Abstract
This review investigates innovative strategies for upcycling agricultural residues into valuable pharmaceutical compounds. The improper disposal of agricultural residues contributes to significant environmental issues, including increased greenhouse gas emissions and ecosystem degradation. Upcycling offers a sustainable solution, transforming these residues into high-value bioproducts (antioxidants, antitumor agents, antidiabetic compounds, anti-inflammatory agents, and antiviral drugs). Nanotechnology and microbial biotechnology have a crucial role in enhancing bioavailability and targeted delivery of bioactive compounds. Advanced techniques like enzymatic hydrolysis, green solvents, microwave processing, pyrolysis, ultrasonic processing, acid and alkaline hydrolysis, ozonolysis, and organosolv processes are explored for their effectiveness in breaking down agricultural waste and extracting valuable compounds. Despite the promising potential, challenges such as variability in residue composition, scalability, and high costs persist. The review emphasizes the need for future research on cost-effective extraction techniques and robust regulatory frameworks to ensure the safety, efficacy, and quality of bioproducts. The upcycling of agricultural residues represents a viable path towards sustainable waste management and production of pharmaceutical compounds, contributing to environmental conservation and public health improvements. This review provides an analysis of the current literature and identifies knowledge gaps, offering recommendations for future studies to optimize the use of agricultural residues in the drug industry.
1. Introduction
The improper disposal of agricultural residues via negligent practices is a significant environmental concern and negatively impacts ecosystems. Untreated and underutilized agricultural residues generate numerous greenhouse gas emissions, leading, through various mechanisms, to intensified climate change and the release of detrimental gaseous byproducts. Upcycling, the process of transforming waste materials into higher-value products, offers a sustainable solution by converting these residues into valuable bioproducts, including pharmaceuticals, such as antioxidants, antitumor agents, antidiabetic compounds, anti-inflammatory agents, and antiviral drugs [1].
The agricultural sector is a significant contributor to greenhouse gases, such as carbon dioxide, nitrous oxide, and methane, and the accelerating global production of residues inflicts a powerful effect on the environment, endangering human health and ecosystem longevity. These recent circumstances make urgent sustainability changes in current agricultural procedures and residue management policies mandatory [2,3]. From a pharmaceutical perspective, nanotechnology and microbial biotechnology are essential in transforming agricultural residues into enzymes and bioactive compounds, with improved bioavailability and targeted delivery to specific physiological sites, extending their applications in drug development [4,5]. Nanoencapsulation of bioactive substances in various states, employing matrices or inert nanostructured materials with unique properties, facilitates advanced drug delivery systems, enabling precise control over their therapeutic effects [6,7].
This study is important as it investigates innovative strategies for upcycling agricultural residues into valuable pharmaceutical compounds, addressing both environmental and economic challenges by transforming waste into high-value products. It highlights the benefits to the pharmaceutical industry through a renewable source of bioactive compounds, aiding in environmental conservation and public health. This review defines and classifies agricultural residues, details their unique properties, and identifies knowledge gaps such as variability in composition and scalability of biotechnological processes. It also recommends future research on cost-effective extraction techniques and regulatory frameworks, providing an in-depth critical analysis of the current literature on the effectiveness and scalability of various upcycling strategies. Previous studies have extensively documented the potential of agricultural residues as sources of bioactive compounds, focusing on their composition, potential applications, and extraction technologies like enzymatic hydrolysis, green solvents, microwave processing, pyrolysis, ultrasonic processing, acid and alkaline hydrolysis, ozonolysis, organosolv processes, and fermentation. These technologies enhance the breakdown of agricultural waste, facilitating the extraction of valuable compounds and promoting environmentally friendly utilization. However, a comprehensive analysis addressing challenges such as variability in residue composition and scalability is needed. Despite the promising applications and advancements in nanotechnology and microbial biotechnology, most research remains confined to laboratory settings, highlighting the necessity for targeted research and policy development to address high costs, regulatory challenges, and environmental impacts.
2. Characterization of Agricultural Residues
Agricultural residues, often regarded as waste, include byproducts generated from the harvesting and processing of crops and livestock, such as crop stalks, fruit peels, animal manure, and food processing waste, with increased concentrations of chemical substances [8,9].
These residues can be categorized into several types based on their origin and characteristics, the major ones being delineated in Table 1 [2,10].

Table 1.
Primary types of agro-waste.
Across the food supply chain, considerable residues result from diverse sectors, including the beverage industry, dairy and ice cream production, and fruit and vegetable processing [11].
Agriculture produces a daily average of 23.7 million food tons worldwide [12]. In the EU and globally, substantial volumes of agricultural and food processing residues are generated. Europe produces around 4,000,000 tons of tomato pomace annually, while the USA generates enormous amounts of orange peel waste. Other notable examples include 50,000–100,000 tons of vegetable oil waste in the UK, 57,000 tons of wheat straw in the USA, and 2,881,500 tons of olive pomace worldwide, highlighting the extensive global impact of these residues [10,13].
Table 2 provides an overview of various waste categories generated from food processing and agricultural activities accompanying specific examples [10].

Table 2.
Agricultural and food processing residues.
Because of their valuable content of bioactive compounds, such as polyphenols, proteins, and carbohydrates, agricultural residues display a great potential for the pharmaceutical industry, as a source material for novel products, and, therefore, they are not considered a waste. Bioproducts are obtained through efficient and cost-effective pretreatment technologies (chemical, physical, and biological) and conversion procedures designed to improve the biochemical characteristics of the agro-residue biomass [14,15].
Lignin, cellulose, and hemicellulose are the three main organic components of agricultural biomass. Depending on their origin, crops and agro-industrial residues are the most substantial categories, being generated in enormous amounts each day, followed by aquaculture waste. As a result of their increasing volumes, there is a recent demand for creating optimal management strategies [16,17,18,19,20,21].
Different analytical characterization methods of agricultural residues are used by researchers, such as spectroscopic techniques [nuclear magnetic resonance (NMR), Fourier-transform infrared spectroscopy (FTIR)], chromatographic techniques [gas chromatography–mass spectrometry (GC–MS), high-performance liquid chromatography (HPLC)], and thermal analysis [thermogravimetric analysis (TGA), differential scanning calorimetry (DSC)]. For example, Rambo et al. investigated agricultural biomass from Brazil and explored the physical and chemical properties using X-ray diffraction (XRD), proximate and ultimate analyses, TGA, calorific value determination, near-infrared (NIR) spectroscopy, ultraviolet (UV) spectroscopy, high-performance anion exchange chromatography–pulsed amperometric detection (HPAEC–PAD), and accelerated solvent extraction (ASE) [22].
To summarize, agricultural residues have unique physicochemical properties; therefore, they are valuable for biofuel production due to their lignocellulosic composition, beneficial for composting and soil amendment because of their high nutrient content, and have pharmaceutical potential due to their bioactive compounds like polyphenols, flavonoids, and other antioxidants.
3. Upcycling Strategies for Agricultural Residues
Innovative pretreatment and conversion processes are imperative for successful agricultural residue conversion into useful biocompounds. Essential techniques such as enzymatic hydrolysis, green solvents, pretreatment, and advanced chemical treatments are used to break down complex biomolecules of lignocellulosic materials into simpler forms that can be utilized in drug development and other pharmaceutical applications [23,24,25].
Table 3 describes several examples of the latest upcycling strategies used to transform agricultural residues into valuable bioproducts with specific applications in the pharmaceutical sector, highlighting how these techniques contribute to the extraction and preparation of bioactive compounds for medicinal purposes.

Table 3.
Valorization techniques for transforming agricultural residues into pharmaceutical bioproducts.
3.1. Relevance for Bioeconomy
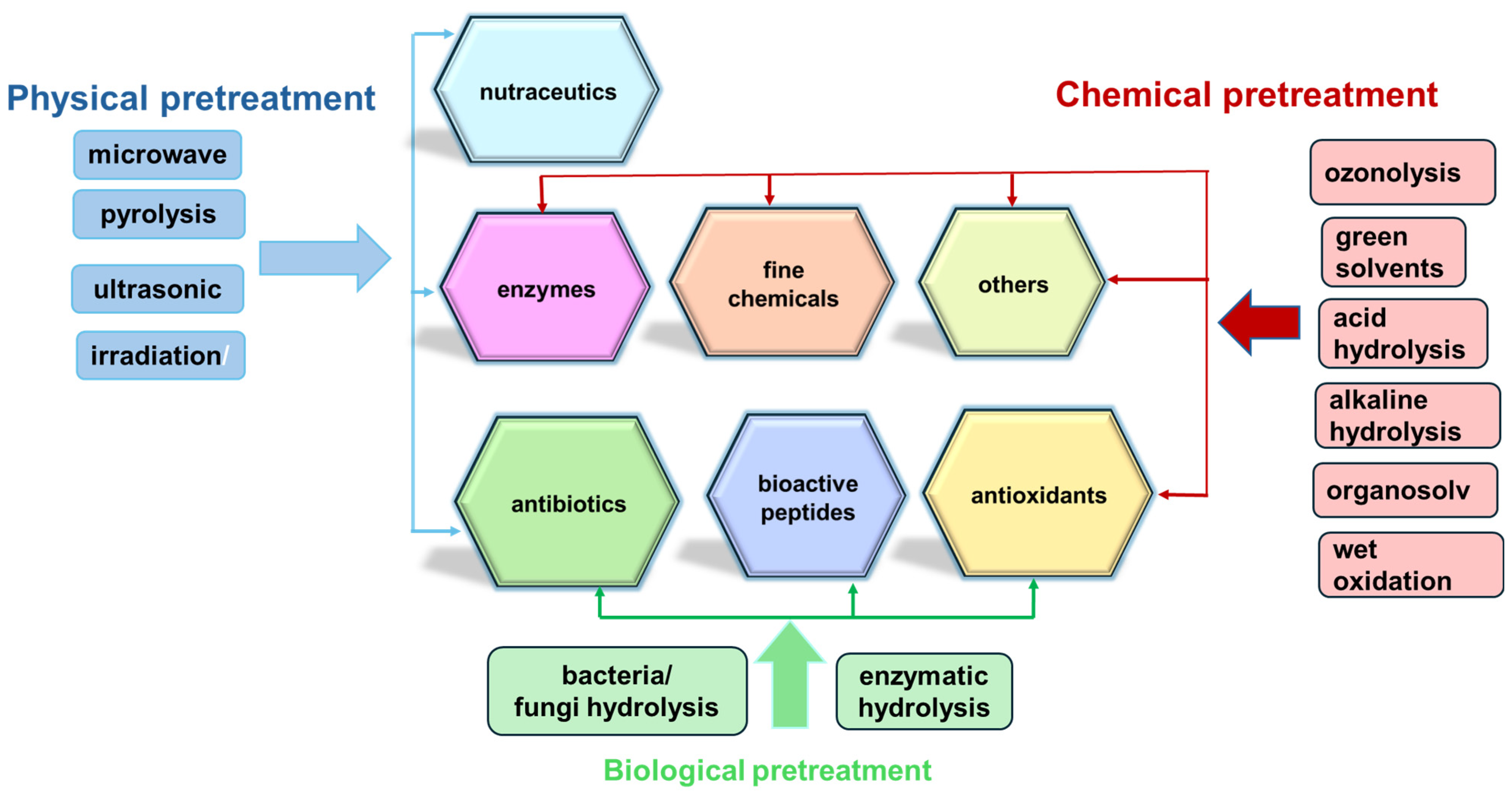
The valorization of agricultural residues into upcycled products, such as bioactive compounds, antioxidants, nutraceuticals, and fine chemicals, represents a sustainable and economically viable alternative to conventional waste management methods like landfilling and incineration [44]. Furthermore, utilizing agro-waste as a substrate in various biotechnological and chemical processes allows for the recovery of a wide range of compounds crucial to the pharmaceutical industry (Figure 1), including antibiotics, industrial enzymes, and bioactive peptides [45].
