Variability in Maize Seed Bacterization and Survival Correlating with Root Colonization by Pseudomonas Isolates with Plant-Probiotic Traits
Abstract
:1. Introduction
2. Results
2.1. Chromosomal Tagging of Pseudomonas Isolates for Facilitating Enumeration in Bacterized Seeds and Visualization in Colonized Roots under Axenic Conditions
2.2. Seed Bacterization Levels Were Strain-Dependent
2.3. Pre-Hydration of Maize Seeds Improved Recovery of Culturable Bacterial Cells
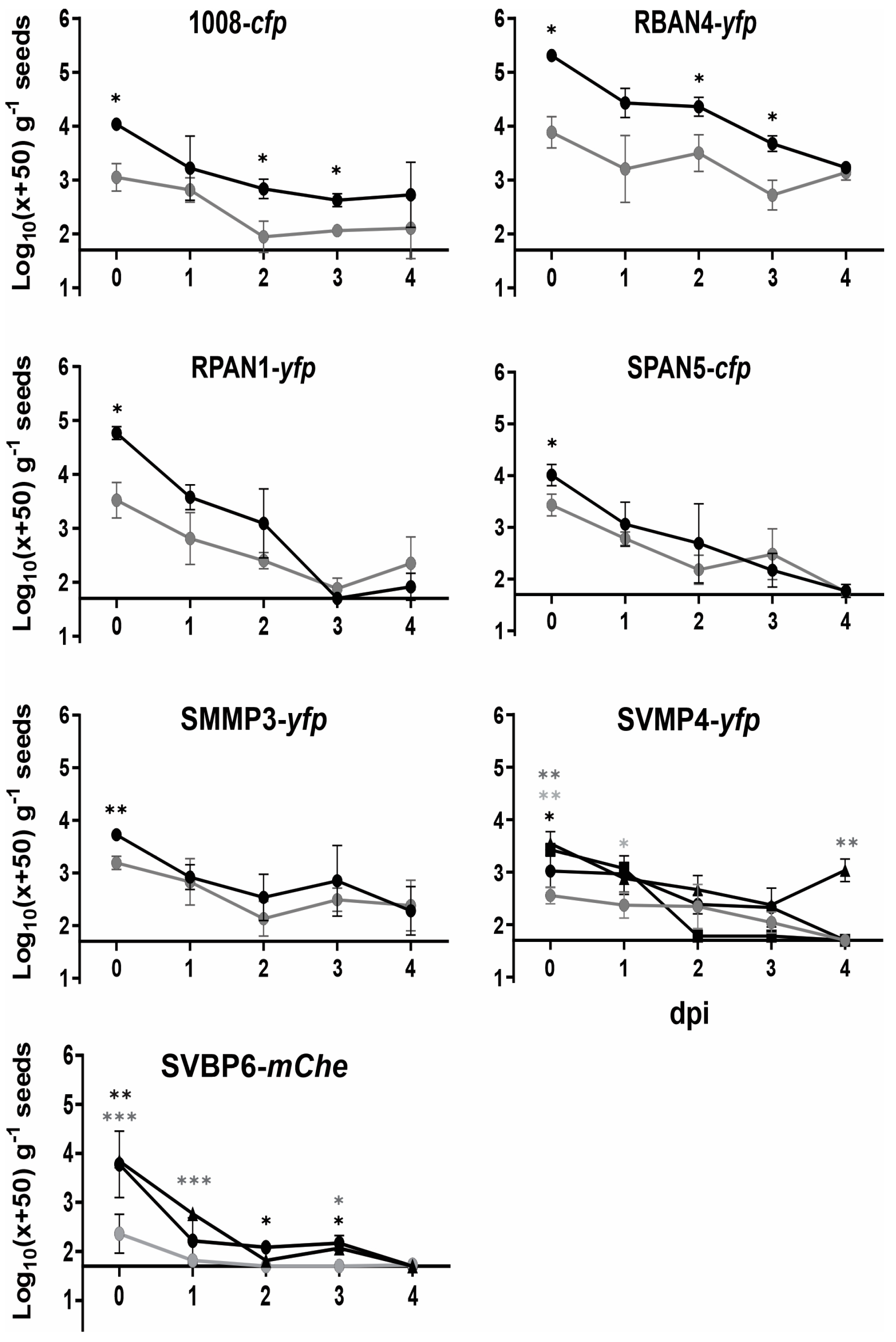
2.4. Bacterization Levels of Maize Seeds Strongly Declined up to 4 dpi
2.5. Colonization Patterns on Maize Seedlings Were Particular and Congruent with Bacterization Levels
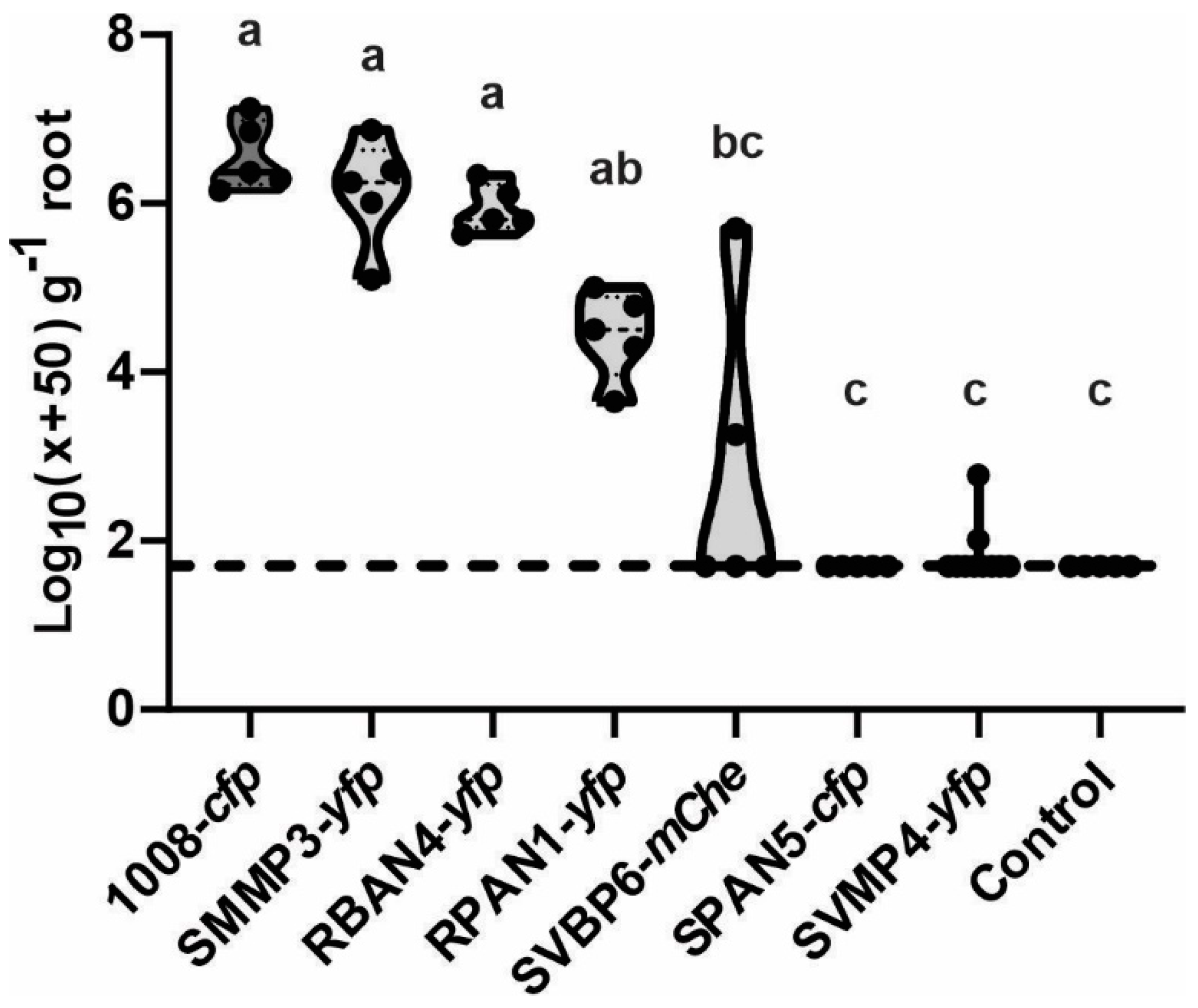
2.6. Maize Root Competitiveness Is Consistent with Seed Bacterization Levels, although Growth Promotion Effects Were Also Detected in Less Competitive Isolates
3. Discussion
3.1. Variability in Maize Seed Bacterization among PGPMs from the Same Bacterial Genus
3.2. Enhancement of Seed Bacterization with a Commercial Bacterial Protectant Additive Was Also Strain-Dependent
3.3. The Incorporation of Trehalose and PVP Ameliorates Seed Bacterization for Isolates Experiencing Higher Dehydration Stress Upon Seed Inoculation
3.4. The Beneficial Effects of Plant-Growth Promoting Microorganisms Might Not Solely Stem from Root Colonization and Competitiveness
4. Materials and Methods
4.1. Bacterial Strains, Growth Condition and Plant Species
4.2. Chromosomal Tagging of Pseudomonas Isolates
| Pseudomonas Isolates (Wild Type) | |||
| Name | Taxonomical Affiliation a | Origin | Reference |
| RBAN4 | P. protegens b | Pasture rhizosphere, Natural Environment, Bengolea, Córdoba, Argentina | [49] |
| SMMP3 | P. chlororaphis subsp. aurantiaca | Bulk soil from soybean plot, Monte Buey, Córdoba, Argentina | [49] |
| SPAN5 | P. chlororaphis | Bulk soil from pasture plot, Pergamino, Buenos Aires, Argentina | [49,97] |
| SVMP4 | P. soli | Bulk soil from pasture plot, Viale, Entre Ríos, Argentina | [49,98] |
| SVBP6 | P. donghuensis | Bulk soil from soybean plot, Viale, Entre Ríos, Argentina | [49,50] |
| RPAN1 | P. chlororaphis subsp. piscium | Pasture rhizosphere, Natural Environment, Pergamino, Buenos Aires, Argentina | [49] |
| 1008 | P. pergaminensis | Wheat rhizosphere from a productive plot, Pergamino, Buenos Aires, Argentina | [58] |
| Pseudomonas derivative strains | |||
