Abscisic Acid Regulates the Occurrence and Recovery of the Striped Leaf Phenotype in Response to Lacking Light at the Base of Sheath in Rice by Modulating Carbohydrate Metabolism
Abstract
:1. Introduction
2. Results
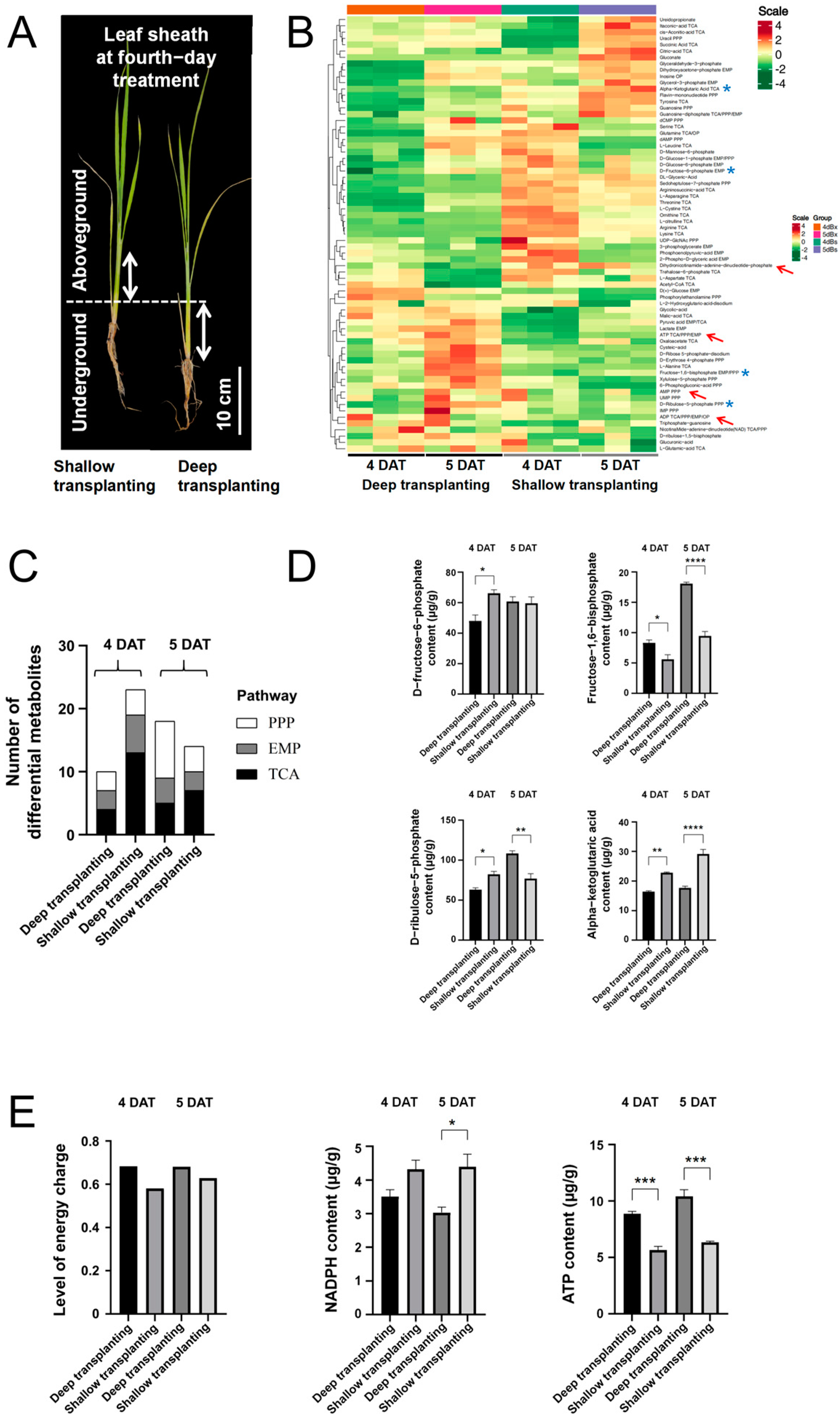
2.1. Dark Shading of the Leaf Sheath Induces the Loss of Greenness in the Leaves of B03S
2.2. Metabolomic Analysis of the Leaf Sheath of B03S
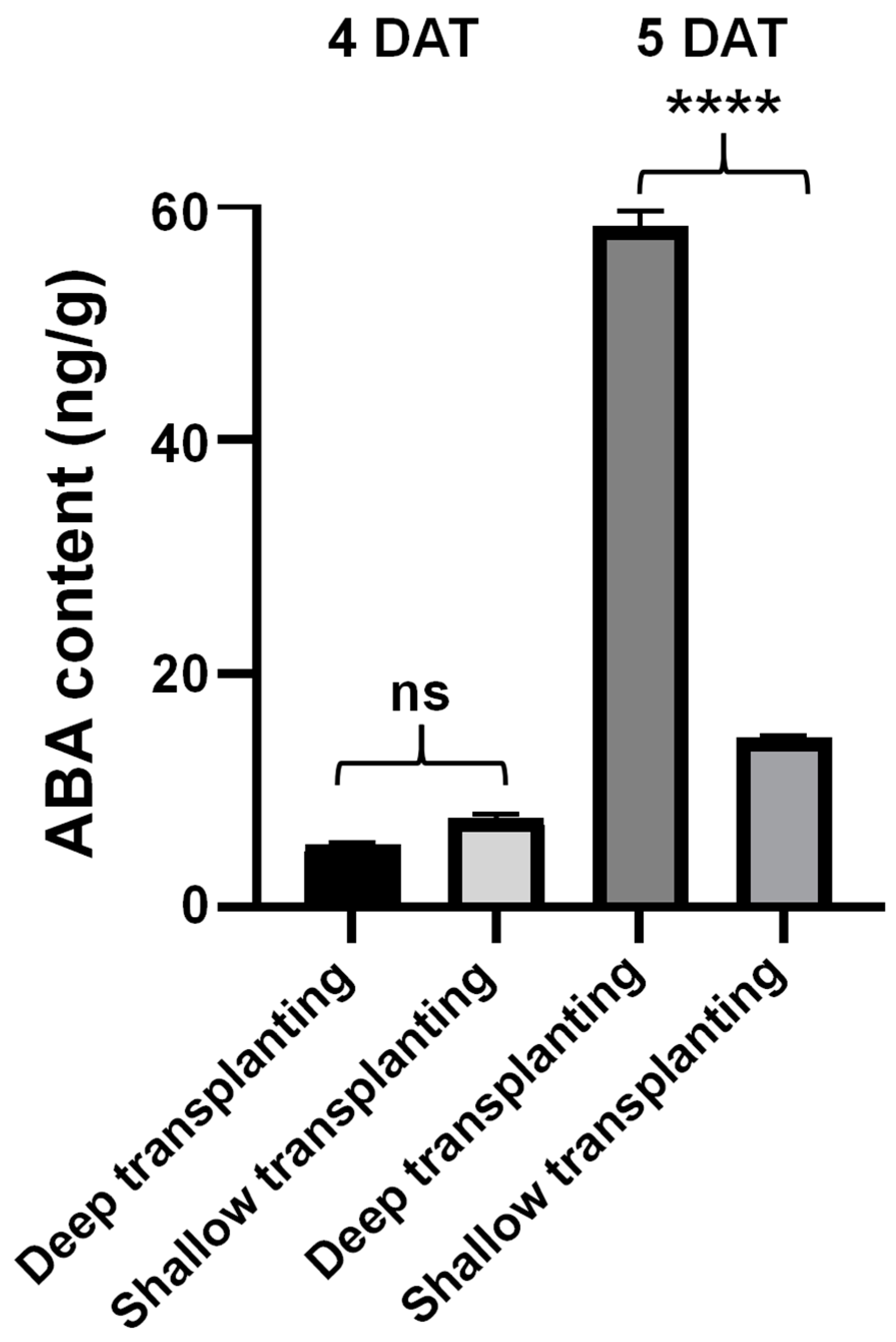
2.3. Analysis of the Abscisic Acid Content in the Leaf Sheath of B03S
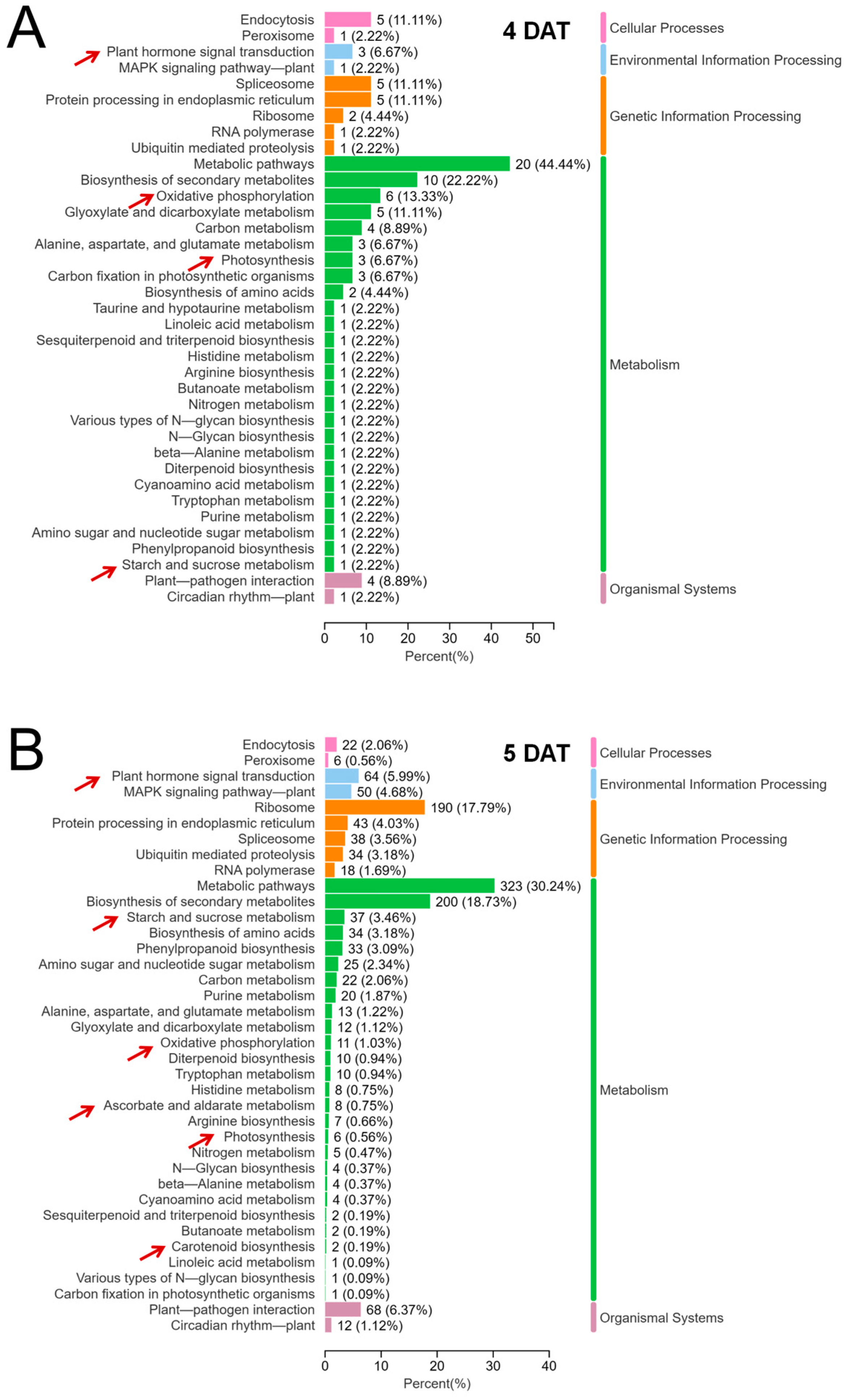
2.4. Transcriptome Sequencing Analysis of the Leaf Sheath of B03S
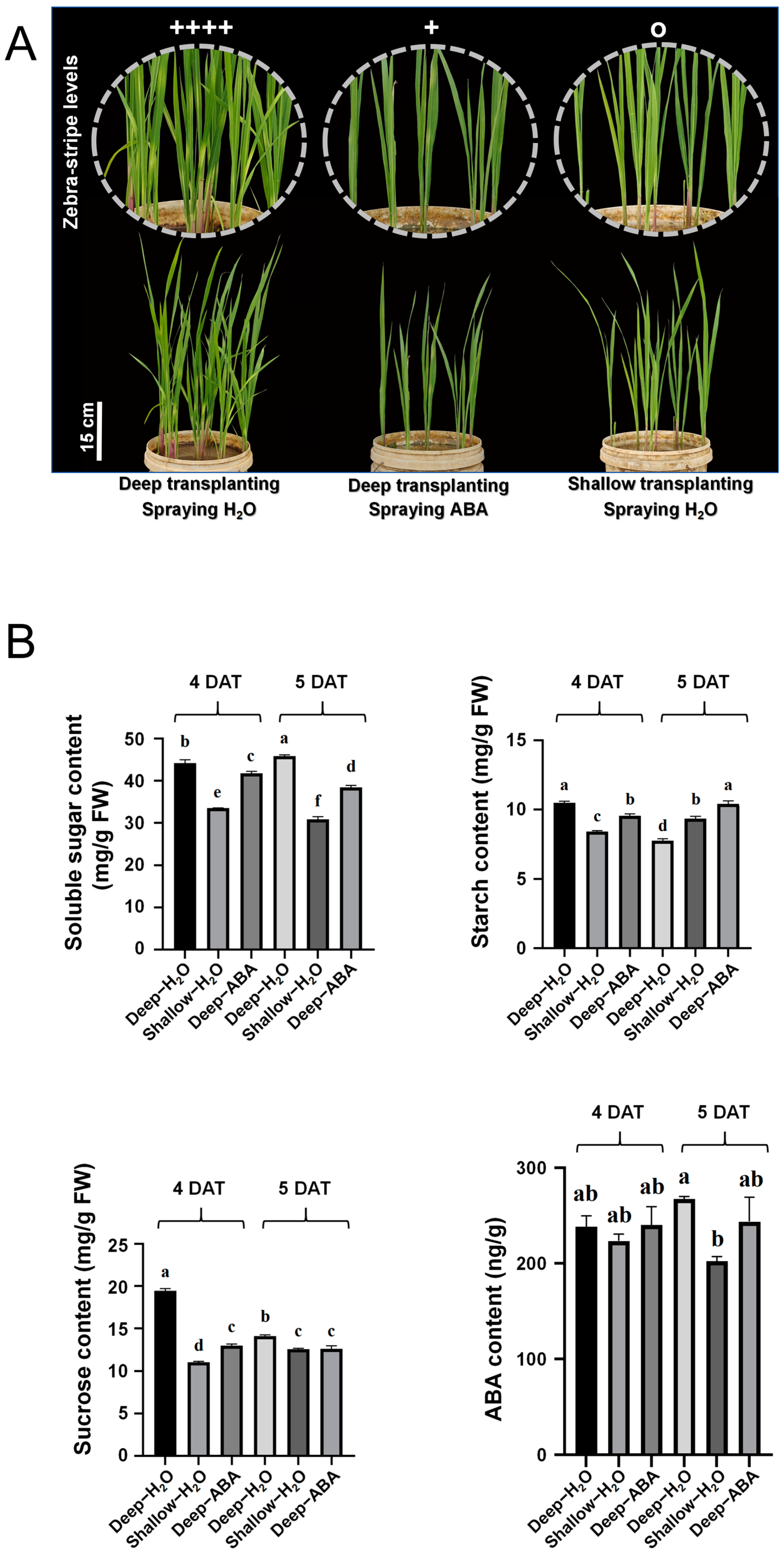
2.5. Evaluation of the Effectiveness of Foliar Spraying
2.6. High ABA Levels Inhibit Sucrose Accumulation in the Leaf Sheath under Dark Shading
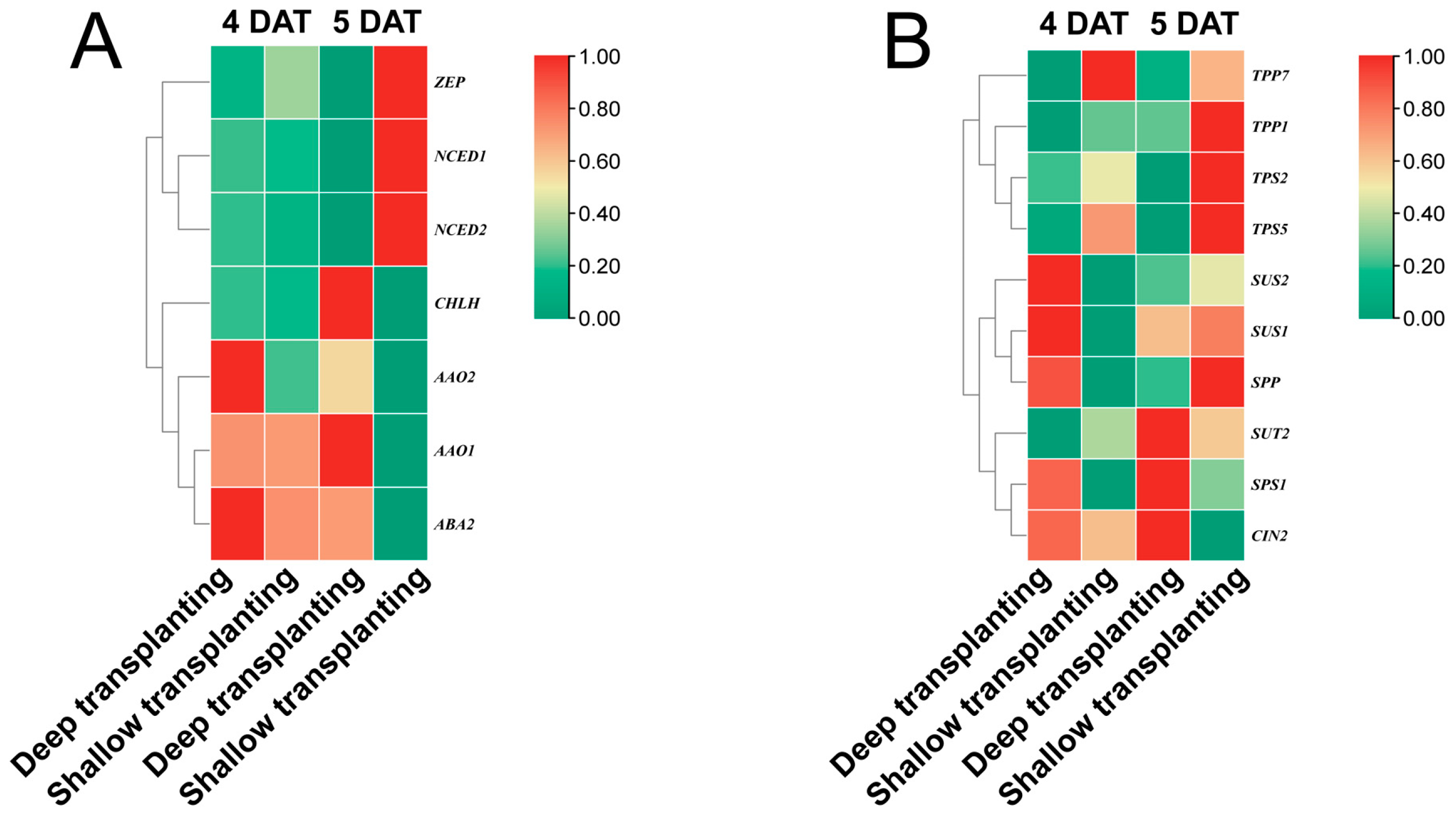
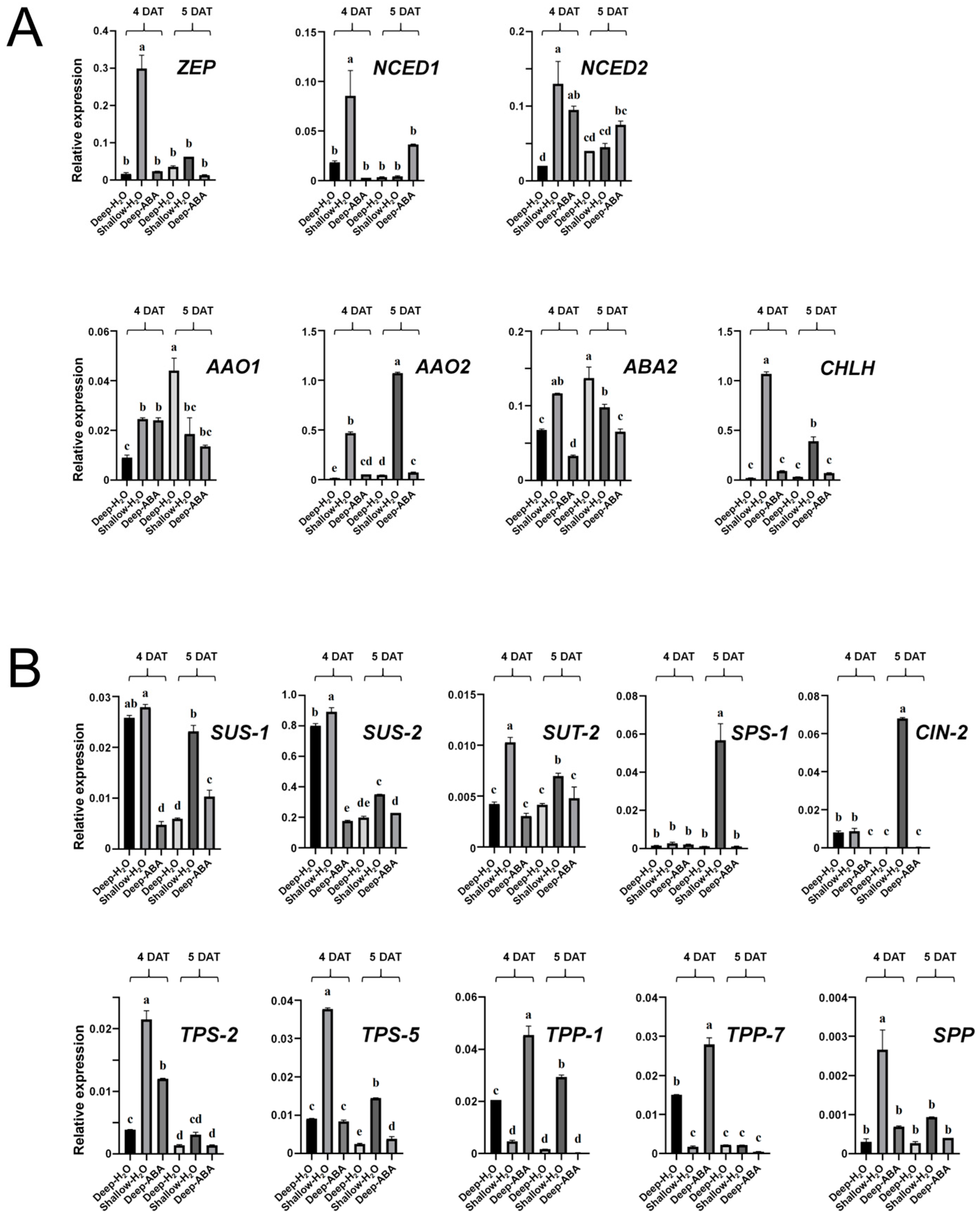
2.7. Verification of the Expression of Genes Involved in the ABA Pathway and the Sucrose Metabolic Pathway in the B03S Leaf Sheath
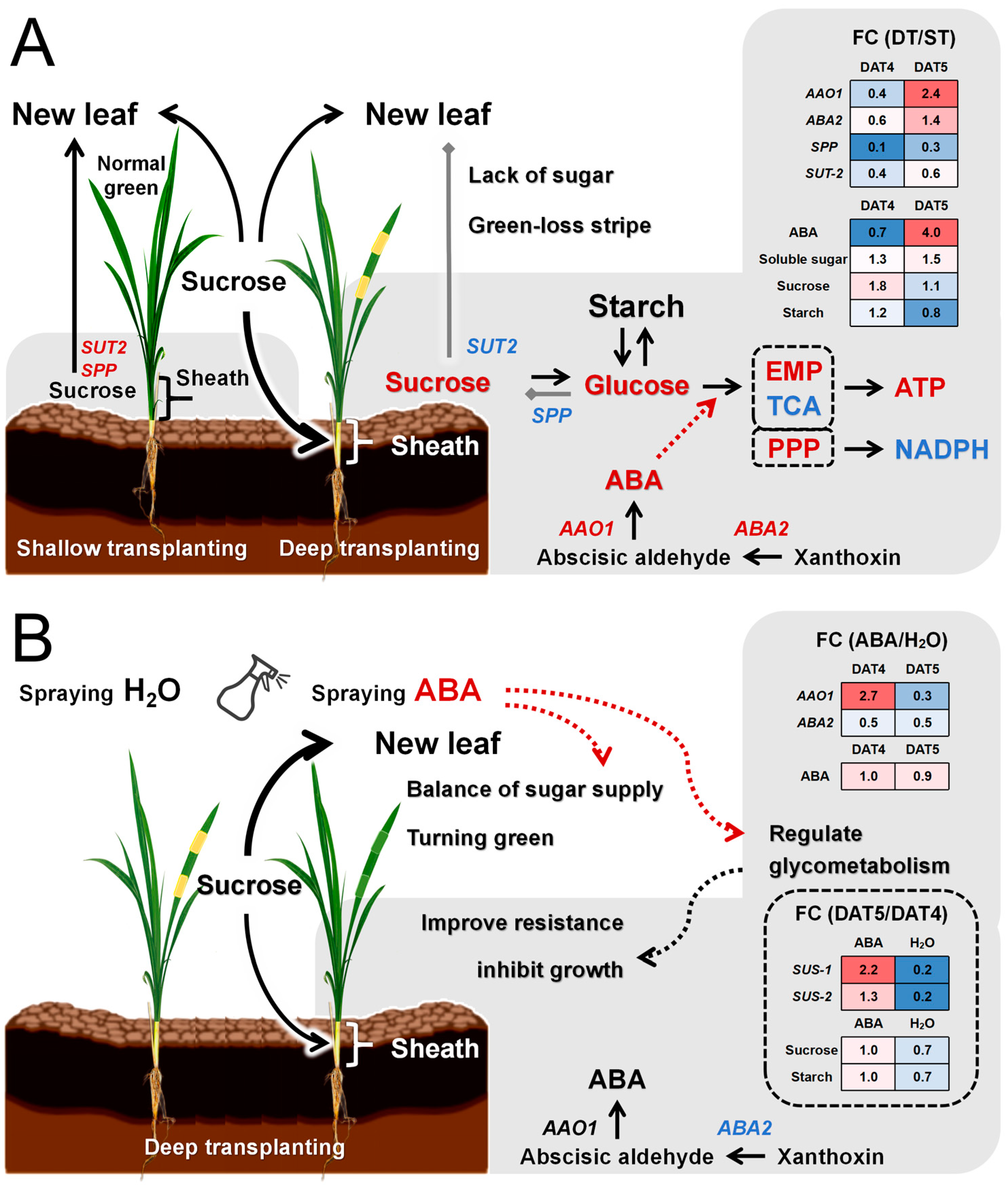
3. Discussion
4. Materials and Methods
4.1. Plant Material and the Leaf Color Phenotype
4.2. Phenotypic Analysis by Transplantation and Spraying Treatments
4.3. UPLC-MS/MS Analysis of Glycometabolism and ABA in the Leaf Sheath
4.4. Transcriptome Sequencing of the Leaf Sheath
4.5. Measurements of Sucrose, Starch, and ABA Contents in the Leaf Sheath
4.6. Real-Time Quantitative PCR-Based Validation of Gene Expression in the Leaf Sheath
4.7. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ahmad, N.; Zaidi, S.S.A.; Mansoor, S. Alternative routes to improving photosynthesis in field crops. Trends Plant Sci. 2020, 25, 958–960. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.M.; Zhou, Y.L.; Xiao, J.W.; Bao, F. Effects of chilling on the structure, function and development of chloroplasts. Front. Plant Sci. 2018, 9, 410990. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.B.; Chen, B.D. Arbuscular mycorrhiza induced putrescine degradation into γ-aminobutyric acid, malic acid accumulation, and improvement of nitrogen assimilation in roots of water-stressed maize plants. Mycorrhiza 2020, 30, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Jalil, S.; Cao, H.; Tsago, Y.; Sunusi, M.; Chen, Z.Y.; Shi, C.H.; Jin, X.L. The purple leaf (pl6) mutation regulates leaf color by altering the anthocyanin and chlorophyll contents in rice. Plants 2020, 9, 1477. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Sakuraba, Y.; Han, S.H.; Yoo, S.C.; Paek, N.C. Mutation of the Arabidopsis NAC016 transcription factor delays leaf senescence. Plant Cell Physiol. 2013, 54, 1660–1672. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.L.; Liu, C.; An, X.Y.; Wu, H.Y.; Feng, Y.; Wang, H.; Sun, D.J. Identification and genetic maping of a novel incompletely dominant yellow leaf color gene, Y1718, on chromosome 2BS in wheat. Euphytica 2017, 213, 141. [Google Scholar] [CrossRef]
- Wang, S.A.; Wang, P.; Gao, L.L.; Yang, R.T.; Li, L.F.; Zhang, E.L.; Wang, Q.; Li, Y.; Yin, Z.F. Characterization and complementation of a chlorophyll-less dominant mutant GL1 in Lagerstroemia indica. DNA Cell Biol. 2017, 36, 354–366. [Google Scholar] [CrossRef] [PubMed]
- Eckhardt, U.; Grimm, B.; Hörtensteiner, S. Recent advances in chlorophyll biosynthesis and breakdown in higher plants. Plant Mol. Biol. 2004, 56, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Horie, Y.; Ito, H.; Kusaba, M.; Tanaka, R.; Tanaka, A. Participation of chlorophyll b reductase in the initial step of the degradation of light-harvesting chlorophyll a/b-protein complexes in Arabidopsis. J. Biol. Chem. 2009, 284, 17449–17456. [Google Scholar] [CrossRef]
- Mei, J.S.; Li, F.F.; Liu, X.R.; Hu, G.C.; Fu, Y.P.; Liu, W.Z. Newly identified CSP41b gene localized in chloroplasts affects leaf color in rice. Plant Sci. 2017, 256, 39–45. [Google Scholar] [CrossRef]
- Chen, H.; Cheng, Z.J.; Ma, X.D.; Wu, H.; Liu, Y.L.; Zhou, K.N.; Chen, Y.L.; Ma, W.W.; Bi, J.C.; Zhang, X.; et al. A knockdown mutation of YELLOW-GREEN LEAF2 blocks chlorophyll biosynthesis in rice. Plant Cell Rep. 2013, 32, 1855–1867. [Google Scholar] [CrossRef] [PubMed]
- Busch, M.; Seuter, A.; Hain, R. Functional analysis of the early steps of carotenoid biosynthesis in tobacco. Plant Physiol. 2002, 128, 439–453. [Google Scholar] [CrossRef] [PubMed]