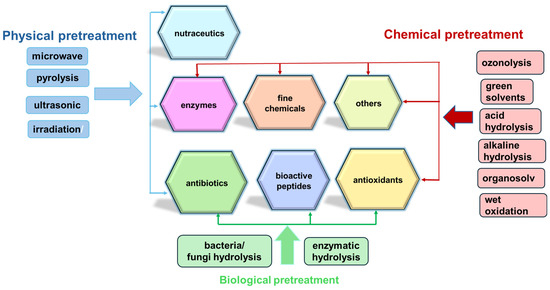
Figure 1.
Schematic representation of valuable products obtained from agriculture residues.
These processes, integrating physical, chemical, and biochemical stages that prevent microbiological hazards, are designed to extract and enhance the utility of bioactive molecules, which are instrumental in drug development and other pharmaceutical applications [11]. Table 3 provides some examples of the effective valorization methods of agricultural residues for valuable bioactive compounds extraction, encompassing cascade or on-site processing of seasonal leftovers, industrial symbiosis, and conversion via green chemical or biotechnological methods. These approaches contribute to diminishing the dependence on fossil resources and support industrial development through sustainable resource management, highlighting the crucial role of integrated biorefining and the exploitation of renewable carbon sources in advancing pharmaceutical, cosmetic, and nutraceutical innovations [46].
3.2. Biorefinery Concept
The efficient upcycling of byproducts generated during biomass production holds significant potential for the pharmaceutical industry. The biorefinery concept, immediately endorsed by the scientific community as a sustainable alternative, involves recycling abundant and inexpensive agricultural wastes to produce commercially useful biomaterials, such as secondary chemicals, single-cell protein (microbial biomass), organic acids, enzymes, and biopolymers through advanced biotechnological techniques, like composting, fermentation, and anaerobic digestion [47,48]. Utilizing residues in chemical synthesis can improve public awareness, promoting a circular economy, with limitless supplies. From a pharmaceutical perspective, the biorefinery notion offers promising environmental benefits, including reduced greenhouse gas emissions, decreased disposable quantity of waste, and diminished reliance on fossil-based sources for raw material production [49,50].
Enzymes, essential components in numerous pharmaceutical processes and products, possess substantial industrial relevance due to their substrate specificity, ability to operate under medium reaction circumstances, minimal byproduct generation, and increased yield efficiency. However, the cost of raw materials accounts for up to a third of the total production expense for enzymes. Consequently, agricultural residue utilization presents a viable strategy for decreasing the costs, amounts of waste, and unfavorable environmental repercussions of their removal [51]. Numerous studies investigate the retrieval of enzymes from agro-residues. For instance, solid-state fermentation using Bacillus coagulans has been employed for lipase from melon waste recovery. Additionally, glucoamylase has been extracted through submerged fermentation with Aspergillus niger, and α-amylase has been obtained from coffee wastes via solid-state fermentation with the fungal strain Neurospora crassa [52,53,54].
The production of organic acids (such as butyric, lactic, acetic, and citric acids) through acidogenesis depends on the agricultural residue content [55]. Simultaneous saccharification and fermentation over two days produced significant quantities of organic acids from cabbage waste, applying Lactobacillus [56].
From a pharmaceutical perspective, single-cell protein derived from microbial fermentation of agricultural residues presents a valuable alternative protein source. Emerging innovative protein solutions are required due to the rising concerns about overpopulation growth and malnutrition. Single-cell protein, extracted from microbial biomass such as fungi, algae, or bacteria, provides a cost-effective alternative to regular protein sources. This can address protein deficiency and improve nutritional outcomes in medical nutrition therapy and dietary supplements [57,58].
Biopolymers from agricultural residues are significant due to their key properties, such as biocompatibility, biostability, biodegradability, and biofunctionality, and a wide range of applications in various industries, including pharmaceuticals, medicine, and cosmetics. An environmentally sustainable solution that not only contributes to reducing plastic waste but also agro-waste, is the production of bioplastics, such as polyhydroxyalkanoate (PHA) and polyhydroxybutyrate (PHB), which are organic polymers that degrade completely into carbon dioxide and water within months [59,60,61].
3.3. Extraction Methods
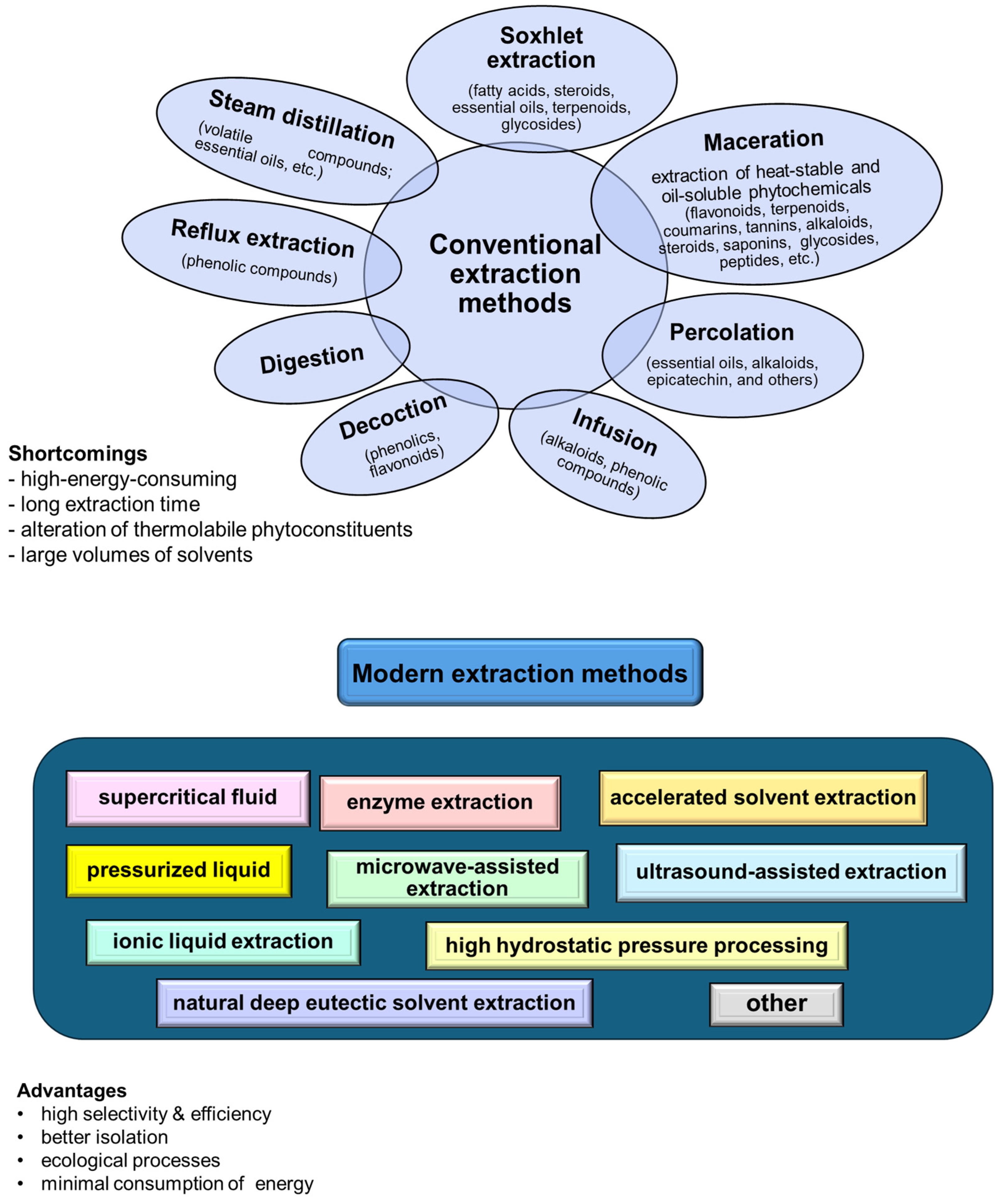
The extraction techniques for valuable compounds from agricultural residues involve both conventional and novel designs (Figure 2).
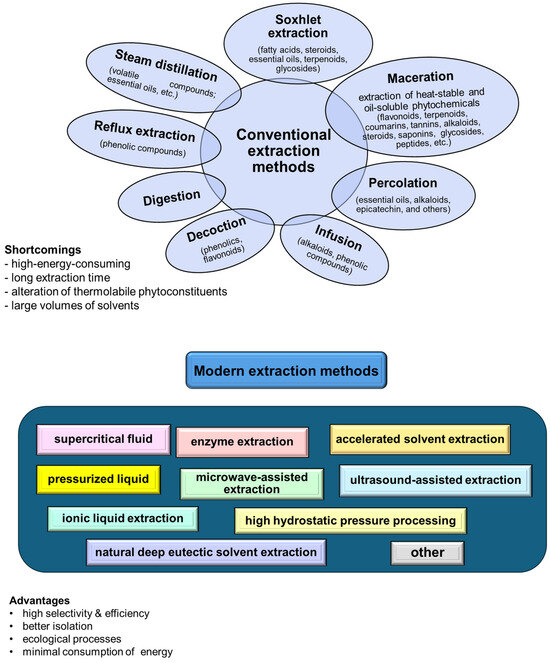
Figure 2.
Schematic representation of the main extraction methods.
Traditional methods, such as hydrodistillation, maceration, and solvent or Soxhlet extraction, use organic solvents and require temperature and agitation conditions [62]. Furthermore, they generally require long extraction times and large solvent volumes, and may result in significant quantities of toxic waste [63].
Nonconventional or modern techniques (including supercritical fluid, pressurized liquid, and enzyme/ultrasound/microwave-assisted extractions) offer more efficient and environmentally friendly alternatives. These methods reduce solvent consumption and extraction time while increasing extraction efficiency and are better suited for preserving the integrity of bioactive compounds [64,65]. The advanced extraction methods are particularly beneficial as they allow for the recovery of valuable biobased molecules, natural biopolymers, and phytochemicals from agricultural residues, which can be used in drugs, cosmetics, nutraceuticals, and other therapeutics formulation. Additionally, eco-friendly solvents, such as deep eutectic solvents and natural deep eutectic solvents, enhance the green guarantees of the extraction processes. These solvents are highly effective in dissolving biomasses and have been successfully applied to extract valuable components from various agricultural residue sources, offering a sustainable approach to waste valorization in the pharmaceutical industry [66,67,68].
4. Innovative Techniques for Recovery of Bioactive Compounds from Agricultural Residues
The substances derived from agro-waste hold remarkable potential for pharmaceutical applications due to their diversified and important properties. These compounds, including polyphenols, vitamins, minerals, fatty acids, and antioxidants, can be isolated from agricultural residues such as fruit peels, vegetable wastes, and other byproducts. They exhibit various pharmacological activities, including antioxidant, anti-inflammatory, antibacterial, and antitumor effects, making them beneficial for developing nutraceuticals, nutritional supplements, and drugs. These biochemicals can enhance health benefits, improve dietary profiles, and provide sustainable alternatives to synthetic compounds in pharmaceutical products. For instance, antioxidants extracted from fruit peels can be used to develop anti-inflammatory and anticancer drugs. At the same time, phenolic compounds from agricultural waste can serve as natural preservatives in pharmaceutical formulations [69,70,71,72,73,74].
Table 4 offers various examples of specific types of agricultural residues, the bioactive compounds derived from them, and their extraction methods, based on pharmaceutical relevance.

Table 4.
Bioactive compounds from agricultural residues.
Advanced techniques, such as microencapsulation and nanoencapsulation, have been demonstrated to notably enhance the bioavailability, solubility, and stability of bioactive compounds derived from agricultural residues and are decisive for their effective utilization in pharmaceutical applications [89,90,91].