| Name | Relevant genetic and/or phenotypic features | Reference | |
| RBAN4-yfp | RBAN4::attTn7-eyfp; Kmr Smr | This study | |
| SMMP3-yfp | SMMP3::attTn7-eyfp; Kmr Smr | This study | |
| SPAN5-cfp | SPAN5::attTn7-ecfp; Kmr Smr | This study | |
| SVMP4-yfp | SVMP4::attTn7-eyfp; Kmr Smr | This study | |
| SVBP6-mChe | SVBP6::attTn7-mCherry; Gmr | This study | |
| RPAN1-yfp | RPAN1::attTn7-eyfp; Gmr | This study | |
| 1008-cfp | 1008::attTn7-ecfp; Kmr Smr | This study | |
| Escherichia coli strains | |||
| Strains | Relevant genetic and/or phenotypic features | Reference | |
| SM10 | thi-1, thr, leu, tonA, lacY, supE, recA::RP4-2-Tc::Mu, λpir. Kmr | [99] | |
| HB101 | Laboratory strain. K12 derivative. F− Pro− Gal− Rec− Smr | [100] | |
| DH5α | Laboratory strain. K12 derivative. F− recA1− endA1− lacZΔM15 | [101] | |
| MT102 | Laboratory strain. araD139 D(ara-leu)7697 Δlac thi hsdR derivate of E. coli K-12 substrain MC1000. Smr Rifr Azider | [102] | |
| Plasmids | |||
| pUX-BF13 | Helper plasmid for Tn7-based transposon mutagenesis containing the transposition functions; R6K-replicon; Apr | [103] | |
| pME497 | Mobilizing plasmid; IncP-1, Tra; RepA(Ts); Apr | [104] | |
| pME9407 | Delivery plasmid for mini-Tn7-mCherry; pME3280a carrying mCherry placed under Plac control; Apr Gmr | [94] | |
| miniTn7(Km, Sm)PA1/04/03–ecfp-a | Delivery plasmid for mini-Tn7-ecfp; pUC19 derivative carrying ecfp under a constitutive promoter (Plac derivative). Not replicative in Pseudomonas. Apr Kmr Smr | [53] | |
| miniTn7(Km, Sm)PA1/04/03–eyfp-a | Delivery plasmid for mini-Tn7-eyfp; pUC19 derivative carrying eyfp under a constitutive promoter (Plac derivative). Not replicative in Pseudomonas. Apr Kmr Smr | [53] | |
4.3. Seed Inoculation and Recuperation of Bacteria from Seeds
4.4. Bacterization of Pre-Hydrated Seeds
4.5. Visualization of Bacterial Root-Colonization Patterns by Confocal Laser Scanning Microscopy (CLSM)
4.6. Root Competitiveness and Plant-Growth Promotion of Maize Grown from Bacterized Seeds
4.7. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Venturi, V.; Keel, C. Signaling in the Rhizosphere. Trends Plant Sci. 2016, 21, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Olanrewaju, O.S.; Ayangbenro, A.S.; Glick, B.R.; Babalola, O.O. Plant Health: Feedback Effect of Root Exudates-Rhizobiome Interactions. Appl. Microbiol. Biotechnol. 2019, 103, 1155–1166. [Google Scholar] [CrossRef] [PubMed]
