Abstract
Plant-specific TEOSINTE BRANCHED1/CYCLOIDEA/PROLIFERATING CELL FACTOR (TCP) proteins play critical roles in plant development and stress responses; however, their functions in chrysanthemum (Chrysanthemum morifolium) have not been well-studied. In this study, we isolated and characterized the chrysanthemum TCP transcription factor family gene CmTCP13, a homolog of AtTCP13. This gene encoded a protein harboring a conserved basic helix–loop–helix motif, and its expression was induced by salinity stress in chrysanthemum plants. Subcellular localization experiments showed that CmTCP13 localized in the nucleus. Sequence analysis revealed the presence of multiple stress- and hormone-responsive cis-elements in the promoter region of CmTCP13. The heterologous expression of CmTCP13 in Arabidopsis plants enhanced their tolerance to salinity stress. Under salinity stress, CmTCP13 transgenic plants exhibited enhanced germination, root length, seedling growth, and chlorophyll content and reduced relative electrical conductivity compared with those exhibited by wild-type (WT) plants. Moreover, the expression levels of stress-related genes, including AtSOS3, AtP5CS2, AtRD22, AtRD29A, and AtDREB2A, were upregulated in CmTCP13 transgenic plants than in WT plants under salt stress. Taken together, our results demonstrate that CmTCP13 is a critical regulator of salt stress tolerance in plants.
1. Introduction
Plants are often subjected to various environmental stresses throughout their life cycle. Soil salinity is a major stress factor affecting plant growth, development, and productivity. It causes osmotic stress, ion imbalance, and oxidative toxicity [1,2,3]. Improving the salt tolerance of plants has become crucial given the increasing soil salinization worldwide. Understanding the mechanisms underlying plant responses to salinity stress is a prerequisite for identifying potential genes that can be used to improve genetic salt tolerance in plants.
Plants adapt to salt stress through various responses, including stress perception, signal transduction, and the expression of stress-related genes and metabolites [4]. Many transcription factors (TFs), such as DREB/CBF, MYB, MYC, and AREB/ABF, play crucial roles in stress responses by regulating the expression of downstream target genes [5]. Overexpression of certain TFs, such as DREB2A and ERF, can enhance plant tolerance to various abiotic stresses including salinity [6,7]. The TCP family is a group of plant-specific TFs named after three initially characterized members, i.e., TEOSINTE BRANCHED 1 (TB1), CYCLOIDEA (CYC), and PROLIFERATING CELL FACTORS (PCFs). These proteins harbor a 59 amino acid noncanonical basic helix–loop–helix (bHLH) motif known as the TCP domain [8]. TCP proteins consist of 24 members in Arabidopsis thaliana [9], 22 in Oryza sativa [10], 33 in Populus euphratica [11], and 66 in Triticum aestivum [12]. Based on the dissimilarities in TCP domains, TCP genes can be classified into two classes, class I (known as PCF or TCP-P) and class II (TCP-C), which are further subdivided into two subclades, CYC/TB1 and CINCINNATA (CIN) [13,14].
TCP proteins play diverse and critical roles in many plant-specific biological processes including seed germination [15], leaf development [16], shoot branching [17], flower development [18,19], circadian rhythms [20], and hormonal pathways [9]. In addition, many TCP family genes coordinate plant responses to environmental stress [8,21]. In rice, OsPCF2 binds directly to the promoter of the O. sativa gene Na+/H+ exchanger 1 (OsNHX1) to upregulate its expression and enhance salt tolerance [22]. The OsTCP19 gene reportedly affects salt and drought stress tolerance positively [23]. Moreover, 46 ZmTCP genes have been identified in maize (Zea mays), and only ZmTCP42, which plays a positive role in drought tolerance, has been well characterized [24]. In Moso bamboo (Phyllostachys edulis), PeTCP10 can be induced by salt stress, and its overexpression enhances the salt tolerance of transgenic Arabidopsis at the vegetative growth stage [25]. Recently, BpTCP20 was reported to confer salt tolerance through the acetyltransferase component of the pyruvate dehydrogenase complex (BpPDCE23)-mediated regulation of acetylation [26].
Chrysanthemum (Chrysanthemum morifolium) is a species of global significance with high ornamental, cultural, and economic value. Salinity stress is a major factor affecting the yield and quality of chrysanthemum and can lead to extensive leaf chlorosis, growth retardation, and, in a few cases, even plant death. Therefore, increasing the salinity tolerance of chrysanthemum plants is essential for achieving stable and sustainable production. Recently, the reference genome of C. morifolium was reported, which laid the foundation for the genetic and molecular breeding of chrysanthemum [27]. Hitherto, there have been limited studies on the functions of TCP proteins in chrysanthemums. Therefore, in this study, we isolated a chrysanthemum TCP family TF gene, CmTCP13, and showed that its expression was induced by salinity stress. Overexpression of CmTCP13 in Arabidopsis significantly enhanced the salinity tolerance of transgenic plants. Through this study, we provide an excellent candidate gene for genetically improving salt tolerance in chrysanthemum plants.
2. Results
2.1. Cloning and Sequence Analysis of CmTCP13
The TCP TF family gene CmTCP13 was cloned from the chrysanthemum cultivar ‘Jinba’. The gene consisted of a 1032 bp open reading frame (ORF) encoding a 343 amino acid protein with a predicted molecular weight of 38.42 kDa and an isoelectric point of 6.63.
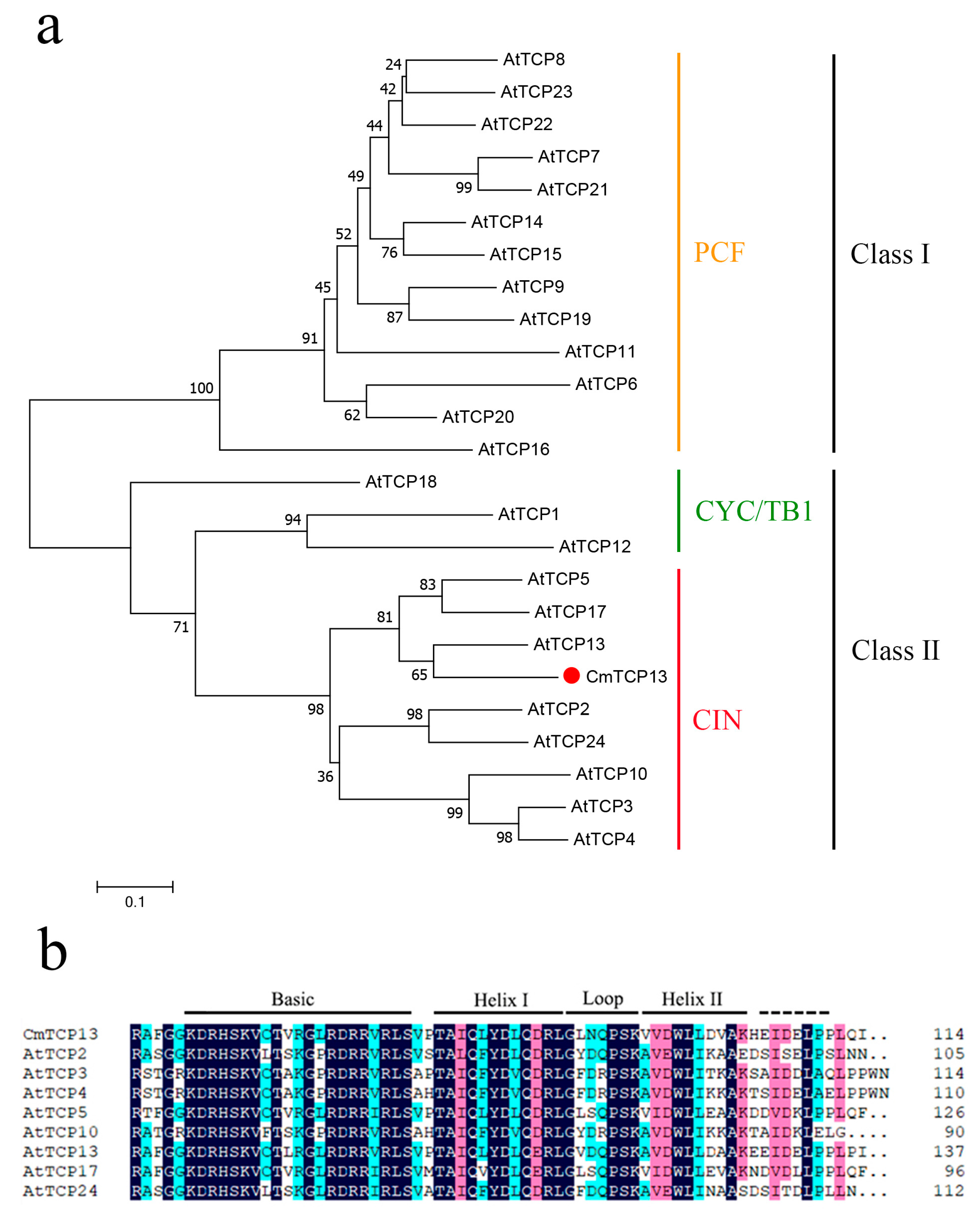
The phylogenetic analysis of CmTCP13 with 24 members of the Arabidopsis TCP family revealed that CmTCP13 is most closely related to AtTCP13 and belongs to the CIN subclass of Class II TCP proteins (Figure 1a). In addition, multiple sequence alignment of CmTCP13 with Arabidopsis CIN TCPs revealed that CmTCP13 harbors a conserved bHLH motif in its N-terminal region (Figure 1b).
