Current Status and Prospects of Pine Wilt Disease Management with Phytochemicals—A Review
Abstract
1. Introduction
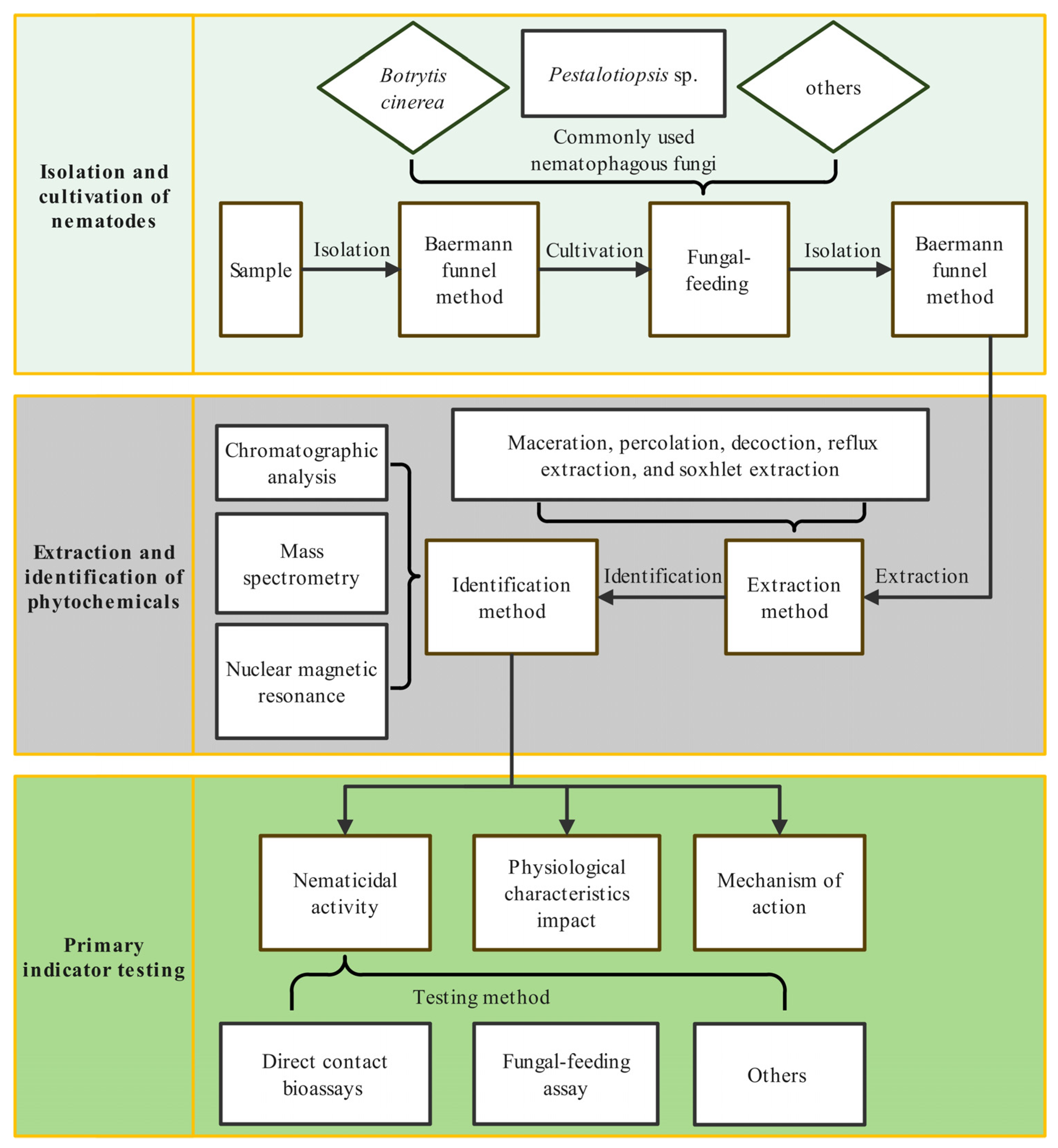
2. Research Analysis of Phytochemicals on Pine Wilt Disease Control
3. Analysis Process of Using Phytochemicals to Control Bursaphelenchus xylophilus
4. Phytochemicals with High Nematicidal Activity against Bursaphelenchus xylophilus
4.1. Alkaloids
4.2. Terpenes
4.3. Phenylpropanoids
4.4. Coumarins
4.5. Flavonoids
4.6. Other Compounds and Extracts
| Cat. | Species | Part | Compound Name | Extractant/ Solvent | Method | Nematicidal Activity | Ref. |
|---|---|---|---|---|---|---|---|
| Alkaloids | Sophora alopecuroides | Leaf | Aloperine | Methylbenzene | FF | LC50/5 d = 0.263 μg/mL | [33] |
| S. alopecuroides | - | Aloperine | - | Injection | 1 year, Control rate = 100% | [35] | |
| S. alopecuroides | - | Aloperine | - | FF | 0.0001 g/mL, Reproductive inhibition rate = 100% (5 d) | [36] | |
| S. alopecuroides | - | Aloperine | Distilled water | DC | 29.8% | [37] | |
| S. alopecuroides | - | Δ11-Dehydroaloperine | Distilled water | DC | 5.8% | [37] | |
| Peganum harmala | Seed | Harmine | Ethanol | DC | LC50/48 h = 135.74 µg/mL | [43] | |
| Piper peepuloides | Fruit | Piperine | DMSO | FF | 50 μg/mL, 100% (6 h) | [47] | |
| Cephalotaxus fortunei | Twig | Drupacine | Ethanol | DC | EC50/54 h = 27.1 µg/mL | [48] | |
| Waltheria indica | Root | 5′-Methoxywaltherio-ne A | Methanol | DC | EC50/72 h = 2.13 μg/mL | [49] | |
| W. indica | Root | Waltherione A | Methanol | DC | EC50/72 h = 3.54 μg/mL | [49] | |
| Clausena lansium | Seed | Lansiumamide B | Methanol | DC | LC50/24 h = 8.38 mg/L | [50] | |
| Orixa japonica | Root | 9-Methoxy-[1,3]dioxolo[4,5-b]-quinoline | Aqueous | DC | LC50/72 h = 12.66 μg/mL | [51] | |
| O. japonica | Root | Skimmianine | Aqueous | DC | LC50/72 h = 15.56 μg/mL | [51] | |
| O. japonica | Root | Kokusaginine | Aqueous | DC | LC50/72 h = 16.66 μg/mL | [51] | |
| O. japonica | Root | 6-Acetonyldihyrochelerythrine | Aqueous | DC | LC50/72 h = 11.27 μg/mL | [51] | |
| O. japonica | Root, bark | (Z)-3-(4-Hydroxybenzylidene)-4-(4-hydroxyphenyl)-1-methylpyrrolidin-2-one | Ethanol | DC | LC50/72 h = 391.50 μg/mL | [74] | |
| Heracleum hemsleyanum | - | Reserpine | Ethanol | DC | LC50/72 h = 489.17 μM | [75] | |
| Sophora flavescens | Root | (–)-N-methylcytisine | Methanol | FF | log(1/ID50) = 7.91 | [99] | |
| S. flavescens | Root | (–)-Anagyrine | Methanol | FF | log(1/ID50) = 7.92 | [99] | |
| S. flavescens | Epigeal | Matrine | Methanol | FF | log(1/ID50) = 6.39 | [100] | |
| Macleaya cordata | Stem, leaf | Sanguinarine | Ethanol | DC | LC50/24 h = 28.52 μg/mL | [101] | |
| M. cordata | Stem, leaf | Chelerytherine | Ethanol | DC | LC50/24 h = 34.50 μg/mL | [101] | |
| M. cordata | Stem, leaf | Allocryptopine | Ethanol | DC | LC50/24 h = 37.45 μg/mL | [101] | |
| S. alopecuroides | Seed | Sophoridine | Chloroform | DC | IC50/24 h = 0.822 μg/mL | [102] | |
| S. alopecuroides | Seed | Oxymatrine | Chloroform | DC | IC50/24 h = 0.722 μg/mL | [102] | |
| S. alopecuroides | Seed | Oxysophocarpine | Chloroform | DC | IC50/24 h = 0.622 μg/mL | [102] | |
| Terpenes | - | - | Citronellol | Triton X-100 | DC | LC50/4 h = 0.245 mg/mL (M) LC50/4 h = 0.235 mg/mL (F) | [56] |
| - | - | Menthol | Triton X-100 | DC | LC50/4 h = 0.985 mg/mL (M) LC50/4 h = 0.894 mg/mL (F) | [56] | |
| - | - | Nerol | Triton X-100 | DC | LC50/4 h = 0.865 mg/mL (M) LC50/4 h = 0.926 mg/mL (F) | [56] | |
| - | - | Geraniol | Triton X-100 | DC | LC50/4 h = 0.540 mg/mL (M) LC50/4 h = 0.415 mg/mL (F) | [56] | |
| - | - | Citral | Triton X-100 | DC | LC50/4 h = 0.187 mg/mL (M) LC50/4 h = 0.139 mg/mL (F) | [56] | |
| - | - | Citronellal | Triton X-100 | DC | LC50/4 h = 0.321 mg/mL (M) LC50/4 h = 0.298 mg/mL (F) | [56] | |
| - | - | Carvacrol | Triton X-100 | DC | LC50/4 h = 0.125 mg/mL (M) LC50/4 h = 0.097 mg/mL (F) | [56] | |
| - | - | Thymol | Triton X-100 | DC | LC50/4 h = 0.119 mg/mL (M) LC50/4 h = 0.110 mg/mL (F) | [56] | |
| Thymus vulgaris | - | Geraniol | Castor oil–ethanol | DC | LC50/24 h = 0.47 mg/mL | [57] | |
| T. vulgaris | - | Thymol | Castor oil–ethanol | DC | LC50/24 h = 1.08 mg/mL | [57] | |
| T. vulgaris | - | Carvacrol | Castor oil–ethanol | DC | LC50/24 h = 1.23 mg/mL | [57] | |
| Trachyspermum ammi, Pimenta dioica, Litsea cubeba | Seed, berry, fruit | Citral | Triton X-100 | DC | LC50/24 h = 0.120 mg/mL | [58] | |
| T. ammi, P. dioica, L. cubeba | Seed, berry, fruit | Neral | Triton X-100 | DC | LC50/24 h = 0.525 mg/mL | [58] | |
| Michelia gioi | Leaf | Parthenolide | Ethyl acetate | DC | 200 μg/mL, 70.4% (48 h) | [59] | |
| Dictamnus dasycarpus | Bark | Evodin | Ethyl acetate | DC | LC50/72 h = 17.91 μg/mL | [60] | |
| D. dasycarpus | Bark | Obacunone | Ethyl acetate | DC | LC50/72 h = 15.99 μg/mL | [60] | |
| D. dasycarpus | Bark | Fraxinellone | Ethyl acetate | DC | LC50/72 h = 9.78 μg/mL | [60] | |
| Euphorbia kansui | Root | 3-O-(2″, 3″-Dimethylbutanoyl)-13-O-dodecanoylingenol | Ethanol | FF | 5 μg, Antinematodal activity | [61] | |
| E. kansui | Root | 3-O-(2″, 3″-Dimethylbutanoyl)-13-O-decanoylingenol | Ethanol | FF | 5 μg, Antinematodal activity | [61] | |
| Magnolia grandiflora | Branch, leaf | 4,5-Epoxy-1(10)E,11(13)-germacradien-12,6-olide | Ethyl acetate | DC | LC50/72 h = 71 µg/mL | [62] | |
| Phenylpropanoids | T. ammi, P. dioica, L. cubeba | Seed, berry, fruit | Methyl isoeugenol | Triton X-100 | DC | LC50/24 h = 0.21 mg/mL | [58] |
| T. ammi, P. dioica, L. cubeba | Seed, berry, fruit | Isoeugenol | Triton X-100 | DC | LC50/24 h = 0.20 mg/mL | [58] | |
| T. ammi, P. dioica, L. cubeba | Seed, berry, fruit | Eugenol | Triton X-100 | DC | LC50/24 h = 0.48 mg/mL | [58] | |
| T. ammi, P. dioica, L. cubeba | Seed, berry, fruit | Methyl eugenol | Triton X-100 | DC | LC50/24 h = 0.517 mg/mL | [58] | |
| Valeriana jatamansi | Root | Cis-asarone | Triton X-100 | DC | 1 mg/mL, 100% (24 h) | [63] | |
| Liquidambar orientalis | Resin | Trans-cinnamyl alcohol | Triton X-100 | DC | 1 mg/mL, 100% (24 h) | [63] | |
| - | - | (E)-cinnamaldehyde | Castor oil–ethanol | DC | LD50/24 h = 0.057 mg/mL | [64] | |
| - | - | α-Methyl-(E)-cinnamaldehyde | Castor oil–ethanol | DC | LD50/24 h = 0.131 mg/mL | [64] | |
