Analysis of Ginkgo biloba Root Exudates and Inhibition of Soil Fungi by Flavonoids and Terpene Lactones
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Material and Root Exudate Collection
2.2. Root Exudate Extraction and Metabolomic Analysis by UPLC-MS/MS
2.3. Inhibitory Efficacy on Fungal Growth
2.4. Detection of Membrane Permeability, Cell Viability, ROS Accumulation, Membrane Permeability, and Autophagy
3. Results
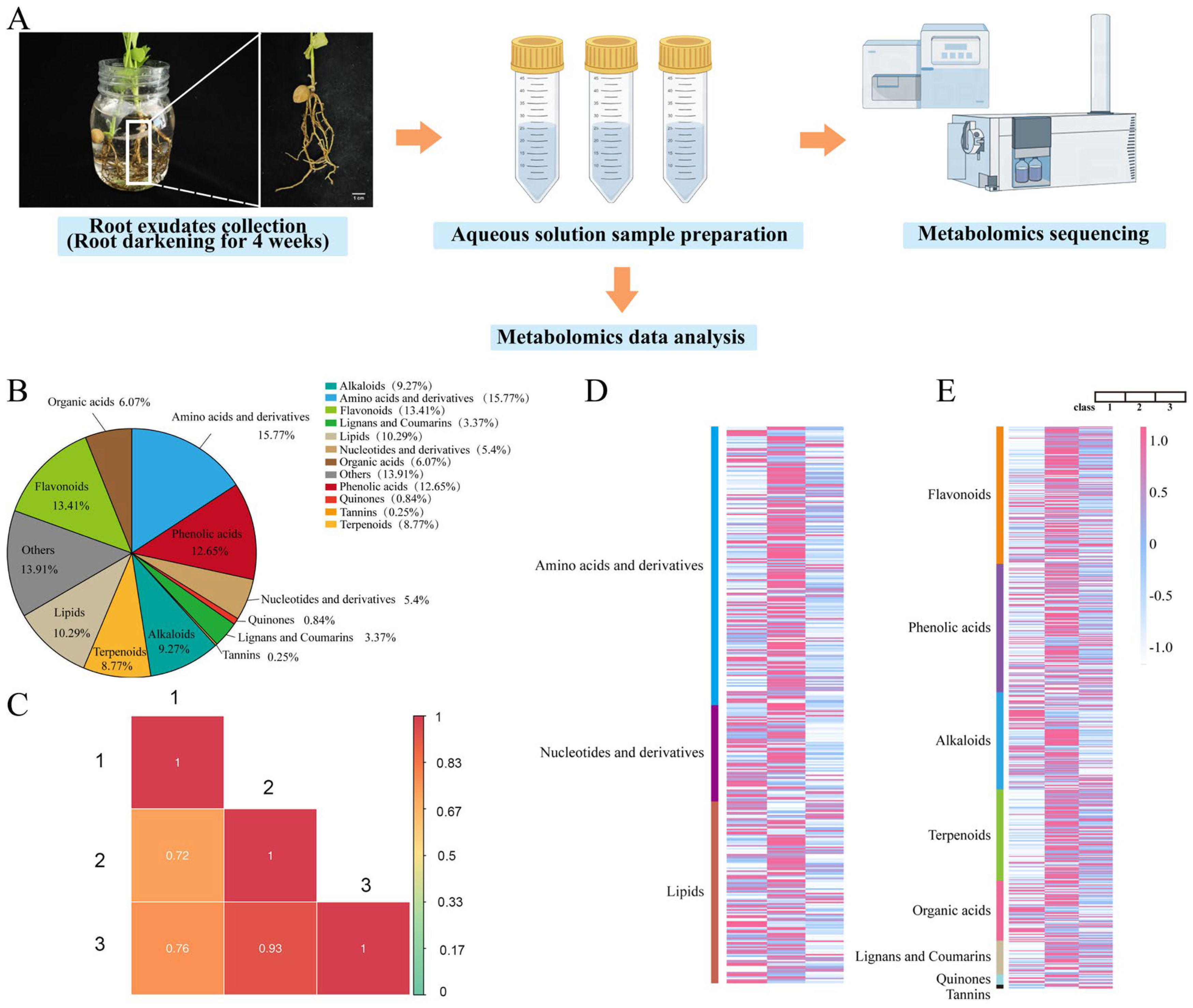
3.1. Collection and Metabolic Profiling of Ginkgo Root Exudates
3.2. Component Analysis of Secondary Metabolites in Ginkgo Root Exudates
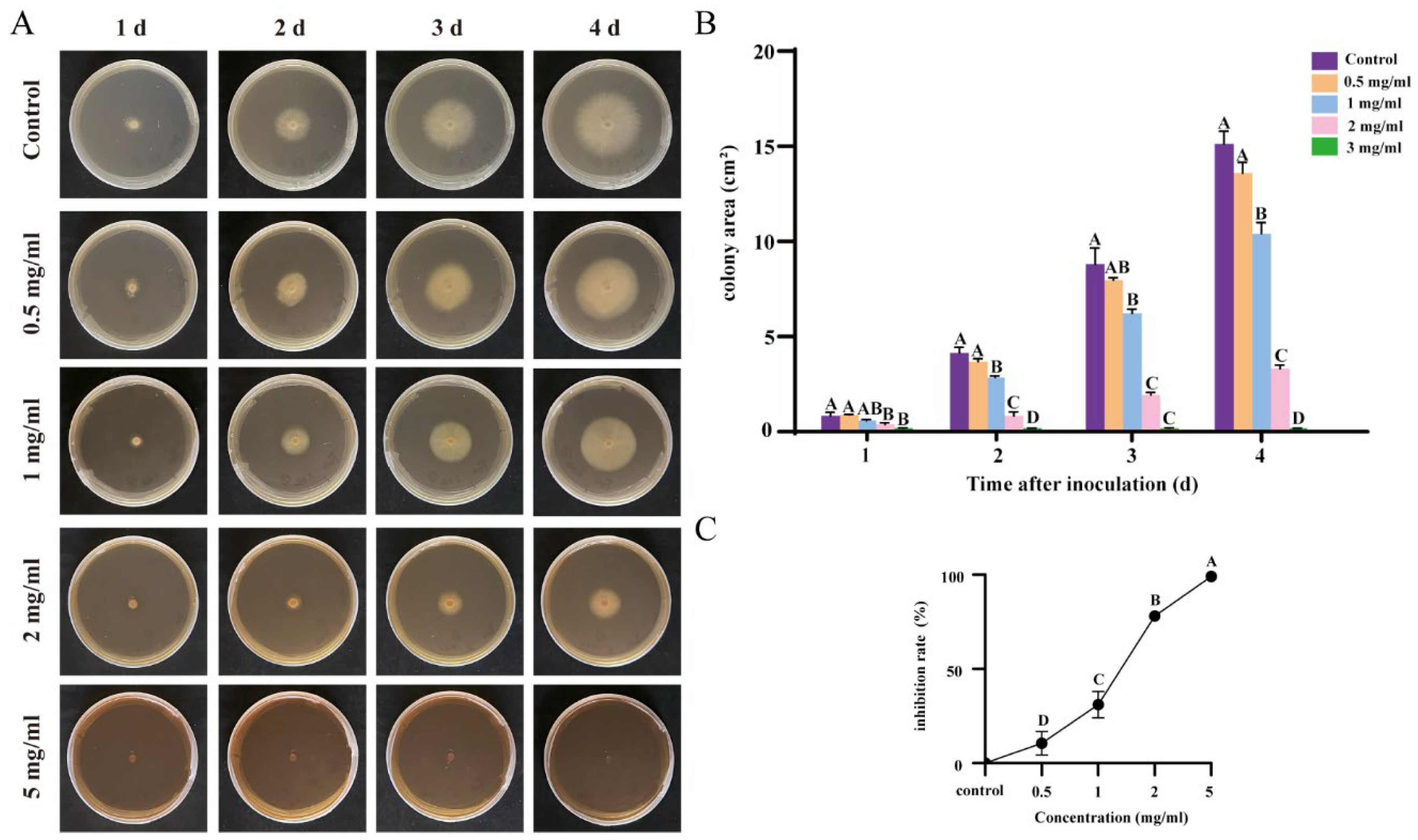
3.3. Effective Inhibition of Foc Growth by Flavonoids and Terpenoids from Ginkgo Extract
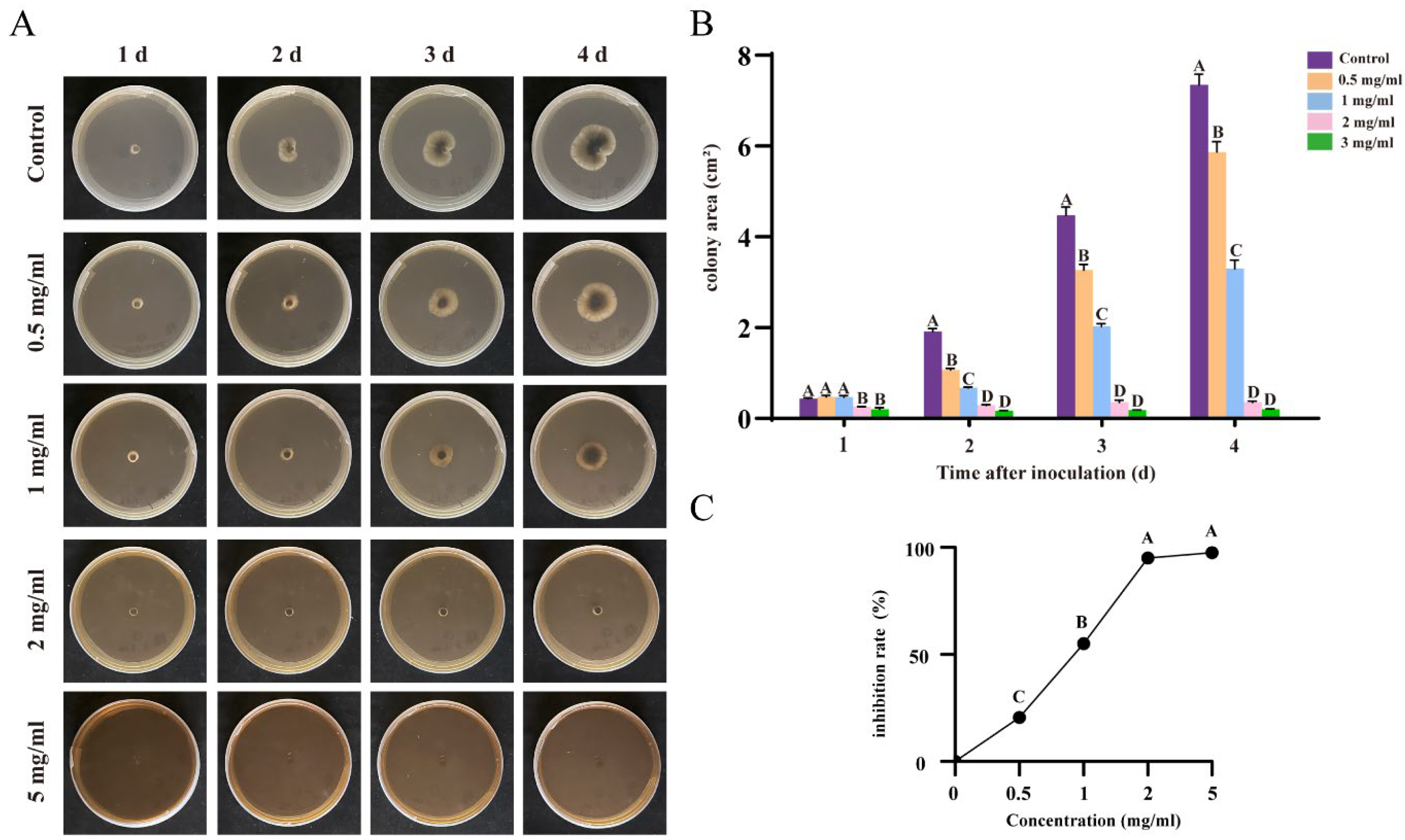
3.4. Flavonoids and Terpenoids from Ginkgo Effectively Inhibit R. solani AG-8
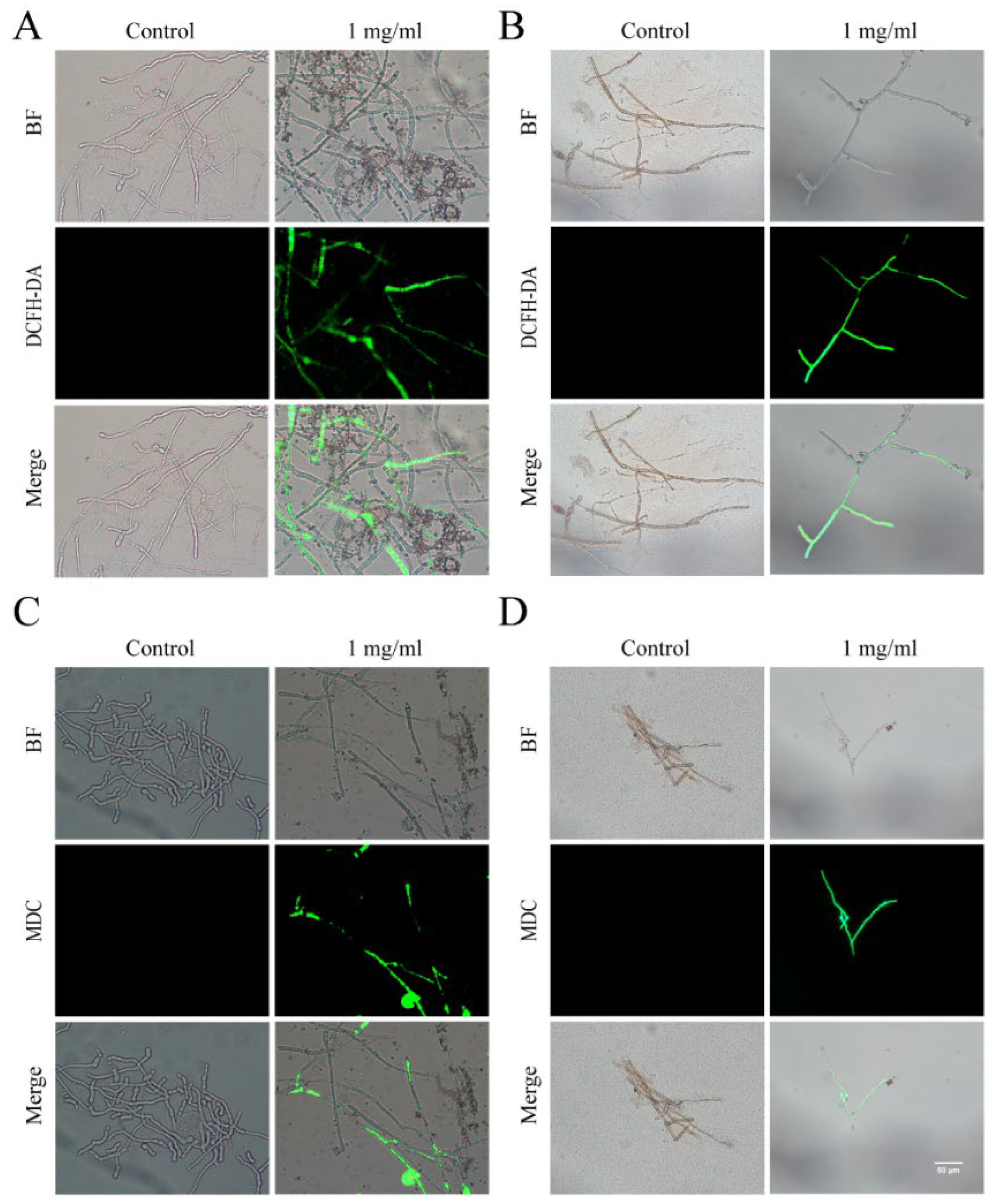
3.5. ROS Accumulation and Autophagy Activity in Foc and R. solani
3.6. Flavonoids and Terpenoids from Ginkgo Enhance the Levels of ROS and Autophagy Activity in Foc and R. solani AG-8
4. Discussion
4.1. Ginkgo Root Exudates
4.2. Significant Secondary Metabolites in Ginkgo Extract Effectively Inhibit Soil-Borne Fungi
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Williams, A.; Langridge, H.; Straathof, A.L.; Muhamadali, H.; Hollywood, K.A.; Goodacre, R.; de Vries, F.T. Root functional traits explain root exudation rate and composition across a range of grassland species. J. Ecol. 2022, 110, 21–33. [Google Scholar] [CrossRef]
- Gargallo-Garriga, A.; Preece, C.; Sardans, J.; Oravec, M.; Urban, O.; Peñuelas, J. Root exudate metabolomes change under drought and show limited capacity for recovery. Sci. Rep. 2018, 8, 12696. [Google Scholar] [CrossRef] [PubMed]