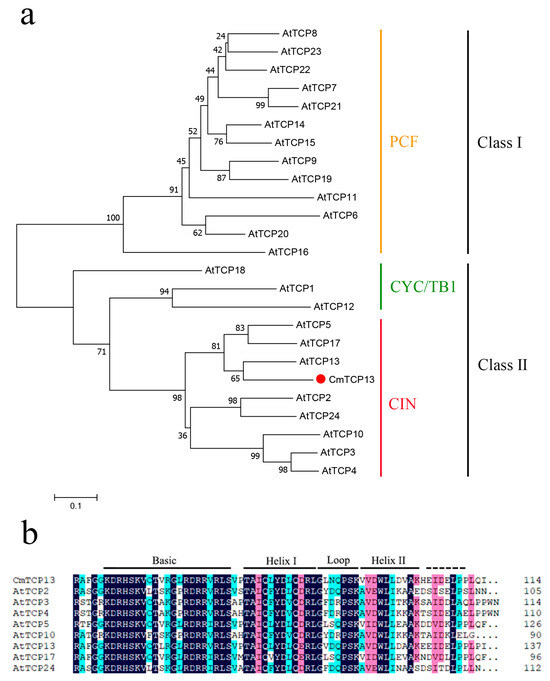
Figure 1.
Characterization of the CmTCP13 polypeptide sequence: (a) Phylogenetic tree of CmTCP13 and other Arabidopsis TCP proteins; (b) Alignment of the amino acid sequences of the bHLH domain. The conserved motifs are highlighted in black lines.
2.2. Subcellular Localization of CmTCP13
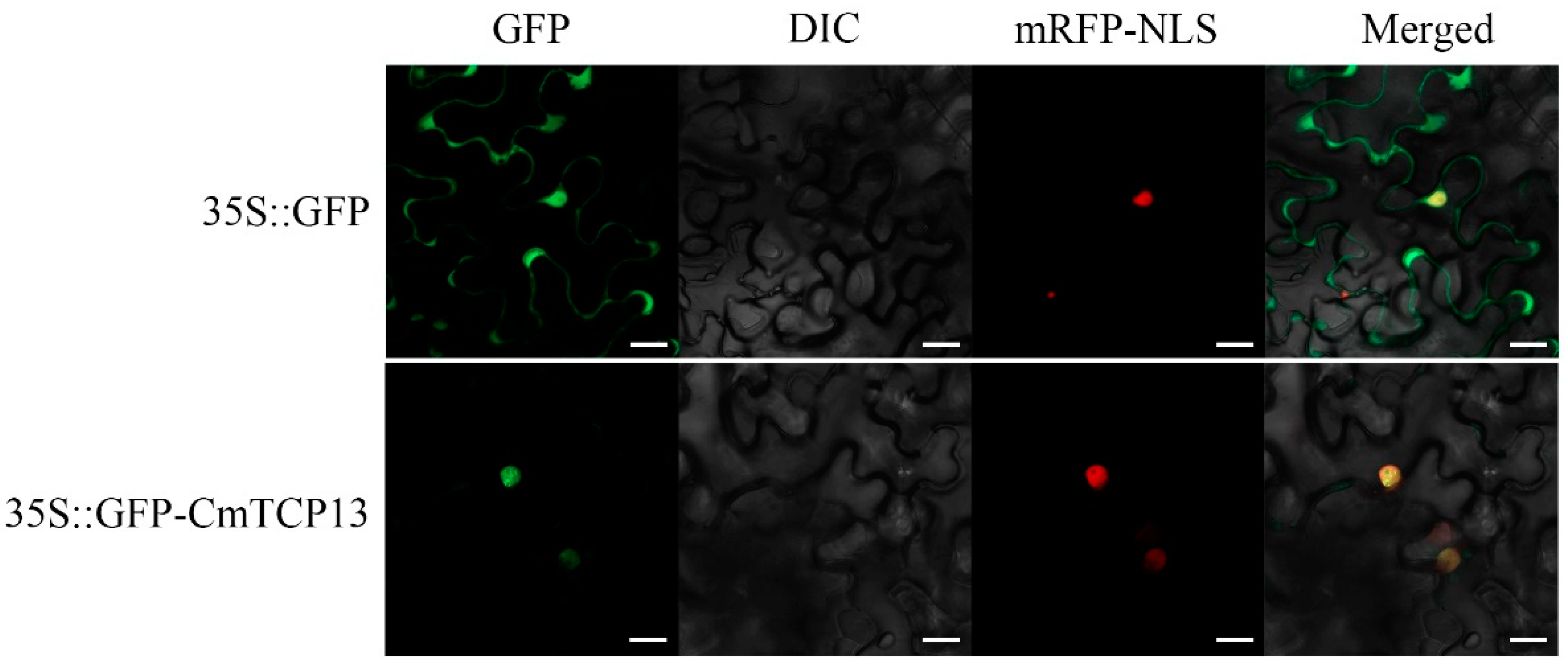
The 35S::GFP control vector and 35S::GFP-CmTCP13 fusion construct were transiently transformed into the epidermal cells of Nicotiana benthamiana together with the 35S::D53-RFP construct as a nuclear marker to explore the subcellular localization of the CmTCP13 protein. The GFP signal of the control 35S::GFP transgene was detected in both the cytoplasm and nucleus of the leaf epidermal cells, whereas the GFP fluorescence of the 35S::GFP-CmTCP13 transgene was specifically detected in the nucleus and co-localized with the nuclear marker D53-mCherry (Figure 2). These results indicate that CmTCP13 is localized in the nucleus.
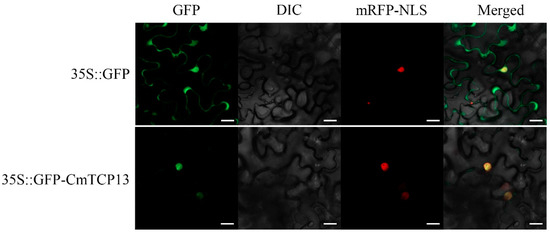
Figure 2.
Subcellular localization of CmTCP13 in tobacco epidermal cells. Green fluorescent protein (GFP): Images under the green fluorescence channel; Differential interference contrast (DIC): Images under bright light; Red fluorescent protein with a nuclear localization signal (mRFP-NLS): D53-mCherry was used as a nuclear marker; Merged: overlay plots. Scale bars represent 20 μm.