- Johnston-Monje, D.; Gutiérrez, J.P.; Lopez-Lavalle, L. Seed-Transmitted Bacteria and Fungi Dominate Juvenile Plant Microbiomes. Front. Microbiol. 2021, 12, 737616. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Adeleke, B.S.; Shi, Y.; Li, C. The Seed Microbiomes of Staple Food Crops. Microb. Biotechnol. 2023, 16, 2236–2249. [Google Scholar] [CrossRef] [PubMed]
- Wassermann, B.; Adam, E.; Cernava, T.; Berg, G. Understanding the Indigenous Seed Microbiota to Design Bacterial Seed Treatments. In Seed Endophytes; Springer International Publishing: Cham, Switzerland, 2019; pp. 83–99. [Google Scholar]
- Wallace, J.G. Maize Seed Endophytes. Mol. Plant Pathol. 2023, 24, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Özkurt, E.; Hassani, M.A.; Sesiz, U.; Künzel, S.; Dagan, T.; Özkan, H.; Stukenbrock, E.H. Seed-Derived Microbial Colonization of Wild Emmer and Domesticated Bread Wheat (Triticum dicoccoides and T. aestivum) Seedlings Shows Pronounced Differences in Overall Diversity and Composition. mBio 2020, 11, 10–128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, C.; Yang, J.; Zhang, R.; Gao, J.; Zhao, X.; Zhao, J.; Zhao, D.; Zhang, X. Insights into Endophytic Bacterial Community Structures of Seeds Among Various Oryza sativa L. Rice Genotypes. J. Plant Growth Regul. 2019, 38, 93–102. [Google Scholar] [CrossRef]
- Morales Moreira, Z.P.; Helgason, B.L.; Germida, J.J. Crop, Genotype, and Field Environmental Conditions Shape Bacterial and Fungal Seed Epiphytic Microbiomes. Can. J. Microbiol. 2021, 67, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Tkalec, V.; Mahnic, A.; Gselman, P.; Rupnik, M. Analysis of Seed-Associated Bacteria and Fungi on Staple Crops Using the Cultivation and Metagenomic Approaches. Folia Microbiol. 2022, 67, 351–361. [Google Scholar] [CrossRef]
- Catroux, G.; Hartmann, A.; Revellin, C. Trends in Rhizobial Inoculant Production and Use. Plant Soil 2001, 230, 21–30. [Google Scholar] [CrossRef]
- O’Callaghan, M. Microbial Inoculation of Seed for Improved Crop Performance: Issues and Opportunities. Appl. Microbiol. Biotechnol. 2016, 100, 5729–5746. [Google Scholar] [CrossRef] [PubMed]
- Idígoras, G. Mejoramiento de Cultivos y Producción de Semillas; Buenos Aires. 2013. Available online: https://www.argentina.gob.ar/sites/default/files/mejoramiento_cultivos_y_produccion_semillas-doc.pdf (accessed on 20 June 2024).
