Influence of Habitat and Effects of Salt Stress on Biochemical and Physiological Parameters of Glycyrrhiza uralensis
Abstract
1. Introduction
2. Results
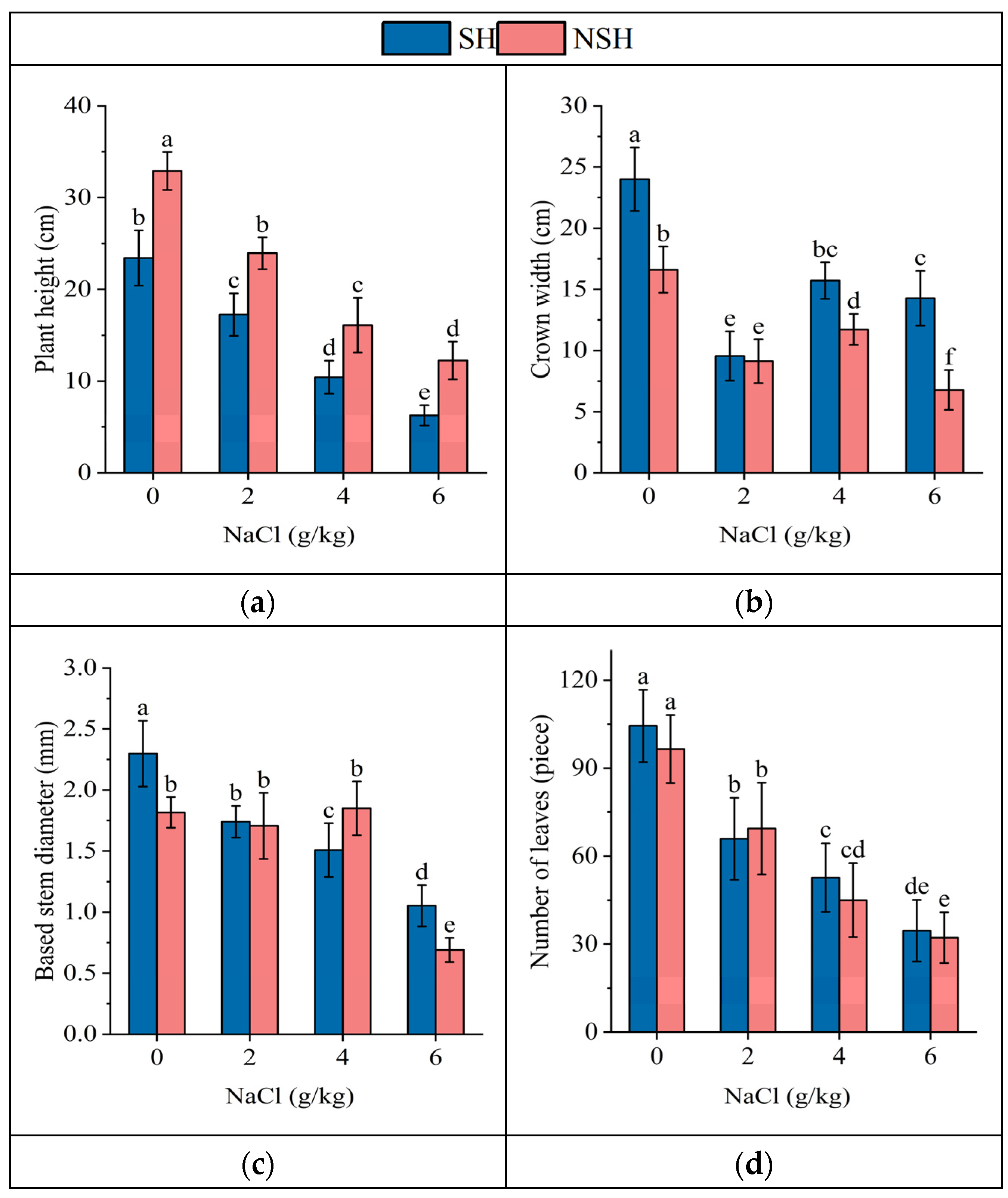
2.1. Growth Indexes of the Seedlings
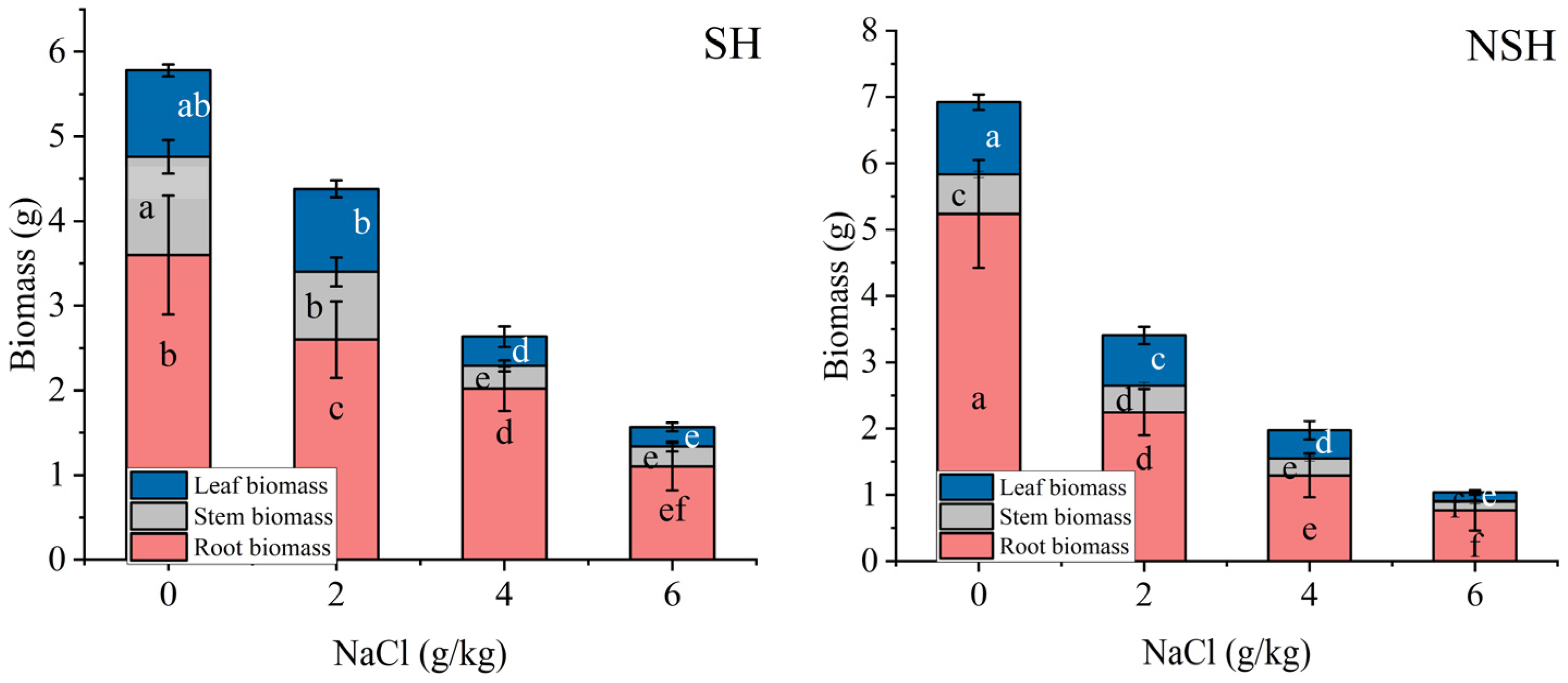
2.2. Biomass Accumulation of the Seedlings
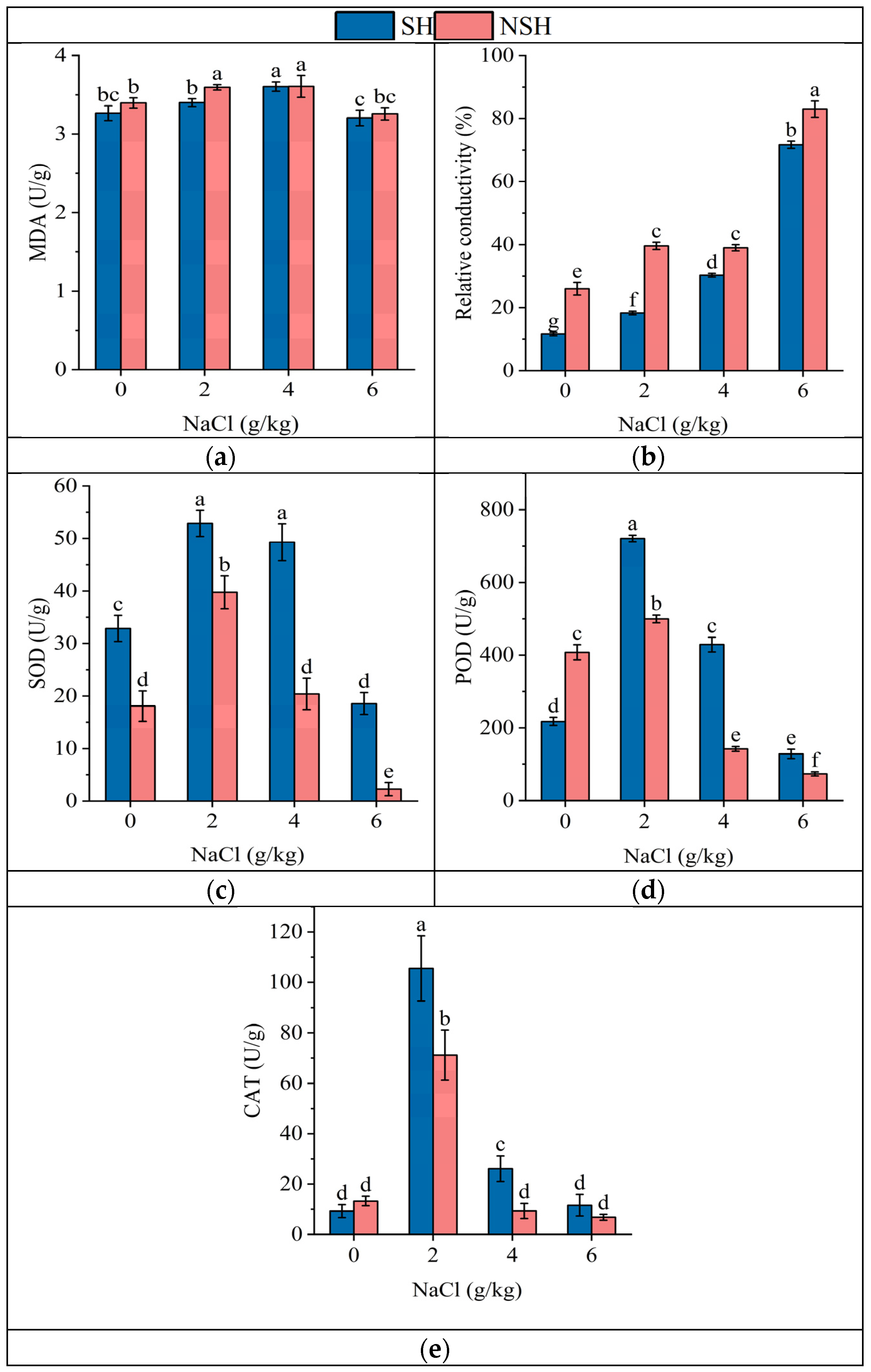
2.3. Antioxidant Capacity of the Seedlings
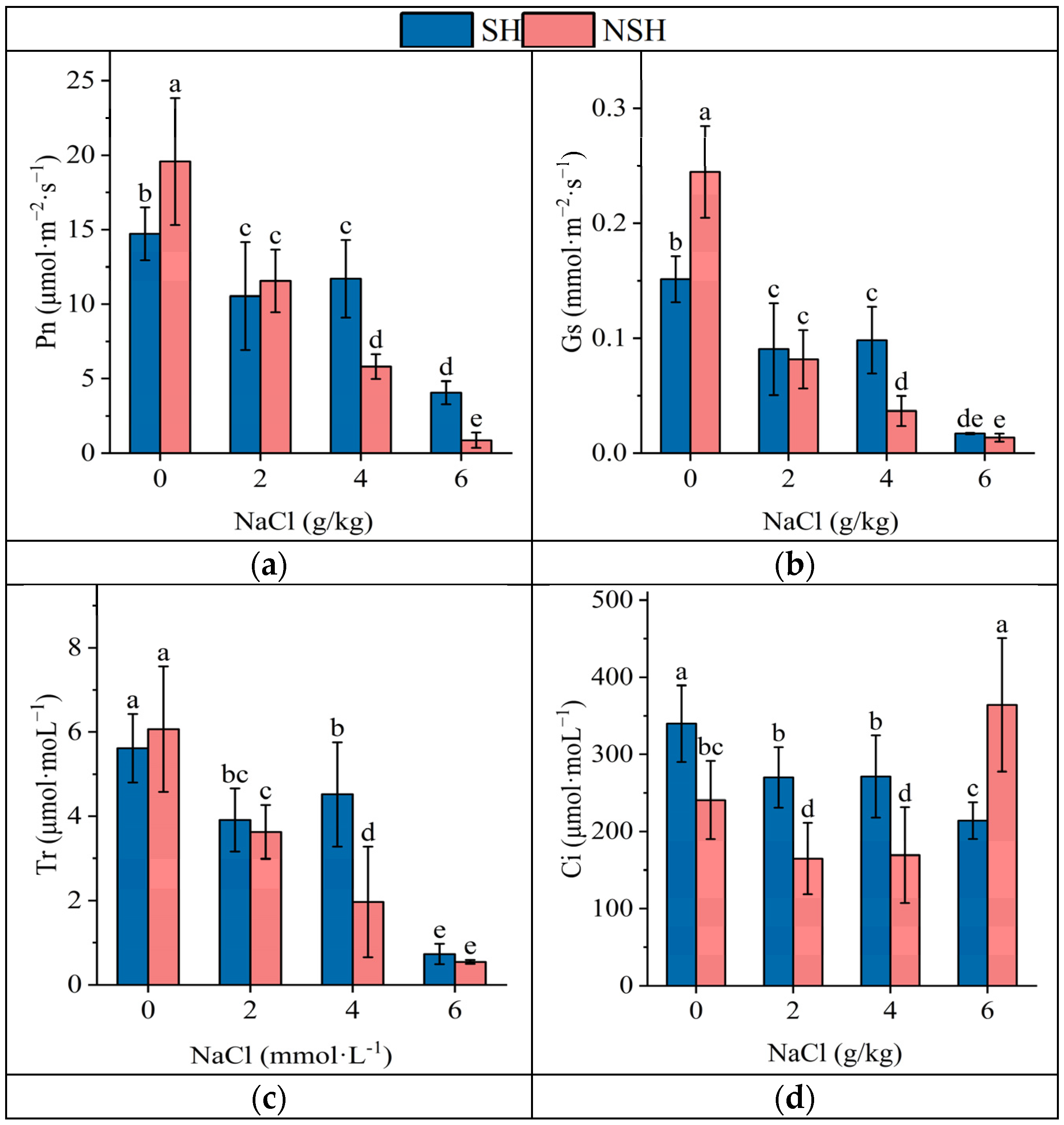
2.4. Photosynthetic Parameters of the Seedlings
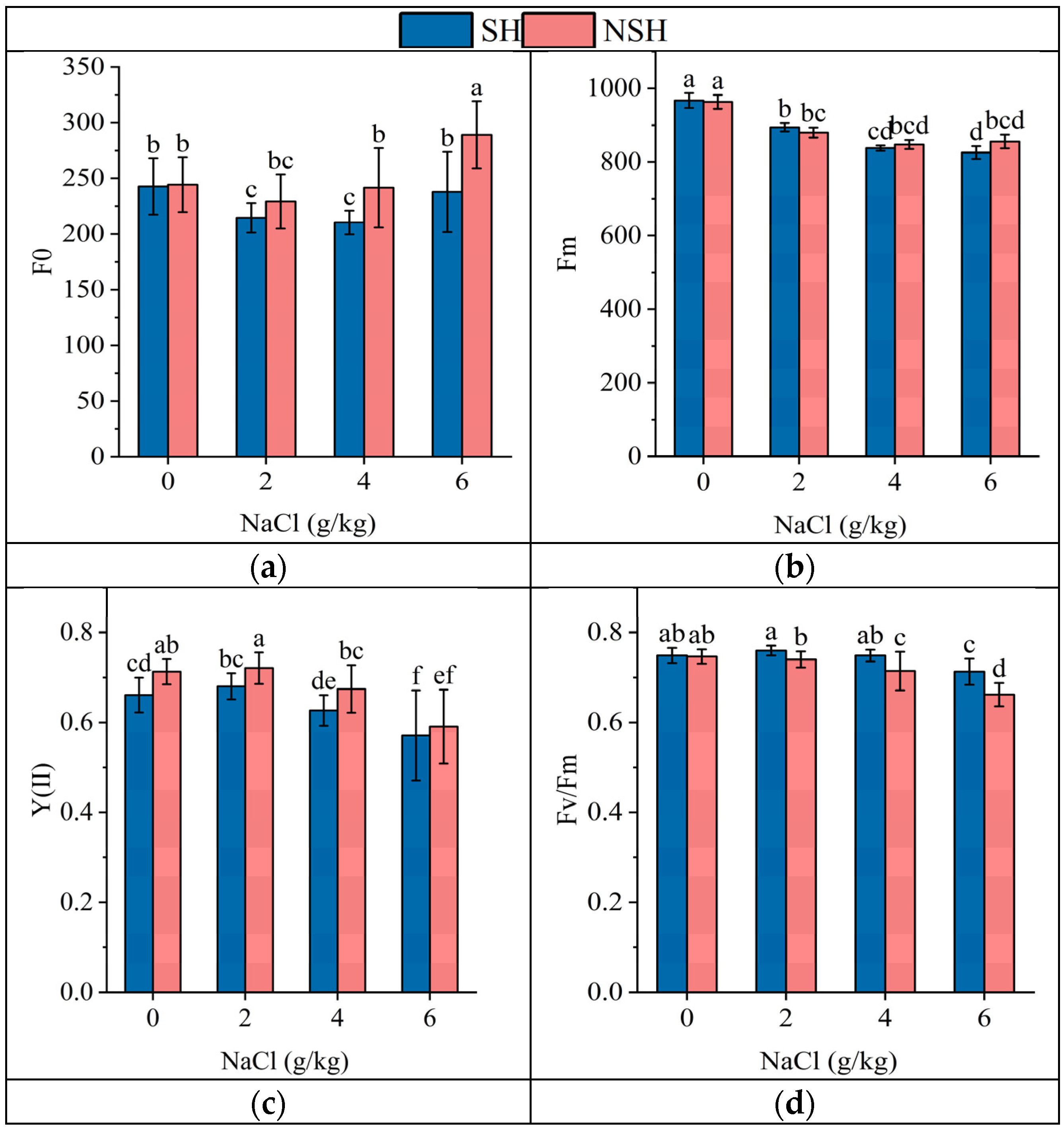
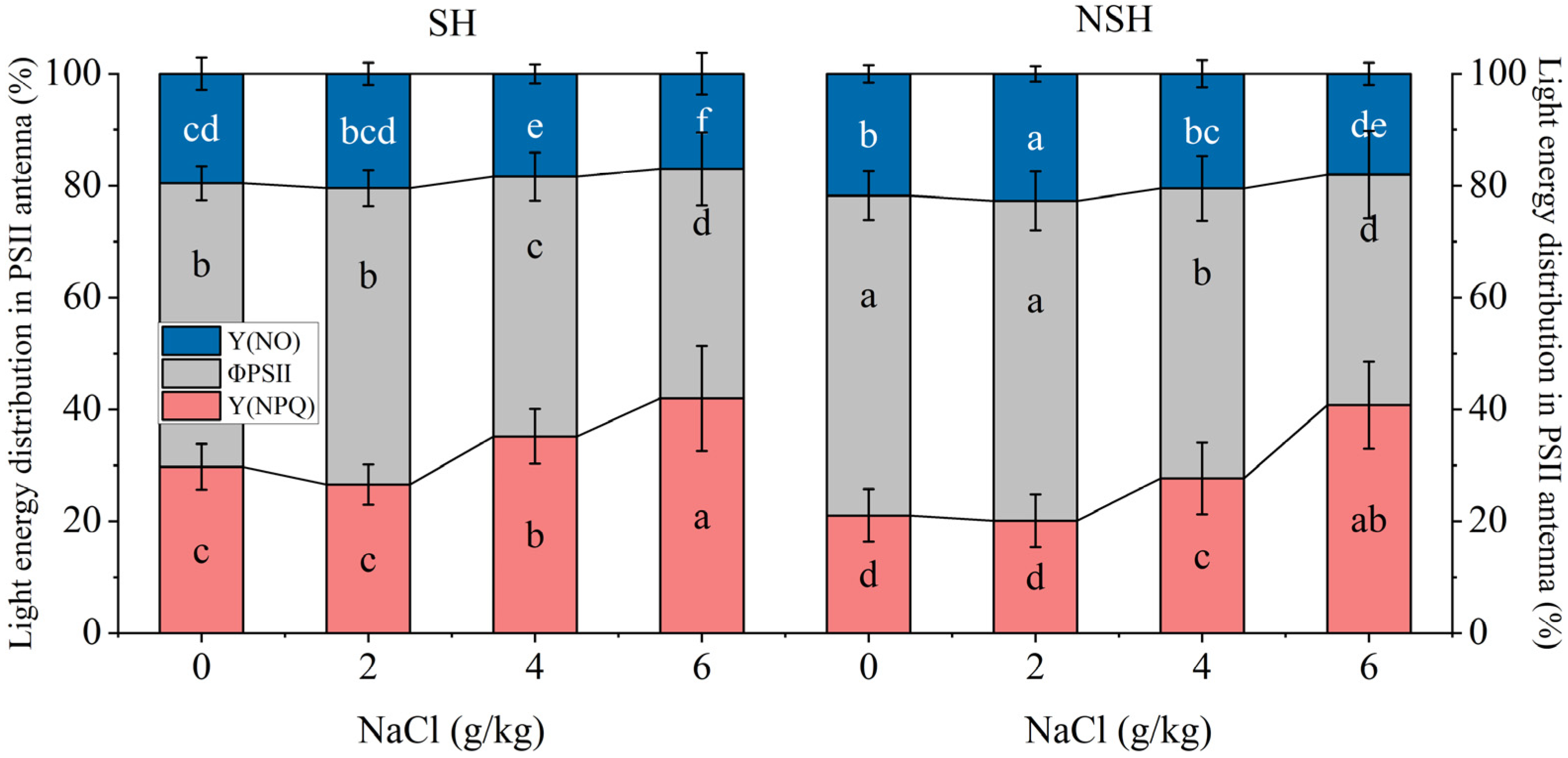
2.5. Fluorescence Parameters of the Seedlings
2.6. Contents of Secondary Metabolites of the Seedlings
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Materials
5.2. Experimental Design
5.3. Determination of Gas Exchange
5.4. Determination of Chlorophyll Fluorescence Parameters
5.5. Determination of Physiological Indexes
5.6. Determination of Growth Indicators
5.7. Determination of Medicinal Components of Roots
5.8. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, Y.X.; Hou, Z.N.; Zhang, X.M.; Yan, K.J.; Liang, Z.S.; He, Q.L. Important changes in germination, seedling tolerance, and active components content due to drought stress on three licorice (Glycyrrhiza) species. Ind. Crops Prod. 2021, 175, 114240. [Google Scholar] [CrossRef]
- Wang, R.; Li, Q.; Ren, S.F.; Wang, S.T. Experimental study on ecological restoration of Glycyrrhiza uralensis in yulin sandy grassland. E3S Web Conf. 2023, 406, 03047. [Google Scholar] [CrossRef]
- Song, L.L.; Wang, J.M.; Wang, Y.M.; Duan, Y.Q.; Zhang, Y.Y.; Li, Y.M.; Qin, L.Y.; He, Q.W.; Ji, L.J.; Zhang, T.Z.; et al. Investigation of the principle of concoction by using the processing excipient Glycyrrhiza uralensis Fisch. juice to reduce the main toxicity of Dioscorea bulbifera L. and enhance its main efficacy as expectorant and cough suppressant. J. Ethnopharmacol. 2024, 319, 117372. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, Y.I.; Hur, M.; Park, W.T.; Moon, Y.H.; Huh, Y.C.; Kim, T.I.; Kang, M.H.; Lang, J.S.; Cho, C.W.; et al. Inhibition by components of Glycyrrhiza uralensis of 3CLpro and HCoV-OC43 proliferation. J. Enzym. Inhib. Med. Chem. 2023, 38, 2242704. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.Y.; Shi, J.J.; Liu, Y.J.; Yu, J.; Li, C.Y.; Tao, F.; Cao, J.F.; Yang, G.J.; Chen, J. The state-of-the-art antibacterial activities of glycyrrhizin: A comprehensive review. Microorganisms 2024, 12, 1155. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.J.; He, L.; Pei, H.Y.; Li, J.M.; Zhao, Y.; Zong, Y.; Kan, H.; He, Z.M.; Du, R. Neuroprotective effect of total flavonoids in stems and leaves of Glycyrrhiza uralensis Fisch. on oxidative stress in HT-22 cells and Caenorhabditis elegans. Aging 2023, 15, 5290–5303. [Google Scholar] [CrossRef]
- Hajirahimkhan, A.; Mbachu, O.; Simmler, C.; Ellis, S.G.; Dong, H.L.; Nikolic, D.; Lankin, D.C.; van Breemen, R.B.; Chen, S.N.; Pauli, G.F.; et al. Estrogen receptor (ER) subtype selectivity identifies 8-Prenylapigenin as an ERβ agonist from Glycyrrhiza inflata and highlights the importance of chemical and biological authentication. J. Nat. Prod. 2018, 81, 966–975. [Google Scholar] [CrossRef]
- Dong, L.; Lee, J.H.; Jo, E.H.; Lee, J.S.; Kim, S.H.; Lee, D.S.; Park, M.C. Effects of varying ratios of Glycyrrhiza uralensis and donkey hide gelatin water extracts on dinitrochlorobenzene-induced atopic dermatitis in NC/Nga mice. Nutrients 2023, 15, 2094. [Google Scholar] [CrossRef] [PubMed]
- Hong, B.; Wang, Y.P.; Hou, Y.J.; Liu, R.H.; Li, W.J. Study on the mechanism of anti-hepatic fibrosis of Glycyrrhiza uralensis-Salvia miltiorrhiza prescription based on serum and urine metabolomics and network pharmacology. J. Chromatogr. B 2022, 1209, 123416. [Google Scholar] [CrossRef]
- Park, J.Y.; Park, W.Y.; Park, J.; Ahn, K.S.; Lee, J.H.; Kwak, H.J.; Um, J.Y. Therapeutic role of Glycyrrhiza uralensis fisher on benign prostatic hyperplasia through 5 alpha reductase regulation and apoptosis. Phytomedicine 2022, 105, 154371. [Google Scholar] [CrossRef]
- Chiyo, N.; Seki, H.; Kanamoto, T.; Ueda, H.; Kojoma, M.; Muranaka, T. Glycyrrhizin production in licorice hairy roots based on metabolic redirection of triterpenoid biosynthetic pathway by genome editing. Plant Cell Physiol. 2023, 65, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Bai, Y.; Zhang, Z.R.; Zhang, Y.F.; Jiang, Y.; Wang, S.T.; Wang, Y.J.; Sun, Z.R. Transcriptomics and metabolomics reveal the primary and secondary metabolism changes in Glycyrrhiza uralensis with different forms of nitrogen utilization. Front. Plant Sci. 2023, 14, 1229253. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.Y.; Wang, Q.L.; Lang, D.Y.; Li, Y.; Zhang, W.J.; Zhang, X.H. Bacillus cereus G2 facilitates N cycle in soil, further improves N uptake and assimilation, and accelerates proline and glycine betaine metabolisms of Glycyrrhiza uralensis subjected to salt stress. J. Agric. Food Chem. 2023, 72, 15485–15496. [Google Scholar] [CrossRef] [PubMed]
