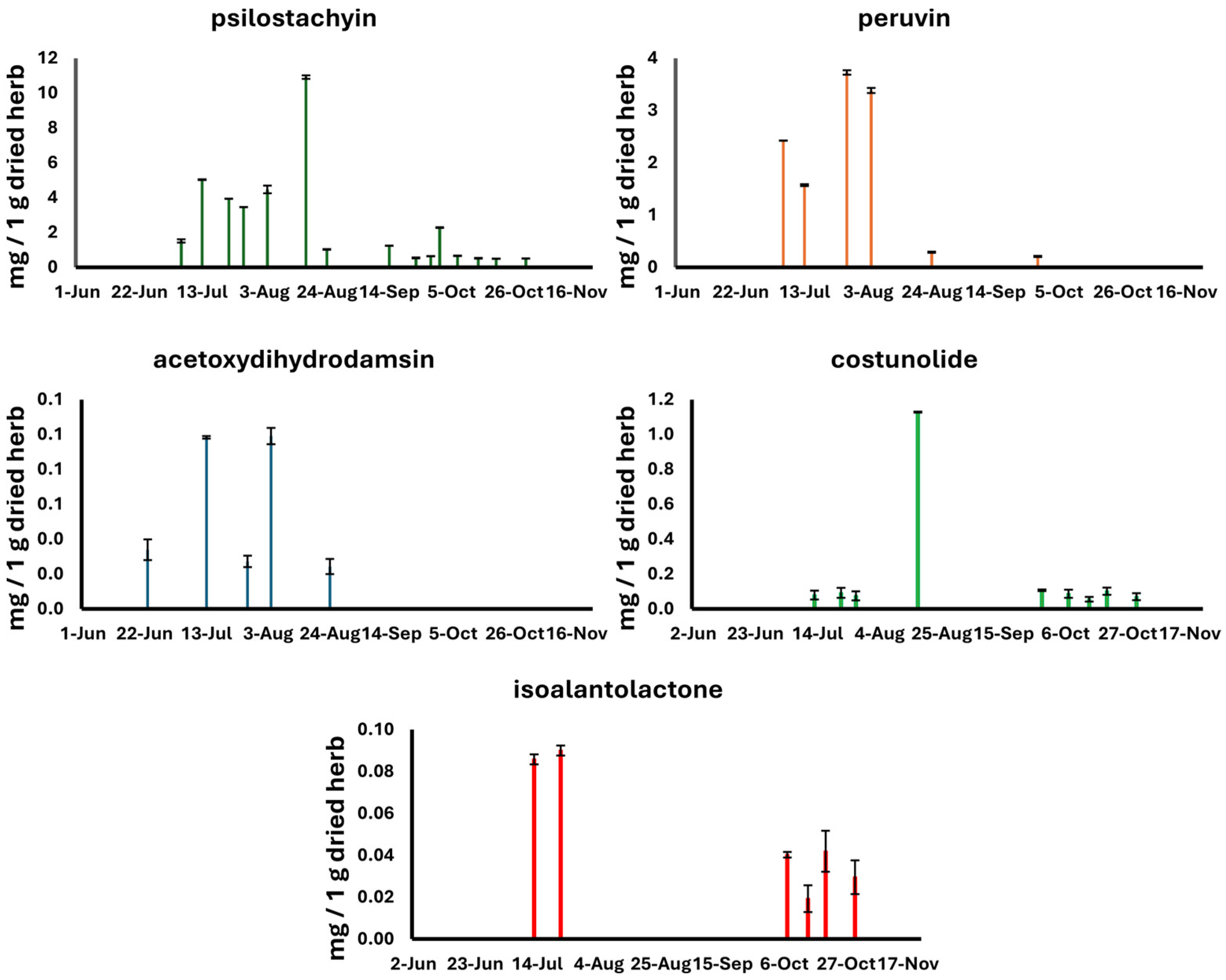
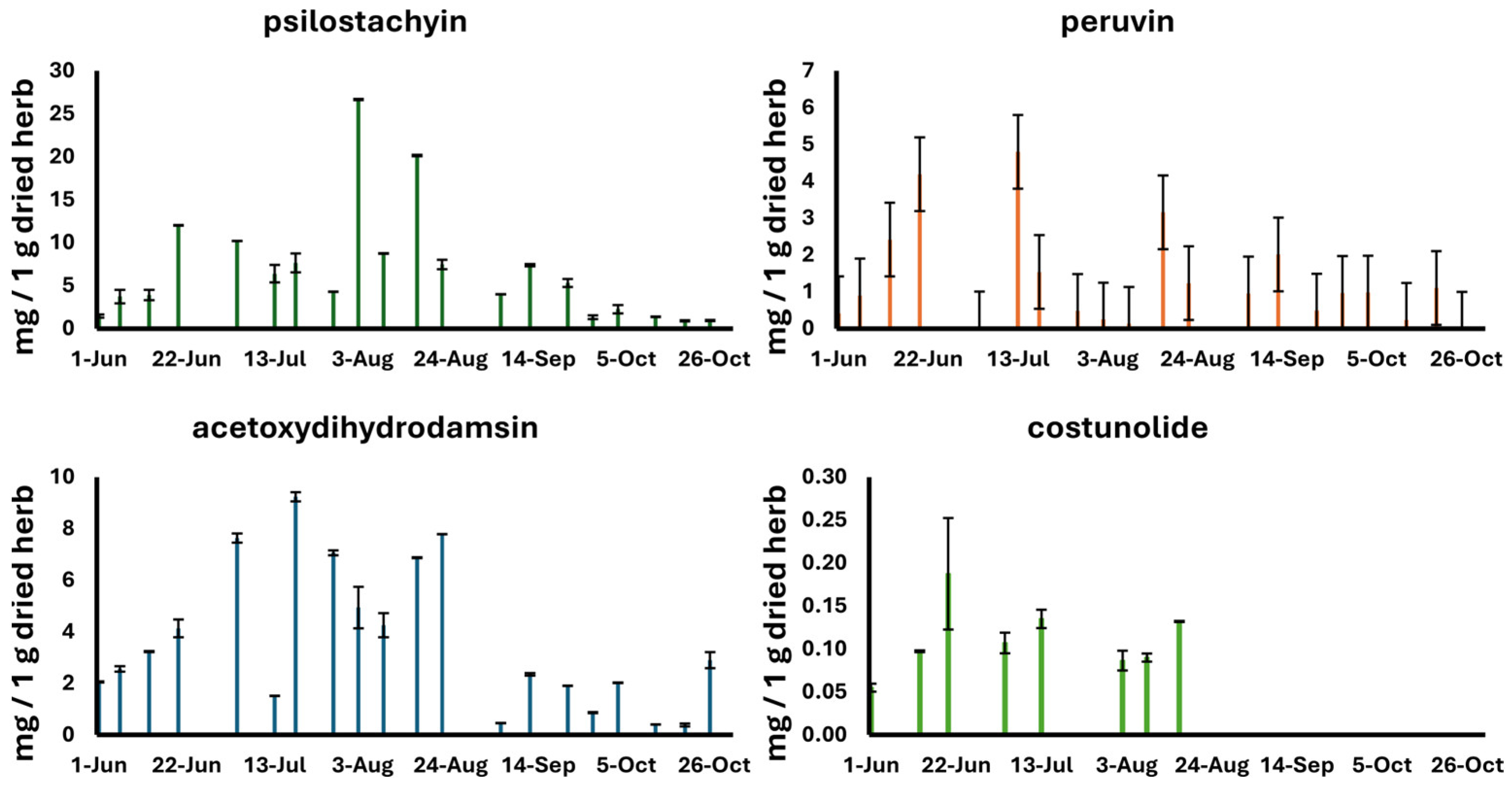
2.2. Quantification of Sesquiterpene Lactones
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. HPLC Analysis and Quantification
4.2.1. Sample Preparation
4.2.2. HPLC Analysis
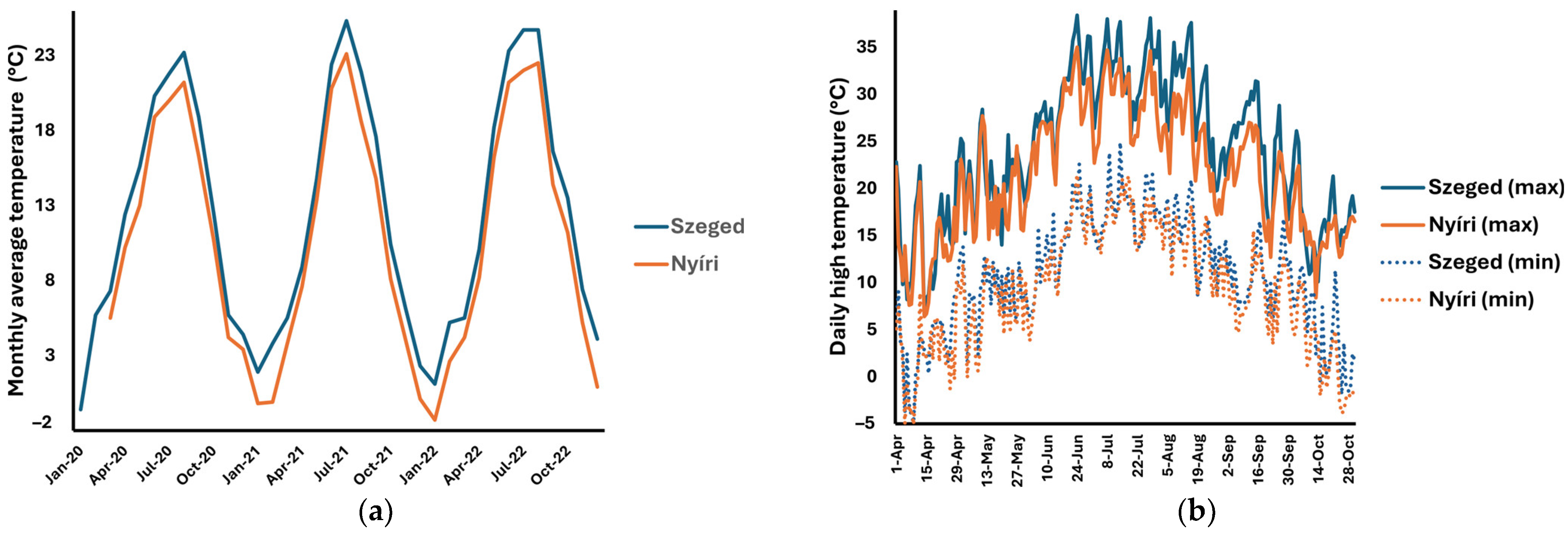
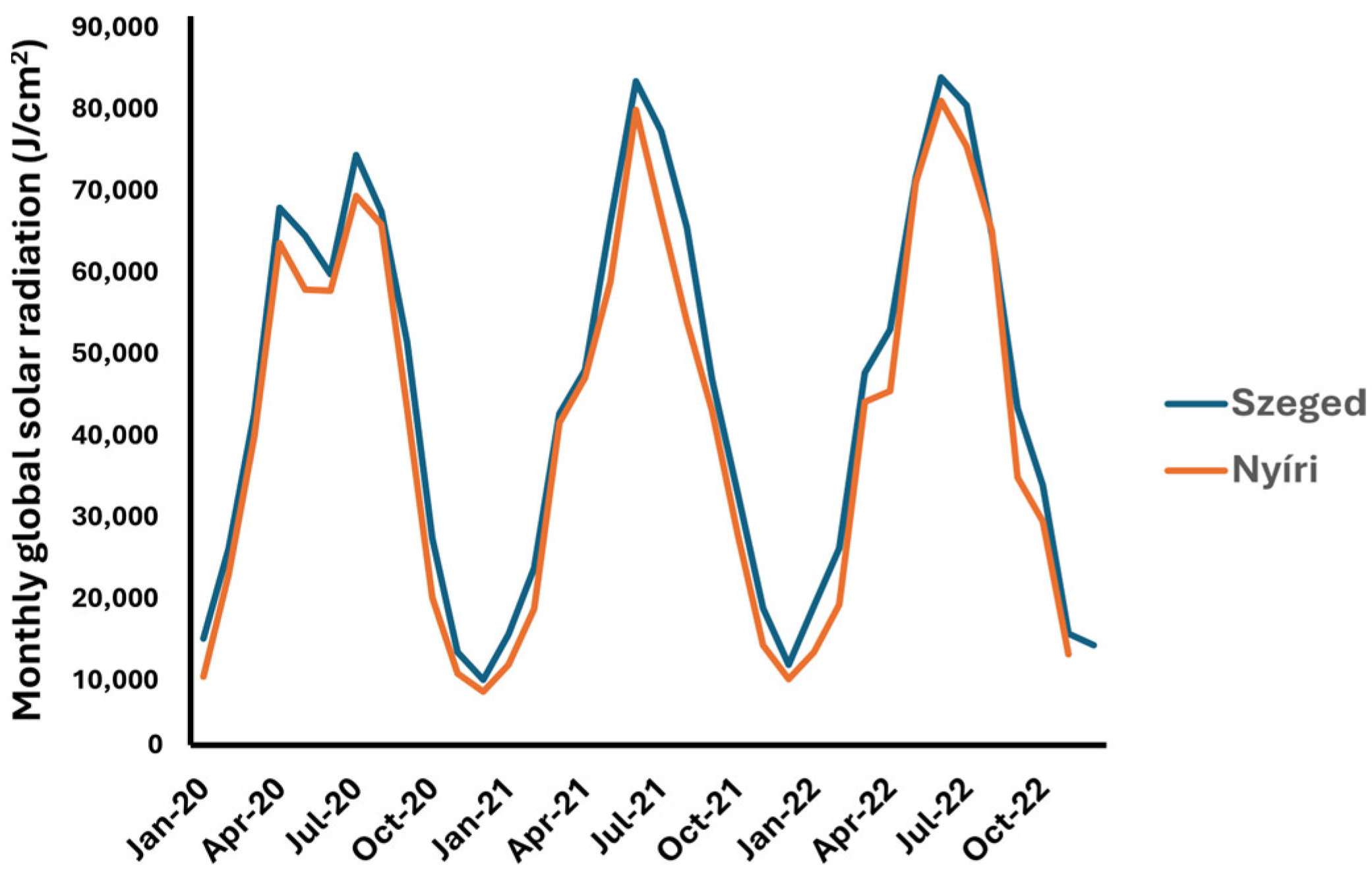
4.3. Meteorological Data
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Payne, W.W. A Re-Evaluation of the Genus Ambrosia (Compositae). J. Arnold Arbor. 1964, 45, 401–438. [Google Scholar] [CrossRef]
- Gerber, E.; Schaffner, U.; Gassmann, A.; Hinz, H.L.; Seier, M.; Müller-Schärer, H. Prospects for Biological Control of Ambrosia Artemisiifolia in Europe: Learning from the Past: Biological Control of Ambrosia in Europe. Weed Res. 2011, 51, 559–573. [Google Scholar] [CrossRef]
- Cunze, S.; Leiblein, M.C.; Tackenberg, O. Range Expansion of Ambrosia Artemisiifolia in Europe Is Promoted by Climate Change. ISRN Ecol. 2013, 2013, 610126. [Google Scholar] [CrossRef]
- Lu, S.; Luo, X.; Han, L.; Yang, J.; Jin, J.; Yang, J. Genetic Patterns Reveal Differences between the Invasion Processes of Common Ragweed in Urban and Non-Urban Ecosystems. Glob. Ecol. Conserv. 2022, 38, e02214. [Google Scholar] [CrossRef]
- McGoey, B.V.; Stinchcombe, J.R. Introduced Populations of Ragweed Show as Much Evolutionary Potential as Native Populations. Evol. Appl. 2021, 14, 1436–1449. [Google Scholar] [CrossRef] [PubMed]
- Heyl, F.W. Analysis of Ragweed Pollen. J. Am. Chem. Soc. 1917, 39, 1470–1476. [Google Scholar] [CrossRef]