The primary distinction between microencapsulation and nanoencapsulation lies in particle size, with microencapsulation typically ranging from 1 μm to 1 mm, and nanoencapsulation involving even smaller particles. Both techniques rely on particle dimension and distribution homogeneity for effectiveness. The supercritical carbon dioxide use in encapsulation processes offers meaningful advantages in designing particle size and managing drug-loading procedures under various conditions. Nanoencapsulation, in particular, prevents the degradation of active pharmaceutical ingredients and improves the accuracy of drug delivery by enabling proper cell entry through surface coating or conjugation. Additionally, nanoencapsulated pharmaceuticals can be labeled with fluorescent probes, assessing therapeutic efficacy during preclinical and clinical research [92,93,94,95]. These methods involve capturing the bioactive molecules within protective matrices, which defend them from degradation during processing and storage and, in addition, facilitate controlled release and targeted delivery in the human body. Moreover, novel carriers such as nanoparticles and liposomes can improve the absorption and bioactivity of the active substances, thereby maximizing their therapeutic potential. Additionally, these compounds’ incorporation into biodegradable polymers ensures sustained release, further increasing their efficacy [1,96].
Table 5 provides a few examples of encapsulation techniques for the phytochemicals retrieved from agro-residue.

Table 5.
Extracted bioactive compounds and their encapsulation method.
In addition to the encapsulation approaches, emulsion-based systems successfully increase the efficiency and stability of compounds derived from agricultural residues for pharmaceutical applications. For instance, mango peel phenolics were effectively encapsulated in water-in-oil-in-water emulsions using various surfactants. Moreover, the use of oil-in-water excipient emulsions prepared by microfluidics has been shown to increase the total phenolic content and lycopene bioaccessibility in tomato pomace [103,104].
Biotechnological approaches, particularly fermentation, are extensively utilized to valorize agricultural residues by converting them into functional components. Fermentation is one of the oldest and most efficient methods for enhancing the content and bioavailability of bioactive compounds through anaerobic metabolism. This process is highly favored in both scientific and industrial fields due to its low energy consumption, minimal water generation, and cost-effectiveness. Three main types of fermentation—solid-state, submerged, and liquid—are employed based on the desired product. Among these, solid-state and submerged fermentation are most used in current research and industry for the extraction of bioactive compounds. Recent advancements in fermentation processes have focused on producing important bioactive compounds, such as antioxidants, which are increasingly recognized for their nutritional and health benefits, making this approach particularly relevant for pharmaceutical applications [105,106,107,108].
Table 6 displays certain examples of fermentation methods used on specific agricultural wastes.

Table 6.
Fermentation of agricultural residues.
Due to these modern technologies, the pharmaceutical industry can develop more efficient and stable formulations, therefore exploiting the full potential of the agricultural residues [10].
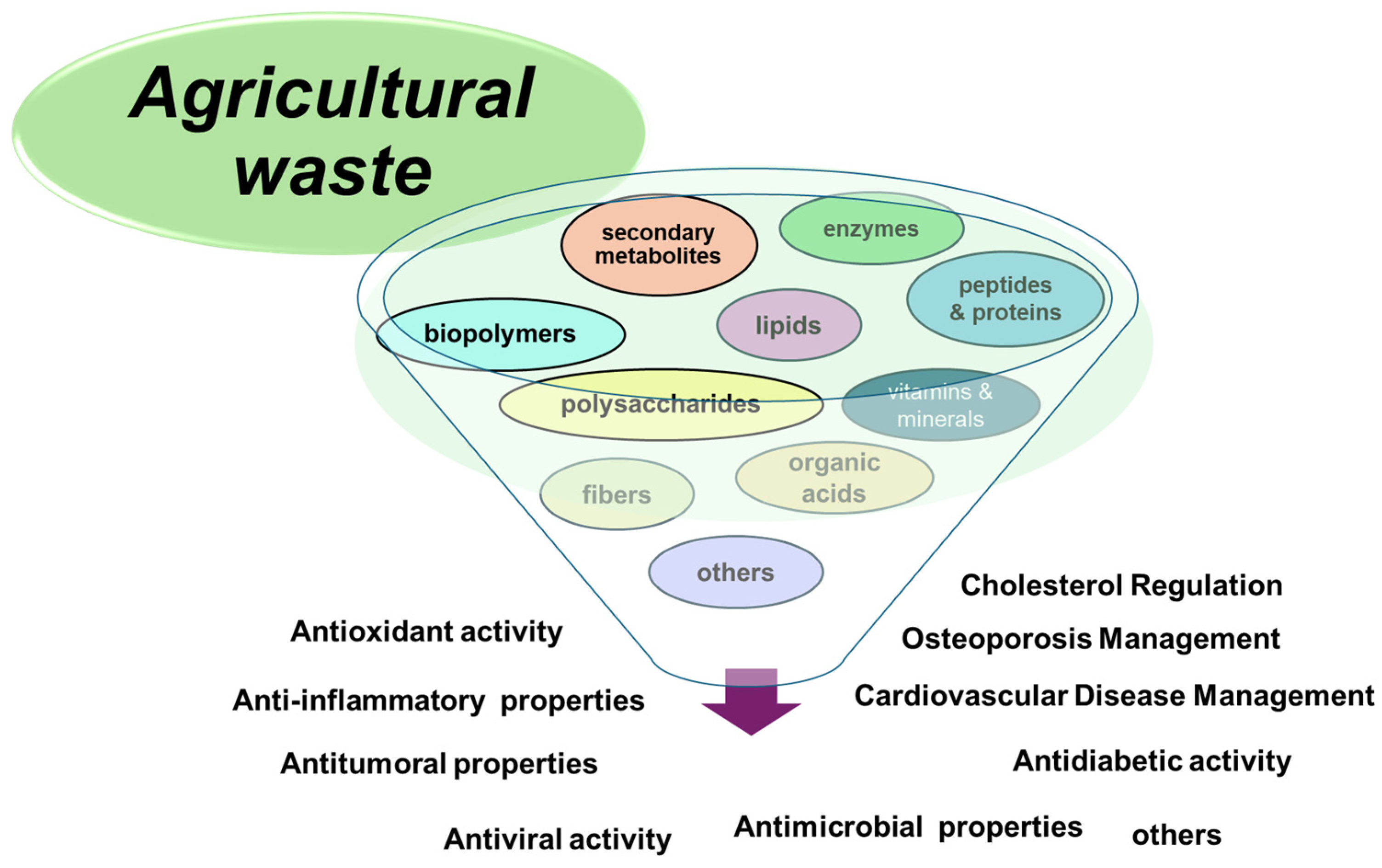
5. Pharmaceutical Applications of Upcycled Agricultural Residues
Pharmaceutical applications of upcycled agricultural residues represent a novel and challenging domain for researchers globally. Annually, billions of tons of agricultural wastes are produced, offering substantial potential for utilization across various industries. However, not all agricultural products are suitable for use due to their toxicity; for instance, β-hydroxy acids pose potential health risks to humans [113]. Despite these limitations, numerous agricultural residues from fruits and vegetables exhibit numerous pharmaceutical advantages in chronic diseases such as diabetes, cardiovascular diseases, and cancer management and treatment strategy [114,115]. Waste from the agriculture industry also can enhance the body’s absorption of various pharmaceuticals. These residues are an excellent source of essential nutrients and phytochemicals. The phenolic compounds are valuable for their therapeutic applications, including anti-inflammatory, antibacterial, antioxidant, and anticancer properties. Moreover, their antibacterial effect is enhanced by various antibiotics produced from microbes cultivated on agricultural waste [70,74].
Extensive research has been conducted on the bioactive compounds present in agricultural residues. For example, the industrial processing of oranges and lemons generates millions of tons of waste annually, rich in hydroxycinnamic acids and flavonoids [116]. Potato peels, a prevalent vegetable residue, contain chlorogenic acid as the most abundant phenolic acid and have numerous potential applications [117]. Lignocellulosic agricultural byproducts, such as wheat and rice bran, spent coffee grounds, and wheat straw residues, are recognized as clean sources of phenolic compounds, offering antioxidant and antimicrobial properties suitable for various functions [116].
Figure 3 shows several pharmaceutical applications of agricultural residues.
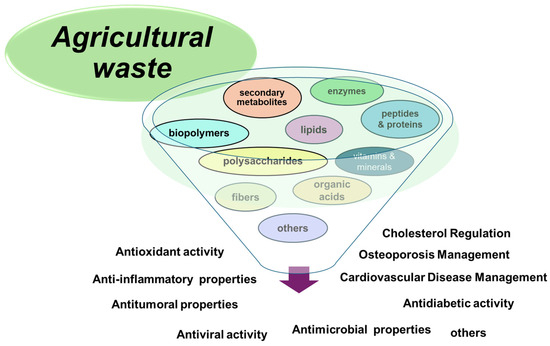
Figure 3.
The main biomedical applications of agricultural waste.
Table 7 offers some examples of agricultural byproducts, according to their relevance in the pharmaceutical field [113].

Table 7.
Agricultural residues and their primary pharmaceutical applications.
5.1. Antioxidants
Antioxidant activity is defined as the inhibitory effect of certain compounds in protecting the organism from free radicals. These radicals, which can originate within the body or be produced by external sources such as pollution, pose significant risks, including cancer and degenerative diseases such as ocular diseases, Alzheimer’s disease, and atherosclerosis [118,119,120]. The mechanism involves the radicals attacking macromolecules within the organism, leading to cellular damage, including damage to proteins and deoxyribonucleic acid (DNA) [121].
The human body can obtain natural antioxidants from fruits and vegetables as valuable sources of health-beneficial phytochemical compounds. Phytochemicals derived from agricultural residues possess antioxidant capacities and are beneficial in managing chronic diseases associated with oxidative stress [120,122]. Agricultural residues from fruits can be utilized to obtain phytochemical compounds with antioxidant properties. Phenolic acids are among the most potent antioxidant elements [113,119]. For instance, phenolic acids with high absorption in the digestive tract can be extracted from the peels of oranges, lemons, grapefruits, grapes, apples, and bananas [122]. From vegetables such as sugarcane, maize, potatoes, soybeans, and tomatoes, various phenolic acids can be extracted. These acids are present in the bagasse, pollen, husks, peels, seeds, leaves, and pulp [113]. For example, potatoes contain a significant quantity of phenolic acids, and it is projected that by 2030, potato peel waste will reach up to 8000 kilotons globally [123]. Furthermore, studies indicate that red beet is among the top vegetables as regards the antioxidant provision [124]. Benzoic acid, gallic acid, and vanillic acid are among the most common phenolic acids, playing a crucial role in neurodegenerative disease treatment associated with oxidative stress and the body’s immune response. Their mechanism of action involves breaking the chain reactions and capturing free radicals from reactive oxygen species [125,126].
A meaningful volume of residue discarded from olives consists of their leaves and seeds, rich in biomolecules such as oleuropein, hydroxytyrosol, and tyrosol [127]. However, a study determined that while olive leaves exhibit antioxidant properties, chestnut and plum leaves possess significantly greater antioxidant activity than olive leaves [128]. Additionally, vitamins, carotenoids, and flavonoids exhibit antioxidant properties, each employing different mechanisms to defend against free radicals. These compounds are found in various parts of fruits (such as peels, seeds, pulp, skin, leaves, flowers, and stems) and vegetables (including pomace, leaves, bagasse, cobs, fiber, and seeds) that are considered waste [129].
Duda-Chodak and Tarko examined the antioxidant capabilities of fruit seeds and peels, demonstrating that the peels exhibit the highest antioxidant activity and polyphenol concentration [130]. Pomegranate farming generates enormous quantities of residues containing punicalin, punicalagin, and ellagitannins, which exhibit potent antioxidants [131]. Overall, phenolic compounds are especially significant because of their well-documented beneficial results on human health, including their roles in cancer and cardiovascular disease avoidance. These actions are attributed to their ability to act as effective antioxidants, counteracting oxidative stress considered the principal cause of various inflammatory and degenerative conditions [132].
5.2. Antitumor Agents
The antitumor mechanism is closely linked to antioxidant activity, as phenolic acids originate from fruit and vegetable residues, combat free radicals, and protect the body against cancer, particularly colon adenocarcinoma (Table 8) [133,134].

Table 8.
Examples of agricultural residues with anticancer activity.