- Egamberdieva, D.; Ma, H.; Bellingrath-Kimura, S.D. Biochar amendments improve licorice (Glycyrrhiza uralensis Fisch.) growth and nutrient uptake under salt stress. Plants 2021, 10, 2135. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.C.; Cao, M.Y.; Lai, K.Y.; Sun, K.; Zhang, L.; Gao, P.C.; Zhang, Y.; Yan, B.B.; Guo, L.P. Unveiling key metabolic pathways in Bacillus subtilis-mediated salt tolerance enhancement in Glycyrrhiza uralensis Fisch. through multi-omics analysis. Environ. Exp. Bot. 2023, 219, 105631. [Google Scholar] [CrossRef]
- Shen, Z.H.; Cheng, X.J.; Li, X.; Deng, X.Y.; Dong, X.X.; Wang, S.M.; Pu, X.Z. Effects of silicon application on leaf structure and physiological characteristics of Glycyrrhiza uralensis Fisch. and Glycyrrhiza inflata Bat. under salt treatment. BMC Plant Biol. 2022, 22, 390. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.P.; Chang, N.; Zhang, T.C. Increased epigenetic diversity and transient epigenetic memory in response to salinity stress in Thlaspi arvense. Ecol. Evol. 2020, 10, 11622–11630. [Google Scholar] [CrossRef] [PubMed]
- Marinoni, L.D.; Richard, G.A.; Bustos, D.; Taleisnik, E.L.; Pensiero, J.F.; Zabala, J.M. Differential response of Trichloris ecotypes from different habitats to drought and salt stress. Theor. Exp. Plant Physiol. 2020, 32, 213–229. [Google Scholar] [CrossRef]
- Gu, J.J.; Yao, S.X.; Ma, M. Maternal effects of habitats induce stronger salt tolerance in early-stage offspring of Glycyrrhiza uralensis from salinized habitats compared with those from non-salinized habitats. Biology 2024, 13, 52. [Google Scholar] [CrossRef]
- Guo, X.; Ahmad, N.; Zhao, S.Z.; Zhao, C.Z.; Zhong, W.; Wang, X.J.; Li, G.H. Effect of salt stress on growth and physiological properties of Asparagus seedlings. Plants 2022, 11, 2836. [Google Scholar] [CrossRef]
- Pan, T.; Liu, M.; Kreslavski, V.D.; Zharmukhamedov, S.K.; Nie, C.; Yu, M.; Kuznetsov, V.V.; Allakhverdiev, S.I.; Shabala, S. Non-stomatal limitation of photosynthesis by soil salinity. Crit. Rev. Environ. Sci. Technol. 2020, 51, 791–825. [Google Scholar] [CrossRef]
- Wang, K.K.; Zhu, J.; Xu, X.W.; Li, T.J.; Wang, X.; Warner, T.A.; Cheng, T.; Zhu, Y.; Cao, W.X.; Yao, X.; et al. Quantitative monitoring of salt stress in rice with solar-induced chlorophyll fluorescence. Eur. J. Agron. 2023, 150, 126954. [Google Scholar] [CrossRef]