- Vranova, V.; Rejsek, K.; Skene, K.R.; Janous, D.; Formanek, P. Methods of collection of plant root exudates in relation to plant metabolism and purpose: A review. J. Plant Nutr. Soil Sci. 2013, 176, 175–199. [Google Scholar] [CrossRef]
- Baetz, U.; Martinoia, E. Root exudates: The hidden part of plant defense. Trends Plant Sci. 2014, 19, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Alfaro-Cuevas, R.; López-Bucio, J. Trichoderma spp. improve growth of Arabidopsis seedlings under salt stress through enhanced root development, osmolite production, and Na+ elimination through root exudates. Mol. Plant-Microbe Interact. 2014, 27, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Balyan, G.; Pandey, A.K. Root exudates, the warrior of plant life: Revolution below the ground. S. Afr. J. Bot. 2024, 164, 280–287. [Google Scholar] [CrossRef]
- Sun, H.; Jiang, S.; Jiang, C.; Wu, C.; Gao, M.; Wang, Q. A review of root exudates and rhizosphere microbiome for crop production. Environ. Sci. Pollut. 2021, 28, 54497–54510. [Google Scholar] [CrossRef]
- Zhao, M.; Zhao, J.; Yuan, J.; Hale, L.; Wen, T.; Huang, Q.; Vivanco, J.M.; Zhou, J.; Kowalchuk, G.A.; Shen, Q. Root exudates drive soil-microbe-nutrient feedbacks in response to plant growth. Plant Cell Environ. 2021, 44, 613–628. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Wang, H.; Dsouza, M.; Lou, J.; He, Y.; Dai, Z.; Brookes, P.C.; Xu, J.; Gilbert, J.A. Geographic patterns of co-occurrence network topological features for soil microbiota at continental scale in eastern China. ISME J. 2016, 10, 1891–1901. [Google Scholar] [CrossRef]
- Yang, Y.; Li, T.; Wang, Y.; Cheng, H.; Chang, S.X.; Liang, C.; An, S. Negative effects of multiple global change factors on soil microbial diversity. Soil Biol. Biochem. 2021, 156, 108229. [Google Scholar] [CrossRef]