- Lamichhane, J.R.; Corrales, D.C.; Soltani, E. Biological Seed Treatments Promote Crop Establishment and Yield: A Global Meta-Analysis. Agron. Sustain. Dev. 2022, 42, 45. [Google Scholar] [CrossRef]
- Ali, M.A.; Ilyas, F.; Arshad, M.; Hussain, S.; Iqbal, M.; Ahmad, S.; Saboor, A.; Mustafa, G.; Ahmed, N. Microbial Inoculation of Seeds for Better Plant Growth and Productivity. In Priming and Pretreatment of Seeds and Seedlings; Springer Singapore: Singapore, 2019; pp. 523–550. [Google Scholar]
- Global Market Analysis, Foreign Agricultural Service. World Agricultural Production; Foreign Agricultural Service: Washington, DC, USA, 2024. [Google Scholar]
- Kumar, B.; Dube, H. Seed Bacterization with a Fluorescent Pseudomonas for Enhanced Plant Growth, Yield and Disease Control. Soil Biol. Biochem. 1992, 24, 539–542. [Google Scholar] [CrossRef]
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; van der Putten, W.H. Going Back to the Roots: The Microbial Ecology of the Rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, S.; Sathya, A.; Vijayabharathi, R.; Varshney, R.K.; Gowda, C.L.L.; Krishnamurthy, L. Plant Growth Promoting Rhizobia: Challenges and Opportunities. Biotech 2014, 5, 355–377. [Google Scholar] [CrossRef] [PubMed]
- Deaker, R.; Roughley, R.; Kennedy, I. Legume Seed Inoculation Technology—A Review. Soil Biol. Biochem. 2004, 36, 1275–1288. [Google Scholar] [CrossRef]
- Silva Araujo, R.; Purin da Cruz, S.; Souchie, E.; Martin, T.; Shigueyoshi Nakatani, A.; Nogueira, M.; Hungria, M. Preinoculation of Soybean Seeds Treated with Agrichemicals up to 30 Days before Sowing: Technological Innovation for Large-Scale Agriculture. Int. J. Microbiol. 2017, 2017, 5914786. [Google Scholar] [CrossRef]
- Roughley, R.J.; Gemell, L.G.; Thompson, J.A.; Brockwell, J. The Number of Bradyrhizobium sp. (Lupinus) Applied to Seed and Its Effect on Rhizosphere Colonization, Nodulation and Yield of Lupin. Soil Biol. Biochem. 1993, 25, 1453–1458. [Google Scholar] [CrossRef]
- Hume, D.J.; Blair, D.H. Effect of Numbers of Bradyrhizobium japonicum Applied in Commercial Inoculants on Soybean Seed Yield in Ontario. Can. J. Microbiol. 1992, 38, 588–593. [Google Scholar] [CrossRef]
- Elegba, M.; Rennie, R. Effect of Different Inoculant Adhesive Agents on Rhizobial Survival, Nodulation, and Nitrogenase (Acetylene-Reducing) Activity of Soybeans (Glycine max (L.) Merrill). Can. J. Soil Sci. 1984, 64, 631–636. [Google Scholar] [CrossRef]
- Mahmood, A.; Turgay, O.; Farooq, M.; Hayat, R. Seed Biopriming with Plant Growth Promoting Rhizobacteria: A Review. FEMS Microbiol. Ecol. 2016, 98, fiw112. [Google Scholar] [CrossRef] [PubMed]
- Temprano, F.J.; Albareda, M.; Camacho, M.; Daza, A.; Santamaría, C.; Rodríguez-Navarro, D.N.; Rodríguez-Navarro, N.D.; Rodríguez-Navarro, D.N. Survival of Several Rhizobium/Bradyrhizobium Strains on Different Inoculant Formulations and Inoculated Seeds. Int. Microbiol. 2002, 5, 81–86. [Google Scholar] [CrossRef]
- Herrmann, L.; Lesueur, D. Challenges of Formulation and Quality of Biofertilizers for Successful Inoculation. Appl. Microbiol. Biotechnol. 2013, 97, 8859–8873. [Google Scholar] [CrossRef]
- Streeter, J.G. Effect of Trehalose on Survival of Bradyrhizobium japonicum during Desiccation. J. Appl. Microbiol. 2003, 95, 484–491. [Google Scholar] [CrossRef]
- Ruhal, R.; Kataria, R.; Choudhury, B. Trends in Bacterial Trehalose Metabolism and Significant Nodes of Metabolic Pathway in the Direction of Trehalose Accumulation. Microb. Biotechnol. 2013, 6, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, J.K.; Bhat, R. Why Is Trehalose an Exceptional Protein Stabilizer? J. Biol. Chem. 2003, 278, 26458–26465. [Google Scholar] [CrossRef]
- Craig, K.; Johnson, B.R.; Grunden, A. Leveraging Pseudomonas Stress Response Mechanisms for Industrial Applications. Front. Microbiol. 2021, 12, 660134. [Google Scholar] [CrossRef]
- Hengge-Aronis, R.; Klein, W.; Lange, R.; Rimmele, M.; Boos, W. Trehalose Synthesis Genes Are Controlled by the Putative Sigma Factor Encoded by RpoS and Are Involved in Stationary-Phase Thermotolerance in Escherichia coli. J. Bacteriol. 1991, 173, 7918–7924. [Google Scholar] [CrossRef] [PubMed]
- De Virgilio, C.; Hottiger, T.; Dominguez, J.; Boller, T.; Wiemken, A. The Role of Trehalose Synthesis for the Acquisition of Thermotolerance in Yeast. Eur. J. Biochem. 1994, 219, 179–186. [Google Scholar] [CrossRef]
- Reina-Bueno, M.; Argandoña, M.; Nieto, J.J.; Hidalgo-García, A.; Iglesias-Guerra, F.; Delgado, M.J.; Vargas, C. Role of Trehalose in Heat and Desiccation Tolerance in the Soil Bacterium Rhizobium etli. BMC Microbiol. 2012, 12, 207. [Google Scholar] [CrossRef]
- Singleton, P.; Keyser, H.; Sande, E. Development and Evaluation of Liquid Inoculants. In Proceedings of the Inoculants and Nitrogen Fixation of Legumes in Vietnam; Herridge, D., Ed.; ACIAR: Canberra, Australian, 2002; pp. 52–66. [Google Scholar]
- Kuppardt, A.; Chatzinotas, A.; Breuer, U.; van der Meer, J.R.; Harms, H. Optimization of Preservation Conditions of As (III) Bioreporter Bacteria. Appl. Microbiol. Biotechnol. 2009, 82, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Manzanera, M.; García de Castro, A.; Tøndervik, A.; Rayner-Brandes, M.; Strøm, A.R.; Tunnacliffe, A. Hydroxyectoine Is Superior to Trehalose for Anhydrobiotic Engineering of Pseudomonas putida KT2440. Appl. Environ. Microbiol. 2002, 68, 4328–4333. [Google Scholar] [CrossRef] [PubMed]
- Frontera, G. Estrategias de inoculación en soja. In Producción Agroindustrial. Tucumán, November–December 2010; pp. 60–61. Available online: https://www.produccion.com.ar/ver_nota.php?edicion=Nov_Dic2016&numero=187&id=772 (accessed on 20 June 2024).