5.3. Cardiovascular Drugs
Many fruit and vegetable residues include compounds with important activity against cardiovascular diseases. Apple pomace contains uric acid, which exhibits endothelial reactivity against xanthine oxidase, providing protective effects [144]. In addition to its antioxidant properties, resveratrol from grape seeds has demonstrated efficacy in managing cardiovascular diseases by reducing atherosclerosis, acting on the renin–angiotensin system, and enhancing nitric oxide production. Procyanidins extracted from grape pomace have been shown to reduce aortic atherosclerosis [141,142]. Olive leaves consist of compounds such as uvaol, ursolic acid, and oleanolic acid, which have vasodepressor effects and help control hypertension by lowering systolic and diastolic blood pressure [127]. Pineapple peels contain bromelain, which has antithrombotic properties and inhibits platelet aggregation [145]. Flavonoids from various fruit and vegetable residues are also effective in managing atherosclerosis by preventing the oxidation of LDL lipoproteins [146]. Polyphenols exhibit antithrombotic activity, and quercetin from onion residues has been shown to act against atheroma plaque formation [147].
5.4. Antidiabetic Compounds
Several compounds extracted from agricultural residues exhibit significant antidiabetic effects. Chlorogenic acid and quercetin, extracted from apple skins, regulate glucose absorption, thereby reducing postprandial glycemia. Moreover, phytochemicals from apple pomace have been shown to influence hyperglycemia and insulin resistance [144]. Oleuropein, derived from olive leaves, can decrease blood glucose levels [127]. Limonin, found in citrus peels, exhibits anti-diabetic activity by affecting relevant biomarkers [143]. Kaempferol, a flavonol compound, has recently been discovered to reduce hyperglycemia [128]. Bell pepper residues, including seeds, peels, and leaves, contain antioxidant compounds that protect against oxidative damage and enhance the sensitivity of pancreatic β-cells to glucose [148]. Furthermore, catechin, isoflavones, tannic acid, and saponins from various fruit and vegetable residues contribute to glucose transport regulation [147].
5.5. Cholesterol Regulation
Apple pomace contains phloridzin and catechin, which have been shown to decrease triglycerides and LDL-cholesterol levels [144]. Resveratrol from grape seeds also exhibits hypolipidemic activity [141,142]. Compounds found in avocado leaves can reduce LDL cholesterol and total cholesterol [136]. Pomegranate seeds contain punicic acid, which helps decrease hyperlipidemia. Additionally, allyl methyl sulphonate and γ-glutamyl cysteine from raw garlic homogenate inhibit LDL oxidation, reducing fatty streak deposition in blood vessels [149].
5.6. Anti-Inflammatory Agents
The phenolic compounds, flavonoids, and tannins extracted from agricultural wastes exhibit strong anti-inflammatory properties in the human body [146]. Furthermore, polyphenols from fruits and vegetables possess both anti-inflammatory and antioxidant properties, which contribute to their effectiveness as anti-aging agents [147]. Olive leaves consist of quercetin, which has properties beneficial for the treatment of gastric ulcers [127].
5.7. Osteoporosis Management
Citrus peels, such as those from limes and oranges, comprise hesperidin, which has properties that can help decrease the risk of osteoporosis or alleviate its symptoms [145].
5.8. Antimicrobial/Antiviral Drugs
Various agricultural residues from fruits and vegetables exhibit antimicrobial activity against pathogens, including fungi and viruses. For instance, apple pomace contains phenolic compounds that possess antioxidant properties and antiviral activity against the Herpes simplex virus [150]. Research highlights the importance of flavonoids, phenolic acids, and other compounds in apple pomace for combating microbial and viral pathogens such as Paenibacillus larvae, Escherichia coli, Pseudomonas aeruginosa, Streptococcus pyogenes, Enterococcus faecalis, and Staphylococcus aureus [144]. Orange peel has demonstrated activity against S. aureus and Candida albicans [128]. Olive leaves exhibit antimicrobial properties due to compounds like verbascoside, oleuropein, and luteolin, showing higher activity against Bacillus cereus, C. albicans, and E. coli, and lower activity against Klebsiella pneumoniae and P. aeruginosa [151].
Flavonoids and anthocyanins from agricultural residues are recognized as therapeutic compounds with antifungal and antibacterial properties. Tangeretin and hesperidin from citrus peels show antiviral properties against hepatitis B virus by blocking viral fusion in the host organism [145]. Hesperidin also exhibits activity against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by binding to and inhibiting the spread of viral cells [152]. Banana peel contains tannins with antimicrobial properties and other compounds active against S. aureus, Salmonella enteritidis, and E. coli, and also shows biofungicidal activity [153].
Phenolic compounds found in many agricultural residues have bactericidal, antiseptic, and anthelmintic properties. Additionally, saponins from these residues exhibit antimicrobial properties [146].
Various agricultural byproducts are utilized in the synthesis of antibiotics. Researchers found that peanut shells were the most productive substrate for generating tetracycline, followed by corncobs [154].
Utilizing low-cost carbon sources extracted from diverse agricultural residues made antibiotic manufacturing far more affordable, offering remarkable chances for innovation in antibiotics such as neomycin. Additionally, the synthesis of extracellular rifamycin B using solid-state fermentation with oil-pressed cake, an agro-industrial waste, was considered [155]. Another study focused on producing oxytetracycline from cocoyam peels, through solid-state fermentation by Streptomyces speibonae [156].
5.9. Skincare Formulations
Certain fruits and vegetables hold significant importance in the beauty and pharmaceutical industries. For instance, compounds with moisturizing and antioxidant properties can be extracted from rice, orange, and oat bran, which are beneficial for skincare [145].
Skin tone is particularly important in contemporary society, especially for women, who often perceive variations in skin color (ranging from low to high yellow or red tones) as indicators of health and attractiveness. Carotenoids extracted from carrots, tomatoes, and other red vegetables can enhance skin color and improve its appearance [157].
Resveratrol and vitamin C extracted from grape seeds possess the ability to reduce melanin concentration in the skin, making them effective skin-lightening agents. In the pharmaceutical domain, these compounds are particularly important for addressing hyperpigmentation caused by UV radiation or hormonal changes [158].
From cocoa pods, malic acid, rosmarinic acid, and ellagic acid can be extracted. These acids exhibit high antioxidant activity and are included in anti-wrinkle products in the pharmaceutical industry due to their ability to enhance skin hydration [159,160]. Additionally, polyphenolic extracts from apples possess anti-aging properties and can regulate sebum production in acne, thereby reducing dermal inflammation [161].
6. Limitations
Despite the promising prospects, several limitations delay the upcycling of agricultural residues for pharmaceutical applications. One major challenge is the variability in the composition of agricultural residues, which affects the consistency and quality of extracted compounds. Factors such as geographic location, seasonal changes, and agricultural practices contribute to this inconsistency. In addition, scaling up biotechnological processes from laboratory to industrial proportion presents major technical and economic challenges. The high costs associated with advanced extraction technologies and the need for rigorous quality control measures further complicate the commercialization of these bioproducts [162,163].
Another critical limitation is the need for strong regulatory strategies to support the use of agricultural residues in pharmaceutical applications. Ensuring the safety, efficacy, and quality of bioactive compounds necessitates complex guidelines, accurate testing, and standardized protocols. These frameworks are essential to guarantee that the phytochemicals derived from agricultural residues meet the high standards required for pharmaceutical use, eliminating toxic substances and preventing microbial hazards [164].
Environmental concerns are also relevant: while upcycling reduces waste, the systems involved must be managed to minimize environmental impact. Developing green technologies and sustainable practices will be essential for the long-term viability of these initiatives (Table 9).

Table 9.
Knowledge gaps and recommendations for future studies.
7. Conclusions and Future Perspectives
The upcycling of agricultural residues into beneficial pharmaceutical products holds great promise for future developments. Biotechnological methods, such as fermentation and enzymatic hydrolysis, are expected to play a vital role in extracting valuable bioproducts from agricultural waste. The integration of nano- and biotechnological methods is particularly promising due to their low energy requirements and cost-effectiveness, making them ideal for industrial residue valorization [10].
Advances in these domains could lead to the development of unique biocompounds with applications across various industries, including pharmaceuticals. Furthermore, future research should focus on optimizing eco-friendly and cost-effective conversion techniques, exploring underutilized agricultural residues, and identifying novel bioactive molecules with potential health benefits [91,165].
Emphasis should also be placed on creating innovative encapsulation procedures to improve the stability and bioavailability of these compounds, thereby maximizing their therapeutic efficacy. Moreover, supporting collaborations between scientists, the pharmaceutical industry, and government agencies will be important in accelerating the laboratory-scale innovations translation to commercial-scale applications.
Agricultural residue upcycling presents a promising avenue for sustainable waste management and valuable pharmaceutical product production. This review highlights the momentous potential of agricultural byproducts as sources of bioactive compounds, which can be utilized in various therapeutic applications, including antioxidants, anticancer agents, and anti-inflammatory drugs, as well as in antibiotic manufacturing. Progress in biotechnological and nanotechnological methods has facilitated the efficient extraction and enhancement of these compounds, making their industrial application more feasible and cost-effective. Despite the challenges associated with the variability in residue composition, scalability, and regulatory frameworks, the continued development of eco-friendly extraction techniques and robust administrative standards will be crucial. Future research should focus on optimizing these processes, exploring underutilized residues, and encouraging interdisciplinary collaborations to fully access the potential of agricultural waste. By addressing these challenges, the pharmaceutical industry can fundamentally benefit from the sustainable and innovative use of agricultural residues, contributing to both environmental conservation and public health advancements.
Author Contributions
Conceptualization, L.E.B., A.R. and C.B.; methodology, A.-E.S., A.B. and C.-V.M.; writing—original draft preparation, L.E.B., A.R., A.-E.S. and G.D.M.; writing—review and editing, L.E.B., A.R., A.-E.S. and G.D.M.; supervision, L.E.B., A.-E.S. and C.B.; funding acquisition, A.-E.S. and C.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a grant from the European Research Executive Agency, Topic: HORIZON-MSCA-2022-SE-01-01, Type of action: HORIZON TMA MSCA Staff Exchanges, Project: 101131420—Exploiting the multifunctional properties of polyphenols: From wastes to high value products, Acronym: PHENOCYCLES.