- Fierer, N. Embracing the unknown: Disentangling the complexities of the soil microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Baquerizo, M.; Maestre, F.T.; Reich, P.B.; Trivedi, P.; Osanai, Y.; Liu, Y.R.; Hamonts, K.; Jeffries, T.C.; Singh, B.K. Carbon content and climate variability drive global soil bacterial diversity patterns. Ecol. Monogr. 2016, 86, 373–390. [Google Scholar] [CrossRef]
- Bahram, M.; Hildebrand, F.; Forslund, S.K.; Anderson, J.L.; Soudzilovskaia, N.A.; Bodegom, P.M.; Bengtsson-Palme, J.; Anslan, S.; Coelho, L.P.; Harend, H.; et al. Structure and function of the global topsoil microbiome. Nature 2018, 560, 233–237. [Google Scholar] [CrossRef]
- Li, H.; Li, C.; Song, X.; Liu, Y.; Gao, Q.; Zheng, R.; Li, J.; Zhang, P.; Liu, X. Impacts of continuous and rotational cropping practices on soil chemical properties and microbial communities during peanut cultivation. Sci. Rep. 2022, 12, 2758. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Baquerizo, M.; Guerra, C.A.; Cano-Díaz, C.; Egidi, E.; Wang, J.T.; Eisenhauer, N.; Singh, B.K.; Maestre, F.T. The proportion of soil-borne pathogens increases with warming at the global scale. Nat. Clim. Change 2020, 10, 550–554. [Google Scholar] [CrossRef]
- Koike, S.T. Crown rot of strawberry caused by Macrophomina phaseolina in California. Plant Dis. 2008, 92, 1253. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Zhao, J.; Wen, T.; Zhao, M.; Li, R.; Goossens, P.; Huang, Q.; Bai, Y.; Vivanco, J.M.; Kowalchuk, G.A.; et al. Root exudates drive the soil-borne legacy of aboveground pathogen infection. Microbiome 2018, 6, 156. [Google Scholar] [CrossRef]
- Hu, L.; Robert, C.A.; Cadot, S.; Zhang, X.I.; Ye, M.; Li, B.; Manzo, D.; Chervet, N.; Steinger, T.; Van Der Heijden, M.G.; et al. Root exudate metabolites drive plant-soil feedbacks on growth and defense by shaping the rhizosphere microbiota. Nat. Commun. 2018, 9, 2738. [Google Scholar] [CrossRef]