- Heydari, A.; Naraghi, L. Application of Antagonistic Bacteria for the Promotion of Cotton Seedlings Growth Characteristics. Int. J. Agric. Crop Sci. 2014, 7, 1267–1273. [Google Scholar]
- Manikandan, R.; Saravanakumar, D.; Rajendran, L.; Raguchander, T.; Samiyappan, R. Standardization of Liquid Formulation of Pseudomonas fluorescens Pf1 for Its Efficacy against Fusarium Wilt of Tomato. Biol. Control 2010, 54, 83–89. [Google Scholar] [CrossRef]
- Seong, K.-Y.; Ryu, O.-R.; Choi, W.-Y. Effect of Trehalose on the Viability of Fluorescent Pseudomonas, Strain SSL3. Korean J. Soil Sci. Fertil. 2000, 33, 292–301. [Google Scholar]
- Shah-Smith, D.A.; Burns, R.G. Shelf-Life of a Biocontrol Pseudomonas putida Applied to Sugar Beet Seeds Using Commercial Coatings. Biocontrol Sci. Technol. 1997, 7, 65–74. [Google Scholar] [CrossRef]
- O’Callaghan, M.; Swaminathan, J.; Lottmann, J.; Wright, D.A.; Jackson, T.A. Seed Coating with Biocontrol Strain Pseudomonas Fluorescens F113. N. Z. Plant Prot. 2006, 59, 80–85. [Google Scholar] [CrossRef]
- Kuz’mina, L.; Melent’ev, A. The Effect of Seed Bacterization by Bacillus Cohn Bacteria on Their Colonization of the Spring Wheat Rhizosphere. Mikrobiologiya 2003, 72, 268–274. [Google Scholar]
- Juhnke, M.E.; Mathre, D.E.; Sands, D.C. Relationship between Bacterial Seed Inoculum Density and Rhizosphere Colonization of Spring Wheat. Soil Biol. Biochem. 1989, 21, 591–595. [Google Scholar] [CrossRef]
- Wang, N.; Wang, T.; Chen, Y.; Wang, M.; Lu, Q.; Wang, K.; Dou, Z.; Chi, Z.; Qiu, W.; Dai, J.; et al. Microbiome Convergence Enables Siderophore-Secreting-Rhizobacteria to Improve Iron Nutrition and Yield of Peanut Intercropped with Maize. Nat. Commun. 2024, 15, 839. [Google Scholar] [CrossRef]
- Mendes, R.; Kruijt, M.; de Bruijn, I.; Dekkers, E.; van der Voort, M.; Schneider, J.H.M.; Piceno, Y.M.; DeSantis, T.Z.; Andersen, G.L.; Bakker, P.A.H.M.; et al. Deciphering the Rhizosphere Microbiome for Disease-Suppressive Bacteria. Science 2011, 332, 1097–1100. [Google Scholar] [CrossRef]
- Zheng, Y.; Han, X.; Zhao, D.; Wei, K.; Yuan, Y.; Li, Y.; Liu, M.; Zhang, C.-S. Exploring Biocontrol Agents from Microbial Keystone Taxa Associated to Suppressive Soil: A New Attempt for a Biocontrol Strategy. Front. Plant Sci. 2021, 12, 655673. [Google Scholar] [CrossRef] [PubMed]
- Agaras, B.; Scandiani, M.; Luque, A.; Fernández, L.; Farina, F.; Carmona, M.; Gally, M.; Romero, A.; Wall, L.; Valverde, C. Quantification of the Potential Biocontrol and Direct Plant Growth Promotion Abilities Based on Multiple Biological Traits Distinguish Different Groups of Pseudomonas spp. Isolates. Biol. Control 2015, 90, 173–186. [Google Scholar] [CrossRef]
- Agaras, B.C.; Iriarte, A.; Valverde, C.F. Genomic Insights into the Broad Antifungal Activity, Plant-Probiotic Properties, and Their Regulation, in Pseudomonas donghuensis Strain SVBP6. PLoS ONE 2018, 13, e0194088. [Google Scholar] [CrossRef]
- Muzio, F.M.; Agaras, B.C.; Masi, M.; Tuzi, A.; Evidente, A.; Valverde, C. 7-Hydroxytropolone Is the Main Metabolite Responsible for the Fungal Antagonism of Pseudomonas donghuensis Strain SVBP6. Environ. Microbiol. 2020, 22, 2550–2563. [Google Scholar] [CrossRef] [PubMed]
- Agaras, B.C.; Noguera, F.; González Anta, G.; Wall, L.; Valverde, C. Biocontrol Potential Index of Pseudomonads, Instead of Their Direct-Growth Promotion Traits, Is a Predictor of Seed Inoculation Effect on Crop Productivity under Field Conditions. Biol. Control 2020, 143, 104209. [Google Scholar] [CrossRef]
- Lambertsen, L.; Sternberg, C.; Molin, S. Mini-Tn7 Transposons for Site-Specific Tagging of Bacteria with Fluorescent Proteins. Environ. Microbiol. 2004, 6, 726–732. [Google Scholar] [CrossRef]
- Choi, K.-H.H.; Schweizer, H.P. Mini-Tn7 Insertion in Bacteria with Single AttTn7 Sites: Example Pseudomonas aeruginosa. Nat. Protoc. 2006, 1, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Koch, B.; Jensen, L.E.; Nybroe, O. A Panel of Tn7-Based Vectors for Insertion of the Gfp Marker Gene or for Delivery of Cloned DNA into Gram-Negative Bacteria at a Neutral Chromosomal Site. J. Microbiol. Methods 2001, 45, 187–195. [Google Scholar] [CrossRef]
- Leslie, S.B.; Israeli, E.; Lighthart, B.; Crowe, J.H.; Crowe, L.M. Trehalose and Sucrose Protect Both Membranes and Proteins in Intact Bacteria during Drying. Appl. Environ. Microbiol. 1995, 61, 3592–3597. [Google Scholar] [CrossRef]
- Agaras, B.C.; Wall, L.G.; Valverde, C. Influence of Agricultural Practices and Seasons on the Abundance and Community Structure of Culturable Pseudomonads in Soils under No-till Management in Argentina. Plant Soil 2014, 382, 117–131. [Google Scholar] [CrossRef]
