Two New Steroidal Saponins with Potential Anti-Inflammatory Effects from the Aerial Parts of Gnetum formosum Markgr.
Abstract
:1. Introduction
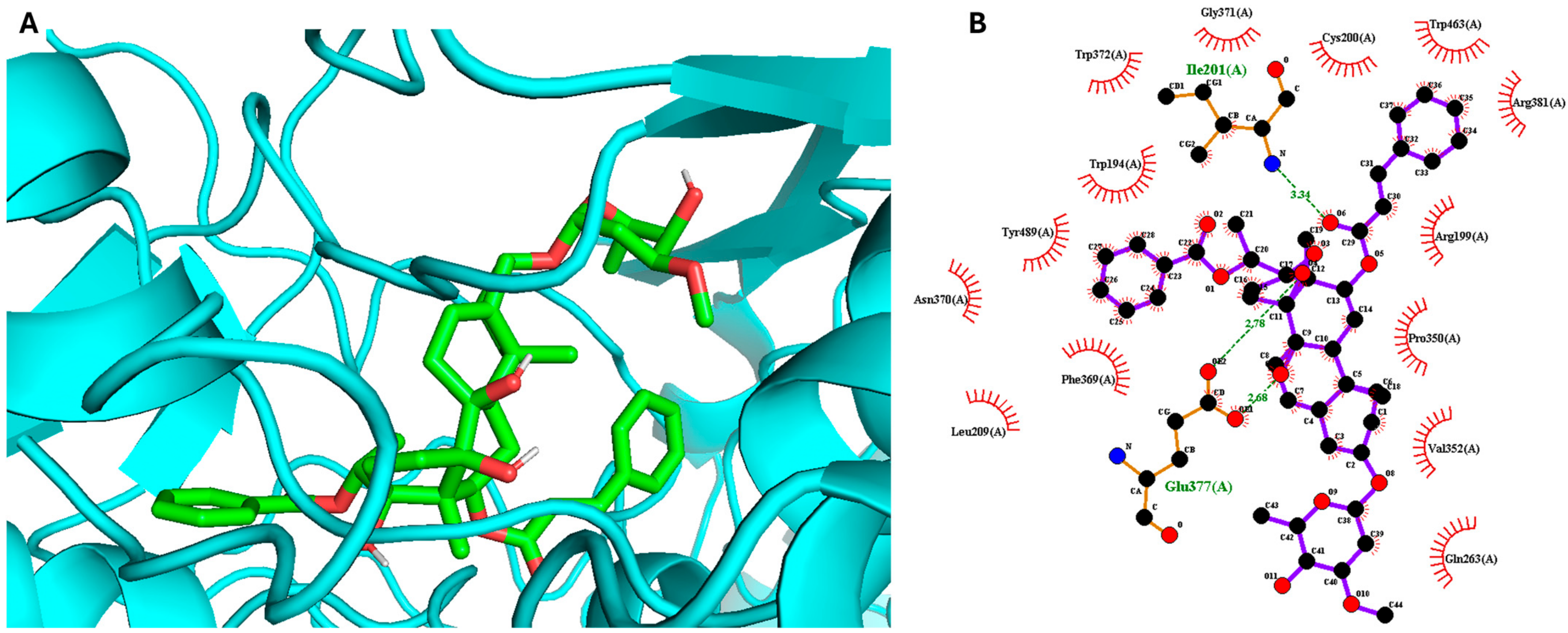
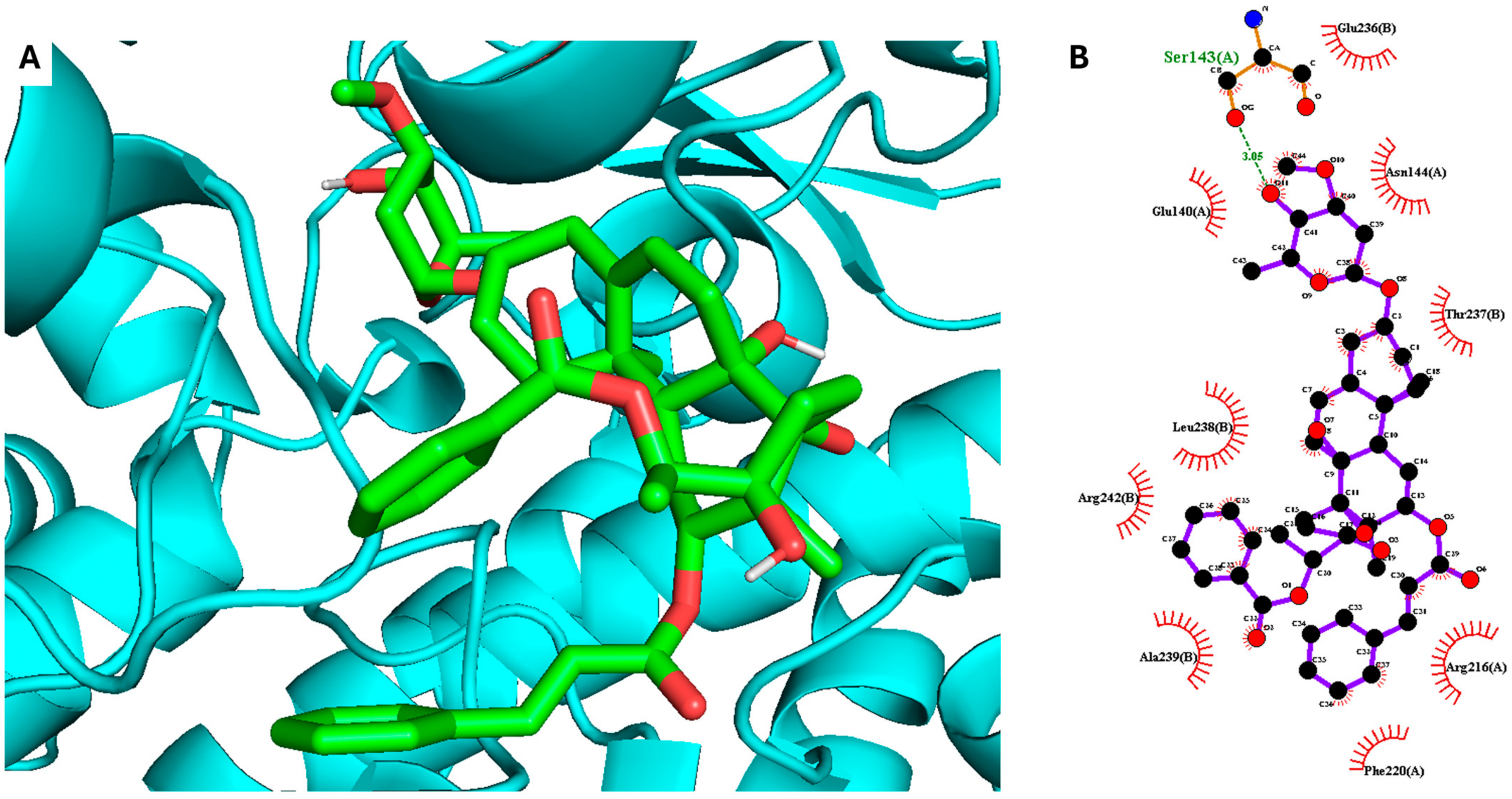
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Botanical Specimens
3.3. Extraction and Isolation
3.4. Acidic Decomposition and Analysis of Sugar Moieties’ Absolute Configuration
3.5. MTT and Measurement of NO Production
3.6. Molecular Docking Simulation
3.7. Molecular Dynamics Simulation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Soares, C.L.R.; Wilairatana, P.; Silva, L.R.; Moreira, P.S.; Barbosa, N.M.M.V.; da Silva, P.R.; Coutinho, H.D.M.; de Menezes, I.R.A.; Felipe, C.F.B. Biochemical aspects of the inflammatory process: A narrative review. Biomed. Pharmacother. 2023, 168, 115764. [Google Scholar] [CrossRef]
- Jiang, H.; Ji, P.; Shang, X.; Zhou, Y. Connection between osteoarthritis and nitric oxide: From pathophysiology to therapeutic target. Molecules 2023, 28, 1683. [Google Scholar] [CrossRef] [PubMed]
- Vinh, L.B.; Heo, M.; Phong, N.V.; Ali, I.; Koh, Y.S.; Kim, Y.H.; Yang, S.Y. Bioactive compounds from Polygala tenuifolia and their inhibitory effects on lipopolysaccharide-stimulated pro-inflammatory cytokine production in bone marrow-derived dendritic cells. Plants 2020, 9, 1240. [Google Scholar] [CrossRef] [PubMed]
- Duyen, N.T.; Vinh, L.B.; Phong, N.V.; Khoi, N.M.; Long, P.Q.; Hien, T.T.; Dat, N.T.; Lee, K.Y. Steroid glycosides isolated from Paris polyphylla var. chinensis aerial parts and paris saponin II induces G1/S-phase MCF-7 cell cycle arrest. Carbohydr. Res. 2022, 519, 108613. [Google Scholar] [PubMed]
- Liu, Z.; Vinh, L.B.; Tuan, N.Q.; Lee, H.; Kim, E.; Kim, Y.-C.; Sohn, J.H.; Yim, J.H.; Lee, H.-J.; Lee, D.-S. Macrosphelides from antarctic fungus Pseudogymnoascus sp. (strain SF-7351) and their neuroprotective effects on BV2 and HT22 cells. Chem.-Biol. Interact. 2023, 385, 110718. [Google Scholar] [CrossRef] [PubMed]