- Vivas, M.; Wingfield, M.J.; Slippers, B. Maternal effects should be considered in the establishment of forestry plantations. For. Ecol. Manag. 2020, 460, 117909. [Google Scholar] [CrossRef]
- Galloway, L.F. Response to natural environmental heterogeneity: Maternal effects and selection on life-history characters and plasticities in Mimulus guttatus. Evolution 1995, 49, 1095–1107. [Google Scholar] [CrossRef] [PubMed]
- Van Rossum, F.; Le Pajolec, S. Maternal effects and inbreeding depression in post-translocation progeny of Campanula glomerata. Plant Biol. 2024, 26, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Aycan, M.; Baslam, M.; Ozdemir, B.; Asiloglu, R.; Mitsui, T.; Yildiz, M. Direct contribution of the maternal genotype on the transgenerational salinity tolerance in wheat (Triticum aestivum L.). Environ. Exp. Bot. 2021, 192, 104648. [Google Scholar] [CrossRef]
- El-Keblawy, A.; Sanjay, G.; Arvind, B.; Mahmoud, T. Effects of maternal salinity on salt tolerance during germination of Suaeda aegyptiaca, a facultative halophyte in the Arab Gulf desert. Plant Species Biol. 2016, 32, 45–53. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, C.Z.; Kang, M.P.; Li, X.Y. The relationship of the main root-shoot morphological characteristics and biomass allocation of Saussurea salsa under different habitat conditions in Sugan lake wetland on the northern margin of the Qinghai-Tibet Plateau. Ecol. Indic. 2021, 128, 107836. [Google Scholar] [CrossRef]
- Liu, G.; Gao, Y.; Huang, F.F.; Yuan, M.Y.; Peng, S.L. The invasion of coastal areas in south China by Ipomoea cairica may be accelerated by the ecotype being more locally adapted to salt stress. PLoS ONE 2016, 11, e0149262. [Google Scholar] [CrossRef]
- Matilde, A.C.; Ken, S.M.; Tonya, D.F.; Kelsey, E.M.; Sergey, V.N.; Eric, J.V.W.; Douglas, R.C. Population differentiation for germination and early seedling root growth traits under saline conditions in the annual legume Medicago truncatula (fabaceae). Botany 2014, 101, 488–498. [Google Scholar] [CrossRef]
- Li, J.M.; Liu, H.Y.; Yan, M.; Du, L.S. No evidence for local adaptation to salt stress in the existing populations of invasive Solidagocanadensis in China. PLoS ONE 2017, 12, e0175252. [Google Scholar] [CrossRef]