| - | - | (E)-4-methoxycinnamaldehyde | Castor oil–ethanol | DC | LD50/24 h = 0.262 mg/mL | [64] | |
| - | - | (E)-2-methoxycinnamaldehyde | Castor oil–ethanol | DC | LD50/24 h = 0.270 mg/mL | [64] | |
| - | - | Ethyl cinnamate | Castor oil–ethanol | DC | LC50/24 h = 0.114 mg/mL | [64] | |
| - | - | Methyl cinnamate | Castor oil–ethanol | DC | LC50/24 h = 0.163 mg/mL | [64] | |
| - | - | Allyl cinnamate | Castor oil–ethanol | DC | LC50/24 h = 0.195 mg/mL | [64] | |
| - | - | Ethyl α-cyanocinnamate | Castor oil–ethanol | DC | LC50/24 h = 0.333 mg/mL | [64] | |
| Cinnamomum verum | Bark | Cinnamyl acetate | Triton X-100 | DC | LC50/4 h = 32.81 µL/L | [65] | |
| Kaempferia galanga | - | Ethyl ρ-methoxy cinnamate | Methanol | DC | LC50/72 h = 2.81 mg/L | [66] | |
| K. galanga | - | Ethyl cinnamate | Methanol | DC | LC50/72 h = 29.7 mg/L | [66] | |
| Zanthoxylum armatum | Fruit | Methyl trans-cinnamate | Distilled water (Triton X-100) | DC | 2.0 mg/mL, 100% | [67] | |
| Z. armatum | Fruit | Ethyl trans-cinnamate | Distilled water (Triton X-100) | DC | 2.0 mg/mL, 100% | [67] | |
| K. galanga | Root | Ethyl trans-cinnamate | Methanol | DC | 60 μg/mL, 100% (4 h) | [68] | |
| K. galanga | Root | Ethyl ρ-methoxycinnamate | Methanol | DC | 60 μg/mL, 100% (4 h) | [68] | |
| Zostera marina | - | Rosmarinic acid | Ethanol | DC | LC50/24 h = 1.18 mg/g | [69] | |
| Coumarins | Heracleum candicans | Root | 8-Geranyloxypsoralen | Ethanol | DC | LC50/72 h = 117.5 mg/L | [71] |
| H. candicans | Root | Imperatorin | Ethanol | DC | LC50/72 h = 179.0 mg/L | [71] | |
| H. candicans | Root | Heraclenin | Ethanol | DC | LC50/72 h = 148.7 mg/L | [71] | |
| Stellera chamaejasme | Root | Umbelliferone | Ethanol | DC | LC50/72 h = 3.3 μM | [72] | |
| S. chamaejasme | Root | Daphnoretin | Ethanol | DC | LC50/72 h = 65.3 μM | [72] | |
| Ficus carica | Leaf | Psoralen | Ethanol | DC | LC50/72 h = 115.03 μg/mL | [73] | |
| Hansenia weberbaueriana | Root | Columbianetin | Ethanol | DC | LC50/72 h = 21.83–103.44 µg/mL | [74] | |
| H. weberbaueriana | Root | Isoimperatorin | Ethanol | DC | LC50/72 h = 17.21–30.91 µg/mL | [74] | |
| H. hemsleyanum | - | Columbianadin | Ethyl acetate | DC | LC50/72 h = 406.74 μM | [75] | |
| F. carica | Leaf | Psoralen | Ethanol | DC | LC50/72 h = 463.32 μM | [75] | |
| F. carica | Leaf | Bergapten | Ethanol | DC | LC50/72 h = 430.08 μM | [75] | |
| Angelica pubescens | Root | Osthole | Ethyl acetate | DC | LC50/72 h = 489.17 μM | [76] | |
| A. pubescens | Root | Xanthotoxin | Ethyl acetate | DC | LC50/72 h = 435.66 μM | [76] | |
| Cnidium monnieri, Angelica dahurica | Fruit (C), root (A) | Osthole | Ethanol | DC | LC50/72 h = 64.93 µg/mL | [77] | |
| C. monnieri, A. dahurica | Fruit (C), root (A) | Xanthotoxin | Ethanol | DC | LC50/72 h = 54.68 µg/mL | [77] | |
| C. monnieri, A. dahurica | Fruit (C), root (A) | Cindimine | Ethanol | DC | LC50/72 h = 24.73 µg/mL | [77] | |
| C. monnieri, A. dahurica | Fruit (C), root (A) | Isopimpinellin | Ethanol | DC | LC50/72 h = 92.16 µg/mL | [77] | |
| C. monnieri, A. dahurica | Fruit (C), root (A) | Marmesin | Ethanol | DC | LC50/72 h = 122.96 µg/mL | [77] | |
| C. monnieri, A. dahurica | Fruit (C), root (A) | Isoimperatorin | Ethanol | DC | LC50/72 h = 43.08 µg/mL | [77] | |
| C. monnieri, A. dahurica | Fruit (C), root (A) | Imperatorin | Ethanol | DC | LC50/72 h = 35.72 µg/mL | [77] | |
| C. monnieri, A. dahurica | Fruit (C), root (A) | Bergapten | Ethanol | DC | LC50/72 h = 52.07 µg/mL | [77] | |
| Flavonoids | S. chamaejasme | Root | (+)-Chamaejasmine | Ethanol | DC | LC50/72 h = 4.7 μM | [72] |
| S. chamaejasme | Root | Ruixianglangdusu B | Ethanol | DC | LC50/72 h = 15.7 μM | [72] | |
| S. chamaejasme | Root | Chamaejasmenin C | Ethanol | DC | LC50/72 h = 2.7 μM | [72] | |
| S. chamaejasme | Root | 7-Methoxyneochamaejasmin A | Ethanol | DC | LC50/72 h = 167.3 μM | [72] | |
| S. chamaejasme | Root | Chamaechromone | Ethanol | DC | LC50/72 h = 36.7 μM | [72] | |
| S. chamaejasme | Root | Isosikokianin A | Ethanol | DC | LC50/72 h = 2200 μM | [72] | |
| - | - | Rotenone | Acetone | DC | LC50/120 h = 1.86 μg/mL | [79] | |
| - | - | 2-Phenyl chromone | DMSO | FF | LC50/24 h = 100 μM | [83] | |
| Polyphenols | Punica granatum | Rind | Punicalin | Aqueous | DC | LC50/72 h = 826.96 μM | [75] |
| P. granatum | Rind | Corilagin | Aqueous | DC | LC50/72 h = 868.28 μM | [75] | |
| P. granatum | Bark | Punicalagin | Aqueous | DC | LC50/72 h = 307.08 μM | [75] | |
| Naphthoquinones | - | - | 1,4-Naphthoquinones | DMSO | DC | LC50/48 h = 100 ppm | [103] |
| - | - | Juglone | DMSO | DC | LC50/48 h = 57 ppm | [103] | |
| - | - | Plumbagin | DMSO | DC | LC50/48 h = 104 ppm | [103] | |
| Polyacetylenes | H. weberbaueriana | Root | Falcarinol | Ethanol | DC | LC50/72 h = 7.42 µg/mL | [74] |
| H. weberbaueriana | Root | Falcarindiol | Ethanol | DC | LC50/72 h = 0.95 µg/mL | [74] | |
| Glycosides | Liriope muscari | Root | 1,4-Epoxy-cis-eudesm-6-O-β-D-glucopyranoside | Ethanol | DC | LC50/72 h = 339.76 μg/mL | [88] |
| L. muscari | Root | 1β,6β-Dihydroxy-cis-eudesm-3-ene-6-O-β-D-glucopyranoside | Ethanol | DC | LC50/72 h = 82.84 μg/mL | [88] | |
| L. muscari | Root | 1α,6β-Dihydroxy-cis-eudesm-3-ene-6-O-β-D-glucopyranoside | Ethanol | DC | LC50/72 h = 153.39 μg/mL | [88] | |
| L. muscari | Root | 1α, 6β-dihydroxy-5, 10-bis-epi-eudesm-4(15)-ene-6-O-β-D-glucopyranoside | Ethanol | DC | LC50/72 h = 465.68 μg/mL | [88] | |
| Sulfides | Allium sativum | Bulb | Diallyl disulphide | Triton X-100 | DC | LC50/4 h = 37.06 µL/L | [65] |
| A. sativum | Bulb | Diallyl trisulphide | Triton X-100 | DC | LC50/4 h = 2.79 µL/L | [65] | |
| Allium cepa | - | Dipropyl trisulfide | Ethanol | DC | LC50/24 h = 5.01 μg/mL | [90] | |
| A. cepa | - | Methyl propyl trisulfide | Ethanol | DC | LC50/24 h = 16.60 μg/mL | [90] | |
| - | - | Allyl isothiocyanate | Distilled water | DC | LC50/24 h = 0.000271% | [91] | |
| A. cepa | - | Propyl trisulphide | Triton X-100 | DC | LC50/24 h = 5 µg/mL | [104] | |
| A. cepa | - | Methyl propyl trisulphide | Triton X-100 | DC | LC50/24 h = 22.9 µg/mL | [104] | |
| Aliphatic compounds | Coriandrum sativum | Herb | Trans-2-decenal | Triton X-100 | DC | 1 mg/mL, 100% (24 h) | [63] |