- Tuan Anh, H.L.; Le Ba, V.; Do, T.T.; Phan, V.K.; Pham Thi, H.Y.; Bach, L.G.; Tran, M.H.; Tran Thi, P.A.; Kim, Y.H. Bioactive compounds from Physalis angulata and their anti-inflammatory and cytotoxic activities. J. Asian Nat. Prod. Res. 2021, 23, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Ba Vinh, L.; Jang, H.-J.; Viet Phong, N.; Dan, G.; Won Cho, K.; Ho Kim, Y.; Young Yang, S. Bioactive triterpene glycosides from the fruit of Stauntonia hexaphylla and insights into the molecular mechanism of its inflammatory effects. Bioorganic Med. Chem. Lett. 2019, 29, 2085–2089. [Google Scholar] [CrossRef] [PubMed]
- Leonti, M. Traditional medicines and globalization: Current and future perspectives in ethnopharmacology. Front. Pharmacol. 2013, 4, 92. [Google Scholar] [CrossRef] [PubMed]
- Ernst, E. The efficacy of herbal medicine—An overview. Fundam. Clin. Pharmacol. 2005, 19, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Prayoga, D.K.; Aulifa, D.L.; Budiman, A.; Levita, J. Plants with anti-ulcer activity and mechanism: A review of preclinical and clinical studies. Drug Des. Dev. Ther. 2024, 18, 193–213. [Google Scholar] [CrossRef] [PubMed]
- Stratton, C.F.; Newman, D.J.; Tan, D.S. Cheminformatic comparison of approved drugs from natural product versus synthetic origins. Bioorganic Med. Chem. Lett. 2015, 25, 4802–4807. [Google Scholar] [CrossRef] [PubMed]
- Iliya, I.; Tanaka, T.; Iinuma, M.; Ali, Z.; Furasawa, M.; Nakaya, K.-i.; Shirataki, Y.; Murata, J.; Darnaedi, D. Stilbene derivatives from two species of Gnetaceae. Chem. Pharm. Bull. 2002, 50, 796–801. [Google Scholar] [CrossRef] [PubMed]
- Do, T.L. Vietnamese Medicinal Plants and Herbs; Medical Publishing House: Hanoi, Vietnam, 2004. [Google Scholar]