2.3. Transcript Profile of CmTCP13 in Response to Salinity
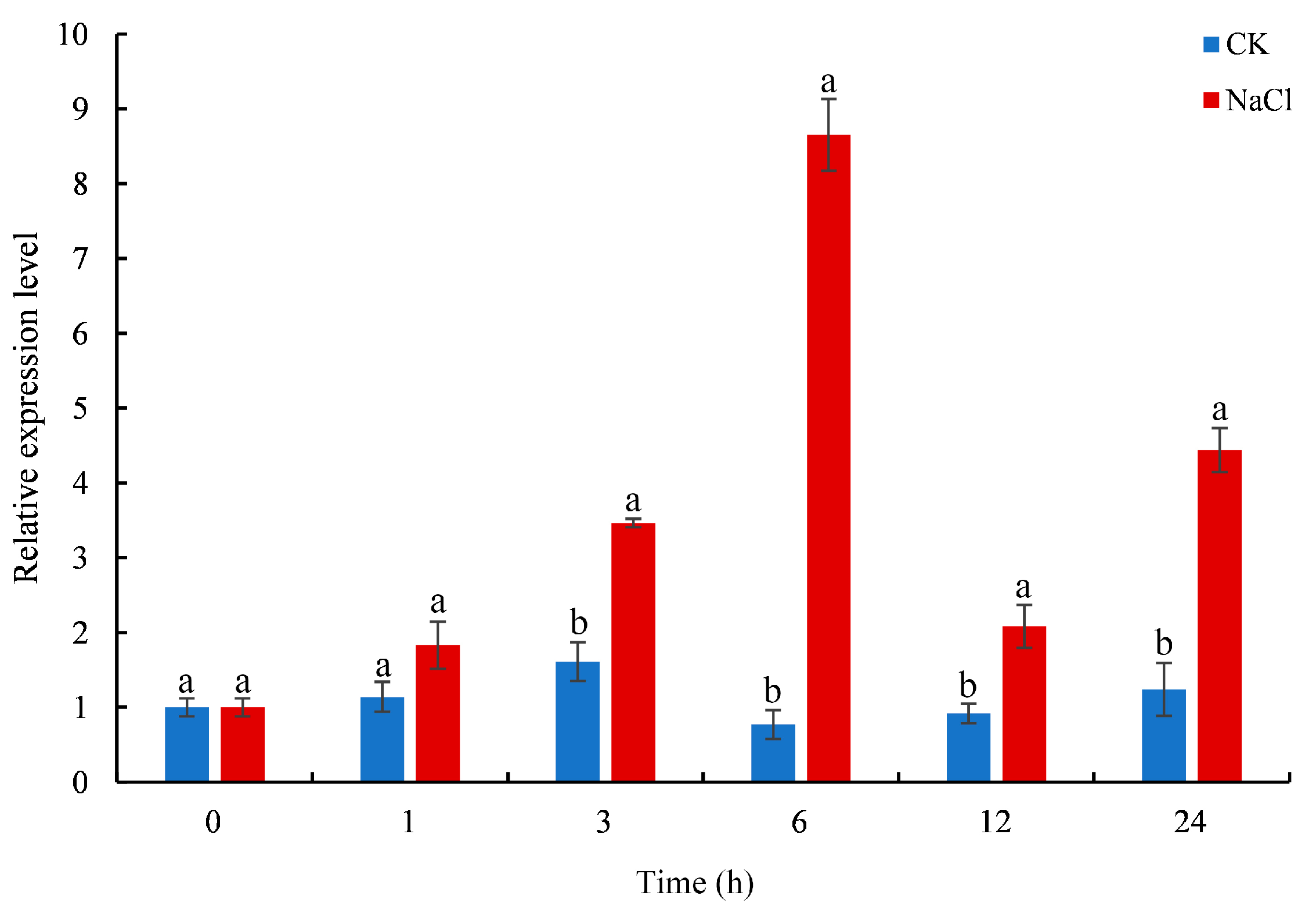
The expression levels of CmTCP13 in chrysanthemum ‘Jinba’ under salinity stress conditions were evaluated by quantitative real-time polymerase chain reaction (qRT-PCR). CmTCP13 transcript levels increased considerably and peaked at 6 h after salt treatment. Subsequently, CmTCP13 expression levels decreased but remained significantly (p < 0.05) higher than those of the untreated control, showing a 3.58-fold increase at 24 h after the treatment (Figure 3). This finding indicates that CmTCP13 may be involved in regulating the salt stress response.
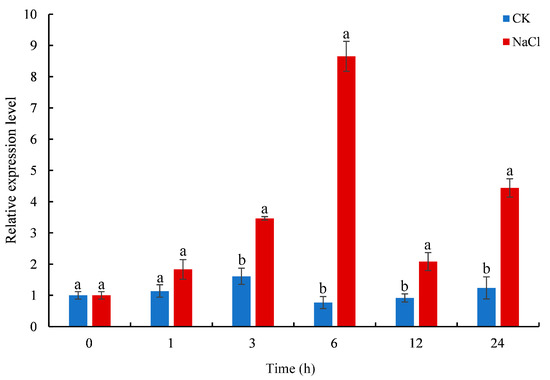
Figure 3.
Expression pattern of CmTCP13 in chrysanthemum ‘Jinba’ under salinity treatment as assayed by qRT-PCR. Values represent means ± standard deviation (SD) with three biological replicates. Letters above bars indicate statistically significant differences between the control (CK) and NaCl treatment (p < 0.05).
2.4. Cis-Acting Regulatory Element Analysis of CmTCP13 Promoter Sequence
We cloned the 2405 bp promoter sequence upstream of CmTCP13 and analyzed its cis-acting elements using the PlantCARE database. The CmTCP13 promoter contained multiple hormone-responsive elements, such as abscisic acid (ABA) response element (ABRE), methyl jasmonate (MeJA) response elements (CGTCA and TGACG motifs), ethylene response element (ERE), auxin response elements (AuxRR-core and TGA-element), and gibberellin and salicylic acid (SA) response elements (P-box and TCA-element, respectively) (Table 1). In addition, certain cis-acting elements are induced by adversity stress, such as anaerobic responsive element (ARE), low-temperature response (LTR) element, MYB binding site (MBS), wound-responsive (WUN) motif, GT-1 motif, and MYB and MYC recognition sites, among which the GT-1 motif is a salinity stress-inducible element (Table 1). Moreover, the CmTCP13 promoter contained light-responsive elements, including Box 4, G-box, I-box, GA-motif, and GATA-motif, and a circadian rhythm regulatory cis-element (circadian) (Table 1). These results indicate that CmTCP13 is widely involved in plant responses to hormones and abiotic stress.

Table 1.
Cis-acting element analysis of CmTCP13 gene promoter.
2.5. CmTCP13 Overexpression in Arabidopsis Enhanced Salinity Tolerance
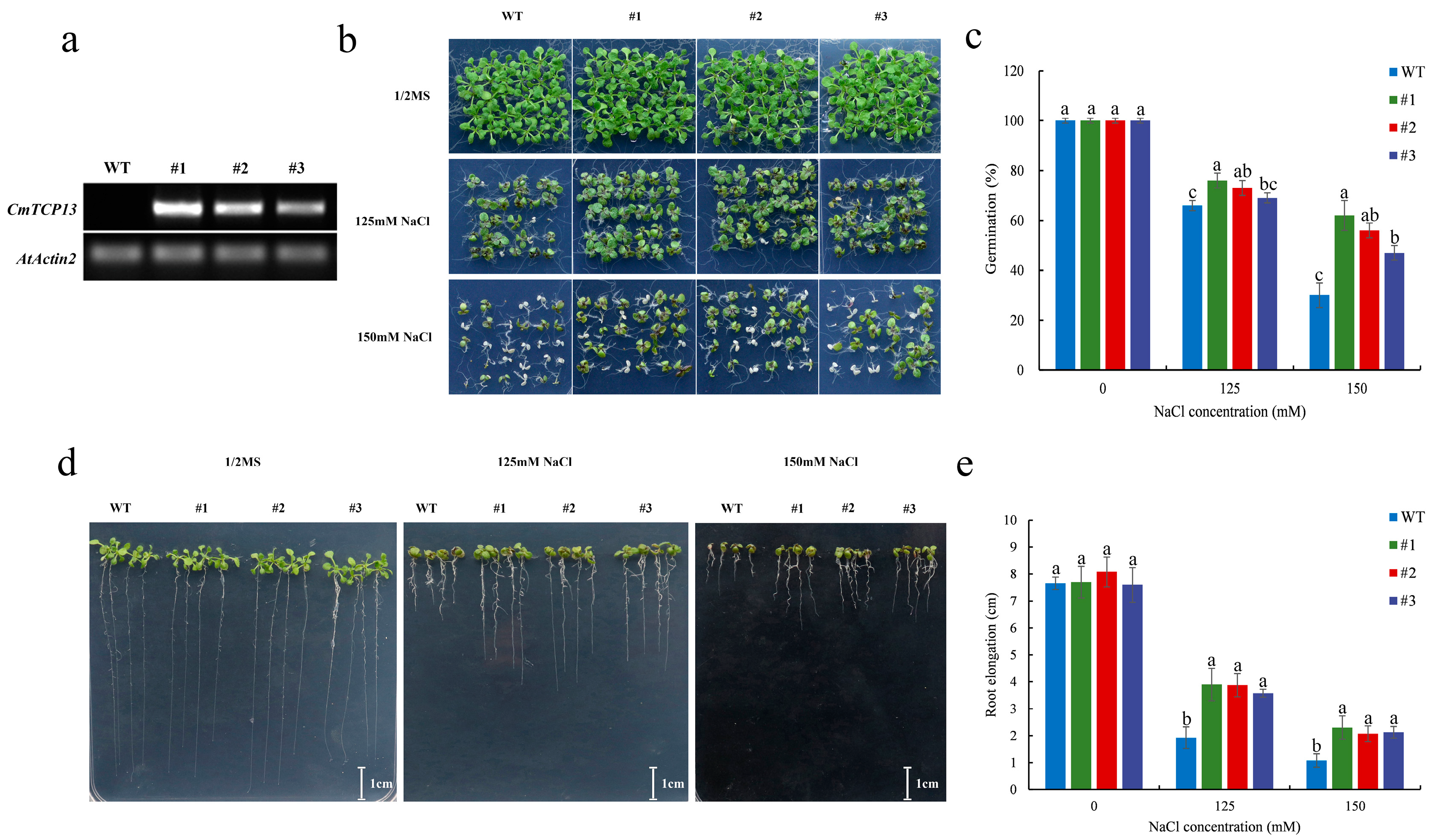
To explore the function of CmTCP13 in Arabidopsis, three transgenic lines from the T3 generation were selected. CmTCP13 transgene transcription was detected in the transgenic lines (#1, #2, and #3) but not in wild-type (WT) plants (Figure 4a). In addition, we also explored the expression levels of Arabidopsis AtTCP13 in CmTCP13 transgenic Arabidopsis lines. The results showed no significant change in the expression levels of endogenous AtTCP13 in the transgenic plants relative to those in WT plants (Figure S1). Salinity stress tolerance was compared between the three T3 transgenic lines and WT plants. No apparent phenotypic differences were observed when seedlings were germinated on a half-strength Murashige and Skoog (1/2MS) medium. However, when these lines were germinated on 1/2MS medium containing 125 and 150 mM NaCl, the seeds from the three transgenic lines showed higher germination than that of WT seeds (Figure 4b,c). On the 150 mM NaCl medium, WT seed germination was only 30.00%, whereas that of the #1, #2, and #3 transgenic line seeds was 62.22, 55.56, and 46.67%, respectively (Figure 4c). We further tested the root growth of the three transgenic lines and WT plants under salt stress. Root length between the transgenic and WT plants on 1/2MS medium did not differ; however, in the presence of 125 and 150 mM NaCl, the transgenic lines exhibited a considerable increase in root length compared with that of WT plants (Figure 4d,e).