- Singh, B.; Kaur, P.; Singh, R.D.; Ahuja, P.S. Biology and chemistry of Ginkgo biloba. Fitoterapia 2008, 79, 401–418. [Google Scholar] [CrossRef]
- Oh, T.S.; Koo, H.M.; Yoon, H.R.; Jeong, N.S.; Kim, Y.J.; Kim, C.H. Antifungal action of Ginkgo biloba outer seedcoat on rice sheath blight. Plant Pathol. J. 2015, 31, 61. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Takeshita, S.; Kimura, F.; Ohno, O.; Suenaga, K. A novel substance with allelopathic activity in Ginkgo biloba. J. Plant Physiol. 2013, 170, 1595–1599. [Google Scholar] [CrossRef]
- Sati, P.; Dhyani, P.; Bhatt, I.D.; Pandey, A. Ginkgo biloba flavonoid glycosides in antimicrobial perspective with reference to extraction method. J. Tradit. Complement. Med. 2019, 9, 15–23. [Google Scholar] [CrossRef]
- Ražná, K.; Sawinska, Z.; Ivanišová, E.; Vukovic, N.; Terentjeva, M.; Stričík, M.; Kowalczewski, P.Ł.; Hlavačková, L.; Rovná, K.; Žiarovská, J.; et al. Properties of Ginkgo biloba L.: Antioxidant characterization, antimicrobial activities, and genomic microRNA based marker fingerprints. Int. J. Mol. Sci. 2020, 21, 3087. [Google Scholar] [CrossRef]
- Guo, Z.; Qin, Y.; Lv, J.; Wang, X.; Ye, T.; Dong, X.; Du, N.; Zhang, T.; Piao, F.; Dong, H.; et al. High red/far-red ratio promotes root colonization of Serratia plymuthica A21-4 in tomato by root exudates-stimulated chemotaxis and biofilm formation. Plant Physiol. Biochem. 2024, 206, 108245. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, N.; Wang, K.; Xian, Q.; Dong, J.; Qi, X.; Chen, X. Chitinase Chi 2 positively regulates cucumber resistance against Fusarium oxysporum f. sp. cucumerinum. Genes 2022, 13, 62. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Stummer, B.E.; Guo, Q.; Zhang, W.; Zhang, X.; Zhang, L.; Harvey, P.R. Quantification of Pseudomonas protegens FD6 and Bacillus subtilis NCD-2 in soil and the wheat rhizosphere and suppression of root pathogenic Rhizoctonia solani AG-8. Biol. Control 2021, 154, 104504. [Google Scholar] [CrossRef]