- Pérez, G.R.M. Anti-Inflammatory Activity of Ambrosia artemisiaefolia and Rhoeo spathacea. Phytomedicine 1996, 3, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Robles-Zepeda, R.E.; Coronado-Aceves, E.W.; Velázquez-Contreras, C.A.; Ruiz-Bustos, E.; Navarro-Navarro, M.; Garibay-Escobar, A. In Vitro Anti-Mycobacterial Activity of Nine Medicinal Plants Used by Ethnic Groups in Sonora, Mexico. BMC Complement. Altern. Med. 2013, 13, 329. [Google Scholar] [CrossRef]
- Möller, H.; Spirén, A.; Svensson, Å.; Gruvberger, B.; Hindsén, M.; Bruze, M. Contact Allergy to the Asteraceae Plant Ambrosia Artemisiifolia L. (Ragweed) in Sesquiterpenelactone-Sensitive Patients in Southern Sweden: Ragweed Contact Allergy. Contact Dermat. 2002, 47, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Kovács, B.; Hohmann, J.; Csupor-Löffler, B.; Kiss, T.; Csupor, D. A Comprehensive Phytochemical and Pharmacological Review on Sesquiterpenes from the Genus Ambrosia. Heliyon 2022, 8, e09884. [Google Scholar] [CrossRef]
- Laughlin, J.C. The influence of distribution of antimalarial constituents in Artemisia annua L. on time and method of harvest. Acta Hortic. 1995, 67–74. [Google Scholar] [CrossRef]
- Kiss, T.; Szabó, A.; Oszlánczi, G.; Lukács, A.; Tímár, Z.; Tiszlavicz, L.; Csupor, D. Repeated-Dose Toxicity of Common Ragweed on Rats. PLoS ONE 2017, 12, e0176818. [Google Scholar] [CrossRef] [PubMed]
- Amrehn, E.; Heller, A.; Spring, O. Capitate Glandular Trichomes of Helianthus Annuus (Asteraceae): Ultrastructure and Cytological Development. Protoplasma 2014, 251, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Champagne, A.; Boutry, M. Proteomics of Terpenoid Biosynthesis and Secretion in Trichomes of Higher Plant Species. Biochim. Et Biophys. Acta (BBA) Proteins Proteom. 