Antioxidative and Metabolic Responses in Canola: Strategies with Wood Distillate and Sugarcane Bagasse Ash for Improved Growth under Abiotic Stress
Abstract
:1. Introduction
2. Results
2.1. K and Na Ions
2.1.1. Exchangeable Sodium Percentage in the Soil
2.1.2. Concentration of Na and K Ions
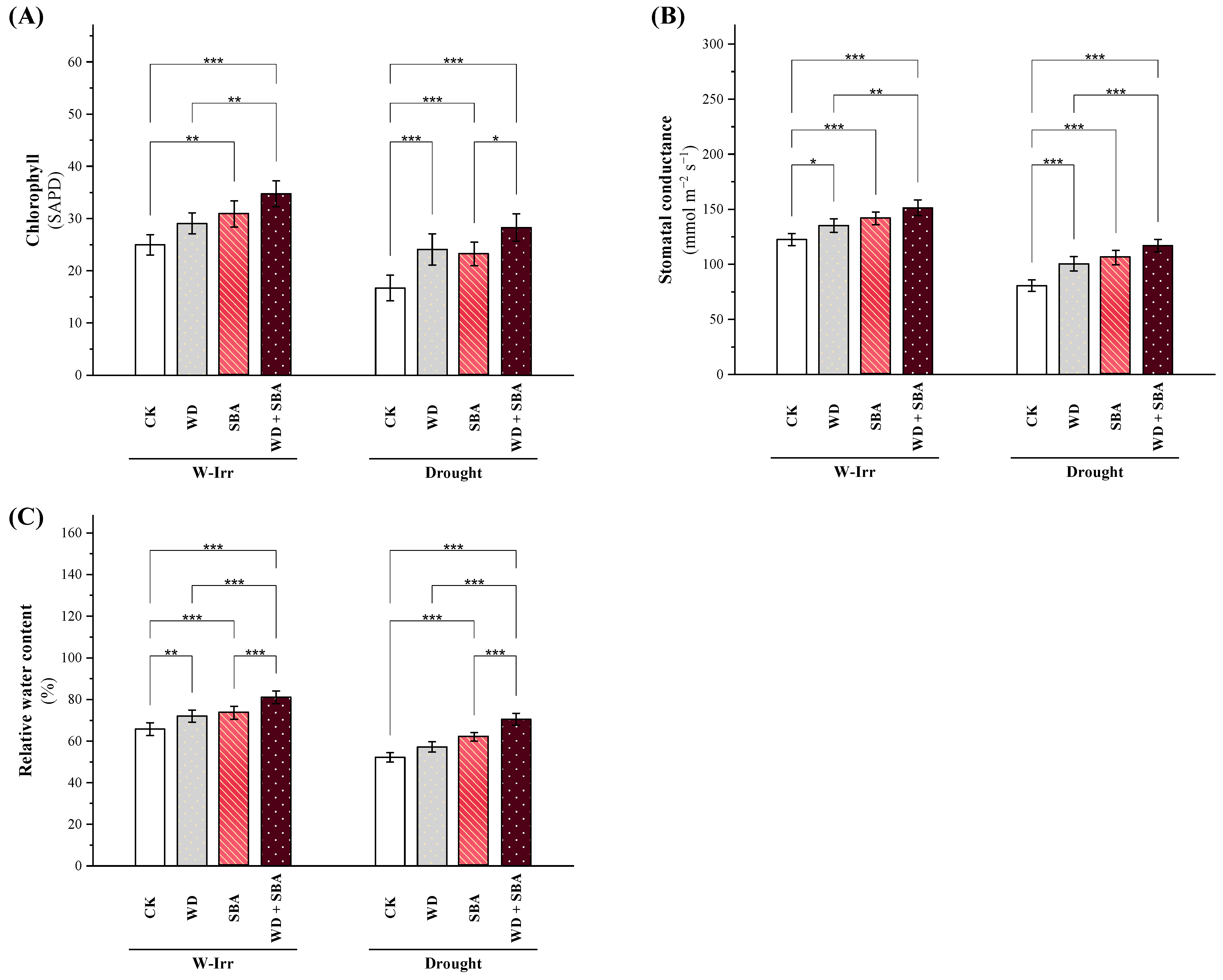
2.2. Physiological Attributes
2.2.1. Chlorophyll Content and Water Relations
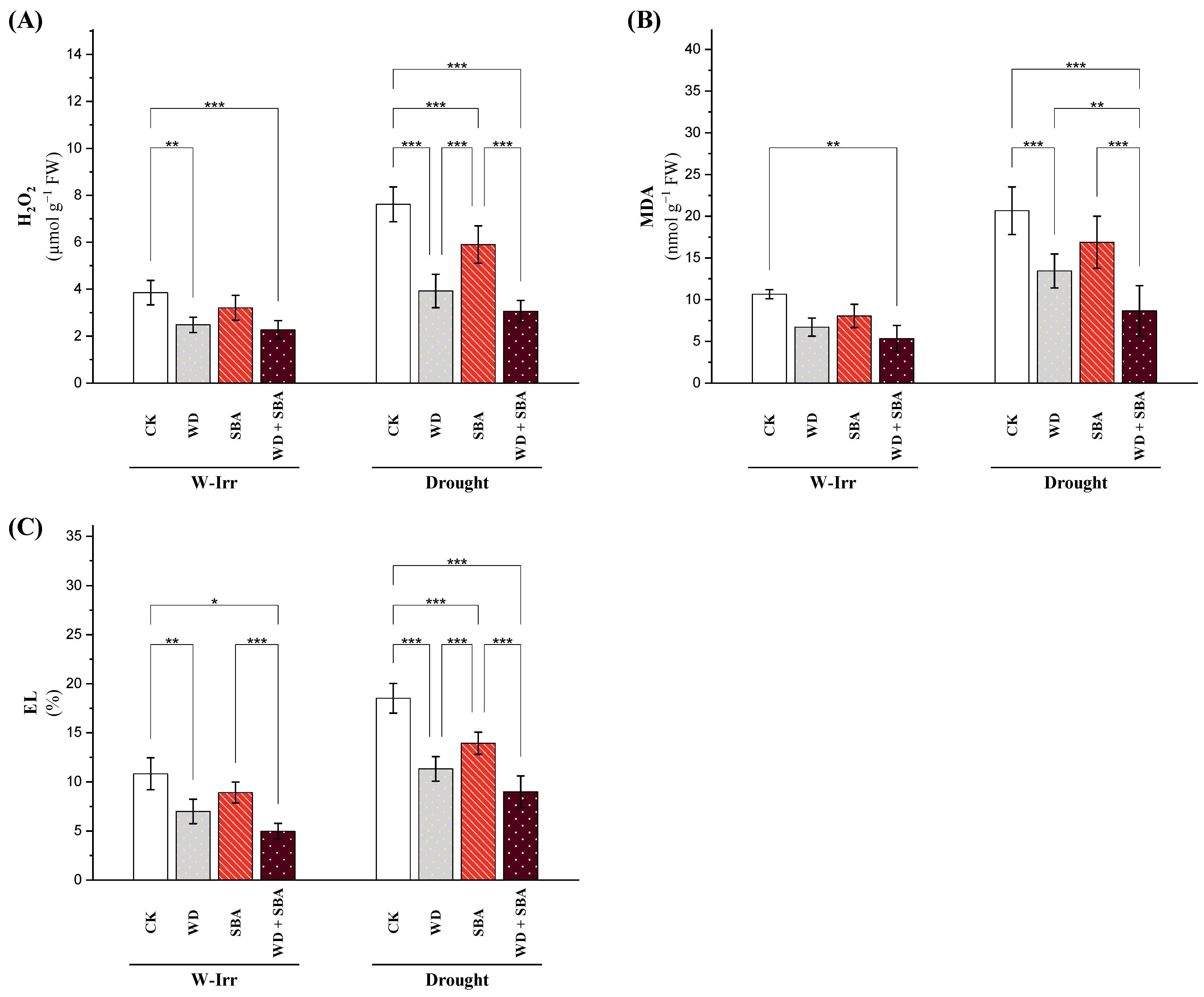
2.2.2. Oxidative Stress Indicators
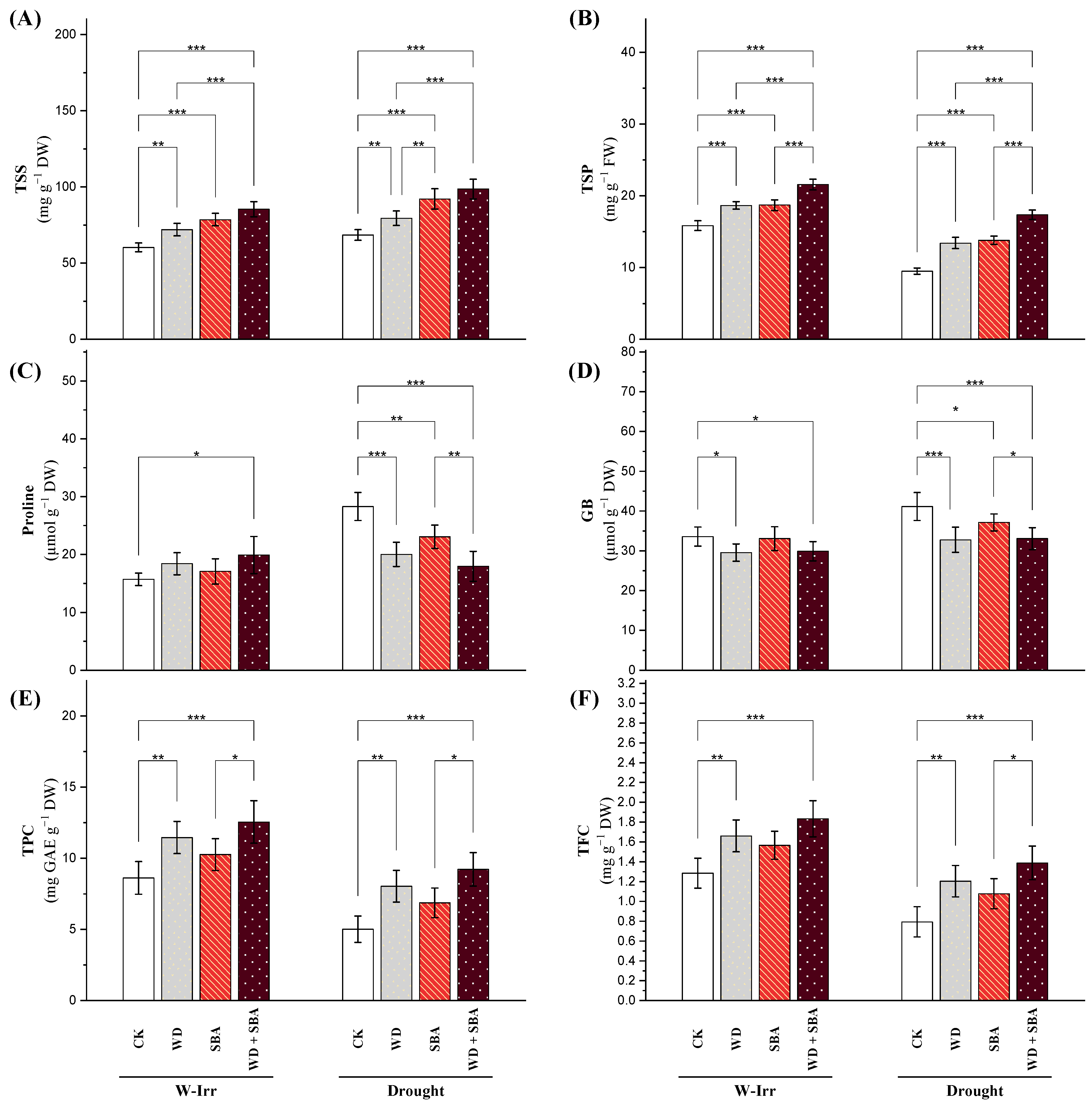
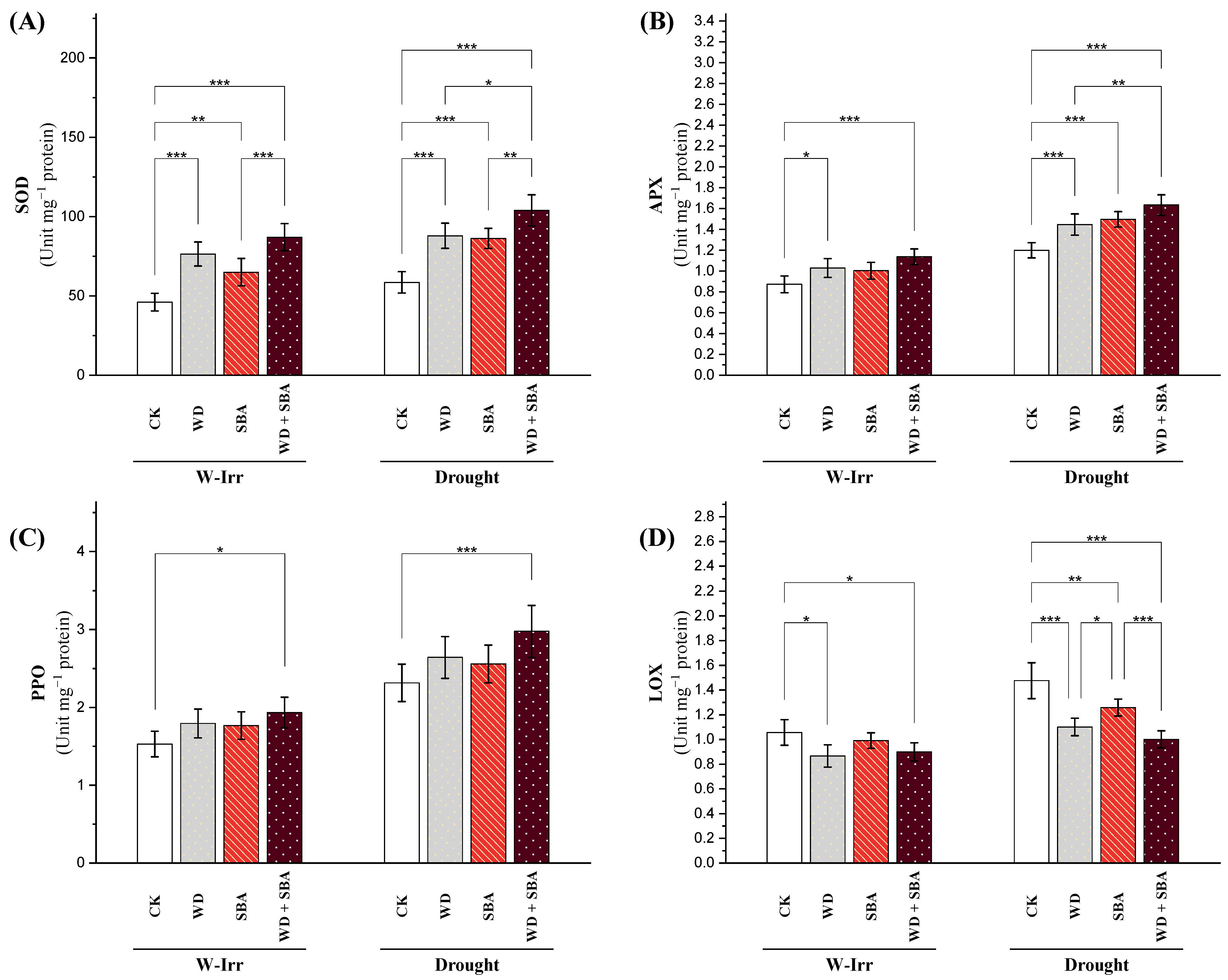
2.2.3. Antioxidant Defense System
Non-Enzymatic Antioxidants
Enzymatic Antioxidants
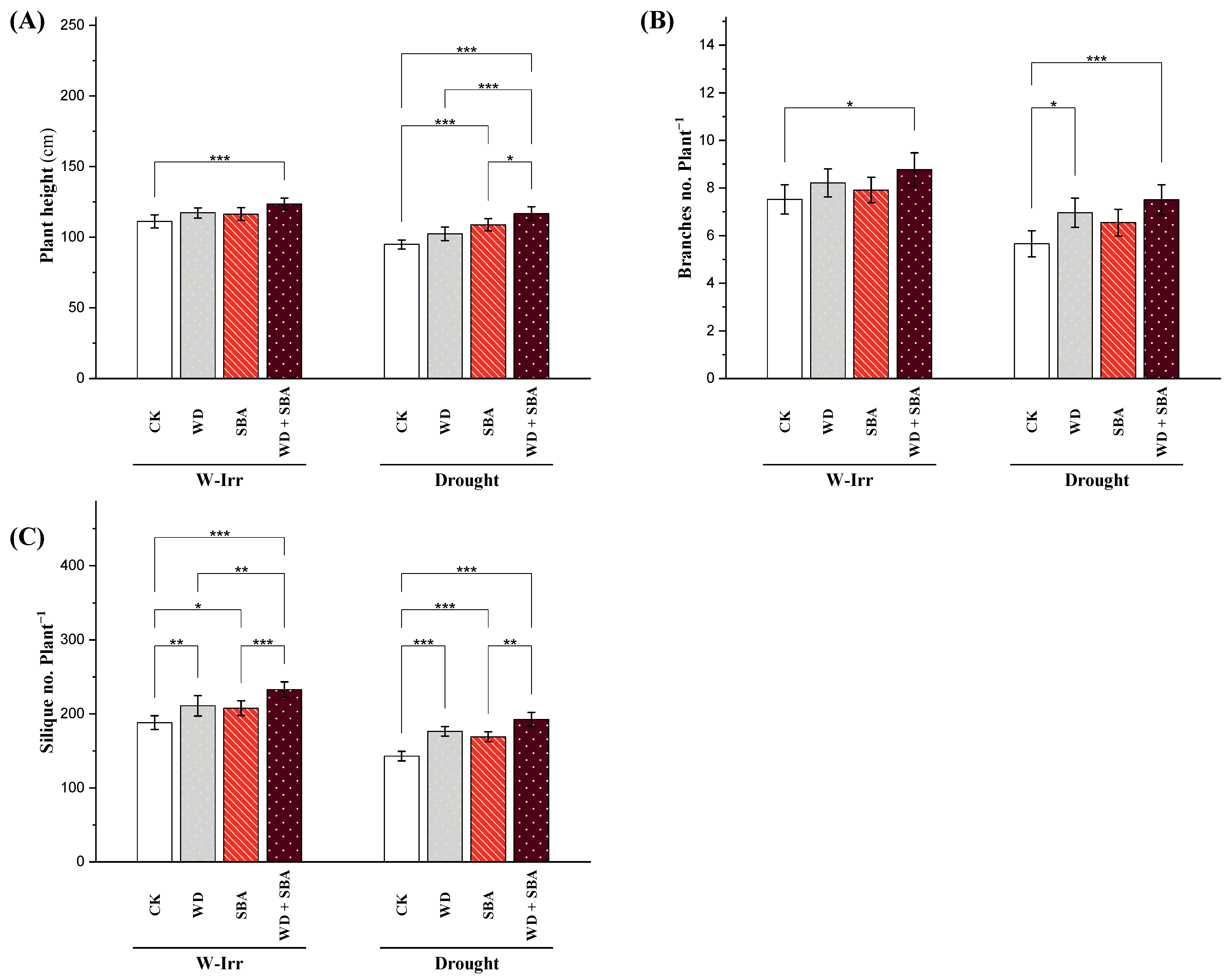
2.3. Plant Height, Number of Branches and Siliques
2.4. Yield Attributes
2.5. Relationship between Physiological and Yield Attributes
3. Discussion
4. Materials and Methods
4.1. Experimental Layout
4.1.1. SBA Characterization
4.1.2. Wood Distillate Characterization
4.2. Measurements
4.2.1. Exchangeable Sodium Percentage (ESP)
4.2.2. Leaf Na+ and K+ Determination
4.2.3. Chlorophyll Content (SPAD Reading)
4.2.4. Determination of Relative Water Content and Stomatal Conductance
4.2.5. Electrolyte Leakage, Lipid Peroxidation, and H2O2
4.2.6. Proline, GB, TSS, TSP, TPC, and TFC
Glycine Betaine (GB, µmol g−1 DW)
4.2.7. SOD, APX, PPO, and LOX
Superoxide Dismutase (SOD Units mg−1 Protein)
4.2.8. Attributes of Vegetative Growth and Oil Yield
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, M.; Gupta, S.K.; Mondal, A.K. Production and Trade of Major World Oil Crops. In Technological Innovations in Major World Oil Crops; Volume 1: Breeding; Gupta, S.K., Ed.; Springer: New York, NY, USA, 2012; pp. 1–15. [Google Scholar]
- Zhu, K.; Gu, S.; Liu, J.; Luo, T.; Khan, Z.; Zhang, K.; Hu, L. Wood vinegar as a complex growth regulator promotes the growth, yield, and quality of rapeseed. Agronomy 2021, 11, 510. [Google Scholar] [CrossRef]
- FAO. FAOSTAT Database. 2023. Available online: https://www.fao.org/faostat/en/#data/QC (accessed on 25 June 2024).