- Yang, Y.; Xiang, Y.; Xu, M. From red to green: The propidium iodide-permeable membrane of Shewanella decolorationis S12 is repairable. Sci. Rep. 2015, 5, 18583. [Google Scholar] [CrossRef]
- Jones, K.H.; Senft, J.A. An improved method to determine cell viability by simultaneous staining with fluorescein diacetate-propidium iodide. J. Histochem. Cytochem. 1985, 33, 77–79. [Google Scholar] [CrossRef]
- Pathak, J.; Chatterjee, A.; Singh, S.P.; Sinha, R.P. Detection of Reactive Oxygen Species (ROS) in cyanobacteria using the oxidant-sensing probe 2′, 7′-dichlorodihydrofluorescein diacetate (DCFH-DA). Bio-Protocol 2017, 7, e2545. [Google Scholar]
- Contento, A.L.; Xiong, Y.; Bassham, D.C. Visualization of autophagy in Arabidopsis using the fluorescent dye monodansylcadaverine and a GFP-AtATG8e fusion protein. Plant J. 2005, 42, 598–608. [Google Scholar] [CrossRef]
- Akaberi, M.; Baharara, H.; Amiri, M.S.; Moghadam, A.T.; Sahebkar, A.; Emami, S.A. Ginkgo biloba: An update review on pharmacological, ethnobotanical, and phytochemical studies. Pharmacol. Res.-Mod. Chin. Med. 2023, 9, 100331. [Google Scholar] [CrossRef]
- Guo, J.; Tang, W.; Tang, W.; Gao, T.; Yuan, M.; Wu, Y.; Wang, G. Research progress on the types, functions, biosynthesis, and metabolic regulation of Ginkgo terpenoids. Plant Physiol. Biochem. 2024, 212, 108754. [Google Scholar] [CrossRef] [PubMed]
- Mao, D.; Zhong, L.; Zhao, X.; Wang, L. Function, biosynthesis, and regulation mechanisms of flavonoids in Ginkgo biloba. Fruit Res. 2023, 3, 18. [Google Scholar] [CrossRef]