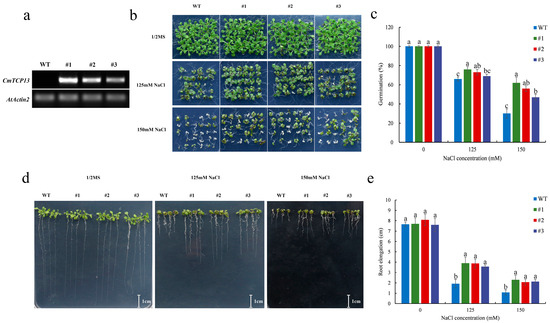
Figure 4.
Phenotypic comparison of the growth of the WT and CmTCP13-expressing transgenic Arabidopsis plants under salt stress. (a) RT-PCR analyses of CmTCP13 expression in WT and T3 transgenic plants. AtActin2 was the endogenous reference gene. (b,c) Germination of WT and transgenic seeds grown for 10 days on 1/2MS medium containing 125 and 150 mM NaCl. (d,e) Root elongation of WT and transgenic plants after 9 days of growth on 1/2MS medium with different NaCl concentrations. Data represent means ± standard error (SE), and different letters indicate significant differences between WT and transgenic plants at p < 0.05, as determined by Duncan’s test.
In addition, for the salinity tolerance assay, 3-week-old WT and transgenic plants were sequentially watered with 100, 200, and 300 mM NaCl at 4-day intervals. After the salinity treatment, the three transgenic lines exhibited relatively lower sensitivity to salt stress (Figure 5a). The percentage survival of the #1, #2, and #3 CmTCP13 transgenic lines was 96.30, 92.59, and 88.89%, respectively, which was significantly (p < 0.05) higher than that of the WT plants (29.63%) (Figure 5b). These results indicate that CmTCP13 overexpression enhances salinity tolerance in Arabidopsis.

Figure 5.
Salinity tolerance of the WT and CmTCP13-expressing transgenic Arabidopsis plants. (a) Phenotype of WT and CmTCP13 overexpressing seedlings after irrigation with increasing NaCl concentrations. (b) Survival rates of the WT and CmTCP13 transgenic plants under salt stress. Values are presented as means ± SE (n = 45), and statistical differences are determined using Duncan’s test (p < 0.05).
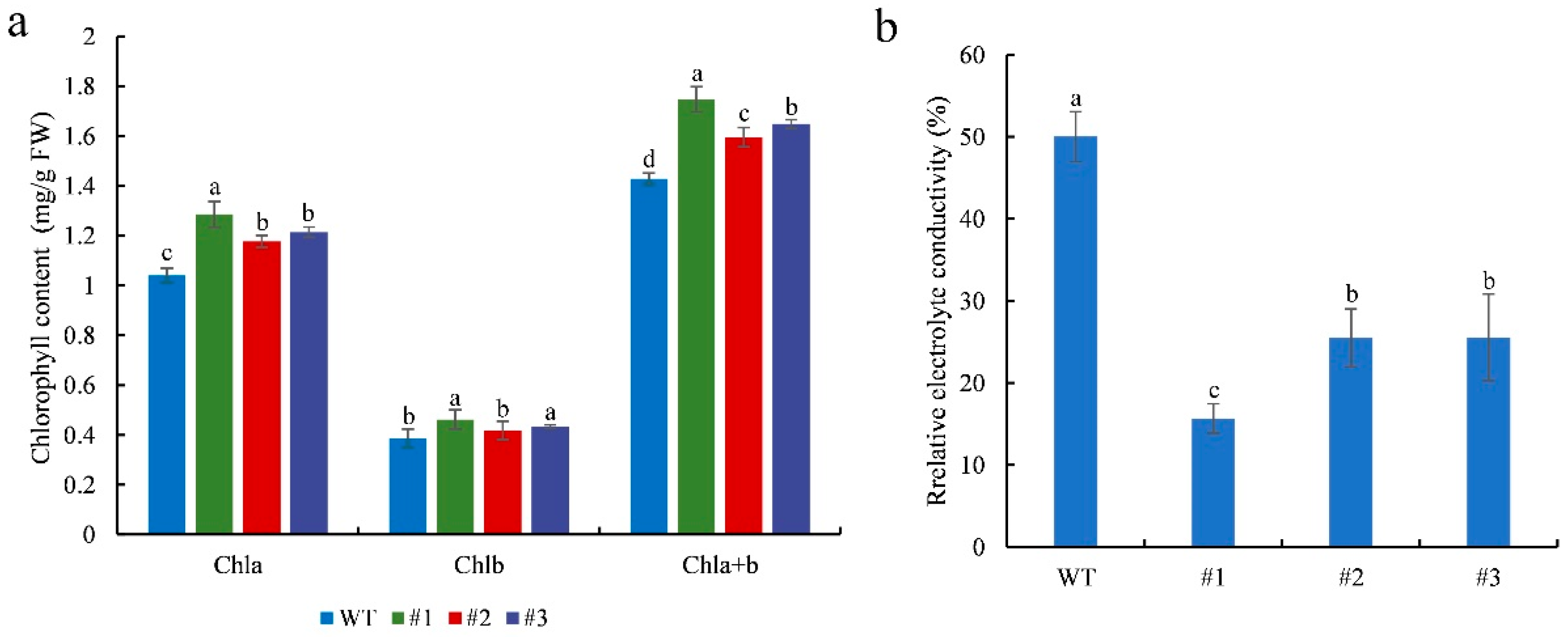
Chlorophyll content and electrolyte leakage were measured in WT and transgenic plants after 7 days of salinity stress. The leaf chlorophyll content of the treated transgenic plants was significantly (p < 0.05) higher than that of the treated WT plants (Figure 6a). These results indicate that CmTCP13 transgene overexpression delays chlorophyll degradation. In contrast, relative electrolyte conductivity was significantly (p < 0.05) lower in transgenic plants than in WT plants (Figure 6b), indicating that CmTCP13 overexpression protected plant membranes from damage under salinity stress.
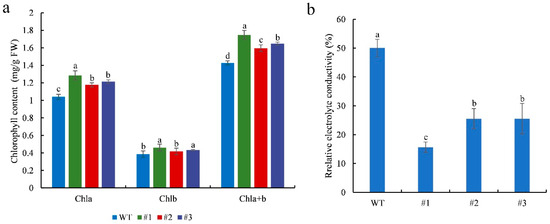
Figure 6.
Physiological effects of salinity treatment on the WT and CmTCP13-expressing transgenic Arabidopsis plants: (a) Leaf chlorophyll content; FW, fresh weight; Chla, chlorophyll a content; Chlb, chlorophyll b content; Chla+b, total chlorophyll content. (b) Leaf relative electrolyte conductivity. Data represent means ± SE of three replicates, and different letters indicate significant differences between WT and the three transgenic lines at p < 0.05, as determined by Duncan’s test.