| C. sativum | Herb | Octanal | Triton X-100 | DC | 1 mg/mL, 89.0% (24 h) | [63] | |
| C. sativum | Herb | Nonanal | Triton X-100 | DC | 1 mg/mL, 95.8% (24 h) | [63] | |
| C. sativum | Herb | Decanal | Triton X-100 | DC | 1 mg/mL, 100% (24 h) | [63] | |
| C. sativum | Herb | Undecanal | Triton X-100 | DC | 1 mg/mL, 98.7% (24 h) | [63] | |
| C. sativum | Herb | Dodecanal | Triton X-100 | DC | 1 mg/mL, 86.3% (24 h) | [63] | |
| C. sativum | Herb | Trans-2-decen-1-ol | Triton X-100 | DC | 0.2 mg/mL, 98% (24 h) | [63] | |
| C. sativum | Herb | Decanol | Triton X-100 | DC | 0.2 mg/mL, 100% (24 h) | [63] | |
| - | - | 3-Methylbutyl tiglate | Ethanol | DC | LC50/48 h = 0.0218 mg/mL | [92] | |
| - | - | Isobutyl 2-methylbutanoate | Ethanol | DC | LC50/48 h = 0.0284 mg/mL | [92] | |
| - | - | 3-Methylbutyl 2-methylbutanoate | Ethanol | DC | LC50/48 h = 0.0326 mg/mL | [92] | |
| - | - | 3-Methyl-2-butenyl 2-methylbutanoate | Ethanol | DC | LC50/48 h = 0.0402 mg/mL | [92] | |
| - | - | Pentyl 2-methylbutanoate | Ethanol | DC | LC50/48 h = 0.0480 mg/mL | [92] | |
| - | - | Trans-2-hexenal | Distilled water | DC | LC10/48 h = 0.162 μL/L | [93] | |
| Thiophenes | O. japonica | Root | α-Terthienyl | Aqueous | DC | LC50/72 h = 1.95 μg/mL | [51] |
| Eclipta prostrata | - | Terthiophene | Methanol | DC | 1.00 ppm, 92.8% (24 h) | [105] | |
| Aromatic compounds | L. orientalis | Resin | Benzaldehyde | Triton X-100 | DC | 1 mg/mL, 94.1% (24 h) | [63] |
| Z. armatum | Fruit | Methyl salicylate | Distilled water (Triton X-100) | DC | 2.0 mg/mL, 100% | [67] | |
| Z. armatum | Fruit | Ethyl salicylate | Distilled water (Triton X-100) | DC | 2.0 mg/mL, 100% | [67] | |
| Lactones | Conioselinum tenuissimum | Root | (Z)-Ligustilide | Ethanol | DC | LC50/24 h = 0.24 mg/mL | [95] |
| Camptothecin derivatives | - | - | 7-CH2C6H5-camptothecin (CPT) | Acetone | DC | LC50/24 h = 2.28 mg/L | [38] |
| - | - | 7-CHO-CPT | Acetone | DC | LC50/24 h = 2.21 mg/L | [38] | |
| - | - | 7-CH2OC-OC6H5-CPT | Acetone | DC | LC50/24 h = 1.37 mg/L | [38] | |
| - | - | 10-CH2OCOC6H5-CPT | Acetone | DC | LC50/24 h = 1.68 mg/L | [38] | |
| - | - | 20-CH2OCOC6H5-CPT | Acetone | DC | LC50/24 h = 0.31 mg/L | [38] | |
| - | - | 20-F-CPT | Acetone | DC | LC50/24 h = 1.71 mg/L | [38] | |
| - | - | 20-(S)-CPT | Acetone | DC | LC50/24 h = 12.18 mg/L | [38] | |
| - | - | 7-(1-(4-methoxybenzoyl)piperazin-4-yl)-methyl-camptothecin | Acetone | DC | LC50/24 h = 6.34 mg/L | [39] | |
| - | - | 7-(1-(2-methoxybenzoyl)piperazin-4-yl)methyl-camptothecin | Acetone | DC | LC50/24 h = 6.53 mg/L | [39] | |
| - | - | N-(2,4,6-trimethoxybenzyl)-β-carboline-3-carbohydrazide | Acetone | DC | LC50/24 h = 42.49 μg/mL | [44] | |
| Harmine derivatives | - | - | Harmine quaternary ammonium derivatives 10 | Distilled water (2% DMSO) | DC | LC50/48 h = 1.63 μg/mL | [46] |
| - | - | Harmine quaternary ammonium derivatives 11 | Distilled water (2% DMSO) | DC | LC50/48 h = 1.63 μg/mL | [46] | |
| - | - | Harmine quaternary ammonium derivatives 12 | Distilled water (2% DMSO) | DC | LC50/48 h = 1.75 μg/mL | [46] | |
| - | - | Harmine quaternary ammonium derivatives 13 | Distilled water (2% DMSO) | DC | LC50/48 h = 1.44 μg/mL | [46] | |
| Nitrile derivatives | - | - | 4-Methoxycinnamonitrile | Castor oil–ethanol | DC | LC50/24 h = 0.224 mg/mL | [64] |
| - | - | Cinnamonitrile | Castor oil–ethanol | DC | LC50/24 h = 0.448 mg/mL | [64] | |
| - | - | Cinnamyl bromide | Castor oil–ethanol | DC | LC50/24 h = 0.502 mg/mL | [64] | |
| Aliphatic derivatives | - | - | C9-C11 alkanols, C10-C11 2E-alkenols, C8-C9 2E-alkenals, C9-C10 alkanoic acids | Triton X-100 | DC | 0.125 mg/mL, >80% (48 h) | [94] |
| Matrine derivatives | - | - | Sophocarpine | Methanol | FF | log(1/ID50) = 7.78 | [100] |
| - | - | Sophoramine | Methanol | FF | log(1/ID50) = 6.68 | [100] | |
| - | - | Cytisine | Methanol | FF | log(1/ID50) = 8.23 | [100] | |
| - | - | N-Nethylcytisine | Methanol | FF | log(1/ID50) = 7.91 | [100] | |
| - | - | Anagyrine | Methanol | FF | log(1/ID50) = 7.54 | [100] | |
| - | - | Sparteine | Methanol | FF | log(1/ID50) = 7.96 | [100] | |
| 3-Acylbarbituric acid analogues | - | - | 3-Acylbarbituric acid analogues-18 | Ethanol | DC | 10 µg/mL, 93.4% | [106] |
| Sulfonate derivatives | - | - | Sulfonate derivatives of maltol 3M | Acetone | DC | LC50/24 h = 1.1762 mg/L | [107] |
| - | - | Sulfonate derivatives of maltol 3N | Acetone | DC | LC50/24 h = 1.2384 mg/L | [107] | |
| Extracts | P. harmala | Seed | Fraction A6 | Ethanol | DC | LC50/24 h = 86.02 μg/mL | [43] |
| E. prostrata | Whole plant | - | Methanol | DC | LC50/24 h = 0.36 μg/mL | [96] | |
| Paeonia × suffruticosa | Root | - | Triton X-100 | DC | LC50/4 h = 0.26 mg/mL | [97] | |
| Perilla frutescens | Leaf | - | Triton X-100 | DC | LC50/4 h = 0.41 mg/mL | [97] | |
| Boswellia serrata | Resin | - | Triton X-100 | DC | LC50/4 h = 0.21 mg/mL | [97] | |
| Schizonepeta tenuifolia | Whole plant | - | Triton X-100 | DC | LC50/4 h = 0.41 mg/mL | [97] | |
| Thymbra capitata | Aerial part | - | Aqueous | DC | LC100/24 h = 0.375 μL/mL | [98] | |
| Satureja montana | Aerial part | - | Aqueous | DC | LC100/24 h = 0.374 μL/mL | [98] | |
| Ruta graveolens | Aerial part | - | Aqueous | DC | LC100/24 h = 0.358 μL/mL | [98] | |
| Origanum vulgare | Aerial part | - | Aqueous | DC | LC100/24 h = 1.606 μL/mL | [98] | |
| Cymbopogon citratus | Leaf | - | Aqueous | DC | LC100/24 h = 1.059 μL/mL | [98] | |
| Pinellia ternata | Tuber | Total alkaloids | Chloroform | DC | IC50/24 h = 16.18 μg/mL | [102] | |
| S. alopecuroides | Seed | Total alkaloids | Chloroform | DC | IC50/24 h = 0.622 μg/mL | [102] | |
| Artemisia capillaris | Leaf, stem | - | Methanol | FF | 20 mg, Propagation Rate = 0.1% | [108] | |
| Cirsium japonicum | Root | - | Methanol | FF | 20 mg, Propagation Rate = 0.1% | [108] | |
| Coreopsis lanceolata | Flower | - | Methanol | FF | 20 mg, Propagation Rate = 0.2% | [108] | |
| Erigeron annuus | Root | - | Methanol | FF | 20 mg, Propagation Rate = 0.1% | [108] | |
| Sauropus androgynus | Shoot | - | Methanol | FF | 0.625 mg/cotton ball, Minimum effective dose | [109] | |