- Cazzonelli, C.I.; Pogson, B.J. Source to sink: Regulation of carotenoid biosynthesis in plants. Trends Plant Sci. 2010, 15, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.J.; Zhang, H.Q.; Yue, W.; Feng, H.; Liu, J.L.; Xiao, X.; Shu, Z.F.; Wei, L.; Wang, G.H.; Wang, G.L. Mapped clone and functional analysis of leaf-color gene Ygl7 in a rice hybrid (Oryza sativa L. ssp. indica). PLoS ONE 2014, 9, e99564. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; Hu, Y.; Zhu, Y.F.; Baloch, A.W.; Jia, X.M.; Guo, A.X. Transcriptional and physiological analyses of short-term Iron deficiency response in apple seedlings provide insight into the regulation involved in photosynthesis. BMC Genom. 2018, 19, 461. [Google Scholar] [CrossRef] [PubMed]
- Hao, Q.Y.; Zhang, G.W.; Zuo, X.L.; He, Y.; Zeng, H.L. Cia zeaxanthin biosynthesis, OsZEP and OsVDE regulate striped leaves occurring in response to deep transplanting of rice. Int. J. Mol. Sci. 2022, 23, 8340. [Google Scholar] [CrossRef] [PubMed]
- Richardson, A.E.; Cheng, J.; Johnston, R.; Kennaway, R.; Conlon, B.R.; Rebocho, A.B.; Kong, H.; Scanlon, M.J.; Hake, S.; Coen, E. Evolution of the grass leaf by primordium extension and petiole-lamina remodeling. Science 2021, 374, 1377–1381. [Google Scholar] [CrossRef] [PubMed]
- Ishimaru, K.; Kosone, M.; Sasaki, H.; Kashiwagi, T. Leaf contents differ depending on the position in a rice leaf sheath during sink-source transition. Plant Physiol. Biochem. 2004, 42, 855–860. [Google Scholar] [CrossRef]
- Tsuno, Y.; Sato, T.; Miyamoto, H.; Harada, N. Studies on CO2 Uptake and CO2 Evolution in Each Part of Crop Plants: II. Photosynthetic activity in the leaf sheath and ear of rice plant. Jpn. J. Crop Sci. 1975, 44, 287–292. [Google Scholar] [CrossRef]
- Sakuraba, Y.; Kim, D.; Han, S.H.; Kim, S.H.; Piao, W.; Yanagisawa, S.; An, G.; Paek, N.C. Multilayered regulation of membrane-bound ONAC054 is essential for abscisic acid-induced leaf senescence in rice. Plant Cell 2020, 32, 630–649. [Google Scholar] [CrossRef]
- Teng, K.Q.; Li, J.Z.; Liu, L.; Han, Y.C.; Du, Y.X.; Zhang, J.; Sun, H.Z.; Zhao, Q.Z. Exogenous ABA induces drought tolerance in upland rice: The role of chloroplast and ABA biosynthesis-related gene expression on photosystem II during PEG stress. Acta Physiol. Plant. 2014, 36, 2219–2227. [Google Scholar] [CrossRef]