2.6. CmTCP13 Overexpression Altered the Expression of Stress-Responsive Genes
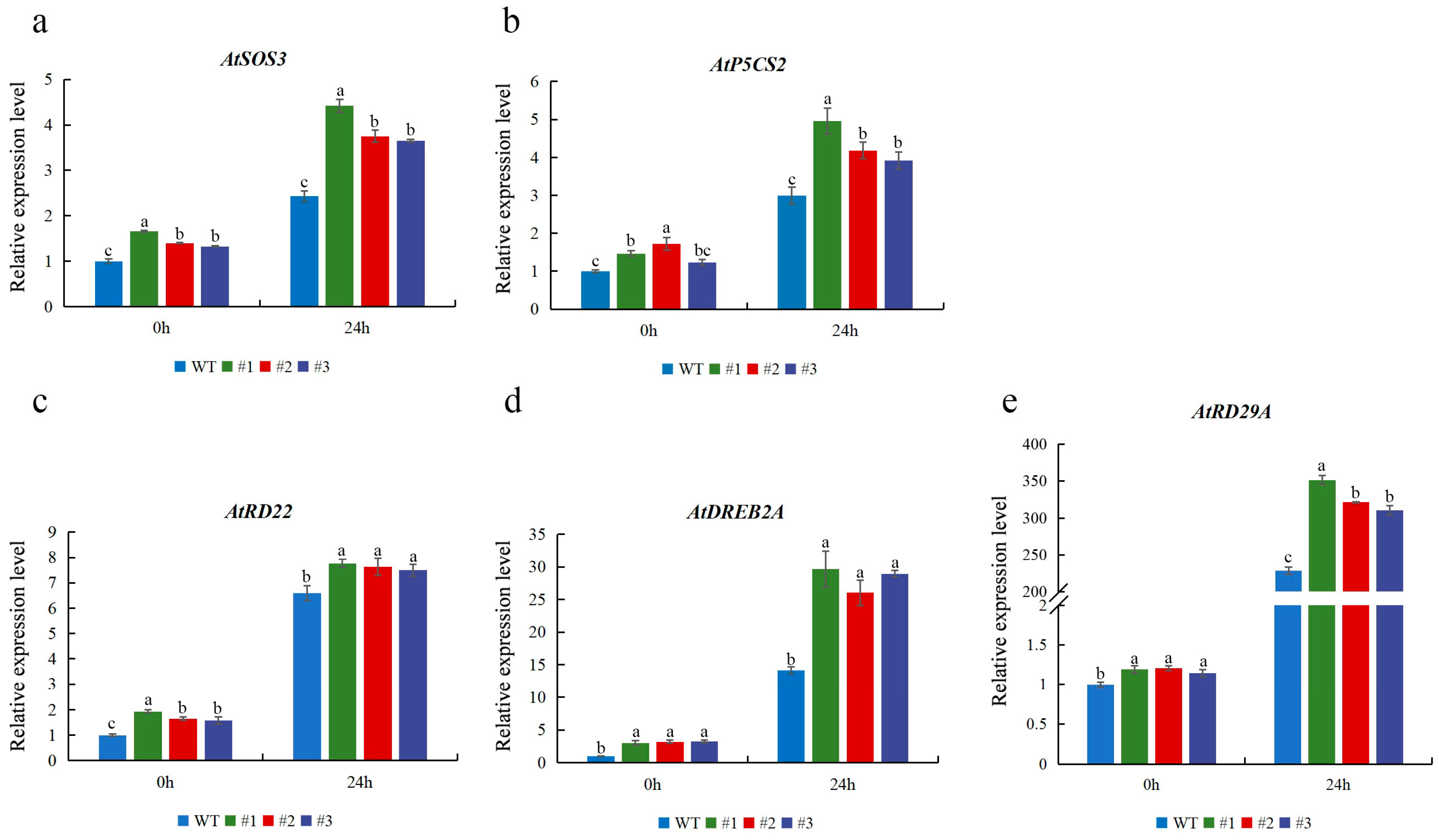
To better elucidate the possible role of CmTCP13 in response to salt stress, the expression of several stress-related genes was studied in CmTCP13 transgenic lines and WT plants. When subjected to salinity stress, the expression of ion homeostasis-related genes, including AtSOS3, was upregulated in the transgenic plants (Figure 7a). The expression levels of AtP5CS2, which was implicated in osmotic regulation, were also significantly induced in the transgenic lines compared with those in WT plants (Figure 7b). In addition, the expression levels of other stress-responsive genes, including AtRD22, AtRD29A, and AtDREB2A, were significantly upregulated in the transgenic plants under salt stress (Figure 7c–e). These data indicated that CmTCP13 may enhance salinity tolerance in Arabidopsis by inducing the expression of salt stress-related genes.
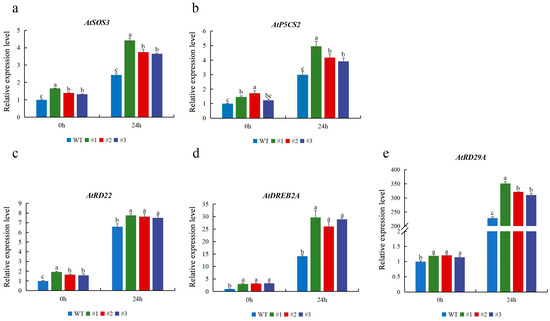
Figure 7.
Expression of stress-related genes AtSOS3 (a), AtP5CS2 (b), AtRD22 (c), AtDREB2A (d), AtRD29A (e), in the WT and CmTCP13-expressing transgenic Arabidopsis plants. Values represent means ± SE of three replicates, and significant differences between WT and transgenic plants are determined by Duncan’s test (p < 0.05).
3. Discussion
Plant-specific TCP proteins are involved in many processes of plant growth and development [8,28]. Furthermore, TCP genes have been identified and characterized in various plant species, including Arabidopsis, P. euphratica, Solanum lycopersicum, and Sorghum [11,14,29,30]. However, few studies have reported the functions of TCP family members in chrysanthemum plants. The CmTCP20 gene is reportedly involved in regulating petal elongation and root development in chrysanthemum [31,32]. The heterologous expression of CmTCP14 from chrysanthemum suppresses organ size and delays senescence in Arabidopsis [33]. Hitherto, no studies have investigated the role of TCP genes in abiotic stress resistance regulation in chrysanthemum. In this study, we isolated a TCP family member CmTCP13 with a conserved noncanonical bHLH motif in the N-terminal region. This domain has been implicated in DNA binding, nuclear targeting, and protein–protein interactions [8,34,35]. Phylogenetic analysis revealed that CmTCP13 belongs to the class II CIN TCP family and is most closely related to AtTCP13. A previous study showed that AtTCP13 exhibits stress-inducible expression and plays a key role in regulating plant growth in leaves and roots under dehydration stress conditions [36]. Here, we found that CmTCP13 was significantly induced by salt stress, similar to PeTCP10 in Phyllostachys edulis [25] and GbTCP4 in Gossypium barbadense [37]. Phylogenetic analysis and expression patterns indicated that CmTCP13, a TF, may be involved in the salinity stress response in chrysanthemum plants.
To further elucidate the mechanisms governing the upstream transcriptional regulation of the CmTCP13 gene, a 2405 bp promoter region upstream of the gene was isolated from chrysanthemum ‘Jinba’. The analysis of the promoter sequence has shown the existence of various hormone and stress response cis-elements, such as ABRE, CGTCA-motif, TGACG-motif, ERE, AuxRR-core, TCA-element, ARE, LTR, MBS, MYB, and GT-1 motif. ABRE is an ABA-response element. Notably, ABA regulates many aspects of plant growth and development and plays a crucial role in plant responses to drought, salt stress, and other adversity stresses [5,38,39]. Jasmonic acid is also an important plant growth regulator, which is implicated in plant growth and development and stress resistance [40,41]. The CmTCP13 promoter region has two MeJA-responsive elements, i.e., the CGTCA and TGACG motifs. In addition, plant hormones such as auxin, ethylene, gibberellin, and SA have been reported to play critical roles in plant responses to biotic and abiotic stresses [42,43,44,45]. The CmTCP13 promoter region also contained ethylene- (ERE), auxin-(AuxRR-core), gibberellin-(P-box), and SA-(TCA-element)-responsive elements. Of the other cis-elements, ARE is an anaerobically inducible element present in the promoter region of the maize Adh gene [46], LTR is a low-temperature response element, the WUN-motif is a trauma response element, and MBS and MYB are the binding elements of stress-related MYB proteins. The GT-1 motif with the consensus sequence GAAAAA was first identified in soybean as a salt- and pathogen-responsive element in the soybean calmodulin isoform-4 (SCaM-4) promoter. The interaction between the GT-1 motif and a GT-1-like TF plays a role in salt- and pathogen-induced SCaM-4 gene expression in both soybean and Arabidopsis [47]. In the CmTCP13 promoter region, we found seven salt-responsive GT-1 motifs, four of which were on the +ve DNA strand and three were on the −ve/complementary DNA strand (Table 1). The higher abundance of the salt-inducible GT-1 motif suggested that transcript expression of CmTCP13 was highly induced by salinity stress.
In the present study, Arabidopsis plants overexpressing the CmTCP13 transgene exhibited enhanced tolerance to salt stress. This result was supported by the higher germination, longer root lengths, and higher survival rates of the transgenic plants after salt treatment. Studies have reported that changes in physiology and biochemistry may be closely associated with resistance in transgenic plants [48]. The roots of the CmTCP13 transgenic plants were longer than those of the WT plants, suggesting that CmTCP13 may increase resistance by modulating the root system. Electrolyte leakage and chlorophyll content are commonly used indicators of plant membrane damage under salinity stress [1,5]. Relative leaf electrolyte leakage in the CmTCP13 overexpressing plants was lower than in the WT plants under salinity stress, indicating that CmTCP13 enhanced salinity tolerance by maintaining plant membrane integrity. Chlorophyllase activity was elevated under salt stress, leading to a decrease in chlorophyll content [49]. We found that the chlorophyll content of CmTCP13 overexpressing plants was higher than that of WT plants under salinity stress, suggesting that CmTCP13 might respond to salinity stress by regulating chlorophyll degradation. A higher chlorophyll content is associated with enhanced photosynthetic activity and increased disease resistance [50].