- Guan, T.C.; Song, J.; Wang, Y.A.; Guo, L.Y.; Yuan, L.; Zhao, Y.D.; Gao, Y.; Lin, L.R.; Wang, Y.L.; Wei, J.Y. Expression and characterization of recombinant bifunctional enzymes with glutathione peroxidase and superoxide dismutase activities. Free Radic. Biol. Med. 2017, 110, 188–195. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, X.Y.; Baskin, C.C.; Wang, L.Y.; Li, Y.H. Seasonal germination responses of seeds in fruits of the cold desert shrub Zygophyllum xanthoxylonburied in the field. Arid Land Res. Manag. 2020, 34, 425–444. [Google Scholar] [CrossRef]
- Mohamed, E.; Kasem, A.M.M.A.; Gobouri, A.A.; Elkelish, A.; Azab, E. Influence of maternal habitat on salt tolerance during germination and growth in Zygophyllum coccineum. Plants 2020, 9, 1504. [Google Scholar] [CrossRef] [PubMed]
- Osei-Kwarteng, M.; Ayipio, E.; Moualeu-Ngangue, D.; Buck-Sorlin, G.; Stutzel, H. Interspecific variation in leaf traits, photosynthetic light response, and whole-plant productivity in amaranths (Amaranthus spp. L.). PLoS ONE 2022, 17, e0270674. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Q.; Kaiser, E.; Li, T.; Marcelis, L.F.M. NaCl affects photosynthetic and stomatal dynamics by osmotic effects and reduces photosynthetic capacity by ionic effects in tomato. J. Exp. Bot. 2022, 73, 3637–3650. [Google Scholar] [CrossRef] [PubMed]
- Shirahmadi, S.; Esna-Ashari, M.; Aliniaeifard, S.; Akbari, G.A. Natural variation for salt tolerance among basil accessions from iran based on fluorescence transient and morphological and growth characteristics. J. Agric. Sci. Technol. 2022, 24, 183–198. [Google Scholar]
- Banks, J.M. Continuous excitation chlorophyll fluorescence parameters: A review for practitioners. Tree Physiol. 2017, 37, 1128–1136. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Xie, S.Q.; Yan, S.; Xu, W.G.; Chen, J.W. Light energy partitioning and photoprotection from excess light energy in shade-tolerant plant Amorphophallus xiei under steady-state and fluctuating high light. Acta Physiol. Plant. 2021, 43, 125. [Google Scholar] [CrossRef]
- Zunzunegui, M.; Sanchez, J.M.; Barradas, M.C.D. Different tolerance to salinity of two populations of Oenothera drummondii with contrasted biogeographical origin. Plant Physiol. Biochem. 2021, 166, 999–1000. [Google Scholar] [CrossRef]