Data Availability Statement
Data described in the manuscript will be made publicly and freely available without restriction at: https://docs.google.com/document/d/1yIATe2CRm1fEokLH8Qr7ZfasOw50KQKQ/edit?usp=sharing&ouid=106104952021876684289&rtpof=true&sd=true (accessed on 8 June 2024).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bala, S.; Garg, D.; Sridhar, K.; Inbaraj, B.S.; Singh, R.; Kamma, S.; Tripathi, M.; Sharma, M. Transformation of agro-waste into value-added bioproducts and bioactive compounds: Micro/nano formulations and application in the agri-food-pharma sector. Bioengineering 2023, 10, 152. [Google Scholar] [CrossRef] [PubMed]
- Koul, B.; Yakoob, M.; Shah, M.P. Agricultural waste management strategies for environmental sustainability. Environ. Res. 2022, 206, 112285. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.; Cain, M.; Frame, D.; Pierrehumbert, R. Agriculture’s contribution to climate change and role in mitigation is distinct from predominantly fossil CO2-emitting sectors. Front. Sustain. Food Syst. 2021, 4, 518039. [Google Scholar] [CrossRef] [PubMed]
- Balan, A.; Murthy, V.V.; Kadeppagari, R.K. Chapter 3: Immobilized enzymes for bioconversion of waste to wealth. In Biotechnology for Zero Waste: Emerging Waste Management Techniques; Hussain, C.M., Kadeppagari, R.K., Eds.; Wiley-VCH GmbH: Hoboken, NJ, USA, 2022; pp. 33–46. [Google Scholar] [CrossRef]
- Gupta, V.K.; Nguyen, Q.D.; Liu, S.; Taherzadeh, M.J.; Sirohi, R. Microbes in valorisation of biomass to value-added products. Bioresour. Technol. 2022, 347, 126738. [Google Scholar] [CrossRef] [PubMed]
- Bala, S.; Sharma, M.; Dashora, K.; Siddiqui, S.; Diwan, D.; Tripathi, M. Nanomaterials based sustainable bioenergy production systems: Current trends and future prospects. Nanofabrication 2022, 7, 314–324. [Google Scholar] [CrossRef]
- Pateiro, M.; Gómez, B.; Munekata, P.E.S.; Barba, F.J.; Putnik, P.; Kovačević, D.B.; Lorenzo, J.M. Nanoencapsulation of promising bioactive compounds to improve their absorption, stability, functionality and the appearance of the final food products. Molecules 2021, 26, 1547. [Google Scholar] [CrossRef]
- Obi, F.O.; Ugwuishiwu, B.O.; Nwakaire, J.N. Agricultural waste concept, generation, utilization and management. Niger. J. Technol. 2016, 35, 957–964. [Google Scholar] [CrossRef]
- Dwivedi, S.; Tanveer, A.; Yadav, S.; Anand, G.; Yadav, D. Chapter 23: Agro-wastes for cost effective production of industrially important microbial enzymes: An overview. In Microbial Biotechnology: Role in Ecological Sustainability and Research; Chowdhary, P., Mani, S., Chaturvedi, P., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2022; pp. 435–460. [Google Scholar] [CrossRef]
- Capanoglu, E.; Nemli, E.; Tomas-Barberan, F. Novel approaches in the valorization of agricultural wastes and their applications. J. Agric. Food Chem. 2022, 70, 6787–6804. [Google Scholar] [CrossRef]
- Baiano, A. Recovery of biomolecules from food wastes—A review. Molecules 2014, 19, 14821–14842. [Google Scholar] [CrossRef]
- Duque-Acevedo, M.; Belmonte-Ureña, L.J.; Cortés-García, F.J.; Camacho-Ferre, F. Agricultural waste: Review of the evolution, approaches and perspectives on alternative uses. Glob. Ecol. Conserv. 2020, 22, e00902. [Google Scholar] [CrossRef]
- Ravindran, R.; Jaiswal, A.K. Exploitation of food industry waste for high-value products. Trends Biotechnol. 2016, 34, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Chojnacka, K.; Gorazda, K.; Witek-Krowiak, A.; Moustakas, K. Recovery of fertilizer nutrients from materials—Contradictions, mistakes and future trends. Renew. Sustain. Energy Rev. 2019, 110, 485–498. [Google Scholar] [CrossRef]
- Reshmy, R.; Philip, E.; Madhavan, A.; Sirohi, R.; Pugazhendhi, A.; Binod, P.; Awasthi, M.K.; Vivek, N.; Kumar, V.; Sindhu, R. Lignocellulose in future biorefineries: Strategies for cost-effective production of biomaterials and bioenergy. Bioresour. Technol. 2022, 344, 126241. [Google Scholar] [CrossRef] [PubMed]
- Donner, M.; Gohier, R.; de Vries, H. A new circular business model typology for creating value from agro-waste. Sci. Total Environ. 2020, 716, 137065. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, S.; Abdul Khalil, H.P.S.; Abd Hamid, S.; Albadn, Y.M.; Suriani, A.B.; Kamaruzzaman, S.; Mohamed, A.; Allaq, A.A.; Yahya, E.B. Insights into agricultural-waste-based nano-activated carbon fabrication and modifications for wastewater treatment application. Agriculture 2022, 12, 1737. [Google Scholar] [CrossRef]
- Popa, V.I. Chapter 1: Biomass and sustainability. In Sustainability of Biomass through Bio-Based Chemistry, 1st ed.; Popa, V.I., Ed.; CRC Press–Taylor & Francis Group: Boca Raton, FL, USA, 2021; pp. 1–33. [Google Scholar] [CrossRef]
- Aravani, V.P.; Sun, H.; Yang, Z.; Liu, G.; Wang, W.; Anagnostopoulos, G.; Syriopoulos, G.; Charisiou, N.D.; Goula, M.A.; Kornaros, M.; et al. Agricultural and livestock sector’s residues in Greece & China: Comparative qualitative and quantitative characterization for assessing their potential for biogas production. Renew. Sustain. Energy Rev. 2022, 154, 111821. [Google Scholar] [CrossRef]
- Segneanu, A.E.; Macarie, C.; Ungureanu, M.; Balcu, I.; Gherman, V.; Grozescu, I. Comparative study on enzymatic hydrolysis of cellulose. Dig. J. Nanomater. Biostruct. 2013, 8, 1061–1068. [Google Scholar]
- Macarie, C.A.; Segneanu, A.E.; Balcu, I.; Pop, R.; Burtica, G.; Ungureanu, M.; Grozescu, I. Use of alkaline lyophilization process for lignocellulosic biomass pretreatment. Dig. J. Nanomater. Biostruct. 2012, 7, 1577–1586. [Google Scholar]
- Rambo, M.K.D.; Schmidt, F.L.; Ferreira, M.M.C. Analysis of the lignocellulosic components of biomass residues for biorefinery opportunities. Talanta 2015, 144, 696–703. [Google Scholar] [CrossRef]
- Dey, T.; Bhattacharjee, T.; Nag, P.; Ritika; Ghati, A.; Kuila, A. Valorization of agro-waste into value added products for sustainable development. Bioresour. Technol. Rep. 2021, 16, 100834. [Google Scholar] [CrossRef]
- de Souza, L.; Shivakumar, S. Conversion of agro-industrial wastes for the manufacture of bio-based plastics. In Bioplastics for Sustainable Development; Kuddus, M., Roohi, Eds.; Springer: Singapore, 2021; pp. 177–204. [Google Scholar] [CrossRef]
- Saravanan, A.; Senthil Kumar, P.; Jeevanantham, S.; Karishma, S.; Vo, D.-V.N. Recent advances and sustainable development of biofuels production from lignocellulosic biomass. Bioresour. Technol. 2022, 344, 126203. [Google Scholar] [CrossRef] [PubMed]
- Usmani, Z.; Sharma, M.; Diwan, D.; Tripathi, M.; Whale, E.; Jayakody, L.N.; Moreau, B.; Thakur, V.K.; Tuohy, M.; Gupta, V.K. Valorization of sugar beet pulp to value-added products: A review. Bioresour. Technol. 2022, 346, 126580. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, Q.; Dong, L.; Zhang, J. Cleaner agricultural production in drinking-water source areas for the control of non-point source pollution in China. J. Environ. Manag. 2021, 285, 112096. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, Q.; Zhang, Z.; Jing, Y.; Hu, J.; He, C.; Lu, C. A review on biological recycling in agricultural waste-based biohydrogen production: Recent developments. Bioresour. Technol. 2022, 347, 126595. [Google Scholar] [CrossRef] [PubMed]
- Naik, G.P.; Poonia, A.K.; Chaudhari, P.K. Pretreatment of lignocellulosic agricultural waste for delignification, rapid hydrolysis, and enhanced biogas production: A review. J. Indian Chem. Soc. 2021, 98, 100147. [Google Scholar] [CrossRef]
- Yin, X.; Wei, L.; Pan, X.; Liu, C.; Jiang, J.; Wang, K. The pretreatment of lignocelluloses with green solvent as biorefinery preprocess: A minor review. Front. Plant Sci. 2021, 12, 670061. [Google Scholar] [CrossRef] [PubMed]
- Das, L.; Achinivu, E.C.; Barcelos, C.A.; Sundstrom, E.; Amer, B.; Baidoo, E.E.K.; Simmons, B.A.; Sun, N.; Gladden, J.M. Deconstruction of woody biomass via protic and aprotic ionic liquid pretreatment for ethanol production. ACS Sustain. Chem. Eng. 2021, 9, 4422–4432. [Google Scholar] [CrossRef]
- Kumar, N.; Gautam, R.; Stallings, J.D.; Coty IV, G.G.; Lynam, J.G. Secondary agriculture residues pretreatment using deep eutectic solvents. Waste Biomass Valoriz. 2021, 12, 2259–2269. [Google Scholar] [CrossRef]
- Ndayisenga, F.; Yu, Z.; Zheng, J.; Wang, B.; Liang, H.; Phulpoto, I.A.; Habiyakare, T.; Zhou, D. Microbial electrohydrogenesis cell and dark fermentation integrated system enhances biohydrogen production from lignocellulosic agricultural wastes: Substrate pretreatment towards optimization. Renew. Sustain. Energy Rev. 2021, 145, 111078. [Google Scholar] [CrossRef]
- Indriani, D.W.; Susilo, B.; Mashur. The effect of microwave power on lignocellulose content, physical and chemical characteristics of biomass: A review. IOP Conf. Ser. Earth Environ. Sci. 2021, 924, 012069. [Google Scholar] [CrossRef]
- Usmani, Z.; Sharma, M.; Tripathi, M.; Nizami, A.S.; Gong, L.; Nguyen, Q.D.; Reddy, M.S.; Thakur, V.K.; Gupta, V.K. Converting biowaste streams into energy–leveraging microwave assisted valorization technologies for enhanced conversion. J. Energy Inst. 2023, 107, 101161. [Google Scholar] [CrossRef]
- Balcu, I.; Segneanu, A.E.; Mirica, M.C.; Iorga, M.I.; Macarie, C.; Martagiu, R. Microwaves effect over biomass hydrolysis. Environ. Eng. Manag. J. 2009, 8, 741–746. [Google Scholar]
- Alcazar-Ruiz, A.; Ortiz, M.L.; Sanchez-Silva, L.; Dorado, F. Catalytic effect of alkali and alkaline earth metals on fast pyrolysis pre-treatment of agricultural waste. Biofuels Bioprod. Biorefin. 2021, 15, 1473–1484. [Google Scholar] [CrossRef]
- Zhang, D.; Jiang, B.; Luo, Y.; Fu, X.; Kong, H.; Shan, Y.; Ding, S. Effects of ultrasonic and ozone pretreatment on the structural and functional properties of soluble dietary fiber from lemon peel. J. Food Process Eng. 2022, 45, e13916. [Google Scholar] [CrossRef]
- Periyasamy, S.; Karthik, V.; Kumar, P.S.; Isabel, J.B.; Temesgen, T.; Hunegnaw, B.M.; Melese, B.B.; Mohamed, B.A.; Vo, D.-V.N. Chemical, physical and biological methods to convert lignocellulosic waste into value-added products. A review. Environ. Chem. Lett. 2022, 20, 1129–1152. [Google Scholar] [CrossRef]