At the gene transcription level, the CmTCP13 overexpressing plants exhibited an upregulation of stress-related genes, such as AtSOS3, AtP5CS2, AtRD22, AtRD29A, and AtDREB2A. The SOS3 protein is involved in the salt overly sensitive (SOS) signaling pathway in plants and is a key regulator of ion homeostasis and salt tolerance [51]. The overexpression of SOS1 and SOS3 increases salinity tolerance in transgenic Arabidopsis [52]. The SOS3 protein can interact with SOS2, and the SOS3/SOS2 kinase complex regulates the transport activity of SOS1, which encodes a plasma membrane Na+/H+ antiporter responsible for the exclusion of Na+ from cells [53,54]. The AtP5CS gene has been implicated in proline synthesis and transport in response to salt or osmotic stress [55,56]. The RD29A gene reportedly has a role in stress-related detoxification, thereby reducing stress-related injuries [57]. DREB proteins regulate the expression of several stress-responsive genes in response to biotic or abiotic stresses [58]. In our study, the increased expression of CmTCP13 increased the transcript levels of salinity-induced stress response genes, including AtSOS3, AtP5CS2, AtRD29A, and AtDREB2A. Taken together, these results indicate that CmTCP13 acts as a positive regulator of the salt response pathway by improving the expression of stress-responsive genes.
In summary, CmTCP13, a TCP family TF gene isolated from chrysanthemum, was significantly induced by salinity stress. CmTCP13 overexpression in the transgenic Arabidopsis plants enhanced salt stress resistance compared with that in WT plants. These results suggest that CmTCP13 positively regulates stress-related genes to enhance salt tolerance. In the future, the production of CmTCP13-overexpressing transgenic chrysanthemum plants will provide deeper insights into the mechanisms underlying the role of CmTCP13 in salinity stress response.
4. Materials and Methods
4.1. Plant Materials and Salinity Stress Treatments
The chrysanthemum cultivar ‘Jinba’ was planted in the Nanjing Botanical Garden, Mem. Sun Yat-sen (118°49′55″ E, 32°3′32″ N), Nanjing, China. Uniformly rooted cuttings were grown in a 1:1 mix of garden soil and vermiculite and cultured in a greenhouse (day/night temperature: 25 °C/20 °C; photoperiod: 16 h; and relative humidity: 70%). Chrysanthemum seedlings were subjected to salinity stress at the 6–8 leaf stage by watering with 200 mM NaCl, whereas the control plants were treated with water (CK). We collected the third leaf from the apex of the experimental and control treatment plants (three plants each from both groups) at 0, 1, 3, 6, 12, and 24 h after salinity stress treatment. A. thaliana (ecotype Col-0) and transgenic plants were planted in a 1:3 mix of soilrite and vermiculite under a 16 h photoperiod with a day/night temperature of 22 °C/18 °C. After 3 weeks of growth, the WT and transgenic lines were subjected to 200 mM NaCl treatment, and three plants were harvested at 0 and 24 h after the salt treatment.
4.2. Isolation and Sequence Analysis of CmTCP13
Total RNA was isolated from chrysanthemum leaves using an RNA extraction kit (Huayueyang, Beijing, China), following the manufacturer’s protocol. Approximately 1 μg of total RNA was used to synthesize the first cDNA strand using SuperScript III Reverse Transcriptase (Invitrogen, Carlsbad, CA, USA). Based on the CL6155 contig 2 sequence in the chrysanthemum ‘Jinba’ transcriptome [59], the specific primer pair (CmTCP13-F/R; Table S1) was designed to amplify the CmTCP13 coding sequence. To verify the CmTCP13 nucleotide sequence, the PCR product was inserted into a pEASY-Blunt vector (TransGen Biotech, Beijing, China) for sequencing. The amino acid sequences of Arabidopsis TCP family members were acquired from the TAIR database (http://www.arabidopsis.org/, accessed on 16 July 2024), and a phylogenetic tree was constructed using the MEGA 7 software [60] based on the neighbor-joining algorithm with 1000 bootstrap replicates. Multiple sequence alignments of CmTCP13 and CIN AtTCP proteins were performed using the DNAMAN 6.0 software.
4.3. Subcellular Localization of CmTCP13
To generate the 35S::GFP-CmTCP13 fusion construct, the CmTCP13 ORF (lacking the termination codon) was amplified using a KOD FX kit (Toyobo, Osaka, Japan) with the primer pair CmTCP13-R4-F/R (Table S1) harboring the XhoI and SmaI recognition sites. After purification, the amplicon was introduced into the pORE-R4 vector (35S::GFP) using the Gateway method, resulting in the plasmid 35S::GFP-CmTCP13. The Agrobacterium tumefaciens strain GV3101 carrying the pORE-R4 or pORE-R4-CmTCP13 constructs was co-infiltrated with the p19 strain into the leaves of 5-week-old Nicotiana benthamiana as previously reported [61]. After culturing for 48–72 h at 22 °C, the GFP fluorescence signals were detected using a confocal laser scanning microscope (LSM 800, Carl Zeiss, Oberkochen, Germany).
4.4. Ectopic Expression of CmTCP13 in Arabidopsis
The 35S::CmTCP13 (pORE-R4-CmTCP13) construct was introduced into the A. tumefaciens strain EHA105 and then transformed into A. thaliana using the floral dip method [62]. Transformed progenies were selected using 1/2MS medium with 35 mg/mL kanamycin and advanced by self-pollination to obtain T3 transgenic plants. The T3 homozygous progenies were validated by RT-PCR using the primer pair CmTCP13-RT-F/R (Table S1). The expression of endogenous AtTCP13 in the CmTCP13 transgenic Arabidopsis lines was investigated by qRT-PCR using the primer pair AtTCP13-F/R (Table S1). The transcript levels of the AtActin2 gene were used as a reference.
4.5. Cis-Acting Element Analysis in the Promoter Region
Genomic DNA was isolated from the fresh leaves of chrysanthemum ‘Jinba’ using a modified cetyltrimethylammonium bromide method [63]. The promoter fragment of CmTCP13 was obtained from the chrysanthemum reference genome (http://210.22.121.250:8880/asteraceae/homePage, accessed on 12 June 2024), and full-length verification primers were designed to amplify the CmTCP13 promoter from genomic DNA. The primer pair used (CmTCP13pro-F/R) is shown in Table S1. The cis-acting element analysis of the promoter sequence was performed using PlantCARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/, accessed on 25 June 2024).
4.6. Salinity Tolerance Assay of Transgenic Arabidopsis Plants
For the germination assay, 30 seeds of the WT and CmTCP13 overexpression plants each were sown on 1/2MS medium containing two different concentrations of NaCl, i.e., 125 and 150 mM. The germination was scored when the cotyledon remained green after 10 days. The assay was repeated thrice. For root length estimation, seeds were grown on 1/2MS medium plates placed vertically for 3 days and then transferred to 1/2MS medium plates containing the different NaCl concentrations (0, 125, and 150 mM). Root length was measured 9 days later. Three biological replicates were used for each experiment. In addition, for the salinity tolerance test, 90 plants each of 3-week-old WT and CmTCP13 transgenic lines were watered for 12 days at 4-day intervals, first with 100 mM NaCl, then 200 mM NaCl, and finally with 300 mM NaCl, following Li et al. [64], and the survival rate was documented.
4.7. Measurements of Electrolyte Leakage and Chlorophyll Content
Three-week-old WT and CmTCP13 transgenic lines were watered with 200 mM NaCl, and leaves were collected to measure electrolyte leakage and chlorophyll levels 7 days post-treatment. The electrical conductivity of the leaf tissue was measured using a DDS-307 conductivity meter, as previously reported [65]. The chlorophyll content was determined using a slightly modified method [66]. Briefly, 100 mg (fresh weight) of the rosette leaves of the WT and transgenic lines were collected and immersed in 5 mL 95% ethanol for 48 h in the dark, after which the chlorophyll content was determined using a DU 800 UV/Vis spectrophotometer (Beckman Coulter, Indianapolis, IN, USA) by scanning at 663 and 645 nm.