- Yamburenko, M.V.; Zubo, Y.O.; Vanková, R.; Kusnetsov, V.V.; Kulaeva, O.N.; Börner, T. Abscisic acid represses the transcription of chloroplast genes. J. Exp. Bot. 2013, 64, 4491–4502. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.X.; Bai, Q.; Xu, P.Z.; Wu, T.K.; Guo, D.M.; Peng, Y.B.; Zhang, H.Y.; Deng, X.S.; Chen, X.Q.; Luo, M.; et al. Mutation in rice Abscisic Acid2 results in cell death, enhanced disease-resistance, altered seed dormancy and development. Front. Plant Sci. 2018, 9, 405. [Google Scholar] [CrossRef]
- Seo, M.; Aoki, H.; Koiwai, H.; Kamiya, Y.; Nambara, E.; Koshiba, T. Comparative studies on the Arabidopsis aldehyde oxidase (AAO) gene family revealed a major role of AAO3 in ABA biosynthesis in seeds. Plant Cell Physiol. 2004, 45, 1694–1703. [Google Scholar] [CrossRef] [PubMed]
- Nurbekova, Z.; Srivastava, S.; Standing, D.; Kurmanbayeva, A.; Bekturova, A.; Soltabayeva, A.; Oshanova, D.; Turečková, V.; Strand, M.; Biswas, M.S.; et al. Arabidopsis aldehyde oxidase 3, known to oxidize abscisic aldehyde to abscisic acid, protects leaves from aldehyde toxicity. Plant J. 2021, 108, 1439–1455. [Google Scholar] [CrossRef] [PubMed]
- Long, H.T.; Zheng, Z.; Zhang, Y.J.; Xing, P.Z.; Wan, X.R.; Zheng, Y.X.; Li, L. An abscisic acid (ABA) homeostasis regulated by its production, catabolism and transport in peanut leaves in response to drought stress. PLoS ONE 2019, 14, e0213963. [Google Scholar] [CrossRef] [PubMed]
- Arif, M.A.; Alseekh, S.; Harb, J.; Fernie, A.; Frank, W. Abscisic acid, cold and salt stimulate conserved metabolic regulation in the moss Physcomitrella patens. Plant Biol. J. 2018, 20, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.Z.; Hou, L.Y.; Meng, J.J.; You, H.W.; Li, Z.; Gong, Z.Z.; Yang, S.H.; Shi, Y.T. The antagonistic action of abscisic acid and cytokinin signaling mediates drought stress response in Arabidopsis. Mol. Plant 2018, 11, 970–982. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.C.Q.; de Souza, G.A.; Pimenta, T.M.; Brito, F.A.L.; Picoli, E.A.T.; Zsögön, A.; Ribeiro, D.M. Salt stress inhibits germination of Stylosanthes humilis seeds through abscisic acid accumulation and associated changes in ethylene production. Plant Physiol. Biochem. 2018, 130, 399–407. [Google Scholar] [CrossRef]