2016, 1864, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Taglialatela-Scafati, O.; Pollastro, F.; Minassi, A.; Chianese, G.; De Petrocellis, L.; Di Marzo, V.; Appendino, G. Sesquiterpenoids from Common Ragweed (Ambrosia Artemisiifolia L.), an Invasive Biological Polluter. Eur. J. Org. Chem. 2012, 2012, 5162–5170. [Google Scholar] [CrossRef]
- Božičević, A.; De Mieri, M.; Nassenstein, C.; Wiegand, S.; Hamburger, M. Secondary Metabolites in Allergic Plant Pollen Samples Modulate Afferent Neurons and Murine Tracheal Rings. J. Nat. Prod. 2017, 80, 2953–2961. [Google Scholar] [CrossRef]
- Sturgeon, C.M.; Craig, K.; Brown, C.; Rundle, N.T.; Andersen, R.J.; Roberge, M. Modulation of the G2 Cell Cycle Checkpoint by Sesquiterpene Lactones Psilostachyins A and C Isolated from the Common Ragweed Ambrosia artemisiifolia. Planta Med. 2005, 71, 938–943. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, E.; Culvenor, C.; Loder, J. Psilostachyin, a Cytotoxic Constituent of Ambrosia Artemsisiifolia L. Aust. J. Chem. 1968, 21, 1109. [Google Scholar] [CrossRef]
- Silva, G.L.; Oberti, J.C.; Herz, W. Sesquiterpene Lactones and other Constituents of Argentine Ambrosia Species. Phytochemistry 1992, 31, 859–861. [Google Scholar] [CrossRef]
- Błoszyk, E.; Rychłewska, U.; Szczepanska, B.; Buděšínský, M.; Drożdż, B.; Holub, M. Sesquiterpene Lactones of Ambrosia artemisiifolia L. and Ambrosia trifida L. Species. Collect. Czech. Chem. Commun. 1992, 57, 1092–1102. [Google Scholar] [CrossRef]
- Payne, W.W. Biochemistry and Species Problems in Ambrosia (Asteraceae-Ambrosieae). Plant Syst. Evol. 1976, 125, 169–178. [Google Scholar] [CrossRef]
- Payne, W.W. The Morphology of the Inflorescence of Ragweeds (Ambrosia-Franseria: Compositae). Am. J. Bot. 1963, 50, 872–880. [Google Scholar] [CrossRef]