- Zuo, J.W.; Meng, T.; Wang, Y.Y.; Tang, W.J. A review of the antiviral activities of glycyrrhizic acid, glycyrrhetinic acid and glycyrrhetinic acid monoglucuronide. Pharmaceuticals 2023, 16, 641. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhou, J.; Zhang, C. Salt stress decreases seedling growth and development but increases quercetin and kaempferol content in Apocynum venetum. Plant Biol. 2020, 22, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.H.; Zhang, W.J.; Li, Y.T. Silicon improves salt tolerance of Glycyrrhiza uralensis Fisch. by ameliorating osmotic and oxidative stresses and improving phytohormonal balance. Environ. Sci. Pollut. Res. 2018, 25, 25916–25932. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.P.; Ma, X.M.; Zhao, Z.L.; Ma, M. Exogenous betaine enhances salt tolerance of Glycyrrhiza uralensis through multiple pathways. BMC Plant Biol. 2024, 24, 165. [Google Scholar] [CrossRef] [PubMed]
- Kuhlgert, S.; Austic, G.; Zegarac, R.; Osei-Bonsu, I.; Hoh, D.; Chilvers, M.I.; Roth, M.G.; Bi, K.; TerAvest, D.; Weebadde, P.; et al. MultispeQ Beta: A tool for large-scale plant phenotyping connected to the open PhotosynQ network. R. Soc. Open Sci. 2016, 3, 160592. [Google Scholar] [CrossRef] [PubMed]
- Grishina, A.; Sherstneva, O.; Grinberg, M.; Zdobnova, T.; Ageyeva, M.; Khlopkov, A.; Sukhov, V.; Brilkina, A.; Vodeneev, V. Pre-symptomatic detection of viral infection in tobacco leaves using PAM fluorometry. Plants 2021, 10, 2782. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.F.; Men, Y.H.; Li, Z.M.; Zou, Q.Y.; Ji, J.W. Chlorophyll fluorescence imaging as a tool for analyzing the effects of chilling injury on tomato seedlings. Sci. Hortic. 2019, 246, 490–497. [Google Scholar] [CrossRef]
- Yu, K.S.; Shiva, R.B.; Lee, J.G. Monitoring of salinity, temperature, and drought stress in grafted watermelon seedlings using chlorophyll fluorescence. Front. Plant Sci. 2021, 12, 786309. [Google Scholar] [CrossRef]
- Khan, I.; Awan, S.A.; Raza, M.A.; Rizwan, M.; Tariq, R.; Ali, S.; Huang, L.K. Silver nanoparticles improved the plant growth and reduced the sodium and chlorine accumulation in pearl millet: A life cycle study. Environ. Sci. Pollut. Res. 2021, 28, 13721–13724. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Wang, P.l.; Zhang, T.Q.; Li, Y.B.; Wang, Y.Y.; Gao, C.Q. Comprehensive analysis of BpHSP genes and their expression under heat stresses in Betula platyphylla. Environ. Exp. Bot. 2018, 152, 167–176. [Google Scholar] [CrossRef]