- Sun, W.-C.; Wu, J.-Y.; Fang, Y.; Liu, Q.; Yang, R.-Y.; Ma, W.-G.; Li, X.-C.; Zhang, J.-J.; Zhang, P.-F.; Cao, J.-M.; et al. Growth and development characteristics of winter rapeseed northern- extended from the cold and arid regions in China. Acta Agron. Sin. 2010, 36, 2124–2134. [Google Scholar] [CrossRef]
- Onacik-Gür, S.; Żbikowska, A. Effect of high-oleic rapeseed oil oleogels on the quality of short-dough biscuits and fat migration. J. Food Sci. Technol. 2020, 57, 1609–1618. [Google Scholar] [CrossRef]
- Hamzei, J.; Soltani, J. Deficit irrigation of rapeseed for water-saving: Effects on biomass accumulation, light interception and radiation use efficiency under different N rates. Agric. Ecosys. Environ. 2012, 155, 153–160. [Google Scholar] [CrossRef]
- Wu, W.; Ma, B.L.; Whalen, J.K. Enhancing Rapeseed Tolerance to Heat and Drought Stresses in a Changing Climate: Perspectives for Stress Adaptation from Root System Architecture. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2018; Volume 151, pp. 87–157. [Google Scholar]
- Zamani, S.; Nezami, M.T.; Habibi, D.; Khorshidi, M.B. Effect of quantitative and qualitative performance of four canola cultivars (Brassica napus L.) to salinity conditions. Adv. Environ. Biol. 2010, 4, 422–428. [Google Scholar]
- Tesfamariam, E.H.; Annandale, J.G.; Steyn, J.M. Water stress effects on winter canola growth and yield. Agron. J. 2010, 102, 658–666. [Google Scholar] [CrossRef]
- Chaves, M.M.; Oliveira, M.M. Mechanisms underlying plant resilience to water deficits: Prospects for water-saving agriculture. J. Exp. Bot. 2004, 55, 2365–2384. [Google Scholar] [CrossRef]
- Osman, H.S.; Gowayed, S.M.; Elbagory, M.; Omara, A.E.; El-Monem, A.M.A.; Abd El-Razek, U.A.; Hafez, E.M. Interactive impacts of beneficial microbes and Si-Zn nanocomposite on growth and productivity of soybean subjected to water deficit under salt-affected soil conditions. Plants 2021, 10, 1396. [Google Scholar] [CrossRef]