- Vinh, L.B.; Han, Y.K.; Park, S.Y.; Kim, Y.J.; Phong, N.V.; Kim, E.; Ahn, B.-G.; Jung, Y.W.; Byun, Y.; Jeon, Y.H. Identification of triterpenoid saponin inhibitors of interleukin (IL)-33 signaling from the roots of Astragalus membranaceus. J. Funct. Foods 2023, 101, 105418. [Google Scholar] [CrossRef]
- Cao, T.Q.; Phong, N.V.; Kim, J.H.; Gao, D.; Anh, H.L.T.; Ngo, V.-D.; Vinh, L.B.; Koh, Y.S.; Yang, S.Y. Inhibitory effects of cucurbitane-type triterpenoids from Momordica charantia fruit on lipopolysaccharide-stimulated pro-inflammatory cytokine production in bone marrow-derived dendritic cells. Molecules 2021, 26, 4444. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.M.N.; Le, H.S.; Le, B.V.; Kim, Y.H.; Hwang, I. Anti-allergic effect of inotodiol, a lanostane triterpenoid from chaga mushroom, via selective inhibition of mast cell function. Int. Immunopharmacol. 2020, 81, 106244. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sun, H.; Ye, Y.; Chen, F.; Tu, J.; Pan, Y. Three new immunomodulating C21-steroidal glycosides from the stems of Stephanotis mucronata. Chem. Biodivers. 2005, 2, 1701–1711. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xu, N.; Zhou, Y.; Qiao, L.; Cao, J.; Yao, Y.; Hua, H.; Pei, Y. Steroidal glycosides from the roots of Cynanchum amplexicaule Sieb. et Zucc. Steroids 2008, 73, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-B.; Su, S.-S.; Tang, M.-X.; Zhao, D.; Chen, G.; Chen, S.-F.; Wang, H.-F.; Pei, Y.-H. Two new pregnane steroidal glycosides from Cynanchum taihangense. Nat. Prod. Res. 2021, 35, 2308–2314. [Google Scholar] [CrossRef] [PubMed]