- Payne, W.W.; Scora, R.W.; Kumamoto, J. The Volatile Oils of Ambrosia (Compositae: Ambrosieae). Brittonia 1972, 24, 189. [Google Scholar] [CrossRef]
- He, M.; He, C.-Q.; Ding, N.-Z. Abiotic Stresses: General Defenses of Land Plants and Chances for Engineering Multistress Tolerance. Front. Plant Sci. 2018, 9, 1771. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, T.; Guo, Q.; Zou, Q.; Yang, F.; Lu, D.; Liu, J. Effect of Soil Moisture Regimes in the Early Flowering Stage on Inflorescence Morphology and Medicinal Ingredients of Chrysanthemum Morifolium Ramat. Cv. ‘Hangju.’ Sci. Hortic. 2020, 260, 108849. [Google Scholar] [CrossRef]
- Yadav, R.K.; Sangwan, R.S.; Sabir, F.; Srivastava, A.K.; Sangwan, N.S. Effect of Prolonged Water Stress on Specialized Secondary Metabolites, Peltate Glandular Trichomes, and Pathway Gene Expression in Artemisia Annua L. Plant Physiol. Biochem. 2014, 74, 70–83. [Google Scholar] [CrossRef]
- Banyai, W.; Mii, M.; Supaibulwatana, K. Enhancement of Artemisinin Content and Biomass in Artemisia Annua by Exogenous GA3 Treatment. Plant Growth Regul. 2011, 63, 45–54. [Google Scholar] [CrossRef]
- Pinke, G.; Karácsony, P.; Czúcz, B.; Botta-Dukát, Z. Environmental and Land-Use Variables Determining the Abundance of Ambrosia Artemisiifolia in Arable Fields in Hungary. Preslia 2011, 83, 219–235. [Google Scholar]
- Case, M.J.; Stinson, K.A. Climate Change Impacts on the Distribution of the Allergenic Plant, Common Ragweed (Ambrosia Artemisiifolia) in the Eastern United States. PLoS ONE 2018, 13, e0205677. [Google Scholar] [CrossRef]