- Nehela, Y.; Mazrou, Y.S.A.; Alshaal, T.; Rady, A.M.S.; El-Sherif, A.M.A.; Omara, A.E.-D.; Abd El-Monem, A.M.; Hafez, E.M. The integrated amendment of sodic-saline soils using biochar and plant growth-promoting rhizobacteria enhances maize (Zea mays L.) resilience to water salinity. Plants 2021, 10, 1960. [Google Scholar] [CrossRef]
- Ghobadi, M.; Bakhshandeh, M.; Fathi, G.; Gharineh, M.H.; Alami-Said, K.; Naderi, A.; Ghobadi, M.E. Short and long periods of water stress during different growth stages of canola (Brassica napus L.): Effect on yield, yield components, seed oil and protein contents. J. Agron. 2006, 5, 336–341. [Google Scholar] [CrossRef]
- Hafez, E.M.; Osman, H.S.; Gowayed, S.M.; Okasha, S.A.; Omara, A.E.-D.; Sami, R.; Abd El-Monem, A.M.; Abd El-Razek, U.A. Minimizing the adversely impacts of water deficit and soil salinity on maize growth and productivity in response to the application of plant growth-promoting rhizobacteria and silica nanoparticles. Agronomy 2021, 11, 676. [Google Scholar] [CrossRef]
- Ondrasek, G.; Rathod, S.; Manohara, K.K.; Gireesh, C.; Anantha, M.S.; Sakhare, A.S.; Parmar, B.; Yadav, B.K.; Bandumula, N.; Raihan, F.; et al. Salt Stress in Plants and Mitigation Approaches. Plants 2022, 11, 717. [Google Scholar] [CrossRef]
- Hafez, E.M.; Osman, H.S.; El-Razek, U.A.A.; Elbagory, M.; Omara, A.E.-D.; Eid, M.A.; Gowayed, S.M. Foliar-applied potassium silicate coupled with plant growth-promoting rhizobacteria improves growth, physiology, nutrient uptake and productivity of faba bean (Vicia faba L.) irrigated with saline water in salt-affected soil. Plants 2021, 10, 894. [Google Scholar] [CrossRef] [PubMed]
- Hergert, G.W.; Margheim, J.F.; Pavlista, A.D.; Martin, D.L.; Supalla, R.J.; Isbell, T.A. Yield, irrigation response, and water productivity of deficit to fully irrigated spring canola. Agric. Water Manag. 2016, 168, 96–103. [Google Scholar] [CrossRef]
- Aslam, M.M.; Farhat, F.; Siddiqui, M.A.; Yasmeen, S.; Khan, M.T.; Sial, M.A.; Khan, I.A. Exploration of physiological and biochemical processes of canola with exogenously applied fertilizers and plant growth regulators under drought stress. PLoS ONE 2021, 16, e0260960. [Google Scholar] [CrossRef]