- Chang, B.; Qiu, X.; Yang, Y.; Zhou, W.; Jin, B.; Wang, L. Genome-wide analyses of the GbAP2 subfamily reveal the function of GbTOE1a in salt and drought stress tolerance in Ginkgo biloba. Plant Sci. 2024, 342, 112027. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.; Ma, K.; Lu, Z.; Lu, J.; Cui, J.; Wang, L.; Jin, B. Physiological, transcriptomic, and metabolic responses of Ginkgo biloba L. to drought, salt, and heat stresses. Biomolecules 2020, 10, 1635. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Dong, L.; Zhang, W.; Liao, Y.; Wang, L.; Wang, Q.; Ye, J.; Xu, F. Ginkgo biloba GbbZIP08 transcription factor is involved in the regulation of flavonoid biosynthesis. J. Plant Physiol. 2023, 287, 154054. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, Y.; Zhang, J.; Wang, S. Advances in the chemical constituents and chemical analysis of Ginkgo biloba leaf, extract, and phytopharmaceuticals. J. Pharm. Biomed. 2021, 193, 113704. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Yang, K.; Li, Y.; Xu, F.; Cheng, S.; Zhang, W.; Liao, Y.; Yang, X.; Wang, L.; Wang, Q. Genome-wide transcriptome analysis reveals the regulatory network governing terpene trilactones biosynthesis in Ginkgo biloba. Tree Physiol. 2022, 42, 2068–2085. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhao, X.; Jiang, Y.; Jin, B.; Wang, L. Functions of Representative Terpenoids and Their Biosynthesis Mechanisms in Medicinal Plants. Biomolecules 2023, 13, 1725. [Google Scholar] [CrossRef]
- Gutensohn, M.; Hartzell, E.; Dudareva, N. Another level of complex-ity: The role of metabolic channeling and metabolons in plant terpenoid metabolism. Front Plant Sci. 2022, 13, 954083. [Google Scholar] [CrossRef]