- Skálová, H. Performance of Ambrosia Artemisiifolia and Its Potential Competitors in an Experimental Temperature and Salinity Gradient and Implications for Management. MBI 2019, 10, 359–376. [Google Scholar] [CrossRef]
- Béres, I.; Hunyadi, K. Dormancy and germination of common ragweed (Ambrosia elatior L.) seeds in the field in Hungary. Acta Agron. Hung. 1984, 33, 383–387. [Google Scholar]
- Shrestha, A.; Roman, E.S.; Thomas, A.G.; Swanton, C.J. Modeling Germination and Shoot-Radicle Elongation of Ambrosia artemisiifolia. Weed Sci. 1999, 47, 557–562. [Google Scholar] [CrossRef]
- Ianovici, N.; Bîrsan, M.-V. The Influence of Meteorological Factors on the Dynamic of Ambrosia Artemisiifolia Pollen in an Invaded Area. Not. Bot. Horti Agrobo. 2020, 48, 752–769. [Google Scholar] [CrossRef]
- Szentes, D.; Lehoczky, É. The Spreading, Morphology, Biology, Agricultural and Human Health Harmful Effects of Common Ragweed (Ambrosia Artemisiifolia L.). Magy. Gyomkutatás Technológia 2016, 17, 3–24. [Google Scholar]
- Makra, L.; Matyasovszky, I.; Hufnagel, L.; Tusnády, G. The history of ragweed in the world. AEER 2015, 13, 752–769. [Google Scholar] [CrossRef]
- Bassett, I.J.; Crompton, C.W. The Biology of Canadian Weeds: 11. Ambrosia artemisiifolia L. and A. psilostachya DC. Can. J. Plant Sci. 1975, 55, 463–476. [Google Scholar] [CrossRef]
- Battlay, P.; Wilson, J.; Bieker, V.C.; Lee, C.; Prapas, D.; Petersen, B.; Craig, S.; Van Boheemen, L.; Scalone, R.; De Silva, N.P.; et al. Large Haploblocks Underlie Rapid Adaptation in the Invasive Weed Ambrosia artemisiifolia. Nat. Commun. 2023, 14, 1717. [Google Scholar] [CrossRef]
- Kovács, B.; Szemerédi, N.; Kúsz, N.; Kiss, T.; Csupor-Löffler, B.; Tsai, Y.-C.; Rácz, B.; Spengler, G.; Csupor, D. Antiproliferative and Cytotoxic Effects of Sesquiterpene Lactones Isolated from Ambrosia Artemisiifolia on Human Adenocarcinoma and Normal Cell Lines. Pharm. Biol. 2022, 60, 1511–1519. [Google Scholar] [CrossRef]