- Kubo, S.; Kuroda, M.; Yokosuka, A.; Sakagami, H.; Mimaki, Y. Amurensiosides L-P, five new cardenolide glycosides from the roots of Adonis amurensis. Nat. Prod. Commun. 2015, 10, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, G.; Sanogo, R.; Vassallo, A.; Dal Piaz, F.; Autore, G.; Marzocco, S.; De Tommasi, N. Pregnane glycosides from Leptadenia pyrotechnica. J. Nat. Prod. 2006, 69, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, J.R.; Kanwar, R.K.; Burrow, H.; Baratchi, S. Recent advances on the roles of no in cancer and chronic inflammatory disorders. Curr. Med. Chem. 2009, 16, 2373–2394. [Google Scholar] [CrossRef] [PubMed]
- Phong, N.V.; Anh, D.T.N.; Chae, H.Y.; Yang, S.Y.; Kwon, M.J.; Min, B.S.; Kim, J.A. Anti-inflammatory activity and cytotoxicity against ovarian cancer cell lines by amide alkaloids and piperic esters isolated from piper longum fruits: In vitro assessments and molecular docking simulation. Bioorganic Chem. 2022, 128, 106072. [Google Scholar]
- Vinh, L.B.; Nguyet, N.T.M.; Ye, L.; Dan, G.; Phong, N.V.; Anh, H.L.T.; Kim, Y.H.; Kang, J.S.; Yang, S.Y.; Hwang, I. Enhancement of an in vivo anti-inflammatory activity of oleanolic acid through glycosylation occurring naturally in Stauntonia hexaphylla. Molecules 2020, 25, 3699. [Google Scholar] [CrossRef] [PubMed]
- Fischmann, T.O.; Hruza, A.; Niu, X.D.; Fossetta, J.D.; Lunn, C.A.; Dolphin, E.; Prongay, A.J.; Reichert, P.; Lundell, D.J.; Narula, S.K.; et al. Structural characterization of nitric oxide synthase isoforms reveals striking active-site conservation. Nat. Struct. Mol. Biol. 1999, 6, 233–242. [Google Scholar] [CrossRef]
- Orlando, B.J.; Malkowski, M.G. Crystal structure of rofecoxib bound to human cyclooxygenase-2. Acta Crystallogr. Sect. F 2016, 72, 772–776. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, R.A.; Swindells, M.B. Ligplot+: Multiple ligand–protein interaction diagrams for drug discovery. J. Chem. Inf. Model. 2011, 51, 2778–2786. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef]