| Eugenia polyantha | Leaf | - | Methanol | FF | 0.625 mg/cotton ball, Minimum effective dose | [109] | |
| Areca catechu | Fruit | - | Methanol | FF | 0.625 mg/cotton ball, Minimum effective dose | [109] | |
| Piper betle | Leaf | - | Methanol | FF | 0.625 mg/cotton ball, Minimum effective dose | [109] | |
| Piper nigrum | Berry | - | Methanol | FF | 0.625 mg/cotton ball, Minimum effective dose | [109] | |
| Bischofia javanica | Sap | - | Methanol | FF | 0.7 mg/cotton ball, Minimum effective dose | [110] | |
| A. catechu | Seed | - | Methanol | FF | 0.7 mg/cotton ball, Minimum effective dose | [110] | |
| Knema hookeriana | Sap | - | Methanol | FF | 0.7 mg/cotton ball, Minimum effective dose | [110] | |
| Melia azedarach | Bark, fruit | - | Ethanol | DC | 100 mg/kg, 96% | [111] | |
| Paraderris elliptica | Root | - | Acetone | FF | Proliferation rate = 0 | [112] | |
| Nerium oleander | Leaf | - | Ethanol | DC | 1.2 mg/mL, 97.1% | [113] | |
| Tetraena mongolica | Stem, leaf | - | Aqueous | DC | LC50/24 h = 1.18 mg/mL | [114] | |
| Camellia sinensis | Seed | - | Ethanol | DC | LC50/60 h = 0.0119 mg/mL | [115] | |
| C. sinensis | - | - | Ethanol | DC | LC50/60 h = 0.5145 mg/mL | [116] | |
| M. azedarach | - | - | Ethanol | DC | LC50/60 h = 0.6100 mg/mL | [116] | |
| Pterocarya stenoptera | - | - | Ethanol | DC | LC50/60 h = 0.8064 mg/mL | [116] | |
| T. mongolica | - | - | Methanol | DC | 41.25 mg/mL, 92.36% (8 h) | [117] | |
| Yulania cylindrica | - | - | Aqueous | DC | LC50/24 h = 947.10 μL/L | [118] | |
| Torreya grandis | - | - | Aqueous | DC | LC50/24 h = 960.47 μL/L | [118] | |
| Helianthemum ordosicum | - | - | Aqueous | DC | LC50/5 d = 1.21 g/mL | [119] | |
| Ammopiptanthus mongolicus | Stem, leaf | - | Aqueous | DC | LC50/24 h = 0.56 g/L | [120] | |
| P. harmala | Stem, leaf | - | Aqueous | DC | LC50/24 h = 1.39 g/L | [120] | |
| Vincetoxicum mongolicum | - | - | Methanol | DC | 2 g/L, 100% (48 h) | [121] | |
| S. montana | Leaf | - | Aqueous | DC | LC100/24 h = 0.858 mg/mL | [122] | |
| T. capitata | Flower | - | Aqueous | DC | LC100/24 h = 0.985 mg/mL | [122] | |
| Thymus caespititius | Flower | - | Aqueous | DC | LC100/24 h, 0.874 mg/mL | [122] | |
| Tagetes erecta | Root | - | Ethanol | DC | LC50/72 h = 6.3 mg/L | [123] | |
| Camellia oleifera | Camellia cake | - | Aqueous | DC | 10 mg/mL, 100% (48 h) | [124] | |
| Tripterygium wilfordii | Root | - | Aqueous | DC | 10 mg/mL, 88.9% (48 h) | [124] | |
| R. graveolens, S. montana, T. capitata | - | - | Distilled water | DC | LC100/24 h < 0.4 µL/mL | [125] | |
| Foeniculum vulgare | - | - | Ethanol | DC | LC50/24 h = 16.5 mg/mL | [126] | |
| Chromolaena odorata | Aerial part | - | Ethanol | DC | LC50/72 h = 0.6892 g/L | [127] | |
| Ageratina adenophora | Aerial part | - | Ethanol | DC | LC50/72 h = 0.6813 g/L | [127] | |
| Mikania micrantha | Aerial part | - | Ethanol | DC | LC50/72 h = 0.7474 g/L | [127] | |
| Rheum palmatum | Root | - | Aqueous | DC | LC50/72 h = 0.067 g/L | [128] | |
| M. cordata | Leaf | - | Aqueous | DC | 20 mg/mL, 73.3% (72 h) | [129] | |
| Ginkgo biloba | Episperm | - | Aqueous | DC | 20 mg/mL, 84.9% (72 h) | [130] | |
| Zanthoxylum schinifolium | Peel | - | Aqueous | DC | LC50/4 h = 0.625 mg/mL | [131] | |
| Bidens pilosa | - | - | Aqueous | DC | 10 percent solution, 100% (7–8 d) | [132] | |
| B. pilosa | - | - | Aqueous | DC | 10 percent solution, 100% (7–8 d) | [132] | |
| Alternanthera philoxeroides | Whole plant | - | Ethyl acetate | DC | LC50/72 h = 0.5888 g/L | [133] | |
| Causonis japonica | Whole plant | - | Aqueous | DC | LC50/72 h = 0.9693 g/L | [133] | |
| Chamaecyparis pisifera | Stem, leaf | - | Aqueous | DC | LC50/72 h = 2.84 mg/mL | [134] | |
| Chamaecyparis obtusa | Stem, leaf | - | Aqueous | DC | LC50/72 h = 1.76 mg/mL | [134] | |
| Backhousia citriodora | Stem, leaf | - | Distilled water | DC | LC50/72 h = 85.56 μg/mL | [135] |
5. Influence Factors on the Nematicidal Activity of Phytochemicals
6. Research Progress of the Nematicidal Mechanism of Phytochemicals
7. Conclusions and Future Prospects
- (1)
- Comprehensive understanding of PC characteristics is the prerequisite for commercialization and production. Although PCs have drawn increasing interest on the control of PWD caused by the PWN, there are only a few commercial productions and pesticides. By 2023, over a thousand substances and derivatives were proved to exhibit nematicidal activity against the PWN, and more than a hundred have shown significant activity. However, only Sophora flavescens extract, which contains 1% matrine, has been registered as a pesticide for the PWN in China. Many studies assess nematicidal activity in the laboratory and only some simple characteristics are summarized, lacking in-depth research on actual outdoor effects, PC characteristics, and mechanisms of action. For example, some indoor active PCs are hydrophobic and easily degraded in the environment [81]. Therefore, despite ongoing research on the discovery of more plants and active substances, the in-depth comprehensive studies on some excellent ingredients would actually accelerate the procedure for commercializing and producing PC pesticides.
- (2)
- Improving the effectiveness of PCs against the PWN would be the continuous topics and difficulties. It is necessary to optimize the extraction process, which could provide more effective compounds with higher concentrations. The structure–activity relationship among substances is also an important reason for differences in nematicidal effectiveness. Utilizing organic chemistry to understand and modify the structure can potentially enhance the nematicidal effect of substances [18]. The development of nanotechnology in recent years has provided new approaches to improve the control effectiveness. For example, through efficient loading or encapsulation of active ingredients using nanocarriers, the water solubility, stability, and sustained release of substances can be enhanced [141]. Moreover, synergistic enhancement through the combination of PCs and chemical pesticides might be a simple, effective, and low-cost improvement method in the control of the PWN [142], which requires efforts to screen the suitable compounds, ratios, and additives.