- Szigetvári, C.; Benkő, Z.R. Özönnövények; TermészetBÚVÁR Alapítvány: Budapest, Hungary, 2004. [Google Scholar]
- HungaroMet Historical Daily Data for Meteorological Station Szeged-Külterület (No. 58116). Available online: https://odp.met.hu/climate/observations_hungary/daily/historical/HABP_1D_58116_20191130_20231231_hist.zip (accessed on 30 May 2024).
- HungaroMet Historical Monthly Data for Meteorological Station Szeged-Külterület (No. 58116). Available online: https://odp.met.hu/climate/observations_hungary/monthly/historical/HABP_1MO_58116_201911_202401_hist.zip (accessed on 30 May 2024).
- HungaroMet Historical Daily Data for Meteorological Station Hidasnémeti (No. 61104). Available online: https://odp.met.hu/climate/observations_hungary/daily/historical/HABP_1D_61104_20020101_20231231_hist.zip (accessed on 30 May 2024).
- HungaroMet Historical Monthly Data for Meteorological Station Hidasnémeti (No. 61104). Available online: https://odp.met.hu/climate/observations_hungary/monthly/historical/HABP_1MO_61104_200201_202401_hist.zip (accessed on 30 May 2024).
- HungaroMet Historical Daily Data for Meteorological Station Sátoraljaújhely (No. 61709). Available online: https://odp.met.hu/climate/observations_hungary/daily/historical/HABP_1D_61709_20020101_20231231_hist.zip (accessed on 30 May 2024).
- HungaroMet Historical Monthly Data for Meteorological Station Sátoraljaújhely (No. 61709). Available online: https://odp.met.hu/climate/observations_hungary/monthly/historical/HABP_1MO_61709_200201_202401_hist.zip (accessed on 30 May 2024).