- Van Der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J.C. GROMACS: Fast, flexible, and free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Cheng, X.; Swails, J.M.; Yeom, M.S.; Eastman, P.K.; Lemkul, J.A.; Wei, S.; Buckner, J.; Jeong, J.C.; Qi, Y.; et al. CHARMM-GUI input generator for NAMD, GROMACS, AMBER, OpenMM, and CHARMM/OpenMM simulations using the CHARMM36 additive force field. J. Chem. Theory Comput. 2016, 12, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Tariq, L.; Bhat, B.A.; Hamdani, S.S.; Mir, R.A. Phytochemistry, pharmacology and toxicity of medicinal plants. In Medicinal and Aromatic Plants: Healthcare and Industrial Applications; Aftab, T., Hakeem, K.R., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 217–240. [Google Scholar]
- Thomford, N.E.; Senthebane, D.A.; Rowe, A.; Munro, D.; Seele, P.; Maroyi, A.; Dzobo, K. Natural products for drug discovery in the 21st century: Innovations for novel drug discovery. Int. J. Mol. Sci. 2018, 19, 1578. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, P.; Vijayan, V.; Liu, B.; Martinez-Delgado, B.; Matamala, N.; Nikolin, C.; Greite, R.; DeLuca, D.S.; Janciauskiene, S.; Motterlini, R. Distinct metabolic responses to heme in inflammatory human and mouse macrophages–role of nitric oxide. Redox Biol. 2024, 73, 103191. [Google Scholar] [CrossRef] [PubMed]
- Cock, I.E. Terminalia ferdinandiana Exell. extracts reduce pro-inflammatory cytokine and PGE2 secretion, decrease COX-2 expression and down-regulate cytosolic NF-κB levels. Inflammopharmacology 2024, 32, 1839–1853. [Google Scholar] [CrossRef] [PubMed]

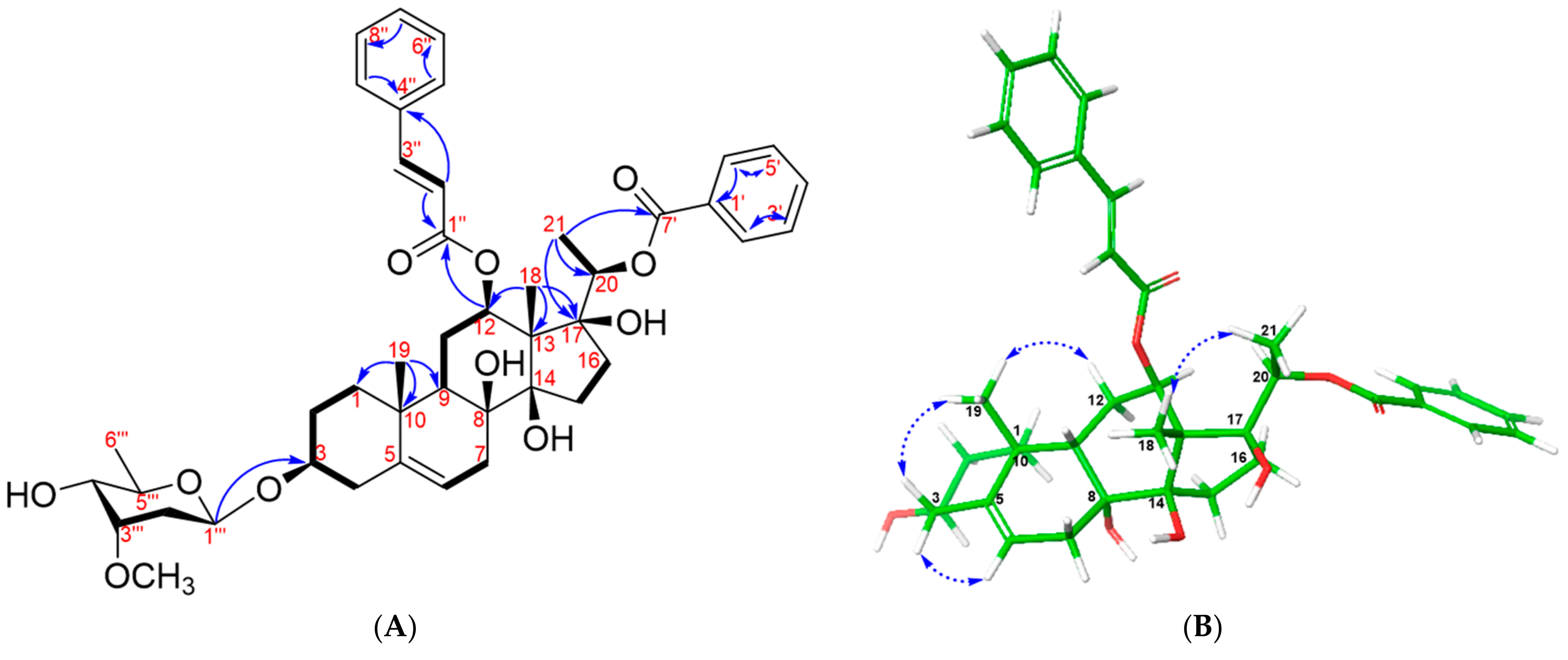



| Position | 1 | 2 | ||
|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | |
| 1 | 39.8, # | 1.13 (1H, m)/1.82 (1H, m) | 39.7 | 1.13 (1H, m)/1.80 (1H, m) |
| 2 | 30.1 | 1.60 (1H, m)/1.87 (1H, m) | 35.2 | 2.17 (1H, m)/2.21 (1H, m) |
| 3 | 79.3 | 3.53 (1H, m) | 79.3 | 3.54 (1H, m) |
| 4 | 39.8, # | 2.23 (1H, m)/2.36 (1H, m) | 39.8 | 2.21 (1H, m)/2.37 (1H, m) |