- Liu, Y.; Xin, H.; Zhang, Y.; Che, F.; Shen, N.; Cui, Y. Leaves, seeds and exocarp of Ginkgo biloba L. (Ginkgoaceae): A Comprehensive Review of Traditional Uses, phytochemistry, pharmacology, resource utilization and toxicity. J. Ethnopharmacol. 2022, 298, 115645. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Yuan, L.; Han, L.; Song, L. Characterization of antioxidant and antibacterial gelatin films incorporated with Ginkgo biloba extract. RSC Adv. 2019, 9, 27449–27454. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ma, K.; Liu, J.; Li, F. Ginkgo biloba extract may alleviate viral myocarditis by suppression of S100A4 and MMP-3. J. Med. Virol. 2019, 91, 2083–2092. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Yang, X.Q.; Yang, Y.B.; Ding, Z.T. Antifungal and antifeedant terpenoids from Paraphaeosphaeria sp. cultured by extract of host Ginkgo biloba. Phytochemistry 2023, 210, 113651. [Google Scholar] [CrossRef] [PubMed]

| Category | Compounds | Number |
|---|---|---|
| Flavonoids | 3-Hydroxy-4′,5,7-Trimethoxyflavanone; Texasin; 5-Hydroxy-3-(2-hydroxybenzyl)-7-methoxychroman-4-one; Kayaflavone*; 4,4′-Dihydroxy-2-methoxychalcone; Echinatin; Sciadopitysin*; 5-hydroxy-7-(1′,2′-dihydroxypropyl)-2-methyl-chromone; 3,7,4′-Trihydroxyflavone; 5,6,3′,4′-Tetrahydroxy-3,7-dimethoxyflavone; 6-Methylflavone | 159 |
| Phenolic acids | Phthalic anhydride; 3,4-Dimethoxyphenyl acetic acid; Ethyl 2,4-dihydroxy-3,6-dimethylbenzoate; 3-Hydroxycinnamic Acid*; 2-Hydroxycinnamic acid*; 4-Nitrophenol; Dimethyl phthalate; 6-O-Feruloyl-β-D-glucose; 4′-Methoxyacetophenone; Ethyl phenylacetate | 150 |
| Quinones | 2-Hydroxy-2,3-dihydronaphthalene-1,4-dione; Danthron; Madeirin; 1,8-Dihydroxyanthraquinone; Hircinol; 8-Hydroxy-2-methoxy-1,4-naphthoquinone; Torachrysone-8-O-glucoside; Aurantio-obtusin-6-O-Glucoside*; 3-Methoxyjuglone; Anthraquinone-2-carboxylic acid; Chrysophanol-9-anthrone; 3-Hydroxydehydroiso-α-Lapachone; | 10 |