- Gong, D.H.; Wang, G.Z.; Si, W.T.; Zhou, Y.; Liu, Z.; Jia, J. Effects of salt stress on photosynthetic pigments and activity of ribulose-1,5-bisphosphate carboxylase/oxygenase in Kalidium foliatum. Russ. J. Plant Physiol. 2018, 65, 98–103. [Google Scholar] [CrossRef]
- Ding, Z.; Kheir, A.M.S.; Ali, O.A.M.; Hafez, E.M.; ElShamey, E.A.; Zhou, Z.; Wang, B.; Lin, X.e.; Ge, Y.; Fahmy, A.E.; et al. A vermicompost and deep tillage system to improve saline-sodic soil quality and wheat productivity. J. Environ. Manag. 2021, 277, 111388. [Google Scholar] [CrossRef]
- Hafez, E.M.; Kheir, A.M.S.; Badawy, S.A.; Rashwan, E.; Farig, M.; Osman, H.S. Differences in physiological and biochemical attributes of wheat in response to single and combined salicylic acid and biochar subjected to limited water irrigation in saline sodic soil. Plants 2020, 9, 1346. [Google Scholar] [CrossRef]
- Omara, A.E.-D.; Hafez, E.M.; Osman, H.S.; Rashwan, E.; El-Said, M.A.A.; Alharbi, K.; Abd El-Moneim, D.; Gowayed, S.M. Collaborative impact of compost and beneficial rhizobacteria on soil properties, physiological attributes, and productivity of wheat subjected to deficit irrigation in salt affected soil. Plants 2022, 11, 877. [Google Scholar] [CrossRef]
- Grewal, A.; Abbey, L.; Gunupuru, L.R. Production, prospects and potential application of pyroligneous acid in agriculture. J. Anal. Appl. Pyrolysis 2018, 135, 152–159. [Google Scholar] [CrossRef]
- Khan, Z.; Khan, M.N.; Zhang, K.; Luo, T.; Zhu, K.; Hu, L. The application of biochar alleviated the adverse effects of drought on the growth, physiology, yield and quality of rapeseed through regulation of soil status and nutrients availability. Ind. Crops Prod. 2021, 171, 113878. [Google Scholar] [CrossRef]
- Lashari, M.S.; Ye, Y.; Ji, H.; Li, L.; Kibue, G.W.; Lu, H.; Zheng, J.; Pan, G. Biochar–manure compost in conjunction with pyroligneous solution alleviated salt stress and improved leaf bioactivity of maize in a saline soil from central China: A 2-year field experiment. J. Sci. Food Agric. 2015, 95, 1321–1327. [Google Scholar] [CrossRef]