- Díaz, M.; Bach, T.; González Anta, G.; Agaras, B.; Wibberg, D.; Noguera, F.; Canciani, W.; Valverde, C. Agronomic Efficiency and Genome Mining Analysis of the Wheat-Biostimulant Rhizospheric Bacterium Pseudomonas pergaminensis sp. nov. Strain 1008T. Front. Plant Sci. 2022, 13, 894985. [Google Scholar] [CrossRef] [PubMed]
- Fukui, R.; Poinar, E.; Bauer, P.; Schroth, M.; Hendson, M.; Wang, X.; Hancock, J. Spatial Colonization Patterns and Interaction of Bacteria on Inoculated Sugar Beet Seed. Phytopathology 1994, 84, 1338–1345. [Google Scholar] [CrossRef]
- Tombolini, R.; van der Gaag, D.J.; Gerhardson, B.; Jansson, J.K. Colonization Pattern of the Biocontrol Strain Pseudomonas chlororaphis MA 342 on Barley Seeds Visualized by Using Green Fluorescent Protein. Appl. Environ. Microbiol. 1999, 65, 3674–3680. [Google Scholar] [CrossRef] [PubMed]
- Molina, L.; Ramos, C.; Duque, E.; Ronchel, M.C.; García, J.; Wyke, L.; Ramos, J. Survival of Pseudomonas putida KT2440 in Soil and in the Rhizosphere of Plants under Greenhouse and Environmental Conditions. Soil Biol. Biochem. 2000, 32, 315–321. [Google Scholar] [CrossRef]
- Rojas-Solís, D.; Hernández-Pacheco, C.E.; Santoyo, G. Evaluation of Bacillus and Pseudomonas to Colonize the Rhizosphere and Their Effect on Growth Promotion in Tomato (Physalis Ixocarpa Brot. Ex Horm.). Rev. Chapingo Ser. Hortic. 2016, XXII, 45–57. [Google Scholar] [CrossRef]
- Martínez-Gil, M.; Yousef-Coronado, F.; Espinosa-Urgel, M. LapF, the Second Largest Pseudomonas putida Protein, Contributes to Plant Root Colonization and Determines Biofilm Architecture. Mol. Microbiol. 2010, 77, 549–561. [Google Scholar] [CrossRef]
- Espinosa-Urgel, M.; Salido, A.; Ramos, J.-L. Genetic Analysis of Functions Involved in Adhesion of Pseudomonas putida to Seeds. J. Bacteriol. 2000, 182, 2363–2369. [Google Scholar] [CrossRef] [PubMed]
- DeFlaun, M.; Marshall, B.; Kulle, E.; Levy, S. Tn5 Insertion Mutants of Pseudomonas fluorescens Defective in Adhesion to Soil and Seeds. Appl. Environ. Microbiol. 1994, 60, 2637–2642. [Google Scholar] [CrossRef]
- Hinsa, S.M.; Espinosa-Urgel, M.; Ramos, J.L.; O’Toole, G.A. Transition from Reversible to Irreversible Attachment during Biofilm Formation by Pseudomonas fluorescens WCS365 Requires an ABC Transporter and a Large Secreted Protein. Mol. Microbiol. 2003, 49, 905–918. [Google Scholar] [CrossRef]
- Yousef-Coronado, F.; Travieso, M.L.; Espinosa-Urgel, M. Different, Overlapping Mechanisms for Colonization of Abiotic and Plant Surfaces by Pseudomonas putida. FEMS Microbiol. Lett. 2008, 288, 118–124. [Google Scholar] [CrossRef]
- Fipke, G.M.; Martin, T.N.; Müller, T.M.; Cunha, V.d.S.; Munareto, J.D.; Schönell, A.T.; Grando, L.F.T.; Rossato, A. da C. Osmoprotectant in Soybean Seeds Can Increase the Inoculation and Co-Inoculation Time in Pre-Sowing. Aust. J. Crop Sci. 2020, 14, 905–912. [Google Scholar] [CrossRef]
- Arora, N.K.; Mishra, J. Prospecting the Roles of Metabolites and Additives in Future Bioformulations for Sustainable Agriculture. Appl. Soil Ecol. 2016, 107, 405–407. [Google Scholar] [CrossRef]
- Tittabutr, P.; Payakapong, W.; Teaumroong, N.; Singleton, P.W.; Boonkerd, N. Growth, Survival and Field Performance of Bradyrhizobial Liquid Inoculant Formulations with Polymeric Additives. ScienceAsia 2007, 33, 69–77. [Google Scholar] [CrossRef]
- Ramette, A.; Frapolli, M.; Fischer-Le Saux, M.; Gruffaz, C.; Meyer, J.-M.; Défago, G.; Sutra, L.; Moënne-Loccoz, Y. Pseudomonas protegens Sp. Nov., Widespread Plant-Protecting Bacteria Producing the Biocontrol Compounds 2,4-Diacetylphloroglucinol and Pyoluteorin. Syst. Appl. Microbiol. 2011, 34, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Troxler, J.; Svercel, M.; Natsch, A.; Zala, M.; Keel, C.; Moënne-Loccoz, Y.; Défago, G. Persistence of a Biocontrol Pseudomonas Inoculant as High Populations of Culturable and Non-Culturable Cells in 200-Cm-Deep Soil Profiles. Soil Biol. Biochem. 2012, 44, 122–129. [Google Scholar] [CrossRef]
- Arana, I.; Muela, A.; Orruño, M.; Seco, C.; Garaizabal, I.; Barcina, I. Effect of Temperature and Starvation upon Survival Strategies of Pseudomonas fluorescens CHA0: Comparison with Escherichia coli. FEMS Microbiol. Ecol. 2010, 74, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Pazos-Rojas, L.A.; Cuellar-Sánchez, A.; Romero-Cerón, A.L.; Rivera-Urbalejo, A.; Van Dillewijn, P.; Luna-Vital, D.A.; Muñoz-Rojas, J.; Morales-García, Y.E.; Bustillos-Cristales, M. del R. The Viable but Non-Culturable (VBNC) State, a Poorly Explored Aspect of Beneficial Bacteria. Microorganisms 2023, 12, 39. [Google Scholar] [CrossRef] [PubMed]