- (3)
- Clarifying the potential PC mechanisms of action would facilitate their further application against the PWN. Most research is relatively limited as yet, mainly involving the effects on enzyme activities, differentially expressed genes, and detoxification regulatory pathways. Although some explorations provide a wealth of theoretical data, these results are insufficient to reveal the complex biological processes. To understand the mechanisms, it is necessary to focus on the target proteins, and the potential aspects on the interaction between active PCs with targets, including target identification and interaction, signal induction and conduction, gene expression and regulation, chemical functional group and activity, synergistic compound and effects, etc. With further development in molecular biology techniques and interdisciplinary intersection, i.e., cytotoxicology, bioinformatics, microimaging, synthetic biology, structural chemistry, nanomaterials science, artificial intelligence, and some others, it will be more efficient and more accurate to explore potential mechanisms.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mota, M.M.; Braasch, H.; Bravo, M.A.; Penas, A.C.; Burgermeister, W.; Metge, K.; Sousa, E. First report of Bursaphelenchus xylophilus in Portugal and in Europe. Nematology 1999, 1, 727–734. [Google Scholar] [CrossRef]
- Abelleira, A.; Picoaga, A.; Mansilla, J.P.; Aguin, O. Detection of Bursaphelenchus xylophilus, causal agent of pine wilt disease on Pinus pinaster in Northwestern Spain. Plant Dis. 2011, 95, 776. [Google Scholar] [CrossRef] [PubMed]
- An, H.; Lee, S.; Cho, S.J. The effects of climate change on pine wilt disease in South Korea: Challenges and prospects. Forests 2019, 10, 486. [Google Scholar] [CrossRef]
- Zhao, B.G.; Futai, K.; Sutherland, J.R.; Takeuchi, Y. Pine Wilt in Japan: From First Incidence to the Present. In Pine Wilt Disease; Springer: Tokyo, Japan, 2008. [Google Scholar]
- Futai, K. Pine wood nematode, Bursaphelenchus xylophilus. Annu. Rev. Phytopathol. 2013, 51, 61–83. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Zhou, J.; Koski, T.M.; Liu, S.; Zhao, L.; Sun, J. Hypoxia-induced tracheal elasticity in vector beetle facilitates the loading of pinewood nematode. eLife 2023, 12, e84621. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.R.; Wu, X.Q.; Sun, H. Pine Wilt Disease. In Forest Microbiology; Academic Press: Pittsburgh, PA, USA, 2023; pp. 169–181. [Google Scholar]
- Takai, K.; Soejima, T.; Suzuki, T.; Kawazu, K. Emamectin benzoate as a candidate for a trunk-injection agent against the pine wood nematode, Bursaphelenchus xylophilus. Pest Manag. Sci. 2000, 56, 937–941. [Google Scholar] [CrossRef]
- Takai, K.; Suzuki, T.; Kawazu, K. Development and preventative effect against pine wilt disease of a novel liquid formulation of emamectin benzoate. Pest Manag. Sci. 2003, 59, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.R.; Lee, S.C.; Lee, D.H. Identification of the aggregation-sex pheromone produced by male Monochamus saltuarius, a major insect vector of the pine wood nematode. J. Chem. Ecol. 2017, 43, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Suh, D.Y.; Jung, J.K.; Lee, S.K.; Seo, S.T. Effect of aerial spraying of thiacloprid on pine sawyer beetles (Monochamus alternatus) and honey bees (Apis mellifera) in pine forests. Entomol. Res. 2021, 51, 83–89. [Google Scholar] [CrossRef]
- Wang, F.; Wen, C.Y. Exploration of the effectiveness of multiple control measures for pine wilt disease caused by Bursaphelenchus xylophilus. J. Green Sci. Technol. 2020, 17, 189–190. [Google Scholar]
- Dong, H.Y. Characteristics and control methods research of pine wilt disease caused by Bursaphelenchus xylophilus in Pinus thunbergii. Agric. Henan 2024, 12, 46–48. [Google Scholar]
- Wang, H.; Qiu, H.J.; Xu, J.; Xiao, B.; Zhang, Y.; Zeng, J.P. Current situation and future prospects of thiacloprid aerial spraying for the control of Monochamus alternatus. World For. Res. 2019, 5, 34–40. [Google Scholar]
- Chitwood, D.J. Nematicides. In Encyclopedia of Agrochemicals; John Wiley & Sons: Hoboken, NJ, USA, 2003; pp. 473–474. [Google Scholar]
- Isman, M.B. Plant essential oils for pest and disease management. Crop Prot. 2000, 19, 603–608. [Google Scholar] [CrossRef]
- Teoh, E.S. Secondary Metabolites of Plants. In Medicinal Orchids of Asia; Springer: Berlin/Heidelberg, Germany, 2016; pp. 59–73. [Google Scholar]
- Faria, J.M.; Barbosa, P.; Vieira, P.; Vicente, C.S.; Figueiredo, A.C.; Mota, M. Phytochemicals as biopesticides against the pinewood nematode Bursaphelenchus xylophilus: A review on essential oils and their volatiles. Plants 2021, 10, 2614. [Google Scholar] [CrossRef] [PubMed]
- Catani, L.; Manachini, B.; Grassi, E.; Guidi, L.; Semprucci, F. Essential oils as nematicides in plant protection—A review. Plants 2023, 12, 1418. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Li, R.; Wang, C.; Yang, H.; Deng, W.; Du, G.; Guo, Q. Nematicidal phytochemicals against pine wood nematode, Bursaphelenchus xylophilus (nematoda: Aphelenchoididae). J. Plant Dis. Prot. 2023, 130, 215–223. [Google Scholar] [CrossRef]
- Kumar, A.; P, N.; Kumar, M.; Jose, A.; Tomer, V.; Oz, E.; Oz, F. Major phytochemicals: Recent advances in health benefits and extraction method. Molecules 2023, 28, 887. [Google Scholar] [CrossRef] [PubMed]
- Arnason, J.T.; Philogène, B.J.R.; Morand, P.; Imire, K.; Iyengar, S.; Duval, F.; Soucy-Breau, C.; Scaiano, J.C.; Werstiuk, N.H.; Hasspieler, B.; et al. Naturally Occurring and Synthetic Thiophenes as Photoactivated Insecticides. In Insecticides of Plant Origin; ACS Publications: Washington, DC, USA, 1989; pp. 164–172. [Google Scholar]
- Hedin, P.A.; Hollingworth, R.M. New Applications for Phytochemical Pest-control Agents. In Phytochemicals for Pest Control; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1997; pp. 1–12. [Google Scholar]
- Chitwood, D.J. Phytochemical based strategies for nematode control. Annu. Rev. Phytopathol. 2002, 40, 221–249. [Google Scholar] [CrossRef] [PubMed]
- Kiyohara, T.; Tokushige, Y. Inoculation experiments of a nematode, Bursaphelenchus sp., onto pine trees. J. Jpn. For. Soc. 1971, 53, 210–218. [Google Scholar]
- Peng, J.; He, L.G. Screening of Bursaphelenchus xylophilus feeding fungi. South. For. Sci. 2018, 46, 56–57+65. [Google Scholar]
- Jiang, T.F. Study on the Efficient Accumulation Mechanism of Jatrorrhizine in the Dedifferentiation Process of Mahonia fortunei Cells. Master’s Thesis, Zhejiang Sci-Tech University, Hangzhou, China, 2023. [Google Scholar]
- Kukula-Koch, W.A.; Widelski, J. Alkaloids. In Pharmacognosy; Academic Press: New York, NY, USA, 2017; pp. 163–198. [Google Scholar]
- Zhao, B.G. Study on alkaloids of Sophora alopecuroides. Acta Pharm. Sin. 1980, 15, 182–183. [Google Scholar]
- Wang, Z.X. Identification of lehmannine alkaloids in Sophora alopecuroides. Chin. Herb. Med. 1986, 17, 284. [Google Scholar]
- Zhang, W. Study on the alkaloid components and isolation of Sophora alopecuroides in Inner Mongolia. J. Inn. Mong. Univ. (Nat. Sci. Ed.) 1986, 25, 661–664. [Google Scholar]
- Wang, Z.X.; Zhang, S.S.; Fang, S.D.; Zhang, R.; Yu, H.G. Structure of Δ″-dehydrosophoridine. Acta Bot. Sin. 1991, 39, 727–728. [Google Scholar]
- Zhao, B.G. Nematicidal activity of aloperine against pine wood nematode. For. Sci. 1996, 32, 243–247. [Google Scholar]
- Zhao, B.G.; Wu, R.Q.; Li, X.P. Field trials of aloperine for the control of pine wilt disease. For. Sci. 1998, 34, 113–117. [Google Scholar]
- Zhao, B.G. Technical and ecological significance of aloperine in the control of pine wilt disease. In Jiangsu Province Plant Disease and Insect Society Communication 2, Proceedings of the Jiangsu Province Pine Wood Nematode Disease Seminar, Zhenjiang, China, 1 January 2003; The Entomological Society of Jiangsu: Nanjing, China; pp. 25–27.