- Lu, X.; Jiang, J.; He, J.; Sun, K.; Sun, Y. Effect of pyrolysis temperature on the characteristics of wood vinegar derived from chinese fir waste: A comprehensive study on its growth regulation performance and mechanism. ACS Omega 2019, 4, 19054–19062. [Google Scholar] [CrossRef] [PubMed]
- Mungkunkamchao, T.; Kesmala, T.; Pimratch, S.; Toomsan, B.; Jothityangkoon, D. Wood vinegar and fermented bioextracts: Natural products to enhance growth and yield of tomato (Solanum lycopersicum L.). Sci. Hortic. 2013, 154, 66–72. [Google Scholar] [CrossRef]
- Ma, J.; Islam, F.; Ayyaz, A.; Fang, R.; Hannan, F.; Farooq, M.A.; Ali, B.; Huang, Q.; Sun, R.; Zhou, W. Wood vinegar induces salinity tolerance by alleviating oxidative damages and protecting photosystem II in rapeseed cultivars. Ind. Crops Prod. 2022, 189, 115763. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Liu, B.; Liu, Q.; Zheng, H.; You, X.; Sun, K.; Luo, X.; Li, F. Comparative study of individual and Co-Application of biochar and wood vinegar on blueberry fruit yield and nutritional quality. Chemosphere 2020, 246, 125699. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Shi, A.; Rensing, C.; Yang, J.; Ni, W.; Xing, S.; Yang, W. Wood vinegar facilitated growth and Cd/Zn phytoextraction of Sedum alfredii Hance by improving rhizosphere chemical properties and regulating bacterial community. Environ. Pollut. 2022, 305, 119266. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Liu, J.; Luo, T.; Zhang, K.; Khan, Z.; Zhou, Y.; Cheng, T.; Yuan, B.; Peng, X.; Hu, L. Wood vinegar impact on the growth and low-temperature tolerance of rapeseed seedlings. Agronomy 2022, 12, 2453. [Google Scholar] [CrossRef]
- Nakhla, D.A.; El-Haggar, S. A proposal to environmentally balanced sugarcane industry in Egypt. Int. J. Agric. Policy Res. 2014, 2, 321–328. [Google Scholar] [CrossRef]
- Patel, H. Environmental valorisation of bagasse fly ash: A review. RSC Adv. 2020, 10, 31611–31621. [Google Scholar] [CrossRef]
- Alabi, D.; Coopoosamy, R.; Naidoo, K.; Arthur, G. Composted bagasse: An impact on agricultural crop production. J. Agric. Sci. Food Res. 2022, 13, 1000483. [Google Scholar]