- Yoshida, T.; Mogami, J.; Yamaguchi-Shinozaki, K. ABA-dependent and ABA-independent signaling in response to osmotic stress in plants. Curr. Opin. Plant Biol. 2014, 21, 133–139. [Google Scholar] [CrossRef]
- Alazem, M.; Lin, N.S. Interplay between ABA signaling and RNA silencing in plant viral resistance. Curr. Opin. Virol. 2020, 42, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gibson, S.I. Sugar and phytohormone response pathways: Navigating a signalling network. J. Exp. Bot. 2004, 55, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Gibson, S.I. Control of plant development and gene expression by sugar signaling. Curr. Opin. Plant Biol. 2005, 8, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Moustakas, M.; Sperdouli, I.; Kouna, T.; Antonopoulou, C.I.; Therios, I. Exogenous proline induces soluble sugar accumulation and alleviates drought stress effects on photosystem II functioning of Arabidopsis thaliana leaves. Plant Growth Regul. 2011, 65, 315–325. [Google Scholar] [CrossRef]
- Rasheed, R.; Wahid, A.; Farooq, M.; Hussain, I.; Basra, S.M.A. Role of proline and glycinebetaine pretreatments in improving heat tolerance of sprouting sugarcane (Saccharum sp.) buds. Plant Growth Regul. 2011, 65, 35–45. [Google Scholar] [CrossRef]
- Sperdouli, I.; Moustakas, M. Interaction of proline, sugars, and anthocyanins during photosynthetic acclimation of Arabidopsis thaliana to drought stress. J. Plant Physiol. 2012, 169, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Qian, Z.J.; Ma, S.M.; Zhou, Y.C.; Patrick, J.W.; Duan, X.W.; Jiang, Y.M.; Qu, H.X. Energy status of ripening and postharvest senescent fruit of litchi (Litchi chinensis Sonn.). BMC Plant Biol. 2013, 13, 55. [Google Scholar] [CrossRef]
- Aroca, R.; Vernieri, P.; Ruiz-Lozano, J.M. Mycorrhizal and non-mycorrhizal Lactuca sativa plants exhibit contrasting responses to exogenous ABA during drought stress and recovery. J. Exp. Bot. 2008, 59, 2029–2041. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ruiz, J.M.; Guinea, M.; Puerto-Galán, L.; Cejudo, F.J. NADPH thioredoxin reductase C is involved in redox regulation of the Mg-chelatase I subunit in Arabidopsis thaliana chloroplasts. Mol. Plant. 2014, 7, 1252–1255. [Google Scholar] [CrossRef]