- Khan, M.U.; Usman, M.; Ashraf, M.A.; Dutta, N.; Luo, G.; Zhang, S. A review of recent advancements in pretreatment techniques of lignocellulosic materials for biogas production: Opportunities and limitations. Chem. Eng. J. Adv. 2022, 10, 100263. [Google Scholar] [CrossRef]
- Rasid, N.S.A.; Shamjuddin, A.; Amin, N.A.S. Chemical and structural changes of ozonated empty fruit bunch (EFB) in a ribbon-mixer reactor. Bull. Chem. React. Eng. Catal. 2021, 16, 383–395. [Google Scholar] [CrossRef]
- Kanrar, B.B.; Singh, S.; Pal, S.K.; Panda, D. Mild-temperature Organosolv treatment of rice-straw: Extracting ability of dimethylformamide and material applications. Int. J. Environ. Sci. Technol. 2022, 20, 3121–3132. [Google Scholar] [CrossRef]
- Awogbemi, O.; Von Kallon, D.V. Pretreatment techniques for agricultural waste. Case Stud. Chem. Environ. Eng. 2022, 6, 100229. [Google Scholar] [CrossRef]
- Kavitha, S.; Kannah, R.Y.; Kumar, G.; Gunasekaran, M.; Banu, J.R. Chapter 1—Introduction: Sources and characterization of food waste and food industry wastes. In Food Waste to Valuable Resources: Applications and Management; Banu, J.R., Kumar, G., Gunasekaran, M., Kavitha, S., Eds.; Academic Press–Elsevier: New York, NY, USA, 2020; pp. 1–13. [Google Scholar] [CrossRef]
- Xiong, X.; Yu, I.K.M.; Tsang, D.C.W.; Bolan, N.S.; Ok, Y.S.; Igalavithana, A.D.; Kirkham, M.B.; Kim, K.H.; Vikrant, K. Value-added chemicals from food supply chain wastes: State-of-the-art review and future prospects. Chem. Eng. J. 2019, 375, 121983. [Google Scholar] [CrossRef]
- Lin, C.S.K.; Koutinas, A.A.; Stamatelatou, K.; Mubofu, E.B.; Matharu, A.S.; Kopsahelis, N.; Pfaltzgraff, L.A.; Clark, J.H.; Papanikolaou, S.; Kwan, T.H.; et al. Current and future trends in food waste valorization for the production of chemicals, materials and fuels: A global perspective. Biofuels Bioprod. Biorefin. 2014, 8, 686–715. [Google Scholar] [CrossRef]
- Kover, A.; Kraljić, D.; Marinaro, R.; Rene, E.R. Processes for the valorization of food and agricultural wastes to value-added products: Recent practices and perspectives. Syst. Microbiol. Biomanuf. 2022, 2, 50–66. [Google Scholar] [CrossRef]
- Nayak, A.; Bhushan, B. An overview of the recent trends on the waste valorization techniques for food wastes. J. Environ. Manag. 2019, 233, 352–370. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, J.; Hoang, S.A.; Bradney, L.; Dutta, S.; Xiong, X.; Tsang, D.C.W.; Ramadass, K.; Vinu, A.; Kirkham, M.B.; Bolan, N.S. A review on the valorisation of food waste as a nutrient source and soil amendment. Environ. Pollut. 2021, 272, 115985. [Google Scholar] [CrossRef] [PubMed]
- Isah, S.; Ozbay, G. Valorization of food loss and wastes: Feedstocks for biofuels and valuable chemicals. Front. Sustain. Food Syst. 2020, 4, 82. [Google Scholar] [CrossRef]
- Ravindran, R.; Jaiswal, A.K. Microbial enzyme production using lignocellulosic food industry wastes as feedstock: A review. Bioengineering 2016, 3, 30. [Google Scholar] [CrossRef] [PubMed]
- Murthy, P.S.; Madhava Naidu, M.; Srinivas, P. Production of α-amylase under solid-state fermentation utilizing coffee waste. J. Chem. Technol. Biotechnol. 2009, 84, 1246–1249. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.; Wang, X.; Ma, H. Glucoamylase production from food waste by Aspergillus niger under submerged fermentation. Process Biochem. 2008, 43, 280–286. [Google Scholar] [CrossRef]
- Alkan, H.; Baysal, Z.; Uyar, F.; Dogru, M. Production of lipase by a newly isolated Bacillus coagulans under solid-state fermentation using melon wastes. Appl. Biochem. Biotechnol. 2007, 136, 183–192. [Google Scholar] [CrossRef]
- Yukesh Kannah, R.; Merrylin, J.; Poornima Devi, T.; Kavitha, S.; Sivashanmugam, P.; Kumar, G.; Rajesh Banu, J. Food waste valorization: Biofuels and value added product recovery. Bioresour. Technol. Rep. 2020, 11, 100524. [Google Scholar] [CrossRef]
- Kim, H.M.; Park, J.H.; Choi, I.S.; Wi, S.G.; Ha, S.; Chun, H.H.; Hwang, I.M.; Chang, J.Y.; Choi, H.J.; Kim, J.C.; et al. Effective approach to organic acid production from agricultural kimchi cabbage waste and its potential application. PLoS ONE 2018, 13, e0207801. [Google Scholar] [CrossRef] [PubMed]
- Spalvins, K.; Ivanovs, K.; Blumberga, D. Single cell protein production from waste biomass: Review of various agricultural byproducts. Agron. Res. 2018, 16, 1493–1508. [Google Scholar]
- Nasseri, A.T.; Rasoul-Amini, S.; Morowvat, M.H.; Ghasemi, Y. Single cell protein: Production and process. Am. J. Food Technol. 2011, 6, 103–116. [Google Scholar] [CrossRef]
- Ranganathan, S.; Dutta, S.; Moses, J.A.; Anandharamakrishnan, C. Utilization of food waste streams for the production of biopolymers. Heliyon 2020, 6, e04891. [Google Scholar] [CrossRef]
- Ramadhan, M.O.; Handayani, M.N. The potential of food waste as bioplastic material to promote environmental sustainability: A review. IOP Conf. Ser. Mater. Sci. Eng. 2020, 980, 012082. [Google Scholar] [CrossRef]
- Bejenaru, C.; Radu, A.; Segneanu, A.-E.; Biţă, A.; Ciocîlteu, M.V.; Mogoşanu, G.D.; Bradu, I.A.; Vlase, T.; Vlase, G.; Bejenaru, L.E. Pharmaceutical applications of biomass polymers: Review of current research and perspectives. Polymers 2024, 16, 1182. [Google Scholar] [CrossRef]
- Bromberger Soquetta, M.; de Marsillac Terra, L.; Peixoto Bastos, C. Green technologies for the extraction of bioactive compounds in fruits and vegetables. CyTA J. Food 2018, 16, 400–412. [Google Scholar] [CrossRef]
- Torres-Valenzuela, L.S.; Ballesteros-Gómez, A.; Rubio, S. Green solvents for the extraction of high added-value compounds from agri-food waste. Food Eng. Rev. 2020, 12, 83–100. [Google Scholar] [CrossRef]
- Galanakis, C.M. Recovery of high added-value components from food wastes: Conventional; emerging technologies and commercialized applications. Trends Food Sci. Technol. 2012, 26, 68–87. [Google Scholar] [CrossRef]
- Zia, S.; Khan, M.R.; Shabbir, M.A.; Maan, A.A.; Khan, M.K.I.; Nadeem, M.; Khalil, A.A.; Din, A.; Aadil, R.M. An inclusive overview of advanced thermal and nonthermal extraction techniques for bioactive compounds in food and food-related matrices. Food Rev. Int. 2022, 38, 1166–1196. [Google Scholar] [CrossRef]
- Jablonský, M.; Škulcová, A.; Malvis, A.; Šima, J. Extraction of value-added components from food industry based and agro-forest biowastes by deep eutectic solvents. J. Biotechnol. 2018, 282, 46–66. [Google Scholar] [CrossRef] [PubMed]
- Florindo, C.; Oliveira, M.M.; Branco, L.C.; Marrucho, I.M. Carbohydrates-based deep eutectic solvents: Thermophysical properties and rice straw dissolution. J. Mol. Liq. 2017, 247, 441–447. [Google Scholar] [CrossRef]
- Fernández, M.L.Á.; Espino, M.; Gomez, F.J.V.; Silva, M.F. Novel approaches mediated by tailor-made green solvents for the extraction of phenolic compounds from agro-food industrial by-products. Food Chem. 2018, 239, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhou, Y.; Yue, W.; Qin, W.; Dong, H.; Vasanthan, T. Nanostructures of protein–polysaccharide complexes or conjugates for encapsulation of bioactive compounds. Trends Food Sci. Technol. 2021, 109, 169–196. [Google Scholar] [CrossRef]
- Guía-García, J.L.; Charles-Rodríguez, A.V.; Reyes-Valdés, M.H.; Ramírez-Godina, F.; Robledo-Olivo, A.; García-Osuna, H.T.; Cerqueira, M.A.; Flores-López, M.L. Micro and nanoencapsulation of bioactive compounds for agri-food applications: A review. Ind. Crop. Prod. 2022, 186, 115198. [Google Scholar] [CrossRef]
- Santini, A.; Cammarata, S.M.; Capone, G.; Ianaro, A.; Tenore, G.C.; Pani, L.; Novellino, E. Nutraceuticals: Opening the debate for a regulatory framework. Br. J. Clin. Pharmacol. 2018, 84, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Banwo, K.; Olojede, A.O.; Adesulu-Dahunsi, A.T.; Verma, D.K.; Thakur, M.; Tripathy, S.; Singh, S.; Patel, A.R.; Gupta, A.K.; Aguilar, C.N.; et al. Functional importance of bioactive compounds of foods with potential health benefits: A review on recent trends. Food Biosci. 2021, 43, 101320. [Google Scholar] [CrossRef]
- Daliri, E.B.-M.; Lee, B.H. Current trends and future perspectives on functional foods and nutraceuticals. In Beneficial Microorganisms in Food and Nutraceuticals; Liong, M.-T., Ed.; Springer International Publishing: Cham, Switzerland, 2015; Volume 27, pp. 221–244. [Google Scholar] [CrossRef]
- Vilas-Boas, A.A.; Pintado, M.; Oliveira, A.L.S. Natural bioactive compounds from food waste: Toxicity and safety concerns. Foods 2021, 10, 1564. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, T.; Kumcuoglu, S.; Tavman, S. Ultrasound-assisted extraction of lycopene and β-carotene from tomato processing wastes. Ital. J. Food Sci. 2017, 29, 186–194. [Google Scholar] [CrossRef]
- Benassi, L.; Alessandri, I.; Vassalini, I. Assessing green methods for pectin extraction from waste orange peels. Molecules 2021, 26, 1766. [Google Scholar] [CrossRef]
- da Rocha, C.B.; Noreña, C.P.Z. Microwave-assisted extraction and ultrasound-assisted extraction of bioactive compounds from grape pomace. Int. J. Food Eng. 2020, 16, 1–10. [Google Scholar] [CrossRef]
- Castillo-Israel, K.A.T.; Baguio, S.F.; Diasanta, M.D.B.; Lizardo, R.C.M.; Dizon, E.I.; Mejico, M.I.F. Extraction and characterization of pectin from Saba banana [Musa ‘saba’ (Musa acuminata × Musa balbisiana)] peel wastes: A preliminary study. Int. Food Res. J. 2015, 22, 202–207. [Google Scholar]
- Carbone, K.; Amoriello, T.; Iadecola, R. Exploitation of kiwi juice pomace for the recovery of natural antioxidants through microwave-assisted extraction. Agriculture 2020, 10, 435. [Google Scholar] [CrossRef]
- Chia, S.L.; Boo, H.C.; Muhamad, K.; Sulaiman, R.; Umanan, F.; Chong, G.H. Effect of subcritical carbon dioxide extraction and bran stabilization methods on rice bran oil. J. Am. Oil Chem. Soc. 2015, 92, 393–402. [Google Scholar] [CrossRef]
- Cardullo, N.; Leanza, M.; Muccilli, V.; Tringali, C. Valorization of agri-food waste from pistachio hard shells: Extraction of polyphenols as natural antioxidants. Resources 2021, 10, 45. [Google Scholar] [CrossRef]
- Wang, H.; Chen, K.; Cheng, J.; Jiang, L.; Yu, D.; Dai, Y.; Wang, L. Ultrasound-assisted three phase partitioning for simultaneous extraction of oil, protein and polysaccharide from pumpkin seeds. LWT 2021, 151, 112200. [Google Scholar] [CrossRef]