- Zhao, B.G.; Zhao, Z.W.; Wang, H.G. Inhibitory effects of alkaloids of Sophora alopecuroids on pine wood nematode (Bursaphelenchus xylophilus). J. Nanjing For. Univ. (Nat. Sci. Ed.) 2006, 30, 129–131. [Google Scholar]
- Li, X.P.; Wu, R.Q.; Xia, M.Z.; Zhao, B.G. Relationship between molecular structure and nematicidal activity of two alkaloids, aloperine and Δ11-dehydroaloperine. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2000, 43, 78. [Google Scholar]
- Zhang, S.Y. Structure-Nematicidal Activity Relationship on Camptothecin Derivants. Master’s Thesis, Zhejiang A&F University, Hangzhou, China, 2011. [Google Scholar]
- Li, Z.B. Synthesis and Nematicidal Activity of Camptothecin Derivatives. Master’s Thesis, Zhejiang A&F University, Hangzhou, China, 2015. [Google Scholar]
- Lin, J.C. Construction and Characterization of Camptothecin Nano Drug Delivery System. Master’s Thesis, Zhejiang A&F University, Hangzhou, China, 2019. [Google Scholar]
- Sobhani, A.M.; Ebrahimi, S.A.; Mahmoudian, M. An in vitro evaluation of human DNA topoisomerase I inhibition by Peganum harmala L. seeds extract and its ß-carboline alkaloids. J. Pharm. Pharm. Sci. 2002, 5, 19–23. [Google Scholar]
- Di Giorgio, C.; Delmas, F.; Ollivier, E.; Elias, R.; Balansard, G.; Timon-David, P. In vitro activity of the beta-carboline alkaloids harmane, harmidine, and harmaline toward parasites of the species Leishmania infantum. Exp. Parasitol. 2004, 106, 67–74. [Google Scholar] [CrossRef]
- Weng, Q.F.; Zhong, G.H.; Hu, M.Y.; Luo, J.J.; Li, X.G. Bioactivities and physiological effects of extracts of Peganum harmala against Bursaphelenchus xylophilus. Sci. Agric. Sin. 2005, 38, 2014–2022. [Google Scholar]
- Chen, X.T. Studies on the Nematocidal Activities of Harmaline Derivatives. Ph.D. Thesis, South China Agricultural University, Guangzhou, China, 2016. [Google Scholar]
- Zhu, S.W. Research on Nematicidal Activity and Mechanism of Harmaline Derivatives. Ph.D. Thesis, South China Agricultural University, Guangzhou, China, 2016. [Google Scholar]
- Xia, Y.; Qi, Y.M.; Yu, X.H.; Wang, B.F.; Cao, R.H.; Jiang, D.X. Nematicidal effect against Bursaphelenchus xylophilus of harmine quaternary ammonium derivatives, inhibitory activity and molecular docking studies on acetylcholinesterase. Eur. J. Plant Pathol. 2019, 153, 239–250. [Google Scholar] [CrossRef]
- Rajasekharan, S.K.; Raorane, C.J.; Lee, J. Nematicidal effects of piperine on the pinewood nematode Bursaphelenchus xylophilus. J. Asia-Pac. Entomol. 2020, 23, 863–868. [Google Scholar] [CrossRef]
- Wen, Y.; Meyer, S.L.; Masler, E.P.; Zhang, F.; Liao, J.; Wei, X.; Chitwood, D.J. Nematotoxicity of drupacine and a cephalotaxus alkaloid preparation against the plant-parasitic nematodes Meloidogyne incognita and Bursaphelenchus xylophilus. Pest Manag. Sci. 2013, 69, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.Y.; Le Dang, Q.; Choi, G.J.; Park, H.W.; Kim, J.C. Control of root-knot nematodes using Waltheria indica producing 4-quinolone alkaloids. Pest Manag. Sci. 2019, 75, 2264–2270. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.N.; Wan, S.Q.; Liu, X.M.; Zhao, F. Active ingredients of seed extracts of Clausena lansium and nematocidal activity against Bursaphelenchus xylophilus. J. South China Agric. Univ. 2009, 30, 23–26. [Google Scholar]
- Liu, X.C. Bioactive Constituents Isolated from Orixa japonica and Toddalia asiatica. Ph.D. Thesis, China Agricultural University, Beijing, China, 2017. [Google Scholar]
- Liu, X.C.; Lai, D.; Liu, Q.Z.; Zhou, L.; Liu, Q.; Liu, Z.L. Bioactivities of a new pyrrolidine alkaloid from the root barks of Orixa japonica. Molecules 2016, 21, 1665. [Google Scholar] [CrossRef] [PubMed]
- Kou, M.Z.; Hui, Y. Design, synthesis and nematicidal activity of new derivatives of matrine. J. Lanzhou Univ. Arts Sci. (Nat. Sci. Ed.) 2023, 37, 83–88. [Google Scholar]
- Li, Y. Function Analysis of Matrine Resistance Genes in Foreign Matter Metabolism Pathway of Bursaphelenchus xylophilus. Master’s Thesis, Northeast Forestry University, Harbin, China, 2021. [Google Scholar]
- Hillier, S.G.; Lathe, R. Terpenes, hormones and life: Isoprene rule revisited. J. Endocrinol. 2019, 242, R9–R22. [Google Scholar] [CrossRef]
- Choi, I.; Kim, J.; Shin, S.; Park, I. Nematicidal activity of monoterpenoids against the pine wood nematode (Bursaphelenchus xylophilus). Russ. J. Nematol. 2007, 15, 35. [Google Scholar]
- Kong, J.O.; Park, I.K.; Choi, K.S.; Shin, S.C.; Ahn, Y.J. Nematicidal and propagation activities of thyme red and white oil compounds toward Bursaphelenchus xylophilus (Nematoda: Parasitaphelenchidae). J. Nematol. 2007, 39, 237. [Google Scholar]
- Park, I.K.; Kim, J.; Lee, S.G.; Shin, S.C. Nematicidal activity of plant essential oils and components from ajowan (Trachyspermum ammi), allspice (Pimenta dioica) and litsea (Litsea cubeba) essential oils against pine wood nematode (Bursaphelenchus xylophilus). J. Nematol. 2007, 39, 275. [Google Scholar] [PubMed]
- Li, G.H.; Hong, L.J.; Li, K.Q.; Zhang, K.Q. Nematicidal active component from Michelia Hedyosperma. Chin. J. Biol. Control 2007, 23, 260. [Google Scholar]
- Yu, J.Y.; Gao, Y.; Kong, Z.; Wang, Y. Nematicidal activity of three limonoids from Dictamnus dasycarps against Bursaphelenchus xylophilus. J. Zhejiang For. Sci. Technol. 2022, 42, 75–79. [Google Scholar]
- Shi, J.X.; Li, Z.X.; Nitoda, T.; Izumi, M.; Kanzaki, H.; Baba, N.; Nakajima, S. Antinematodal activities of ingenane diterpenes from Euphorbia kansui and their derivatives against the pine wood nematode (Bursaphelenchus xylophilus). Z. Naturforschung Sect. C-J. Biosci. 2008, 63, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.J.; Li, G.H.; Zhou, W.; Wang, X.B.; Zhang, K.Q. Screening and isolation of a nematicidal sesquiterpene from Magnolia grandiflora L. Pest Manag. Sci. 2007, 63, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Seo, S.M.; Lee, S.G.; Shin, S.C.; Park, I.K. Nematicidal activity of plant essential oils and components from coriander (Coriandrum sativum), oriental sweetgum (Liquidambar orientalis), and valerian (Valeriana wallichii) essential oils against pine wood nematode (Bursaphelenchus xylophilus). J. Agric. Food Chem. 2008, 56, 7316–7320. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.O.; Lee, S.M.; Moon, Y.S.; Lee, S.G.; Ahn, Y.J. Nematicidal activity of cassia and cinnamon oil compounds and related compounds toward Bursaphelenchus xylophilus (Nematoda: Parasitaphelenchidae). J. Nematol. 2007, 39, 31. [Google Scholar] [PubMed]
- Park, I.K.; Park, J.Y.; Kim, K.H.; Choi, K.S.; Choi, I.H.; Kim, C.S.; Shin, S.C. Nematicidal activity of plant essential oils and components from garlic (Allium sativum) and cinnamon (Cinnamomum verum) oils against the pine wood nematode (Bursaphelenchus xylophilus). Nematology 2005, 7, 767–774. [Google Scholar] [CrossRef]
- Zhang, T.Z.; Zeng, Y.; Zhu, F.W.; Hu, M.Y.; Weng, Q.F. Isolation and identification of nematicide component from Kaempferia galanga L. Acta Bot. Boreali-Occident. Sin. 2010, 12, 2524–2529. [Google Scholar]
- Kim, J.; Seo, S.M.; Park, I.K. Nematicidal activity of plant essential oils and components from Gaultheria fragrantissima and Zanthoxylum alatum against the pine wood nematode, Bursaphelenchus xylophilus. Nematology 2011, 13, 87–93. [Google Scholar]
- Choi, I.H.; Park, J.Y.; Shin, S.C.; Park, I.K. Nematicidal activity of medicinal plant extracts and two cinnamates isolated from Kaempferia galanga L. (Proh Hom) against the pine wood nematode, Bursaphelenchus xylophilus. Nematology 2006, 8, 359–365. [Google Scholar] [CrossRef]
- Wang, J.Y.; Pan, X.R.; Han, Y.; Guo, D.S.; Guo, Q.Q.; Li, R.G. Rosmarinic acid from eelgrass shows nematicidal and antibacterial activities against pine wood nematode and its carrying bacteria. Mar. Drugs 2012, 10, 2729–2740. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.K.; Joshi, H. Coumarin: Chemical and pharmacological profile. J. Appl. Pharm. Sci. 2012, 2, 236–240. [Google Scholar]