- Da Silva, T.I.; Dias, M.G.; De Araujo, N.O.; Santos, M.N.D.; Cruz, R.R.P.; Dias, T.J.; Ribeiro, W.S.; Grossi, J.A.S.; Barbosa, J.G. Spermine reduces the harmful effects of salt stress in Tropaeolum majus. Physiol. Mol. Biol. Plants 2022, 28, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Konôpka, B.; Pajtík, J.; Marusák, R.; Bosela, M.; Lukac, M. Specific leaf area and leaf area index in developing stands of Fagus sylvatica L. and Picea abies Karst. For. Ecol. Manag. 2016, 364, 52–59. [Google Scholar] [CrossRef]
- Jia, T.T.; Chen, B.; Ma, M. Effects of planting density on the growth, taproots yield and quality of Glycyrrhiza uralensis. Legume Res. 2023, 46, 62–68. [Google Scholar] [CrossRef]


| Source | Plant Height | Crown Width | Based Stem Diameter | Number of Leaves | Specific Leaf Area | Root Volume | |
|---|---|---|---|---|---|---|---|
| Habitats | Df | 1 | 1 | 1 | 1 | 1 | 1 |
| MS | 968.1 | 466.1 | 0.4 | 248.5 | 7536.7 | 0.1 | |
| F and p | 196.7 *** | 127.6 *** | 8.7 ** | 1.6 | 30.2 *** | 0.28 | |
| Salinity | Df | 3 | 3 | 3 | 3 | 3 | 3 |
| MS | 1391.6 | 483.2 | 5.0 | 16,564.7 | 8680.9 | 12.8 | |
| F and p | 282.8 *** | 132.3 *** | 125.2 *** | 109.7 *** | 34.7 *** | 70.5 *** | |
| Habitats × Salinity | Df | 3 | 3 | 3 | 3 | 3 | 3 |
| MS | 15.2 | 56.4 | 0.7 | 139.7 | 994.6 | 1.1 | |
| F and p | 3.1 * | 15.4 *** | 16.9 *** | 0.925 | 3.9 * | 5.8 * | |
| Source | Root Biomass | Stem Biomass | Leaf Biomass | Glycyrrhizic Acid | Glycyrrhetinic Acid | |
|---|---|---|---|---|---|---|
| Habitats | Df | 1 | 1 | 1 | 1 | 1 |
| MS | 0.001 | 1.5 | 0.03 | 1879.7 | 3487.3 | |
| F and p | 0.007 | 141.9 *** | 2.9 | 13.9 ** | 97.9 *** | |
| Salinity | Df | 3 | 3 | 3 | 3 | 3 |
| MS | 44.25 | 2.0 | 3.3 | 123,888.6 | 3112.3 | |
| F and p | 239.1 *** | 192.3 *** | 308.5 *** | 915.3 *** | 87.3 *** | |
| Habitats × Salinity | Df | 3 | 3 | 3 | 3 | 3 926.3 *** |
| MS | 6.0 | 0.3 | 0.1 | 25,350.1 | ||
| F and p | 32.6 ** | 30.8 *** | 9.3 *** | 187.3 *** | ||
| Source | SOD | POD | CAT | REC | MDA | |
|---|---|---|---|---|---|---|
| Habitats | Df | 1 | 1 | 1 | 1 | 1 5.4 * |
| MS | 2003.1 | 52,086.7 | 1011.4 | 1162.0 | ||
| F and p | 274.1 *** | 304.3 *** | 24.4 *** | 593.4 *** | ||
| Salinity | Df | 3 | 3 | 3 | 3 | 1 |
| MS | 1382.6 | 266,270.5 | 8663.6 | 3982.4 | 0.188 | |
| F and p | 189.2 *** | 1555.6 *** | 208.9 *** | 2033.6 *** | 25.5 *** | |
| Habitats × Salinity | Df | 3 | 3 | 3 | 3 | 3 |
| MS | 77.7 | 67,686.9 | 414.2 | 44.7 | 0.02 | |
| F and p | 10.6 *** | 395.4 *** | 10 ** | 22.8 *** | 2.1 | |