- Civan, M.; Kumcuoglu, S. Green ultrasound-assisted extraction of carotenoid and capsaicinoid from the pulp of hot pepper paste based on the bio-refinery concept. LWT 2019, 113, 108320. [Google Scholar] [CrossRef]
- Vaszilcsin, C.G.; Segneanu, A.E.; Balcu, I.; Pop, R.; Fitigău, F.; Mirica, M.C. Eco-friendly extraction and separation methods of capsaicines. Environ. Eng. Manag. J. 2010, 9, 971–976. [Google Scholar] [CrossRef]
- Tello, J.; Viguera, M.; Calvo, L. Extraction of caffeine from robusta coffee (Coffea canephora var. robusta) husks using supercritical carbon dioxide. J. Supercrit. Fluids 2011, 59, 53–60. [Google Scholar] [CrossRef]
- Popescu, C.; Fitigau, F.; Segneanu, A.E.; Balcu, I.; Martagiu, R.; Vaszilcsin, C.G. Separation and characterization of anthocyanins by analytical and electrochemical methods. Environ. Eng. Manag. J. 2011, 10, 697–701. [Google Scholar]
- Lasta, H.F.B.; Lentz, L.; Gonçalves Rodrigues, L.G.; Mezzomo, N.; Vitali, L.; Salvador Ferreira, S.R. Pressurized liquid extraction applied for the recovery of phenolic compounds from beetroot waste. Biocatal. Agric. Biotechnol. 2019, 21, 101353. [Google Scholar] [CrossRef]
- Görgüç, A.; Bircan, C.; Yılmaz, F.M. Sesame bran as an unexploited by-product: Effect of enzyme and ultrasound-assisted extraction on the recovery of protein and antioxidant compounds. Food Chem. 2019, 283, 637–645. [Google Scholar] [CrossRef]
- Chaudhari, A.K.; Singh, V.K.; Das, S.; Dubey, N.K. Nanoencapsulation of essential oils and their bioactive constituents: A novel strategy to control mycotoxin contamination in food system. Food Chem. Toxicol. 2021, 149, 112019. [Google Scholar] [CrossRef] [PubMed]
- Comunian, T.; Babazadeh, A.; Rehman, A.; Shaddel, R.; Akbari-Alavijeh, S.; Boostani, S.; Jafari, S.M. Protection and controlled release of vitamin C by different micro/nanocarriers. Crit. Rev. Food Sci. Nutr. 2022, 62, 3301–3322. [Google Scholar] [CrossRef]
- Puttasiddaiah, R.; Lakshminarayana, R.; Somashekar, N.L.; Gupta, V.K.; Inbaraj, B.S.; Usmani, Z.; Raghavendra, V.B.; Sridhar, K.; Sharma, M. Advances in nanofabrication technology for nutraceuticals: New insights and future trends. Bioengineering 2022, 9, 478. [Google Scholar] [CrossRef] [PubMed]
- Abdollahdokht, D.; Gao, Y.; Faramarz, S.; Poustforoosh, A.; Abbasi, M.; Asadikaram, G.; Nematollahi, M.H. Conventional agrochemicals towards nano-biopesticides: An overview on recent advances. Chem. Biol. Technol. Agric. 2022, 9, 13. [Google Scholar] [CrossRef]
- Ghasemi, K.; Tasnim, S.; Mahmud, S. PCM, nano/microencapsulation and slurries: A review of fundamentals, categories, fabrication, numerical models and applications. Sustain. Energy Technol. Assess. 2022, 52, 102084. [Google Scholar] [CrossRef]
- Hosseini, H.; Jafari, S.M. Introducing nano/microencapsulated bioactive ingredients for extending the shelf-life of food products. Adv. Colloid Interface Sci. 2020, 282, 102210. [Google Scholar] [CrossRef]
- Alehosseini, E.; Jafari, S.M. Micro/nano-encapsulated phase change materials (PCMs) as emerging materials for the food industry. Trends Food Sci. Technol. 2019, 91, 116–128. [Google Scholar] [CrossRef]
- Das, C.G.A.; Kumar, V.G.; Dhas, T.S.; Karthick, V.; Kumar, C.M.V. Nanomaterials in anticancer applications and their mechanism of action—A review. Nanomedicine 2023, 47, 102613. [Google Scholar] [CrossRef]
- Moschona, A.; Liakopoulou-Kyriakides, M. Encapsulation of biological active phenolic compounds extracted from wine wastes in alginate–chitosan microbeads. J. Microencapsul. 2018, 35, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Šeregelj, V.; Ćetković, G.; Čanadanović-Brunet, J.; Tumbas Šaponjac, V.; Vulić, J.; Lević, S.; Nedović, V.; Brandolini, A.; Hidalgo, A. Encapsulation of carrot waste extract by freeze and spray drying techniques: An optimization study. LWT 2021, 138, 110696. [Google Scholar] [CrossRef]
- Tumbas Šaponjac, V.; Čanadanović-Brunet, J.; Ćetković, G.; Jakišić, M.; Djilas, S.; Vulić, J.; Stajčić, S. Encapsulation of beetroot pomace extract: RSM optimization, storage and gastrointestinal stability. Molecules 2016, 21, 584. [Google Scholar] [CrossRef] [PubMed]
- Horuz, T.İ.; Belibağlı, K.B. Nanoencapsulation of carotenoids extracted from tomato peels into zein fibers by electrospinning. J. Sci. Food Agric. 2019, 99, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Šeregelj, V.; Ćetković, G.; Čanadanović-Brunet, J.; Tumbas Šaponjac, V.; Vulić, J.; Stajčić, S. Encapsulation and degradation kinetics of bioactive compounds from sweet potato peel during storage. Food Technol. Biotechnol. 2020, 58, 314–324. [Google Scholar] [CrossRef]
- Pashazadeh, H.; Zannou, O.; Ghellam, M.; Koca, I.; Galanakis, C.M.; Aldawoud, T.M.S. Optimization and encapsulation of phenolic compounds extracted from maize waste by freeze-drying, spray-drying, and microwave-drying using maltodextrin. Foods 2021, 10, 1396. [Google Scholar] [CrossRef]
- Velderrain-Rodríguez, G.R.; Acevedo-Fani, A.; González-Aguilar, G.A.; Martín-Belloso, O. Encapsulation and stability of a phenolic-rich extract from mango peel within water-in-oil-in-water emulsions. J. Funct. Foods 2019, 56, 65–73. [Google Scholar] [CrossRef]
- Nemli, E.; Ozakdogan, S.; Tomas, M.; McClements, D.J.; Capanoglu, E. Increasing the bioaccessibility of antioxidants in tomato pomace using excipient emulsions. Food Biophys. 2021, 16, 355–364. [Google Scholar] [CrossRef]
- Torres-León, C.; Chávez-González, M.L.; Hernández-Almanza, A.; Martínez-Medina, G.A.; Ramírez-Guzmán, N.; Londoño-Hernández, L.; Aguilar, C.N. Recent advances on the microbiological and enzymatic processing for conversion of food wastes to valuable bioproducts. Curr. Opin. Food Sci. 2021, 38, 40–45. [Google Scholar] [CrossRef]
- Sadh, P.K.; Kumar, S.; Chawla, P.; Duhan, J.S. Fermentation: A boon for production of bioactive compounds by processing of food industries wastes (by-products). Molecules 2018, 23, 2560. [Google Scholar] [CrossRef]
- Hadj Saadoun, J.; Bertani, G.; Levante, A.; Vezzosi, F.; Ricci, A.; Bernini, V.; Lazzi, C. Fermentation of agri-food waste: A promising route for the production of aroma compounds. Foods 2021, 10, 707. [Google Scholar] [CrossRef] [PubMed]
- Martí-Quijal, F.J.; Khubber, S.; Remize, F.; Tomasevic, I.; Roselló-Soto, E.; Barba, F.J. Obtaining antioxidants and natural preservatives from food by-products through fermentation: A review. Fermentation 2021, 7, 106. [Google Scholar] [CrossRef]
- Schmidt, C.G.; Gonçalves, L.M.; Prietto, L.; Hackbart, H.S.; Furlong, E.B. Antioxidant activity and enzyme inhibition of phenolic acids from fermented rice bran with fungus Rizhopus oryzae. Food Chem. 2014, 146, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Dulf, F.V.; Vodnar, D.C.; Dulf, E.H.; Pintea, A. Phenolic compounds, flavonoids, lipids and antioxidant potential of apricot (Prunus armeniaca L.) pomace fermented by two filamentous fungal strains in solid state system. Chem. Cent. J. 2017, 11, 92. [Google Scholar] [CrossRef] [PubMed]
- Mechmeche, M.; Kachouri, F.; Ksontini, H.; Hamdi, M. Production of bioactive peptides from tomato seed isolate by Lactobacillus plantarum fermentation and enhancement of antioxidant activity. Food Biotechnol. 2017, 31, 94–113. [Google Scholar] [CrossRef]
- Torres-León, C.; Ramírez-Guzmán, N.; Ascacio-Valdés, J.; Serna-Cock, L.; dos Santos Correia, M.T.; Contreras-Esquivel, J.C.; Aguilar, C.N. Solid-state fermentation with Aspergillus niger to enhance the phenolic contents and antioxidative activity of Mexican mango seed: A promising source of natural antioxidants. LWT 2019, 112, 108236. [Google Scholar] [CrossRef]
- Oleszek, M.; Kowalska, I.; Bertuzzi, T.; Oleszek, W. Phytochemicals derived from agricultural residues and their valuable properties and applications. Molecules 2023, 28, 342. [Google Scholar] [CrossRef]
- Sorrentino, C.; Di Gisi, M.; Gentile, G.; Licitra, F.; D’Angiolo, R.; Giovannelli, P.; Migliaccio, A.; Castoria, G.; Di Donato, M. Agri-food by-products in cancer: New targets and strategies. Cancers 2022, 14, 5517. [Google Scholar] [CrossRef] [PubMed]
- Pathak, N.; Singh, S.; Singh, P.; Singh, P.K.; Singh, R.; Bala, S.; Thirumalesh, B.V.; Gaur, R.; Tripathi, M. Valorization of jackfruit waste into value added products and their potential applications. Front. Nutr. 2022, 9, 1061098. [Google Scholar] [CrossRef]
- Fermoso, F.G.; Serrano, A.; Alonso-Fariñas, B.; Fernández-Bolaños, J.; Borja, R.; Rodríguez-Gutiérrez, G. Valuable compound extraction, anaerobic digestion, and composting: A leading biorefinery approach for agricultural wastes. J. Agric. Food Chem. 2018, 66, 8451–8468. [Google Scholar] [CrossRef]
- Joly, N.; Souidi, K.; Depraetere, D.; Wils, D.; Martin, P. Potato by-products as a source of natural chlorogenic acids and phenolic compounds: Extraction, characterization, and antioxidant capacity. Molecules 2021, 26, 177. [Google Scholar] [CrossRef]
- Rahamn, M.M.; Hossain, R.; Herreva-Bravo, J.; Islam, M.T.; Atolani, O.; Adeyemi, O.S.; Owolodun, O.A.; Kambizi, L.; Daştan, S.D.; Calina, D.; et al. Natural antioxidants from some fruits, seeds, foods, natural product, and associated health benefits: An update. Food Sci. Nutr. 2023, 11, 1657–1670. [Google Scholar] [CrossRef]
- Adebukola Adeyanju, A.; Rebecca Oyenihi, O.; Omoniyi Oguntibeju, O. Antioxidant-rich vegetables: Impact on human health. In Vegetable Crops—Health Benefits and Cultivation; Yildirim, E., Ekinci, M., Eds.; IntechOpen: London, UK, 2022; pp. 1–27. [Google Scholar] [CrossRef]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary polyphenols and their role in oxidative stress-induced human diseases: Insights into protective effects, antioxidant potentials and mechanism(s) of action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef]
- Hajhashemi, V.; Vaseghi, G.; Pourfarzam, M.; Abdollahi, A. Are antioxidants helpful for disease prevention? Res. Pharm. Sci. 2010, 5, 1–8. [Google Scholar]
- Arias, A.; Feijoo, G.; Moreira, M.T. Exploring the potential of antioxidants from fruits and vegetables and strategies for their recovery. Innov. Food Sci. Emerg. Technol. 2022, 77, 102974. [Google Scholar] [CrossRef]