| 5 | 140.2 | - | 140.1 | - |
| 6 | 119.8 | 5.35 (1H, m) | 119.8 | 5.37 (1H, t, J = 3.0, 4.8 Hz) |
| 7 | 35.2 | 2.17 (1H, m)/2.22 (1H, m) | 35.6 | 1.57 (1H, m)/2.25 (1H, m) |
| 8 | 75.0 | - | 75.0 | - |
| 9 | 44.7 | 1.61 (1H, m) | 44.7 | - |
| 10 | 38.1 | - | 38.0 | - |
| 11 | 26.1 | 1.67 (1H, m)/2.00 (1H, m) | 26.1 | 1.68 (1H, m)/1.99 (1H, m) |
| 12 | 75.3 | 4.86 (1H, m, H-12) | 75.3 | 4.87 (1H, m) |
| 13 | 57.8 | - | 57.7 | - |
| 14 | 89.6 | - | 89.6 | - |
| 15 | 34.5 | 1.97 (1H, m)/2.03 (1H, m) | 34.5 | 1.89 (1H, m)/2.01 (1H, m) |
| 16 | 34.1 | 1.96 (1H, m)/2.04 (1H, m) | 34.0 | 1.90 (1H, m)/2.00 (1H, m) |
| 17 | 88.6 | - | 88.5 | - |
| 18 | 11.3 | 1.64 (3H, s) | 11.3 | 1.67 (3H, s) |
| 19 | 18.5 | 1.12 (3H, s) | 18.5 # | 1.14 (3H, s) |
| 20 | 76.4 | 4.81 (1H, m) | 76.4 | 4.82 (1H, m) |
| 21 | 15.2 | 1.33 (3H, d, J = 6.0 Hz) | 15.2 | 1.35 (3H, d, J = 6.6 Hz, H-21) |
| 1′ | 131.7 | - | 131.7 | - |
| 2′/6′ | 131.0 | 7.96 (2H, dd, J = 1.2, 8.4 Hz) | 131.0 | 7.98 (1H, dd, J = 1.2, 8.4 Hz) |
| 3′/5′ | 129.8 | 7.35 (2H, m) | 129.5 | 7.35 (1H, m) |
| 4′ | 134.2 | 7.55 (1H, m) | 134.2 | 7.57 (1H, m) |
| 7′ | 167.0 | - | 167.0 | - |
| 1″ | 168.1 | - | 168.1 | - |
| 2″ | 120.1 | 6.07 (1H, d, J = 16.2 Hz) | 120.1 | 6.09 (1H, d, J = 16.2 Hz) |
| 3″ | 145.4 | 7.35 (1H, d, J = 16.2 Hz) | 145.4 | 7.38 (1H, d, J = 16.2 Hz) |
| 4″ | 135.6 | - | 135.6 | - |
| 5″/9″ | 129.2 | 7.24 (2H, t, J = 1.2, 8.4 Hz) | 129.3 | 7.27 (2H, m) |
| 6″/8″ | 129.5 | 7.34 (2H, m) | 129.8 | 7.35 (2H) |
| 7″ | 131.3 | 7.38(1H, m) | 131.3 | 7.40 (1H, m) |
| CymI | ||||
| 1‴ | 97.2 | 4.85 (1H, m) | 97.2 | 4.89 (1H, m) |
| 2‴ | 36.0 | 1.53 (1H, m)/2.16 (1H, m) | 36.7 | 1.56 (1H, m)2.08 (1H, m) |
| 3‴ | 79.2 | 3.60(1H, m) | 78.6 | 3.86 (1H, m) |
| 4‴ | 74.5 | 3.16 (1H, dd, J = 3.0, 9.6 Hz) | 83.8 | 3.24 (1H, dd, J = 3.0, 9.6 Hz) |
| 5‴ | 71.5 | 3.77 (1H, dd, J = 6.6, 9.6 Hz) | 70.0 | 3.83 (1H, dd, J = 6.6, 9.6, Hz) |