| Lignans and Coumarins | Marmin [7-(6′,7′-Dihydroxygeranyloxy)coumarin]; Daphnetin; 1′,2′-epoxy-4-isobutyryl coniferol; (7-Hydroxy-6-methoxycoumarin)*; 6-Hydroxy-7-methoxycoumarin*; Paniculin; Scopoletin; 6-Hydroxy-7-methoxycoumarin*; Sesaminol*; 6-(2′,3′-dihydroxy-3-methylbutyl)-8-prenyl umbelliferone; 4-HydroxyseSamin* | 40 |
| Tannins | 3,3′-O-Dimethylellagic Acid; 3,3′,4-O-Trimethylellagic acid; Procyanidin B1 | 3 |
| Alkaloids | Hordenine; Pseudoephedrine; Octadec-8-enamide; octadecadienoic acid amide; Laurocapram; Choline; Acetohexamide; 8β-acetoxy-12β-hydroxy-lycopodine; 5-Hydroxyindole; Hydroquinine | 110 |
| Terpenoids | Cafestol; 12,13-Dehydrogeranylgeraniol; Curcumenol; ginkgolide B*; Procurcumenol; 14-deoxycoleon U; ginkgolide X; 5α,6α,7β,10β-11α,13-dihydro-4(15)-eudesmene-12,6-olide Tsoongiodendroonolide; eupatorinol | 104 |
| Organic acids | Malonic acid; 3-(3-hydroxy-4-methoxyphenyl)propanoic acid; trans-4-Methylene-2-octyl-5-oxotetrahydrofuran-3-carboxylic acid; Muconic acid; 2-Cyclohexyl-2-hydroxy-2-phenylacetic acid; 4-Tert-butylphenoxyacetic acid; 3-(2-Methoxyphenyl)propanoic acid; 3-Carboxy-4-methyl-5-propyl-2-furanpropionic acid; Azelaic acid; Methanesulfonic acid | 72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Jiang, Y.; Liu, X.; Chen, Y.; Zhang, Q.; Wang, L.; Li, W. Analysis of Ginkgo biloba Root Exudates and Inhibition of Soil Fungi by Flavonoids and Terpene Lactones. Plants 2024, 13, 2122. https://doi.org/10.3390/plants13152122
Wang Y, Jiang Y, Liu X, Chen Y, Zhang Q, Wang L, Li W. Analysis of Ginkgo biloba Root Exudates and Inhibition of Soil Fungi by Flavonoids and Terpene Lactones. Plants. 2024; 13(15):2122. https://doi.org/10.3390/plants13152122
Chicago/Turabian StyleWang, Yawen, Yanbing Jiang, Ximeng Liu, Yadi Chen, Qingxia Zhang, Li Wang, and Weixing Li. 2024. "Analysis of Ginkgo biloba Root Exudates and Inhibition of Soil Fungi by Flavonoids and Terpene Lactones" Plants 13, no. 15: 2122. https://doi.org/10.3390/plants13152122