- Khanal, S.; Karimi, K.; Majumdar, S.; Kumar, V.; Verma, R.; Bhatia, S.K.; Kuca, K.; Esteban, J.; Kumar, D. Sustainable utilization and valorization of potato waste: State of the art, challenges, and perspectives. Biomass Conv. Bioref. 2023. [Google Scholar] [CrossRef]
- Žitňanová, I.; Ranostajová, S.; Sobotová, H.; Demelová, D.; Pecháň, I.; Ďuračková, Z. Antioxidative activity of selected fruits and vegetables. Biologia 2006, 61, 279–284. [Google Scholar] [CrossRef]
- Kumar, A.; Khan, F.; Saikia, D. Phenolic compounds and their biological and pharmaceutical activities. In The Chemistry inside Spices & Herbs: Research and Development; Chaurasia, P.K., Bharati, S.L., Eds.; Bentham Science Publishers: Sharjah, United Arab Emirates, 2022; Volume 1, pp. 206–236. [Google Scholar] [CrossRef]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The role of polyphenols in human health and food systems: A mini-review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef]
- Hashmi, M.A.; Khan, A.; Hanif, M.; Farooq, U.; Perveen, S. Traditional uses, phytochemistry and pharmacology of Olea europaea (olive). Evid.-Based Complement. Alternat. Med. 2015, 2015, 541591. [Google Scholar] [CrossRef]
- Karasawa, M.M.G.; Mohan, C. Fruits as prospective reserves of bioactive compounds: A review. Nat. Prod. Bioprospect. 2018, 8, 335–346. [Google Scholar] [CrossRef]
- Ravimannan, N.; Nisansala, A. Study on antioxidant activity in fruits and vegetables—A review. Int. J. Adv. Res. Biol. Sci. 2017, 4, 93–101. [Google Scholar] [CrossRef]
- Duda-Chodak, A.; Tarko, T. Antioxidant properties of different fruit seeds and peels. Acta Sci. Pol. Technol. Aliment. 2007, 6, 29–36. [Google Scholar]
- Mourtzinos, I.; Goula, A. Chapter 2—Polyphenols in agricultural byproducts and food waste. In Polyphenols in Plants: Isolation, Purification and Extract Preparation, 2nd ed.; Watson, R.R., Ed.; Academic Press–Elsevier: London, UK, 2019; pp. 23–44. [Google Scholar] [CrossRef]
- Jimenez-Lopez, C.; Fraga-Corral, M.; Carpena, M.; García-Oliveira, P.; Echave, J.; Pereira, A.G.; Lourenço-Lopes, C.; Prieto, M.A.; Simal-Gandara, J. Agriculture waste valorisation as a source of antioxidant phenolic compounds within a circular and sustainable bioeconomy. Food Funct. 2020, 11, 4853–4877. [Google Scholar] [CrossRef]
- Rosa, L.S.; Silva, N.J.A.; Soares, N.C.P.; Monteiro, M.C.; Teodoro, A.J. Anticancer properties of phenolic acids in colon cancer—A review. J. Nutr. Food Sci. 2016, 6, 2. [Google Scholar] [CrossRef]
- Kiokias, S.; Proestos, C.; Oreopoulou, V. Phenolic acid of plant origin—A review on their antioxidant activity in vitro (o/w emulsion systems) along with their in vivo health biochemical properties. Foods 2020, 9, 534. [Google Scholar] [CrossRef]
- Syed, D.N.; Chamcheu, J.-C.; Adhami, V.M.; Mukhtar, H. Pomegranate extracts and cancer prevention: Molecular and cellular activities. Anticancer Agents Med. Chem. 2013, 13, 1149–1161. [Google Scholar] [CrossRef]
- Tene Tcheghebe, O.; Nyamen, L.D.; Ngoufong Tatong, F.; Seukep, A.J. Ethnobotanical uses, phytochemical and pharmacological profiles, and toxicity of Persea americana Mill.: An overview. PharmacologyOnLine 2016, 3, 213–221. [Google Scholar]
- Anilkumar, A.; Bhanu, A. In vitro anticancer activity of “Methanolic extract of papaya blackseeds” (MPB) in Hep G2 cell lines and its effect in the regulation of bcl-2, caspase-3 and p53 gene expression. Adv. Cancer Biol. Metastasis 2022, 4, 100025. [Google Scholar] [CrossRef]
- Ilhan-Ayisigi, E.; Budak, G.; Celiktas, M.S.; Sevimli-Gur, C.; Yesil-Celiktas, O. Anticancer activities of bioactive peptides derived from rice husk both in free and encapsulated form in chitosan. J. Ind. Eng. Chem. 2021, 103, 381–391. [Google Scholar] [CrossRef]
- Perveen, S.; Ashfaq, H.; Ambreen, S.; Ashfaq, I.; Kanwal, Z.; Tayyeb, A. Methanolic extract of Citrullus colocynthis suppresses growth and proliferation of breast cells through regulation of cell cycle. Saudi J. Biol. Sci. 2021, 28, 879–886. [Google Scholar] [CrossRef]
- Mandour, Y.M.; Refaat, E.; Hassanein, H.D. Anticancer activity, phytochemical investigation and molecular docking insights of Citrullus colocynthis (L.) fruits. Sci. Rep. 2023, 13, 20038. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.-D.; Luo, M.; Huang, S.-Y.; Saimaiti, A.; Shang, A.; Gan, R.-Y.; Li, H.-B. Effects and mechanisms of resveratrol on aging and age-related diseases. Oxid. Med. Cell. Longev. 2021, 2021, 9932218. [Google Scholar] [CrossRef] [PubMed]
- Bejenaru, L.E.; Biţă, A.; Belu, I.; Segneanu, A.-E.; Radu, A.; Dumitru, A.; Ciocîlteu, M.V.; Mogoşanu, G.D.; Bejenaru, C. Resveratrol: A review on the biological activity and applications. Appl. Sci. 2024, 14, 4534. [Google Scholar] [CrossRef]
- Gualdani, R.; Cavalluzzi, M.M.; Lentini, G.; Habtemariam, S. The chemistry and pharmacology of Citrus limonoids. Molecules 2016, 21, 1530. [Google Scholar] [CrossRef] [PubMed]
- Sha, S.P.; Modak, D.; Sarkar, S.; Roy, S.K.; Sah, S.P.; Ghatani, K.; Bhattacharjee, S. Fruit waste: A current perspective for the sustainable production on pharmacological, nutraceutical, and bioactive resources. Front. Microbiol. 2023, 14, 1260071. [Google Scholar] [CrossRef] [PubMed]
- Osorio, L.L.D.R.; Flórez-López, E.; Grande-Tovar, C.D. The potential of selected agri-food loss and waste to contribute to a circular economy: Applications in the food, cosmetic and pharmaceutical industries. Molecules 2021, 26, 515. [Google Scholar] [CrossRef] [PubMed]
- Kabera, J.N.; Semana, E.; Mussa, A.R.; He, X. Plants secondary metabolites: Biosynthesis, classification, function and pharmacological properties. J. Pharm. Pharmacol. 2014, 2, 377–392. [Google Scholar]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants on human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Anaya-Esparza, L.M.; Mora, Z.V.; Vázquez-Paulino, O.; Ascencio, F.; Villarruel-López, A. Bell peppers (Capsicum annum L.) losses and wastes: Source for food and pharmaceutical applications. Molecules 2021, 26, 5341. [Google Scholar] [CrossRef]
- Prasad, K.; Kaur, R.; Shekhar, S. Antioxidant potential of fruits and vegetables. J. Clin. Nutr. Diet. 2022, 8, 111. [Google Scholar]
- Kauser, S.; Murtaza, M.A.; Hussain, A.; Imran, M.; Kabir, K.; Najam, A.; An, Q.U.; Akram, S.; Fatima, H.; Batool, S.A.; et al. Apple pomace, a bioresource of functional and nutritional components with potential of utilization in different food formulations: A review. Food Chem. Adv. 2024, 4, 100598. [Google Scholar] [CrossRef]
- Pereira, A.P.; Ferreira, I.C.F.R.; Marcelino, F.; Valentão, P.; Andrade, P.B.; Seabra, R.; Estevinho, L.; Bento, A.; Pereira, J.A. Phenolic compounds and antimicrobial activity of olive (Olea europaea L. Cv. Cobrançosa) leaves. Molecules 2007, 12, 1153–1162. [Google Scholar] [CrossRef]
- Meneguzzo, F.; Ciriminna, R.; Zabini, F.; Pagliaro, M. Review of evidence available on hesperidin-rich products as potential tools against COVID-19 and hydrodynamic cavitation-based extraction as a method of increasing their production. Processes 2020, 8, 549. [Google Scholar] [CrossRef]
- Hikal, W.M.; Said-Al Ahl, H.A.H.; Bratovcic, A.; Tkachenko, K.G.; Sharifi-Rad, J.; Kačániová, M.; Elhourri, M.; Atanassova, M. Banana peels: A waste treasure for human being. Evid.-Based Complement. Alternat. Med. 2022, 2022, 7616452. [Google Scholar] [CrossRef] [PubMed]
- Asagbra, A.E.; Sanni, A.I.; Oyewole, O.B. Solid-state fermentation production of tetracycline by Streptomyces strains using some agricultural wastes as substrate. World J. Microbiol. Biotechnol. 2005, 21, 107–114. [Google Scholar] [CrossRef]
- Vastrad, B.M.; Neelagund, S.E. Optimization and production of neomycin from different agro industrial wastes in solid state fermentation. Int. J. Pharm. Sci. Drug Res. 2011, 3, 104–111. [Google Scholar]
- Ezejiofor, T.I.N.; Duru, C.I.; Asagbra, A.E.; Ezejiofor, A.N.; Orisakwe, O.E.; Afonne, J.O.; Obi, E. Waste to wealth: Production of oxytetracycline using Streptomyces species from household kitchen wastes of agricultural produce. Afr. J. Biotechnol. 2012, 11, 10115–10124. [Google Scholar] [CrossRef]
- Appleton, K.M.; McGrath, A.J.; McKinley, M.C.; Draffin, C.R.; Hamill, L.L.; Young, I.S.; Woodside, J.V. The value of facial attractiveness for encouraging fruit and vegetable consumption: Analyses from a randomized controlled trial. BMC Public Health 2018, 18, 298. [Google Scholar] [CrossRef] [PubMed]
- Dini, I.; Laneri, S. The new challenge of green cosmetics: Natural food ingredients for cosmetic formulations. Molecules 2021, 26, 3921. [Google Scholar] [CrossRef]
- Mellou, F.; Varvaresou, A.; Papageorgiou, S. Renewable sources: Applications in personal care formulations. Int. J. Cosmet. Sci. 2019, 41, 517–525. [Google Scholar] [CrossRef]
- Agudelo, C.; Bravo, K.; Ramírez-Atehortúa, A.; Torres, D.; Carrillo-Hormaza, L.; Osorio, E. Chemical and skincare property characterization of the main cocoa byproducts: Extraction optimization by RSM approach for development of sustainable ingredients. Molecules 2021, 26, 7429. [Google Scholar] [CrossRef] [PubMed]
- Zahra, A.; Lim, S.-K.; Seong, H.-A.; Shin, S.-J. Nano-processing of apple pomace for cosmetics ingredients. J. Korea TAPPI 2022, 54, 68–77. [Google Scholar] [CrossRef]
- Gontard, N.; Sonesson, U.; Birkved, M.; Majone, M.; Bolzonella, D.; Celli, A.; Angellier-Coussy, H.; Jang, G.W.; Verniquet, A.; Broeze, J.; et al. A research challenge vision regarding management of agricultural waste in a circular bio-based economy. Crit. Rev. Environ. Sci. Technol. 2018, 48, 614–654. [Google Scholar] [CrossRef]
- Sharma, V.; Tsai, M.-L.; Nargotra, P.; Chen, C.-W.; Kuo, C.-H.; Sun, P.-P.; Dong, C.-D. Agro-industrial food waste as a low-cost substrate for sustainable production of industrial enzymes: A critical review. Catalysts 2022, 12, 1373. [Google Scholar] [CrossRef]
- Federici, F.; Fava, F.; Kalogerakis, N.; Mantzavinos, D. Valorisation of agro-industrial by-products, effluents and waste: Concept, opportunities and the case of olive mill wastewaters. J. Chem. Technol. Biotechnol. 2009, 84, 895–900. [Google Scholar] [CrossRef]
- Das, A.; Nazni, P. Formulation and quality evaluation of (Pennisetum glaucum incorporated) value-added paneer by response surface methodology. Indian J. Dairy Sci. 2021, 74, 131–137. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).