- Shang, Y.; Yan, L.; Liu, Z.Q.; Cao, Z.; Mei, C.; Xin, Q.; Wu, F.Q.; Wang, X.F.; Du, S.Y.; Jiang, T.; et al. The Mg-chelatase H subunit of Arabidopsis antagonizes a group of WRKY transcription repressors to relieve ABA-responsive genes of inhibition. Plant Cell 2010, 22, 1909–1935. [Google Scholar] [CrossRef]
- Sun, M.; Shen, Y. Integrating the multiple functions of CHLH into chloroplast-derived signaling fundamental to plant development and adaptation as well as fruit ripening. Plant Sci. 2023, 338, 111892. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.Q.; Xin, Q.; Cao, Z.; Liu, Z.Q.; Du, S.Y.; Mei, C.; Zhao, C.X.; Wang, X.F.; Shang, Y.; Jiang, T.; et al. The magnesium-chelatase H subunit binds abscisic acid and functions in abscisic acid signaling: New evidence in Arabidopsis. Plant Physiol. 2009, 150, 1940–1954. [Google Scholar] [CrossRef] [PubMed]
- Stein, O.; Granot, D. An overview of sucrose synthases in plants. Front. Plant Sci. 2019, 10, 435701. [Google Scholar] [CrossRef] [PubMed]
- Goren, S.; Lugassi, N.; Stein, O.; Yeselson, Y.; Schaffer, A.A.; David-Schwartz, R.; Granot, D. Suppression of sucrose synthase affects auxin signaling and leaf morphology in tomato. PLoS ONE 2017, 12, e0182334. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Park, W.D. Sink-and vascular-associated sucrose synthase functions are encoded by different gene classes in potato. Plant Cell. 1995, 7, 1369–1385. [Google Scholar] [CrossRef] [PubMed]
- Rezaul, I.M.; Baohua, F.; Tingting, C.; Weimeng, F.; Caixia, Z.; Longxing, T.; Guanfu, F. Abscisic acid prevents pollen abortion under high-temperature stress by mediating sugar metabolism in rice spikelets. Physiol. Plantarum. 2019, 165, 644–663. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.; Park, Y.; Hwang, I. Abscisic acid: Biosynthesis, inactivation, homoeostasis and signalling. Essays Biochem. 2015, 58, 29–48. [Google Scholar] [CrossRef] [PubMed]
- Leng, P.; Yuan, B.; Guo, Y.D. The role of abscisic acid in fruit ripening and responses to abiotic stress. J. Exp. Bot. 2014, 65, 4577–4588. [Google Scholar] [CrossRef] [PubMed]
- Tolnai, Z.; Sharma, H.; Soós, V. D27-like carotenoid isomerases: At the crossroads of strigolactone and abscisic acid biosynthesis. J. Exp. Bot. 2024, 75, 1148–1158. [Google Scholar] [CrossRef]