| Standard | LOD (µg/inj) | LOQ (µg/inj) | Calibration Points | Range Covered (µg/inj) | Regressions Equations | R2 | RSD% of AUC | Tailing Factor | RSD% of RT |
|---|---|---|---|---|---|---|---|---|---|
| psilostachyin (1) | 0.04155 | 0.12590 | 9 | 0.02–20.00 | y = 621593.7970x − 150728.6813 | 0.9970 | 1.00 | 0.876–1.224 | 0.16 |
| peruvin (2) | 0.06877 | 0.20841 | 9 | 0.05–4 | y = 4929518558x − 46339884 | 0.9999 | 0.78 | 0.947–1.216 | 0.11 |
| acetoxydihydrodamsin (3) | 0.02688 | 0.08121 | 10 | 0.02–5 | y = 1178552.7162x + 52571.8051 | 0.9992 | 0.54 | 1.081-1.110 | 0.13 |
| costunolide (4) | 0.17653 | 0.53496 | 8 | 0.02–2.5 | y = 417313.4111x − 32627130 | 0.9999 | 0.78 | 1.108–1.789 | 0.12 |
| isoalantolactone (5) | 0.07902 | 0.23947 | 8 | 0.02–2.5 | y = 851443.9139x + 6909.0185 | 0.9999 | 0.29 | 1.108–1.239 | 0.11 |
| Date of Harvest | Psilostachyin | Peruvin | Acetoxydihydrodamsin | Costunolide | Isoalantolactone |
|---|---|---|---|---|---|
| 2 June 2021 | ND | ND | ND | ND | ND |
| 8 June 2021 | ND | ND | ND | ND | ND |
| 15 June 2021 | ND | ND | ND | ND | ND |
| 23 June 2021 | ND | ND | <LOQ | ND | ND |
| 6 July 2021 | 1.52 ± 0.10 | 2.43 ± 0.00 | ND | ND | ND |
| 13 July 2021 | 5.03 ± 0.02 | 1.57 ± 0.02 | 0.10 ± 0.00 | <LOD | <LOQ |
| 22 July 2021 | 3.94 ± 0.00 | ND | ND | <LOD | <LOQ |
| 27 July 2021 | 3.46 ± 0.01 | 3.73 ± 0.04 | 0.03 ± 0.00 | <LOD | ND |
| 4 August 2021 | 4.47 ± 0.22 | 3.38 ± 0.05 | 0.10 ± 0.01 | ND | ND |
| 17 August 2021 | 10.92 ± 0.10 | ND | ND | 1.13 ± 0.00 | ND |
| 24 August 2021 | 1.03 ± 0.01 | 0.03 ± 0.01 | <LOQ | ND | ND |
| 14 September 2021 | 1.25 ± 0.00 | ND | ND | ND | ND |
| 23 September 2021 | 0.54 ± 0.03 | ND | ND | ND | ND |
| 28 September 2021 | 0.64 ± 0.01 | 0.21 ± 0.01 | ND | <LOD | ND |
| 31 September 2021 | 2.26 ± 0.03 | ND | ND | ND | ND |
| 7 October 2021 | 0.66 ± 0.02 | ND | ND | <LOD | <LOD |
| 14 October 2021 | 0.53 ± 0.02 | ND | ND | <LOD | <LOD |
| 20 October 2021 | 0.50 ± 0.00 | ND | ND | <LOD | <LOD |
| 30 October 2021 | 0.51 ± 0.01 | ND | ND | <LOD | <LOD |
| Date of Harvest | Psilostachyin | Peruvin | Acetoxydihydrodamsin | Costunolide | Isoalantolactone |
|---|---|---|---|---|---|
| 1 June 2021 | 1.46 ± 0.19 | 0.42 ± 0.02 | 2.06 ± 0.02 | <LOD | ND |
| 6 June 2021 | 3.73 ± 0.79 | 0.90 ± 0.01 | 2.60 ± 0.10 | ND | ND |
| 13 June 2021 | 3.91 ± 0.60 | 2.42 ± 0.03 | 3.23 ± 0.02 | <LOD | ND |
| 20 June 2021 | 12.01 ± 0.03 | 4.19 ± 0.04 | 4.13 ± 0.34 | <LOD | ND |
| 4 July 2021 | 10.23 ± 0.00 | <LOD | 7.63 ± 0.18 | <LOD | ND |
| 13 July 2021 | 6.39 ± 1.02 | 4.80 ± 0.00 | 1.51 ± 0.00 | <LOD | ND |
| 18 July 2021 | 7.64 ± 1.11 | 1.54 ± 0.03 | 9.23 ± 0.17 | ND | ND |
| 27 July 2021 | 4.30 ± 0.03 | 0.48 ± 0.01 | 7.06 ± 0.10 | ND | ND |
| 2 August 2021 | 26.66 ± 0.08 | 0.25 ± 0.01 | 4.94 ± 0.81 | <LOD | ND |
| 8 August 2021 | 8.76 ± 0.04 | <LOQ | 4.25 ± 0.47 | <LOD | ND |