4.8. qRT-PCR Analyses
Total RNA was extracted from the leaves of the salinity-stressed chrysanthemum and Arabidopsis plants using an RNA extraction kit (Huayueyang, Beijing, China). First-strand cDNA was synthesized using a PrimeScriptTM RT reagent kit (TaKaRa Bio Inc., Shiga, Japan). A LightCycler 96 Real-Time PCR System (Roche, Basel, Switzerland) was used for qRT-PCR experiments using an SYBR Premix Ex Taq II kit (TaKaRa Bio Inc., Shiga, Japan). The Chrysanthemum CmEF1a and Arabidopsis AtActin2 genes were used as the endogenous controls. Each sample was evaluated based on three biological and three technical replicates. The relative transcript abundances were determined using the 2−ΔΔCT method [67]. All primers used for qRT-PCR are listed in Table S1.
4.9. Statistical Analyses
All statistical analyses were conducted using SPSS v25.0 software (SPSS Inc., Chicago, IL, USA). Significantly different trait values were determined using Duncan’s multiple range test. Statistical significance was considered at p < 0.05.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/plants13152118/s1, Table S1: List of primers used in this study. Figure S1: Expression of endogenous AtTCP13 in the WT and CmTCP13-overexpressing transgenic Arabidopsis plants as assessed by qRT-PCR.
Author Contributions
X.C. conceived and designed the project. T.Z. collected samples. P.L. and Y.W. performed the experiments. Y.L. and H.C. analyzed the data. X.C. wrote the manuscript. H.W. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Natural Science Foundation of Jiangsu Province (BK20210163) and the National Natural Science Foundation of China (32202535, 32002083).
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Deinlein, U.; Stephan, A.B.; Horie, T.; Luo, W.; Xu, G.; Schroeder, J.I. Plant salt-tolerance mechanisms. Trends Plant Sci. 2014, 19, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Koussevitzky, S.; Mittler, R.; Miller, G. ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ. 2012, 35, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Schumaker, K.S.; Zhu, J.-K. Cell signaling during cold, drought, and salt stress. Plant Cell 2002, 14, 165–183. [Google Scholar] [CrossRef] [PubMed]
- Vinocur, B.; Altman, A. Recent advances in engineering plant tolerance to abiotic stress: Achievements and limitations. Curr. Opin. Biotechnol. 2005, 16, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.-K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 2002, 53, 247–273. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, Y.; Maruyama, K.; Osakabe, Y.; Qin, F.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Functional analysis of an Arabidopsis transcription factor, DREB2A, involved in drought-responsive gene expression. Plant Cell 2006, 18, 1292–1309. [Google Scholar] [CrossRef]
- Yao, W.; Wang, S.; Zhou, B.; Jiang, T. Transgenic poplar overexpressing the endogenous transcription factor ERF76 gene improves salinity tolerance. Tree Physiol. 2016, 36, 896–908. [Google Scholar] [CrossRef] [PubMed]
- Martín-Trillo, M.; Cubas, P. TCP genes: A family snapshot ten years later. Trends Plant Sci. 2010, 15, 31–39. [Google Scholar] [CrossRef]
- Nicolas, M.; Cubas, P. TCP factors: New kids on the signaling block. Curr. Opin. Plant Biol. 2016, 33, 33–41. [Google Scholar] [CrossRef]
- Yao, X.; Ma, H.; Wang, J.; Zhang, D. Genome-wide comparative analysis and expression pattern of TCP gene families in Arabidopsis thaliana and Oryza sativa. J. Integr. Plant Biol. 2007, 49, 885–897. [Google Scholar] [CrossRef]
- Ma, X.; Ma, J.; Fan, D.; Li, C.; Jiang, Y.; Luo, K. Genome-wide identification of TCP family transcription factors from Populus euphratica and their involvement in leaf shape regulation. Sci. Rep. 2016, 6, 32795. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhai, Z.; Li, Y.; Geng, S.; Song, G.; Guan, J.; Jia, M.; Wang, F.; Sun, G.; Feng, N. Genome-wide identification and expression profiling of the TCP family genes in spike and grain development of wheat (Triticum aestivum L.). Front. Plant Sci. 2018, 9, 1282. [Google Scholar] [CrossRef]
- Navaud, O.; Dabos, P.; Carnus, E.; Tremousaygue, D.; Hervé, C. TCP transcription factors predate the emergence of land plants. J. Mol. Evol. 2007, 65, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Li, S. The Arabidopsis thaliana TCP transcription factors: A broadening horizon beyond development. Plant Signal. Behav. 2015, 10, e1044192. [Google Scholar] [CrossRef] [PubMed]
- Resentini, F.; Felipo-Benavent, A.; Colombo, L.; Blázquez, M.A.; Masiero, S. TCP14 and TCP15 mediate the promotion of seed germination by gibberellins in Arabidopsis thaliana. Mol. Plant 2014, 8, 482–485. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, L.; Wang, W.; Tian, P.; Wang, W.; Wang, K.; Gao, Z.; Liu, S.; Zhang, Y.; F, I.V. TCP5 controls leaf margin development by regulating KNOX and BEL-like transcription factors in Arabidopsis. J. Exp. Bot. 2021, 72, 1809–1821. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, J.; Li, C.; Deng, X.; Wang, T.; Dong, L. Genome-wide analysis of TCP transcription factor family in sunflower and identification of HaTCP1 involved in the regulation of shoot branching. BMC Plant Biol. 2023, 23, 222. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Sun, M.; Pan, H.; Cheng, T.; Zhang, Q. Two Cyc2CL transcripts (Cyc2CL-1 and Cyc2CL-2) may play key roles in the petal and stamen development of ray florets in chrysanthemum. BMC Plant Biol. 2021, 21, 105. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Irish, V.F. Temporal control of plant organ growth by TCP transcription factors. Curr. Biol. 2015, 25, 1765–1770. [Google Scholar] [CrossRef] [PubMed]
- Giraud, E.; Ng, S.; Carrie, C.; Duncan, O.; Low, J.; Lee, C.P.; Van Aken, O.; Millar, A.H.; Murcha, M.; Whelan, J. TCP transcription factors link the regulation of genes encoding mitochondrial proteins with the circadian clock in Arabidopsis thaliana. Plant Cell 2010, 22, 3921–3934. [Google Scholar] [CrossRef]
- Yao, Y.; Dong, L.; Fu, X.; Zhao, L.; Wei, J.; Cao, J.; Sun, Y.; Liu, J. HrTCP20 dramatically enhance drought tolerance of sea buckthorn (Hippophae rhamnoides L.) by mediating the JA signaling pathway. Plant Physiol. Biochem. 2022, 174, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Almeida, D.M.; Gregorio, G.B.; Oliveira, M.M.; Saibo, N.J. Five novel transcription factors as potential regulators of OsNHX1 gene expression in a salt tolerant rice genotype. Plant Mol. Biol. 2017, 93, 61–77. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, P.; Tyagi, A.K. OsTCP19 influences developmental and abiotic stress signaling by modulating ABI4-mediated pathways. Sci. Rep. 2015, 5, 9998. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Cai, Z.; Du, H.; Wang, H. Genome-wide analysis of TCP family genes in Zea mays L. identified a role for ZmTCP42 in drought tolerance. Int. J. Mol. Sci. 2019, 20, 2762. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, H.; Gao, Y.; Xiong, R.; Wu, M.; Zhang, K.; Xiang, Y. The TCP transcription factor PeTCP10 modulates salt tolerance in transgenic Arabidopsis. Plant Cell Rep. 2021, 40, 1971–1987. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Shi, X.; Wang, Z.; Qu, M.; Gao, C.; Wang, C.; Wang, Y. Acetylation of transcription factor BpTCP20 by acetyltransferase BpPDCE23 modulates salt tolerance in birch. Plant Physiol. 2024, 195, kiae168. [Google Scholar] [CrossRef] [PubMed]
- Song, A.; Su, J.; Wang, H.; Zhang, Z.; Zhang, X.; Van de Peer, Y.; Chen, F.; Fang, W.; Guan, Z.; Zhang, F. Analyses of a chromosome-scale genome assembly reveal the origin and evolution of cultivated chrysanthemum. Nat. Commun. 2023, 14, 2021. [Google Scholar] [CrossRef]
- Manassero, N.G.U.; Viola, I.L.; Welchen, E.; Gonzalez, D.H. TCP transcription factors: Architectures of plant form. Biomol. Concepts 2013, 4, 111–127. [Google Scholar] [CrossRef] [PubMed]
- Parapunova, V.; Busscher, M.; Busscher-Lange, J.; Lammers, M.; Karlova, R.; Bovy, A.G.; Angenent, G.C.; de Maagd, R.A. Identification, cloning and characterization of the tomato TCP transcription factor family. BMC Plant Biol. 2014, 14, 157. [Google Scholar] [CrossRef]