| Source | Habitats | Salinity | Habitats × Salinity |
|---|---|---|---|
| Df | Df | Df | |
| MS | MS | MS | |
| F and p | F and p | F and p | |
| Pn | 1 | 3 | 3 |
| 15.2 | 883.6 | 133.9 | |
| 2.5 | 148.9 *** | 22.6 *** | |
| Gs | 1 | 3 | 3 |
| 0.00007 | 0.1 | 0.02 | |
| 0.1 | 171.9 *** | 24.7 *** | |
| Ci | 1 | 3 | 3 |
| 40,532.7 | 42,533.5 | 97,708.8 | |
| 13.8 *** | 14.5 *** | 33.3 *** | |
| Tr | 1 | 3 | 3 |
| 9.9 | 110.0 | 10.4 | |
| 10.9 ** | 122.0 *** | 11.5 *** | |
| F0 | 1 | 3 | 3 |
| 18,278.0 | 10,808.0 | 3453.5 | |
| 26.1 *** | 15.4 *** | 4.9 ** | |
| Fm | 1 | 3 | 3 |
| 861.4 | 101,694.7 | 2749.9 | |
| 0.2 | 27.7 * | 0.7 | |
| Fv/Fm | 1 | 3 | 3 |
| 0.02 | 0.3 | 0.003 | |
| 37.7 *** | 43.5 *** | 5.3 ** | |
| Y(II) | 1 | 3 | 3 |
| 0.05 | 0.09 | 0.002 | |
| 15.6 *** | 28.3 *** | 0.6 | |
| Y(NO) | 1 | 3 | 3 |
| 0.03 | 0.01 | 0.3 | |
| 45.4 *** | 21.5 *** | 0.8 | |
| Y(NPQ) | 1 | 3 | 3 |
| 0.1 | 0.2 | 1.3 | |
| 39.5 *** | 58.54 *** | 1.3 |
| Secondary Metabolite | Parent Ion (m·z−1) | Daughter Ion (m·z−1) | Ionization Mode | Voltage (V) | Collisional Energy (eV) | Retention Time (min) | Regression Equation | R2 | Linear Over (ng·mL−1) |
|---|---|---|---|---|---|---|---|---|---|
| Glycyrrhizic acid | 821.2 | 350.9 * | – | 62 | 42 | 2.48 | Y = 87.2X − 3.76 | 0.9992 | 1.0–978.0 |
| 113.0 | 62 | 56 | |||||||
| Glycyrrhetinic acid | 469.2 | 355.1 * | – | 100 | 44 | 3.81 | Y = 136.8X − 122.4 | 0.9990 | 1.4–993.2 |
| 409.2 | 100 | 46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, J.; Jia, T.; Ma, M. Influence of Habitat and Effects of Salt Stress on Biochemical and Physiological Parameters of Glycyrrhiza uralensis. Plants 2024, 13, 2108. https://doi.org/10.3390/plants13152108
Gu J, Jia T, Ma M. Influence of Habitat and Effects of Salt Stress on Biochemical and Physiological Parameters of Glycyrrhiza uralensis. Plants. 2024; 13(15):2108. https://doi.org/10.3390/plants13152108
Chicago/Turabian StyleGu, Junjun, Tingting Jia, and Miao Ma. 2024. "Influence of Habitat and Effects of Salt Stress on Biochemical and Physiological Parameters of Glycyrrhiza uralensis" Plants 13, no. 15: 2108. https://doi.org/10.3390/plants13152108
APA StyleGu, J., Jia, T., & Ma, M. (2024). Influence of Habitat and Effects of Salt Stress on Biochemical and Physiological Parameters of Glycyrrhiza uralensis. Plants, 13(15), 2108. https://doi.org/10.3390/plants13152108