- Azadi, N.; Raiesi, F. Sugarcane bagasse biochar modulates metal and salinity stresses on microbial functions and enzyme activities in saline co-contaminated soils. Appl. Soil Ecol. 2021, 167, 104043. [Google Scholar] [CrossRef]
- Raza, Q.-U.-A.; Bashir, M.A.; Rehim, A.; Sial, M.U.; Ali Raza, H.M.; Atif, H.M.; Brito, A.F.; Geng, Y. Sugarcane industrial byproducts as challenges to environmental safety and their remedies: A review. Water 2021, 13, 3495. [Google Scholar] [CrossRef]
- Pandey, A.; Soccol, C.R.; Nigam, P.; Soccol, V.T. Biotechnological potential of agro-industrial residues. I: Sugarcane bagasse. Bioresour. Technol. 2000, 74, 69–80. [Google Scholar] [CrossRef]
- Alharbi, K.; Hafez, E.M.; Omara, A.E.-D.; Nehela, Y. Composted bagasse and/or cyanobacteria-based bio-stimulants maintain barley growth and productivity under salinity stress. Plants 2023, 12, 1827. [Google Scholar] [CrossRef]
- Zafeer, M.K.; Menezes, R.A.; Venkatachalam, H.; Bhat, K.S. Sugarcane bagasse-based biochar and its potential applications: A review. Emergent Mater. 2024, 7, 133–161. [Google Scholar] [CrossRef]
- Xu, N.; Bhadha, J.H.; Rabbany, A.; Swanson, S.; McCray, J.M.; Li, Y.C.; Strauss, S.L.; Mylavarapu, R. Crop nutrition and yield response of bagasse application on sugarcane grown on a mineral soil. Agronomy 2021, 11, 1526. [Google Scholar] [CrossRef]
- Nie, C.; Yang, X.; Niazi, N.K.; Xu, X.; Wen, Y.; Rinklebe, J.; Ok, Y.S.; Xu, S.; Wang, H. Impact of sugarcane bagasse-derived biochar on heavy metal availability and microbial activity: A field study. Chemosphere 2018, 200, 274–282. [Google Scholar] [CrossRef]
- Zafar-ul-Hye, M.; Wasim, M.M.; Munir, T.M.; Aon, M.; Shaaban, M.; Abbas, M.; Hussain, M.; Ahmad, M. Co-application of sugarcane bagasse biochar, farmyard manure and mineral nitrogen improved growth indices of corn grown in alkaline calcareous soil. J. Plant Nutr. 2020, 43, 1293–1305. [Google Scholar] [CrossRef]