| 16 August 2021 | 20.13 ± 0.09 | 3.16 ± 0.08 | 6.87 ± 0.02 | <LOD | ND |
| 22 August 2021 | 7.47 ± 0.56 | 1.24 ± 0.00 | 7.78 ± 0.01 | ND | ND |
| 5 September 2021 | 4.00 ± 0.01 | 0.96 ± 0.01 | 0.46 ± 0.00 | ND | ND |
| 12 September 2021 | 7.39 ± 0.14 | 2.02 ± 0.03 | 2.35 ± 0.05 | ND | ND |
| 21 September 2021 | 5.32 ± 0.48 | 0.49 ± 0.00 | 1.90 ± 0.00 | ND | ND |
| 27 September 2021 | 1.32 ± 0.22 | 0.97 ± 0.00 | 0.86 ± 0.02 | ND | ND |
| 3 October 2021 | 2.26 ± 0.48 | 0.98 ± 0.04 | 2.02 ± 0.01 | ND | ND |
| 12 October 2021 | 1.37 ± 0.03 | 0.24 ± 0.03 | 0.40 ± 0.00 | ND | ND |
| 19 October 2021 | 0.91 ± 0.06 | 1.11 ± 0.16 | 0.39 ± 0.06 | ND | ND |
| 25 October 2021 | 0.94 ± 0.07 | ND | 2.90 ± 0.31 | ND | ND |
| Climatic Factor | Collection Site | Psilostachyin (1) | Peruvin (2) | Acetoxydihydrodamsin (3) | Costunolide (4) | Isoalantolactone (5) |
|---|---|---|---|---|---|---|
| temperature (average) | SZEGED | 0.34 | 0.59 (**) | 0.55 (*) | 0.00 | 0.01 |
| NYÍRI | 0.46 (*) | 0.46 (*) | 0.58 (**) | 0.61 (**) | ND | |
| temperature (minimal) | SZEGED | 0.46 (*) | 0.52 (*) | 0.50 (*) | 0.18 | −0.09 |
| NYÍRI | 0.59 (**) | 0.43 | 0.61 (**) | 0.54 (*) | ND | |
| temperature (maximal) | SZEGED | 0.24 | 0.55 (*) | 0.52 (*) | 0.09 | −0.26 |
| NYÍRI | 0.43 | 0.45 (*) | 0.55 (*) | 0.62 (**) | ND | |
| global radiation (daily) | SZEGED | 0.04 | 0.33 | 0.30 | −0.18 | 0.02 |
| NYÍRI | 0.12 | 0.39 | 0.35 | 0.46 | ND | |
| precipitation (daily) | SZEGED | –0.12 | –0.12 | –0.11 | −0.01 | 0.20 |
| NYÍRI | 0.53 (*) | 0.02 | 0.21 | 0.37 | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovács, B.; Püski, P.; Bajtel, Á.; Ferencz, E.; Csupor-Löffler, B.; Csupor, D.; Kiss, T. Targeted Screening and Quantification of Characteristic Sesquiterpene Lactones in Ambrosia artemisiifolia L. at Different Growth Stages. Plants 2024, 13, 2053. https://doi.org/10.3390/plants13152053
Kovács B, Püski P, Bajtel Á, Ferencz E, Csupor-Löffler B, Csupor D, Kiss T. Targeted Screening and Quantification of Characteristic Sesquiterpene Lactones in Ambrosia artemisiifolia L. at Different Growth Stages. Plants. 2024; 13(15):2053. https://doi.org/10.3390/plants13152053
Chicago/Turabian StyleKovács, Balázs, Péter Püski, Ákos Bajtel, Elek Ferencz, Boglárka Csupor-Löffler, Dezső Csupor, and Tivadar Kiss. 2024. "Targeted Screening and Quantification of Characteristic Sesquiterpene Lactones in Ambrosia artemisiifolia L. at Different Growth Stages" Plants 13, no. 15: 2053. https://doi.org/10.3390/plants13152053
APA StyleKovács, B., Püski, P., Bajtel, Á., Ferencz, E., Csupor-Löffler, B., Csupor, D., & Kiss, T. (2024). Targeted Screening and Quantification of Characteristic Sesquiterpene Lactones in Ambrosia artemisiifolia L. at Different Growth Stages. Plants, 13(15), 2053. https://doi.org/10.3390/plants13152053