- Boursiac, Y.; Léran, S.; Corratgé-Faillie, C.; Gojon, A.; Krouk, G.; Lacombe, B. ABA transport and transporters. Trends Plant Sci. 2013, 18, 325–333. [Google Scholar] [CrossRef]
- Martin-StPaul, N.; Delzon, S.; Cochard, H. Plant resistance to drought depends on timely stomatal closure. Ecol. Lett. 2017, 20, 1437–1447. [Google Scholar] [CrossRef] [PubMed]
- Movahedi, M.; Zoulias, N.; Casson, S.A.; Sun, P.; Liang, Y.K.; Hetherington, A.M.; Gray, J.E.; Chater, C.C. Stomatal responses to carbon dioxide and light require abscisic acid catabolism in Arabidopsis. Interface Focus. 2021, 11, 20200036. [Google Scholar] [CrossRef] [PubMed]
- Su, J.B.; Zhang, M.M.; Zhang, L.; Sun, T.F.; Liu, Y.D.; Lukowitz, W.; Xu, J.; Zhang, S.Q. Regulation of stomatal immunity by interdependent functions of a pathogen-responsive MPK3/MPK6 cascade and abscisic acid. Plant Cell 2017, 29, 526–542. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Li, G.J.; Bressan, R.A.; Song, C.P.; Zhu, J.K.; Zhao, Y. Abscisic acid dynamics, signaling, and functions in plants. J. Integr. Plant Biol. 2020, 62, 25–54. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Bai, J.W.; Tao, X.Y.; Mou, W.S.; Luo, Z.S.; Mao, L.C.; Ban, Z.J.; Ying, T.J.; Li, L. Synergistic effect of abscisic acid and ethylene on color development in tomato (Solanum lycopersicum L.) fruit. Sci. Hortic. 2018, 235, 169–180. [Google Scholar] [CrossRef]
- Galpaz, N.; Wang, Q.; Menda, N.; Zamir, D.; Hirschberg, J. Abscisic acid deficiency in the tomato mutant high-pigment 3 leading to increased plastid number and higher fruit lycopene content. Plant J. 2008, 53, 717–730. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Longhurst, A.D.; Talavera-Rauh, F.; Hokin, S.A.; Barton, M.K. The Arabidopsis transcription factor ABIG1 relays ABA signaled growth inhibition and drought induced senescence. Elife 2016, 5, e13768. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.; Yin, X.M.; Wang, H.T.; Lu, P.Y.; Liu, X.; Gong, C.Y.; Wu, Y. ABA-INDUCED expression 1 is involved in ABA-inhibited primary root elongation via modulating ROS homeostasis in Arabidopsis. Plant Sci. 2021, 304, 110821. [Google Scholar] [CrossRef]