- Wang, X.B.; Li, G.H.; Li, L.; Zheng, L.J.; Huang, R.; Zhang, K.Q. Nematicidal coumarins from Heracleum candicans Wall. Nat. Prod. Res. 2008, 22, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Jin, H.; Liu, Q.; Yan, Z.; Ding, L.; Qin, B. Nematicidal metabolites from roots of Stellera chamaejasme against Bursaphelenchus xylophilus and Bursaphelenchus mucronatus. Pest Manag. Sci. 2014, 70, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Du, G.; He, H.; Xu, H.; Guo, D.; Li, R. Two nematicidal furocoumarins from Ficus carica L. leaves and their physiological effects on pine wood nematode (Bursaphelenchus xylophilus). Nat. Prod. Res. 2016, 30, 1969–1973. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Lai, D.; Liu, Q.Z.; Zhou, L.; Liu, Z.L. Identification of nematicidal constituents of Notopterygium incisum rhizomes against Bursaphelenchus xylophilus and Meloidogyne incognita. Molecules 2016, 21, 1276. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.Q. Isolation and Identification of Nematicidal Constituents from Plants and Their Nematicidal Mechanism against Bursaphelenchus xylophilus. Ph.D. Thesis, Qingdao University, Qingdao, China, 2016. [Google Scholar]
- Guo, Q.Q.; Du, G.C.; Li, Y.X.; Liang, C.Y.; Wang, C.; Zhang, Y.N.; Li, R.G. Nematotoxic coumarins from Angelica pubescens Maxim. f. biserrata Shan et Yuan roots and their physiological effects on Bursaphelenchus xylophilus. J. Nematol. 2018, 50, 559–568. [Google Scholar]
- Feng, J.; Qin, C.; Liu, X.; Li, R.; Wang, C.; Li, C.; Guo, Q. Nematicidal coumarins from Cnidium monnieri fruits and Angelica dahurica roots and their physiological effect on pine wood nematode (Bursaphelenchus xylophilus). Molecules 2023, 28, 4109. [Google Scholar] [CrossRef]
- Cushnie, T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Huang, X.D.; Xia, J.; Wang, E.W.; An, Y.X.; Xu, H.H. The effect of rotenone and six nemacide medicines on Bursaphelenchus xylophilus. Acta Agric. Univ. Jiangxiensis 2006, 28, 204–207. [Google Scholar]
- Xu, H.H.; Huang, J.G. Research progress on rotenone. J. Southwest Agric. Univ. 2001, 23, 140–143. [Google Scholar]
- Hu, L.; Xu, H.H.; Liang, M.L. The characterization of aqueous nanosuspension of rotenone and the bioactivity against Bursaphelenchus Xylophilus. Chin. J. Pestic. Sci. 2005, 7, 171–175. [Google Scholar]
- Hu, L.; Zhan, S.L.; Huang, X.D.; Xia, J.; Xu, H.H. The nematicidal effect of cyclodextrin inclusion complex of rotenone on Bursaphelenchus Xylophilus. J. East China Jiaotong Univ. 2006, 23, 164–168. [Google Scholar]
- Lee, Y.U.; Kawasaki, I.; Lim, Y.; Oh, W.S.; Paik, Y.K.; Shim, Y.H. Inhibition of developmental processes by flavone in Caenorhabditis elegans and its application to the pinewood nematode, Bursaphelenchus xylophilus. Mol. Cells 2008, 26, 171–174. [Google Scholar] [CrossRef]
- Goswami, C.; Pawase, P.A.; Shams, R.; Pandey, V.K.; Tripathi, A.; Rustagi, S.; Darshan, G. A conceptual review on classification, extraction, bioactive potential and role of phytochemicals in human health. Future Foods 2024, 9, 100313. [Google Scholar]
- Campos-Vega, R.; Oomah, B.D. Chemistry and classification of phytochemicals. In Handbook of Plant Food Phytochemicals; Wiley: Hoboken, NJ, USA, 2013; pp. 5–48. [Google Scholar]
- Guo, Q.Q.; Du, G.C.; Qi, H.T.; Zhang, Y.N.; Yue, T.Q.; Wang, J.C.; Li, R.G. A nematicidal tannin from Punica granatum L. rind and its physiological effect on pine wood nematode (Bursaphelenchus xylophilus). Pestic. Biochem. Physiol. 2017, 135, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Bartnik, M.; Facey, P. Glycosides. In Pharmacognosy; Academic Press: New York, NY, USA, 2024; pp. 103–165. [Google Scholar]
- Zhang, H.M.; Wang, G.L.; Bai, C.Q.; Liu, P.; Liu, Z.M.; Liu, Q.Z.; Deng, Z.W. A new eudesmane sesquiterpene glucoside from Liriope muscari fibrous roots. Molecules 2011, 16, 9017–9024. [Google Scholar] [CrossRef]
- Barbieri, R.; Coppo, E.; Marchese, A.; Daglia, M.; Sobarzo-Sánchez, E.; Nabavi, S.F.; Nabavi, S.M. Phytochemicals for human disease: An update on plant-derived compounds antibacterial activity. Microbiol. Res. 2017, 196, 44–68. [Google Scholar] [CrossRef]
- Yang, E.; Lee, J.W.; Chang, P.S.; Park, I.K. Development of chitosan-coated nanoemulsions of two sulfides present in onion (Allium cepa) essential oil and their nematicidal activities against the pine wood nematode, Bursaphelenchus xylophilus. Environ. Sci. Pollut. Res. 2021, 28, 69200–69209. [Google Scholar] [CrossRef] [PubMed]
- Han, S.S.; Tan, Z.Q.; Huang, X.D.; Qian, H.; Chen, B.; Zhao, B.T.; Chen, S.L.; Yan, S.Z. Preliminary study on the action mechanism of ally isothiocyanate against Bursaphelenchus xylophilus. Chin. J. Pestic. Sci. 2020, 22, 48–53. [Google Scholar]
- Seo, S.M.; Kim, J.; Koh, S.H.; Ahn, Y.J.; Park, I.K. Nematicidal activity of natural ester compounds and their analogues against pine wood nematode, Bursaphelenchus xylophilus. J. Agric. Food Chem. 2014, 62, 9103–9108. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xu, S.; Lu, H.; Zhang, D.; Liu, F.; Lin, J.; Mu, W. Effects of the plant volatile trans-2-hexenal on the dispersal ability, nutrient metabolism and enzymatic activities of Bursaphelenchus xylophilus. Pestic. Biochem. Physiol. 2017, 143, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.M.; Kim, J.; Kim, E.; Park, H.M.; Kim, Y.J.; Park, I.K. Structure-activity relationship of aliphatic compounds for nematicidal activity against pine wood nematode (Bursaphelenchus xylophilus). J. Agric. Food Chem. 2010, 58, 1823–1827. [Google Scholar] [CrossRef]
- Lee, J.W.; Nam, I.; Park, J.H.; Huh, M.J.; Park, I.K. Nematicidal activity of (Z)-ligustilide isolated from Angelica tenuissima Nakai root extract against the pine wood nematode Bursaphelenchus xylophilus. J. Asia-Pac. Entomol. 2022, 25, 101957. [Google Scholar] [CrossRef]
- Zhang, T.Z.; Zeng, Y.; Li, H.; Chen, G.J.; Su, J.S.; Weng, Q.F. Photoactivated toxicity of extracts from 14 compositae plants against Bursaphelenchus xylophilus and Culex pipiens pallen. Acta Bot. Boreali-Occident. Sin. 2010, 4, 821–826. [Google Scholar]
- Choi, I.H.; Park, J.Y.; Shin, S.C.; Kim, J.; Park, I.K. Nematicidal activity of medicinal plant essential oils against the pinewood nematode (Bursaphelenchus xylophilus). Appl. Entomol. Zool. 2007, 42, 397–401. [Google Scholar] [CrossRef]
- Faria, J.M.; Barbosa, P.; Bennett, R.N.; Mota, M.; Figueiredo, A.C. Bioactivity against Bursaphelenchus xylophilus: Nematotoxics from essential oils, essential oils fractions and decoction waters. Phytochemistry 2013, 94, 220–228. [Google Scholar] [CrossRef]
- Matsuda, K.; Kimura, M.; Komai, K.; Hamada, M. Nematicidal activities of (–)-N-methylcytisine and (-)-anagyrine from Sophora flavescens against PWN. Agric. Biol. Chem. 1989, 53, 2287–2288. [Google Scholar]
- Matsuda, K.; Yamada, K.; Kimura, M.; Hamada, M. Nematicidal activity of matrine and its derivatives against PWN. J. Agric. Food Chem. 1991, 39, 189–191. [Google Scholar] [CrossRef]
- Wang, K.; Luo, C.; Liu, H.; Xu, J.M.; Sun, W.B.; Zhou, L.G. Nematicidal activity of the alkaloids from Macleaya cordata against certain nematodes. Afr. J. Agric. Res. 2012, 7, 5925–5929. [Google Scholar]
- Wang, W.X.; Wang, K.; Xu, J.M.; Zhou, L.G. Antinematodal activity of the alkaloids from Pinellia ternata and Sophora alopecuroides. Nat. Prod. Res. Dev. 2016, 28, 719. [Google Scholar]
- Cha, D.J.; Kim, J.; Kim, D.S. Nematicidal activities of three naphthoquinones against the pine wood nematode, Bursaphelenchus xylophilus. Molecules 2019, 24, 3634. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.H.; Shin, S.C.; Park, I.K. Nematicidal activity of onion (Allium cepa) oil and its components against the pine wood nematode (Bursaphelenchus xylophilus). Nematology 2007, 9, 231–235. [Google Scholar] [CrossRef]
- Shin, J.H.; Kwon, O.; Lee, C.M.; Lee, S.M.; Choi, Y.H.; Kim, J.H.; Lee, D.W. Nematicidal activity of Eclipta prostrata extract and terthiophene against pine wood nematode, Bursaphelenchus xylophilus. Korean J. Pestic. Sci. 2016, 20, 56–65. [Google Scholar] [CrossRef]
- Seo, S.M.; Lee, H.R.; Lee, J.E.; Jeong, Y.C.; Kwon, H.W.; Moon, J.K.; Park, I.K. Larvicidal and nematicidal activities of 3-acylbarbituric acid analogues against Asian tiger mosquito, Aedes albopictus, and pine wood nematode, Bursaphelenchus xylophilus. Molecules 2017, 22, 1196. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.E.; Sun, D.; Yang, J.M.; Che, Z.P.; Liu, S.M.; Lin, X.M.; Chen, G.Q. Synthesis of sulfonate derivatives of maltol and their biological activity against Phytophthora capsici and Bursaphelenchus xylophilus in vitro. J. Asian Nat. Prod. Res. 2020, 22, 578–587. [Google Scholar] [CrossRef] [PubMed]
- Kawazu, K.; Nishii, Y.; Ishii, K.; Tada, M. A convenient screening method for nematicidal activity. Agric. Biol. Chem. 1980, 44, 631–635. [Google Scholar]