- Bashir, S.; Hussain, Q.; Akmal, M.; Riaz, M.; Hu, H.; Ijaz, S.S.; Iqbal, M.; Abro, S.; Mehmood, S.; Ahmad, M. Sugarcane bagasse-derived biochar reduces the cadmium and chromium bioavailability to mash bean and enhances the microbial activity in contaminated soil. J. Soils Sediments 2018, 18, 874–886. [Google Scholar] [CrossRef]
- Khan, M.I.; Shoukat, M.A.; Cheema, S.A.; Ali, S.; Azam, M.; Rizwan, M.; Qadri, R.; Al-Wabel, M.I. Foliar- and soil-applied salicylic acid and bagasse compost addition to soil reduced deleterious effects of salinity on wheat. Arab. J. Geosci. 2019, 12, 78. [Google Scholar] [CrossRef]
- Theerakulpisut, P.; Madee, P.; Pamuta, D.; Nounjan, N. Exogenous application of spermidine (SPD) and wood vinegar improves salt tolerance in salt-sensitive rice (Oryza sativa L.). Pak. J. Bot. 2021, 53, 1–9. [Google Scholar] [CrossRef]
- Becagli, M.; Arduini, I.; Cantini, V.; Cardelli, R. Soil and foliar applications of wood distillate differently affect soil properties and field bean traits in preliminary field tests. Plants 2023, 12, 121. [Google Scholar] [CrossRef]
- Srisompan, A.; Theerakulpisut, P. The effect of exogenous spermidine and wood vinegar on growth and physiology of rice (Oryza sativa L.) cv. RD6 under salt stress. Curr. Appl. Sci. Technol. 2019, 19, 289–296. [Google Scholar]
- Luo, X.; Wang, Z.; Meki, K.; Wang, X.; Liu, B.; Zheng, H.; You, X.; Li, F. Effect of co-application of wood vinegar and biochar on seed germination and seedling growth. J. Soils Sediments 2019, 19, 3934–3944. [Google Scholar] [CrossRef]
- Alharbi, K.; Osman, H.S.; Rashwan, E.; Hafez, E.M.; Omara, A.E.-D. Stimulating the growth, anabolism, antioxidants, and yield of rice plants grown under salt stress by combined application of bacterial inoculants and nano-silicon. Plants 2022, 11, 3431. [Google Scholar] [CrossRef]
- Mohd Amnan, M.A.; Teo, W.F.A.; Aizat, W.M.; Khaidizar, F.D.; Tan, B.C. Foliar application of oil palm wood vinegar enhances Pandanus amaryllifolius tolerance under drought stress. Plants 2023, 12, 785. [Google Scholar] [CrossRef]