| 6‴ | 18.7 | 1.22 (3H, d, J = 6.6 Hz) | 18.5 # | 1.21 (3H, t, J = 6.6 Hz) |
| 3-OCH3 | 58.1 | 3.43 (3H, s, -OCH3) | 58.1 | 3.47 (3H, #) |
| CymII | ||||
| 1⁗ | 101.2 | 4.80 (1H, dd, J = 1.8, 9.6 Hz) | ||
| 2⁗ | 35.6 | 1.57 (1H, m)/2.25 (1H, m) | ||
| 3⁗ | 79.2 | 3.63 (1H, m) | ||
| 4⁗ | 74.5 | 3.20 (1H, dd, J = 3.0, 9.6 Hz) | ||
| 5⁗ | 71.3 | 3.74 (1H, dd, J = 6.0, 9.6 Hz) | ||
| 6⁗ | 18.7 | 1.25 (3H, d, J = 6.6 Hz) | ||
| 3-OCH3 | 58.5 | 3.48 (3H, m,) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hieu, N.V.; Vinh, L.B.; Phong, N.V.; Cong, P.V.; Dat, N.T.; Dan, N.V.; Duc, N.V.; Tao, H.M.; Tam, L.T.; Anh, L.T.; et al. Two New Steroidal Saponins with Potential Anti-Inflammatory Effects from the Aerial Parts of Gnetum formosum Markgr. Plants 2024, 13, 2100. https://doi.org/10.3390/plants13152100
Hieu NV, Vinh LB, Phong NV, Cong PV, Dat NT, Dan NV, Duc NV, Tao HM, Tam LT, Anh LT, et al. Two New Steroidal Saponins with Potential Anti-Inflammatory Effects from the Aerial Parts of Gnetum formosum Markgr. Plants. 2024; 13(15):2100. https://doi.org/10.3390/plants13152100
Chicago/Turabian StyleHieu, Ngo Van, Le Ba Vinh, Nguyen Viet Phong, Pham Van Cong, Nguyen Tien Dat, Nguyen Van Dan, Ngo Viet Duc, Hoang Minh Tao, Le Thi Tam, Le Tuan Anh, and et al. 2024. "Two New Steroidal Saponins with Potential Anti-Inflammatory Effects from the Aerial Parts of Gnetum formosum Markgr." Plants 13, no. 15: 2100. https://doi.org/10.3390/plants13152100
APA StyleHieu, N. V., Vinh, L. B., Phong, N. V., Cong, P. V., Dat, N. T., Dan, N. V., Duc, N. V., Tao, H. M., Tam, L. T., Anh, L. T., Cuong, N. C., Tai, B. H., Yang, S. Y., & Tuan Anh, H. L. (2024). Two New Steroidal Saponins with Potential Anti-Inflammatory Effects from the Aerial Parts of Gnetum formosum Markgr. Plants, 13(15), 2100. https://doi.org/10.3390/plants13152100