- Mackeen, M.M.; Ali, A.M.; Abdullah, M.A.; Nasir, R.M.; Mat, N.B.; Razak, A.R.; Kawazu, K. Antinematodal activity of some Malaysian plant extracts against the pine wood nematode, Bursaphelenchus xylophilus. Pestic. Sci. 1997, 51, 165–170. [Google Scholar] [CrossRef]
- Alen, Y.; Nakajima, S.; Nitoda, T.; Baba, N.; Kanzaki, H.; Kawazu, K. Antinematodal activity of some tropical rainforest plants against the pine wood nematode, Bursaphelenchus xylophilus. Z. Naturforschung Sect. C-J. Biosci. 2000, 55, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.; Bi, Q.; Zhao, B. Lethal activity of Melia azedarach extract against pine wood nematode. J. For. Eng. 2002, 16, 26–27. [Google Scholar]
- Wen, Y.; Feng, Z.; Xu, H.; Chen, L. Nematicidal activity screening of plant extracts against several plant pathogenic nematodes. J. Huazhong Agric. Univ. 2001, 20, 235–238. [Google Scholar]
- Wang, M.; Cao, F.; Teng, T. Study on fatality of extract of Nerium indicum against Bursaphelenchus xyophilus. Hunan For. Sci. Technol. 2006, 33, 7–8. [Google Scholar]
- Gao, W.F. Study on the Insecticidal and Antifungal Activities of Tetraena mongolica Endemic to China. Master’s Thesis, Tianjin Normal University, Tianjin, China, 2007. [Google Scholar]
- Wu, H.P.; Xu, X.L.; Wang, J. In vitro nematicidal activity of ethanol plant extrocts of tea seed against pine-wood nematode. Plant Quar. 2007, 21, 335–337. [Google Scholar]
- Xu, X.L. Nematicidal Activity of Crude Extraction from Tea Seed. Master’s Thesis, Anhui Agricultural University, Hefei, China, 2007. [Google Scholar]
- Liu, B.; Gao, W.F.; Liu, Q. Separation and bio-active identification of the nematicidal substances from Tetraena mongolica extract. J. Tianjin Norm. Univ. Nat. Sci. Ed. 2008, 28, 12–13. [Google Scholar]
- Shi, D.H. Study on Essential Oils of Several Woody Plants Against Plant Pathogens. Master’s Thesis, Zhejiang A&F University, Hangzhou, China, 2008. [Google Scholar]
- Tian, L.; Liu, Q. Nematicidal activity of extracts of Helianthemum ordosicum against Bursaphelenchus xylophilus. Guihaia 2008, 28, 851–855. [Google Scholar]
- Gao, W.F.; Zhu, G.; Liu, Q. On the nematocidal activity of Ammopiptanthus mongolicus and Peganum harmala L against Bursaphelenchus xylophilus. J. Tianjin Norm. Univ. Nat. Sci. Ed. 2009, 29, 55–57. [Google Scholar]
- Yang, J.; Li, J.; Liu, Q. Bioassay of methanol extracts from Cynanchum komarovii AL. Iljinski on Bursaphelenchus xylophilus. Nat. Prod. Res. Dev. 2009, 5, 840–843. [Google Scholar]
- Barbosa, P.; Lima, A.S.; Vieira, P.; Dias, L.S.; Tinoco, M.T.; Barroso, J.G.; Pedro, L.G.; Figueiredo, A.C.; Mota, M. Nematicidal activity of essential oils and volatiles derived from Portuguese aromatic flora against the pinewood nematode, Bursaphelenchus xylophilus. J. Nematol. 2010, 42, 8. [Google Scholar]
- Ju, Y.W.; Fan, P.F.; Xi, Y.M.; Xue, Z.G. Marigold (Tagetes erecta) extracts to kill Bursaphelenchus xylophilus. J. Zhejiang A&F Univ. 2010, 27, 316–319. [Google Scholar]
- Long, J.X.; Cao, F.X.; Peng, J.Q. Determination of the effects of plant extracts on the activity of Bursaphelenchus xylophilus. North. Hortic. 2010, 22, 148–150. [Google Scholar]
- Faria, J.M.S.; Barbosa, P.; Mota, M.; Figueiredo, A.C. Nematoxic effect of essential oils and their fractions against the pinewood nematode, Bursaphelenchus xylophilus. In Proceedings of the 43rd International Symposium on Essential Oils, Lisbon, Portugal, 5–8 September 2012; p. 72. [Google Scholar]
- Guo, Y.; Qin, L.; Qiao, C.X.; Guo, D.S.; Zhao, A.Y.; Zhao, B.G.; Yue, T.Q.; Li, R.G. Inhibition effects of fennel extract (Foeniculum vulgare Mill) on pine wood nematode and the bacteria it carries. Afr. J. Microbiol. Res. 2012, 6, 1837–1843. [Google Scholar]
- Li, Z.G. Study on the nematicidal effect of extracts from three Asteraceae plants on pine wood nematodes. Hebei For. Sci. Technol. 2012, 2, 1–3. [Google Scholar]
- Jiao, H.; Zhang, L.; Zhao, B.; Liu, D. Toxicity of thirty-six Chinese medicinal herbs against Bursaphelenchus xylophilus. Chin. J. Biol. Control 2014, 30, 239. [Google Scholar]
- Qin, D.; Du, Z.W.; Yan, J.X.; Wu, P. Toxic effect of extracts from leaves of Fig and Macleaya cordata against Bursaphelenchus xylophilus. J. Anhui Agric. Sci. 2014, 42, 7412–7413. [Google Scholar]
- Qin, D.; Fu, Y.K.; Du, Z.W. Toxic action of extracts from leaf and testa of Ginkgo biolaba against Bursaphelenchus xylophilus. J. Anhui Agric. Sci. 2014, 42, 1697–1698. [Google Scholar]
- Shu, C.J.; Zhang, M.L.; Zhao, K.F.; Zhang, F.L.; Zhang, W.M. Prevention activity of Zanthoxylum schinifolium aqueous extract and essential oil against Bursaphelenchus xylophilus. Chin. Wild Plant Resour. 2019, 38, 11–15. [Google Scholar]
- Taba, S.; Ashikaga, K.; Oohama, T.; Ajitomi, A.; Kiyuna, C.; Kinjo, M.; Sekine, K.T. Bidens pilosa extract effects on pine wilt: Causal agents and their natural enemies. For. Sci. 2020, 66, 284–290. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, F. Nematocidal activity of extracts of Alternanthera philoxeroides and Cayratia japonica against Bursaphelenchus xylophilus. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2022, 46, 45. [Google Scholar]
- Zhang, H.; Xu, D.; Lin, X.; Chen, L.J.; Liu, H.K. Nematicidal activity of essential oil from four species of Cupressaceae against Bursaphelenchus xylophilus. J. Zhejiang For. Sci. Technol. 2022, 42, 80–83. [Google Scholar]
- Huang, S.R.; Yang, Y.T.; Cai, T.; Li, J.H.; Ye, C.Y.; Shan, T.J. Chemical composition, nematocidal and antibacterial activities of volatile oil from Backhousia citriodora. J. Zhongkai Univ. Agric. Eng. 2023, 36, 52–58. [Google Scholar]
- Cavaco, T.; Gonçalves, D.; Pombo, A.; Moiteiro, C.; Inácio, M.L.; Faria, J.M. Nematicidal activity of oxygen-containing aliphatic compounds on Bursaphelenchus xylophilus, B. mucronatus and B. fraudulentus. Chem. Proc. 2022, 12, 55. [Google Scholar] [CrossRef]
- Nunes, I.S.; Faria, J.M.S.; Figueiredo, A.C.; Pedro, L.G.; Trindade, H.; Barroso, J.G. Menthol and geraniol biotransformation and glycosylation capacity of Levisticum officinale hairy roots. Planta Medica 2009, 75, 387–391. [Google Scholar] [CrossRef]
- Kang, J.S.; Moon, Y.S.; Lee, S.H.; Park, I.K. Inhibition of acetylcholinesterase and glutathione S-transferase of the pinewood nematode (Bursaphelenchus xylophilus) by aliphatic compounds. Pestic. Biochem. Physiol. 2013, 105, 184–188. [Google Scholar] [CrossRef]
- López-Villamor, A.; Nunes da Silva, M.; Vasconcelos, M.W. Evaluation of plant elicitation with methyl-jasmonate, salicylic acid and benzo (1, 2, 3)-thiadiazole-7-carbothioic acid-S-methyl ester for the sustainable management of the pine wilt disease. Tree Physiol. 2022, 42, 2596–2613. [Google Scholar] [CrossRef] [PubMed]
- Deresa, E.M.; Diriba, T.F. Phytochemicals as alternative fungicides for controlling plant diseases: A comprehensive review of their efficacy, commercial representatives, advantages, challenges for adoption, and possible solutions. Heliyon 2023, 9, e13810. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Wu, H.; Zhang, Y.; Kang, J.; Dong, A.; Liang, W. Advances in stimuli-responsive systems for pesticides delivery: Recent efforts and future outlook. J. Control. Release 2022, 352, 288–312. [Google Scholar] [CrossRef]
- Lee, J.W.; Mwamula, A.O.; Choi, J.H.; Lee, H.W.; Kim, Y.S.; Kim, J.H.; Lee, D.W. The potency of abamectin formulations against the pine wood nematode, Bursaphelenchus xylophilus. Plant Pathol. J. 2023, 39, 290. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Si, G.; Chen, L.; Hu, L.; Cui, G.; Wang, M.; Zhao, D. Current Status and Prospects of Pine Wilt Disease Management with Phytochemicals—A Review. Plants 2024, 13, 2129. https://doi.org/10.3390/plants13152129
Zhang Q, Si G, Chen L, Hu L, Cui G, Wang M, Zhao D. Current Status and Prospects of Pine Wilt Disease Management with Phytochemicals—A Review. Plants. 2024; 13(15):2129. https://doi.org/10.3390/plants13152129
Chicago/Turabian StyleZhang, Quanhong, Guiling Si, Liusheng Chen, Lili Hu, Gaofeng Cui, Min Wang, and Danyang Zhao. 2024. "Current Status and Prospects of Pine Wilt Disease Management with Phytochemicals—A Review" Plants 13, no. 15: 2129. https://doi.org/10.3390/plants13152129
APA StyleZhang, Q., Si, G., Chen, L., Hu, L., Cui, G., Wang, M., & Zhao, D. (2024). Current Status and Prospects of Pine Wilt Disease Management with Phytochemicals—A Review. Plants, 13(15), 2129. https://doi.org/10.3390/plants13152129