- Cramer, G.R.; Quarrie, S.A. Abscisic acid is correlated with the leaf growth inhibition of four genotypes of maize differing in their response to salinity. Funct. Plant Biol. 2002, 29, 111–115. [Google Scholar] [CrossRef]
- Morita, K.; Hatanaka, T.; Misoo, S.; Fukayama, H. Unusual small subunit that is not expressed in photosynthetic cells alters the catalytic properties of Rubisco in rice. Plant Physiol. 2014, 164, 69–79. [Google Scholar] [CrossRef]
- Pan, X.Q.; Welti, R.; Wang, X. Quantitative analysis of major plant hormones in crude plant extracts by high-performance liquid chromatography-mass spectrometry. Nat. Protoc. 2010, 5, 986–992. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Groenke, K.; Takors, R.; Wandrey, C.; Oldiges, M. Simultaneous determination of multiple intracellular metabolites in glycolysis, pentose phosphate pathway and tricarboxylic acid cycle by liquid chromatography-mass spectrometry. J. Chromatogr. A 2007, 1147, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Laurentin, A.; Edwards, C.A. A microtiter modification of the anthrone-sulfuric acid colorimetric assay for glucose-based carbohydrates. Anal. Biochem. 2003, 315, 143–145. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Chen, C.J.; Wu, Y.; Li, J.W.; Wang, X.; Zeng, Z.H.; Xu, J.; Liu, Y.L.; Feng, J.T.; Chen, H.; He, Y.H.; et al. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Yang, S.; Fu, M.; He, Y.; Zeng, H. Abscisic Acid Regulates the Occurrence and Recovery of the Striped Leaf Phenotype in Response to Lacking Light at the Base of Sheath in Rice by Modulating Carbohydrate Metabolism. Plants 2024, 13, 2090. https://doi.org/10.3390/plants13152090
Chen J, Yang S, Fu M, He Y, Zeng H. Abscisic Acid Regulates the Occurrence and Recovery of the Striped Leaf Phenotype in Response to Lacking Light at the Base of Sheath in Rice by Modulating Carbohydrate Metabolism. Plants. 2024; 13(15):2090. https://doi.org/10.3390/plants13152090
Chicago/Turabian StyleChen, Jiasheng, Shaowen Yang, Ming Fu, Ying He, and Hanlai Zeng. 2024. "Abscisic Acid Regulates the Occurrence and Recovery of the Striped Leaf Phenotype in Response to Lacking Light at the Base of Sheath in Rice by Modulating Carbohydrate Metabolism" Plants 13, no. 15: 2090. https://doi.org/10.3390/plants13152090