- Francis, A.; Dhaka, N.; Bakshi, M.; Jung, K.-H.; Sharma, M.K.; Sharma, R. Comparative phylogenomic analysis provides insights into TCP gene functions in Sorghum. Sci. Rep. 2016, 6, 38488. [Google Scholar] [CrossRef]
- Wang, J.; Guan, Y.; Ding, L.; Li, P.; Zhao, W.; Jiang, J.; Chen, S.; Chen, F. The CmTCP20 gene regulates petal elongation growth in Chrysanthemum morifolium. Plant Sci. 2019, 280, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.-M.; Liu, B.-W.; Ma, F.-F.; Sun, X.; Zheng, C.-S. Proteomic profiling of root system development proteins in chrysanthemum overexpressing the CmTCP20 gene. Plant Sci. 2019, 287, 110175. [Google Scholar] [CrossRef]
- Zhang, T.; Qu, Y.; Wang, H.; Wang, J.; Song, A.; Hu, Y.; Chen, S.; Jiang, J.; Chen, F. The heterologous expression of a chrysanthemum TCP-P transcription factor CmTCP14 suppresses organ size and delays senescence in Arabidopsis thaliana. Plant Physiol. Biochem. 2017, 115, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Danisman, S.; van Dijk, A.D.; Bimbo, A.; van der Wal, F.; Hennig, L.; de Folter, S.; Angenent, G.C.; Immink, R.G. Analysis of functional redundancies within the Arabidopsis TCP transcription factor family. J. Exp. Bot. 2013, 64, 5673–5685. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, P.; Das Gupta, M.; Joseph, A.P.; Chatterjee, N.; Srinivasan, N.; Nath, U. Identification of specific DNA binding residues in the TCP family of transcription factors in Arabidopsis. Plant Cell 2010, 22, 1174–1189. [Google Scholar] [CrossRef] [PubMed]
- Urano, K.; Maruyama, K.; Koyama, T.; Gonzalez, N.; Inzé, D.; Yamaguchi-Shinozaki, K.; Shinozaki, K. CIN-like TCP13 is essential for plant growth regulation under dehydration stress. Plant Mol. Biol. 2022, 108, 257–275. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, Y.; Wan, H.; Tang, J.; Ni, Z. The sea-island cotton GbTCP4 transcription factor positively regulates drought and salt stress responses. Plant Sci. 2022, 322, 111329. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yang, Y.-N.; Xue, L.-J.; Zou, M.-J.; Liu, J.-Y.; Chen, F.; Xue, H.-W. Rice ABI5-Like1 regulates abscisic acid and auxin responses by affecting the expression of ABRE-containing genes. Plant Physiol. 2011, 156, 1397–1409. [Google Scholar] [CrossRef]
- Zhang, J.; Jia, W.; Yang, J.; Ismail, A.M. Role of ABA in integrating plant responses to drought and salt stresses. Field Crops Res. 2006, 97, 111–119. [Google Scholar] [CrossRef]
- Wang, Y.; Mostafa, S.; Zeng, W.; Jin, B. Function and mechanism of jasmonic acid in plant responses to abiotic and biotic stresses. Int. J. Mol. Sci. 2021, 22, 8568. [Google Scholar] [CrossRef]
- Wang, J.; Song, L.; Gong, X.; Xu, J.; Li, M. Functions of jasmonic acid in plant regulation and response to abiotic stress. Int. J. Mol. Sci. 2020, 21, 1446. [Google Scholar] [CrossRef]
- Yang, J.; Duan, G.; Li, C.; Liu, L.; Han, G.; Zhang, Y.; Wang, C. The crosstalks between jasmonic acid and other plant hormone signaling highlight the involvement of jasmonic acid as a core component in plant response to biotic and abiotic stresses. Front. Plant Sci. 2019, 10, 1349. [Google Scholar] [CrossRef]
- Song, W.; Shao, H.; Zheng, A.; Zhao, L.; Xu, Y. Advances in roles of salicylic acid in plant tolerance responses to biotic and abiotic stresses. Plants 2023, 12, 3475. [Google Scholar] [CrossRef]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016, 16, 86. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wu, Y.; Dong, G.; Zhu, G.; Zhou, G. Progress of research on the physiology and molecular regulation of sorghum growth under salt stress by gibberellin. Int. J. Mol. Sci. 2023, 24, 6777. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.C.; Howard, E.A.; Dennis, E.S.; Peacock, W.J. DNA sequences required for anaerobic expression of the maize alcohol dehydrogenase 1 gene. Proc. Natl. Acad. Sci. USA 1987, 84, 6624–6628. [Google Scholar] [CrossRef]
- Park, H.C.; Kim, M.L.; Kang, Y.H.; Jeon, J.M.; Yoo, J.H.; Kim, M.C.; Park, C.Y.; Jeong, J.C.; Moon, B.C.; Lee, J.H.; et al. Pathogen- and NaCl-induced expression of the SCaM-4 promoter is mediated in part by a GT-1 box that interacts with a GT-1-like transcription factor. Plant Physiol. 2004, 135, 2150–2161. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Cui, M.-Y.; Hu, Y.; Gao, K.; Xie, Y.-G.; Jiang, Y.; Feng, J.-Y. Ectopic expression of FvWRKY42, a WRKY transcription factor from the diploid woodland strawberry (Fragaria vesca), enhances resistance to powdery mildew, improves osmotic stress resistance, and increases abscisic acid sensitivity in Arabidopsis. Plant Sci. 2018, 275, 60–74. [Google Scholar] [CrossRef]
- Santos, C.V. Regulation of chlorophyll biosynthesis and degradation by salt stress in sunflower leaves. Sci. Hortic. 2004, 103, 93–99. [Google Scholar] [CrossRef]
- Eckhardt, U.; Grimm, B.; Hörtensteiner, S. Recent advances in chlorophyll biosynthesis and breakdown in higher plants. Plant Mol. Biol. 2004, 56, 1–14. [Google Scholar] [CrossRef]
- Sanders, D. Plant biology: The salty tale of Arabidopsis. Curr. Biol. 2000, 10, 486–488. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Chen, Z.-Z.; Zhou, X.-F.; Yin, H.-B.; Li, X.; Xin, X.-F.; Hong, X.-H.; Zhu, J.-K.; Gong, Z. Overexpression of SOS (Salt Overly Sensitive) genes increases salt tolerance in transgenic Arabidopsis. Mol. Plant 2009, 2, 22–31. [Google Scholar] [CrossRef]
- Halfter, U.; Ishitani, M.; Zhu, J.-K. The Arabidopsis SOS2 protein kinase physically interacts with and is activated by the calcium-binding protein SOS3. Proc. Natl. Acad. Sci. USA 2000, 97, 3735–3740. [Google Scholar] [CrossRef]
- Qiu, Q.-S.; Guo, Y.; Dietrich, M.A.; Schumaker, K.S.; Zhu, J.-K. Regulation of SOS1, a plasma membrane Na+/H+ exchanger in Arabidopsis thaliana, by SOS2 and SOS3. Proc. Natl. Acad. Sci. USA 2002, 99, 8436–8441. [Google Scholar] [CrossRef] [PubMed]
- Strizhov, N.; Ábrahám, E.; Ökrész, L.; Blickling, S.; Zilberstein, A.; Schell, J.; Koncz, C.; Szabados, L. Differential expression of two P5CS genes controlling proline accumulation during salt-stress requires ABA and is regulated by ABA1, ABI1 and AXR2 in Arabidopsis. Plant J. 1997, 12, 557–569. [Google Scholar] [CrossRef] [PubMed]
- Amini, S.; Ghobadi, C.; Yamchi, A. Proline accumulation and osmotic stress: An overview of P5CS gene in plants. J. Plant Mol. Breed. 2015, 3, 44–55. [Google Scholar]
- Zhu, J.-K. Plant salt tolerance. Trends Plant Sci. 2001, 6, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.K.; Agarwal, P.; Reddy, M.; Sopory, S.K. Role of DREB transcription factors in abiotic and biotic stress tolerance in plants. Plant Cell Rep. 2006, 25, 1263–1274. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, H.; Ding, L.; Song, A.; Shen, F.; Jiang, J.; Chen, S.; Chen, F. Transcriptomic and hormone analyses reveal mechanisms underlying petal elongation in Chrysanthemum morifolium ‘Jinba’. Plant Mol. Biol. 2017, 93, 593–606. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Wang, F.-Q.; Zhang, Z.-Y. Interactions among mediator subunits of tobacco by bimolecular fluorescence complementation (BiFC) method. J. Agric. Biotechnol. 2012, 20, 38–47. [Google Scholar]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef]
- Murray, M.; Thompson, W.F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980, 8, 4321–4325. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Song, A.; Gao, C.; Wang, L.; Wang, Y.; Sun, J.; Jiang, J.; Chen, F.; Chen, S. Chrysanthemum WRKY gene CmWRKY17 negatively regulates salt stress tolerance in transgenic chrysanthemum and Arabidopsis plants. Plant Cell Rep. 2015, 34, 1365–1378. [Google Scholar] [CrossRef]
- Yadav, N.S.; Shukla, P.S.; Jha, A.; Agarwal, P.K.; Jha, B. The SbSOS1 gene from the extreme halophyte Salicornia brachiata enhances Na+ loading in xylem and confers salt tolerance in transgenic tobacco. BMC Plant Biol. 2012, 12, 188. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Method. Enzymol. 1987, 148, 350–382. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).