- García de Castro, A.; Bredholt, H.; Strøm, A.R.; Tunnacliffe, A. Anhydrobiotic Engineering of Gram-Negative Bacteria. Appl. Environ. Microbiol. 2000, 66, 4142–4144. [Google Scholar] [CrossRef] [PubMed]
- Pazos-Rojas, L.A.; Muñoz-Arenas, L.C.; Rodríguez-Andrade, O.; López-Cruz, L.E.; López-Ortega, O.; Lopes-Olivares, F.; Luna-Suarez, S.; Baez, A.; Morales-García, Y.E.; Quintero-Hernández, V.; et al. Desiccation-Induced Viable but Nonculturable State in Pseudomonas putida KT2440, a Survival Strategy. PLoS ONE 2019, 14, e0219554. [Google Scholar] [CrossRef]
- Vílchez, J.; García-Fontana, C.; Román-Naranjo, D.; González-López, J.; Manzanera, M. Plant Drought Tolerance Enhancement by Trehalose Production of Desiccation-Tolerant Microorganisms. Front. Microbiol. 2016, 7, 01577. [Google Scholar] [CrossRef] [PubMed]
- Matuszewska, E.; Leszczyńska, D.; Kuczyńska-Wiśnik, D.; Algara, M.M.; Stojowska, K.; Augustynowicz, M.; Laskowska, E. Lack of Intracellular Trehalose Affects Formation of Escherichia coli Persister Cells. Microbiology 2015, 161, 786–796. [Google Scholar] [CrossRef]
- Lim, B.R.; Choi, H.J.; Kwon, G.-S.; Joo, W.H. Enhancement of Solvent Tolerance in Pseudomonas sp. BCNU 106 with Trehalose. Lett. Appl. Microbiol. 2015, 61, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Kumaresan, G.; Reetha, D. Survival of Azospirillum Brasilense in Liquid Formulation Amended with Different Chemical Additives. J. Phytol. 2011, 3, 48–51. [Google Scholar]
- Lalucat, J.; Gomila, M.; Mulet, M.; Zaruma, A.; García-Valdés, E. Past, Present and Future of the Boundaries of the Pseudomonas Genus: Proposal of Stutzerimonas Gen. Nov. Syst. Appl. Microbiol. 2022, 45, 126289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Khan, S.; Chousalkar, K.K. Development of PMAxxTM-Based QPCR for the Quantification of Viable and Non-Viable Load of Salmonella From Poultry Environment. Front. Microbiol. 2020, 11, 581201. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Gil, M.; Romero, D.; Kolter, R.; Espinosa-Urgel, M. Calcium Causes Multimerization of the Large Adhesin LapF and Modulates Biofilm Formation by Pseudomonas putida. J. Bacteriol. 2012, 194, 6782–6789. [Google Scholar] [CrossRef]
- Achouak, W.; Conrod, S.; Cohen, V.; Heulin, T. Phenotypic Variation of Pseudomonas brassicacearum as a Plant Root-Colonization Strategy. Mol. Plant-Microbe Interact. 2004, 17, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Sang, M.K.; Kim, K.D. Biocontrol Activity and Root Colonization by Pseudomonas corrugata Strains CCR04 and CCR80 against Phytophthora Blight of Pepper. BioControl 2014, 59, 437–448. [Google Scholar] [CrossRef]
- Chin-A-Woeng, T.F.C.; de Priester, W.; van der Bij, A.J.; Lugtenberg, B.J.J. Description of the Colonization of a Gnotobiotic Tomato Rhizosphere by Pseudomonas fluorescens Biocontrol Strain WCS365, Using Scanning Electron Microscopy. MPMI 1997, 10, 79–86. [Google Scholar] [CrossRef]
- Pliego, C.; de Weert, S.; Lamers, G.; de Vicente, A.; Bloemberg, G.; Cazorla, F.M.; Ramos, C. Two Similar Enhanced Root-Colonizing Pseudomonas Strains Differ Largely in Their Colonization Strategies of Avocado Roots and Rosellinia Necatrix Hyphae. Environ. Microbiol. 2008, 10, 3295–3304. [Google Scholar] [CrossRef] [PubMed]
- Campbell, R.; Greaves, M.P. Anatomy and Community Structure of the Rhizosphere. In The Rhizosphere; Lynch, J.M., Ed.; John Wiley and Sons Ltd.: Hoboken, NJ, USA, 1990; pp. 11–34. ISBN 0-471-92548-9. [Google Scholar]
- Canarini, A.; Kaiser, C.; Merchant, A.; Richter, A.; Wanek, W. Root Exudation of Primary Metabolites: Mechanisms and Their Roles in Plant Responses to Environmental Stimuli. Front. Plant Sci. 2019, 10, 157. [Google Scholar] [CrossRef] [PubMed]
- Validov, S.; Kamilova, F.; Qi, S.; Stephan, D.; Wang, J.J.; Makarova, N.; Lugtenberg, B. Selection of Bacteria Able to Control Fusarium oxysporum f. sp. radicis-lycopersici in Stonewool Substrate. J. Appl. Microbiol. 2007, 102, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Hatzinger, P.B.; Alexander, M. Relationship between the Number of Bacteria Added to Soil or Seeds and Their Abundance and Distribution in the Rhizosphere of Alfalfa. Plant Soil 1994, 158, 211–222. [Google Scholar] [CrossRef]
- Berlanga-Clavero, M.V.; Molina-Santiago, C.; Caraballo-Rodríguez, A.M.; Petras, D.; Díaz-Martínez, L.; Pérez-García, A.; de Vicente, A.; Carrión, V.J.; Dorrestein, P.C.; Romero, D. Bacillus subtilis Biofilm Matrix Components Target Seed Oil Bodies to Promote Growth and Anti-Fungal Resistance in Melon. Nat. Microbiol. 2022, 7, 1001–1015. [Google Scholar] [CrossRef] [PubMed]
- Gould, W.D.; Hagedorn, C.; Bardinelli, T.R.; Zablotowicz, R.M. New Selective Media for Enumeration and Recovery of Fluorescent Pseudomonads from Various Habitats. Appl. Environ. Microbiol. 1985, 49, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Rochat, L.; Péchy-tarr, M.; Baehler, E.; Maurhofer, M.; Keel, C. Combination of Fluorescent Reporters for Simultaneous Monitoring of Root Colonization and Antifungal Gene Expression by a Biocontrol Pseudomonad on Cereals with Flow Cytometry. MPMI 2010, 23, 949–961. [Google Scholar] [CrossRef] [PubMed]
- Green, M.R.; Sambrook, J. Molecular Cloning: A Laboratory Manual, 4th ed.; Cold Spring Harbor Laboratory: Cold Spring Harbor, NY, USA, 2012. [Google Scholar]
- Martínez-García, E.; Aparicio, T.; de Lorenzo, V.; Nikel, P.I. New Transposon Tools Tailored for Metabolic Engineering of Gram-Negative Microbial Cell Factories. Front. Bioeng. Biotechnol. 2014, 2, 46. [Google Scholar] [CrossRef] [PubMed]
- Agaras, B.; Valverde, C. A Mutation in the gltA Gene from a Native Isolate of the Pseudomonas chlororaphis Subgroup Induces a Phenotypic Change Associated with Phenazine Production. Arch. Phytopathol. Plant Prot. 2019, 52, 601–624. [Google Scholar] [CrossRef]
- Garavaglia, M.; Muzlera, A.; Valverde, C. Comparative Genomics and Informational Content Analysis Uncovered Internal Regions of the Core Genes rpoD, pepN and gltX for an MLSA with Genome-Level Resolving Power within the Genus Pseudomonas. Mol. Phylogenet. Evol. 2023, 179, 107663. [Google Scholar] [CrossRef]
- Simon, R.; Priefer, U.; Pühler, A. A Broad Host Range Mobilization System for In Vivo Genetic Engineering: Transposon Mutagenesis in Gram Negative Bacteria. Bio/Technol. 1983, 1, 784–791. [Google Scholar] [CrossRef]
- Boyer, H.W.; Roulland-Dussoix, D. A Complementation Analysis of the Restriction and Modification of DNA in Escherichia coli. J. Mol. Biol. 1969, 41, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Woodcock, D.M.; Crowther, P.J.; Doherty, J.; Jefferson, S.; DeCruz, E.; Noyer-Weidner, M.; Smith, S.S.; Michael, M.Z.; Graham, M.W. Quantitative Evaluation of Escherichia coli Host Strains for Tolerance to Cytosine Methylation in Plasmid and Phage Recombinants. Nucleic Acids Res. 1989, 17, 3469–3478. [Google Scholar] [CrossRef] [PubMed]
- Valcek, A.; Overballe-Petersen, S.; Hansen, F.; Dolejska, M.; Hasman, H. Complete Genome Sequence of Escherichia coli MT102, a Plasmid-Free Recipient Resistant to Rifampin, Azide, and Streptomycin, Used in Conjugation Experiments. Microbiol. Resour. Announc. 2019, 8, e00383-19. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Lies, D.P.; Fu, H.; Roberts, G.P. An Improved Tn7-Based System for the Single-Copy Insertion of Cloned Genes into Chromosomes of Gram-Negative Bacteria. Gene 1991, 109, 167–168. [Google Scholar] [CrossRef] [PubMed]
- Voisard, C.; Rella, M.; Haas, D. Conjugative Transfer of Plasmid RP1 to Soil Isolates of Pseudomonas Fluorescens Is Facilitated by Certain Large RP1 Deletions. FEMS Microbiol. Lett. 1988, 55, 9–13. [Google Scholar] [CrossRef]
- Taurian, T.; Anzuay, M.S.; Angelini, J.G.; Tonelli, M.L.; Ludueña, L.; Pena, D.; Ibáñez, F.; Fabra, A. Phosphate-Solubilizing Peanut Associated Bacteria: Screening for Plant Growth-Promoting Activities. Plant Soil 2010, 329, 421–431. [Google Scholar] [CrossRef]
- Naghili, H.; Tajik, H.; Mardani, K.; Razavi Rouhani, S.M.; Ehsani, A.; Zare, P. Validation of Drop Plate Technique for Bacterial Enumeration by Parametric and Nonparametric Tests. Vet. Res. Forum 2013, 4, 179–183. [Google Scholar] [PubMed]
- Marrero, M.A.; Agaras, B.C.; Wall, L.G.; Valverde, C. Enriquecimiento Diferencial de Pseudomonas Spp. En El Rizoplano de Distintas Especies Cultivadas. Rev. Argent Microbiol. 2015, 47, 132–137. [Google Scholar] [CrossRef]
- Vincent, J. A Manual for Practical Study of Root-Nodule Bacteria. In IBP Handbook; Backwell Scientific Publications: Oxford, UK, 1970; p. 164. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorch, M.G.; Valverde, C.; Agaras, B.C. Variability in Maize Seed Bacterization and Survival Correlating with Root Colonization by Pseudomonas Isolates with Plant-Probiotic Traits. Plants 2024, 13, 2130. https://doi.org/10.3390/plants13152130
Lorch MG, Valverde C, Agaras BC. Variability in Maize Seed Bacterization and Survival Correlating with Root Colonization by Pseudomonas Isolates with Plant-Probiotic Traits. Plants. 2024; 13(15):2130. https://doi.org/10.3390/plants13152130
Chicago/Turabian StyleLorch, Melani G., Claudio Valverde, and Betina C. Agaras. 2024. "Variability in Maize Seed Bacterization and Survival Correlating with Root Colonization by Pseudomonas Isolates with Plant-Probiotic Traits" Plants 13, no. 15: 2130. https://doi.org/10.3390/plants13152130