- Wang, Y.; Qiu, L.; Song, Q.; Wang, S.; Wang, Y.; Ge, Y. Root proteomics reveals the effects of wood vinegar on wheat growth and subsequent tolerance to drought stress. Int. J. Mol. Sci. 2019, 20, 943. [Google Scholar] [CrossRef]
- Yang, J.-F.; Yang, C.-H.; Liang, M.-T.; Gao, Z.-J.; Wu, Y.-W.; Chuang, L.-Y. Chemical composition, antioxidant, and antibacterial activity of wood vinegar from Litchi chinensis. Molecules 2016, 21, 1150. [Google Scholar] [CrossRef]
- Arshad, M.A.C.; Lowery, B.; Grossman, B. Physical Tests for Monitoring Soil Quality; SSSA Special Publications; SSSA: Madison, WI, USA, 2015; pp. 123–141. [Google Scholar] [CrossRef]
- Temminghoff, E.E.; Houba, V.J. Plant Analysis Procedures; Springer: Dordrecht, The Netherlands, 2004; p. 179. [Google Scholar]
- Ling, Q.; Huang, W.; Jarvis, P. Use of a SPAD-502 meter to measure leaf chlorophyll concentration in Arabidopsis thaliana. Photosyn. Res. 2010, 107, 209–214. [Google Scholar] [CrossRef]
- Weatherley, P.E. Studies in the water relations of the cotton plant. I. The field measurement of water deficits in leaves. New Phytol. 1950, 49, 81–97. [Google Scholar] [CrossRef]
- Bajji, M.; Kinet, J.-M.; Lutts, S. The use of the electrolyte leakage method for assessing cell membrane stability as a water stress tolerance test in durum wheat. Plant Growth Regul. 2002, 36, 61–70. [Google Scholar] [CrossRef]
- Du, Z.; Bramlage, W.J. Modified thiobarbituric acid assay for measuring lipid oxidation in sugar-rich plant tissue extracts. J. Agric. Food Chemist. 1992, 40, 1566–1570. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Grieve, C.M.; Grattan, S.R. Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 1983, 70, 303–307. [Google Scholar] [CrossRef]
- Sadasivam, S.; Manickam, A. Biochemical Methods, 3rd ed.; New Age International Publishers: New Delhi, India, 2010. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analyt. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Baydar, N.G.; Özkan, G.; Sağdiç, O. Total phenolic contents and antibacterial activities of grape (Vitis vinifera L.) extracts. Food Control 2004, 15, 335–339. [Google Scholar] [CrossRef]
- Chang, C.-C.; Yang, M.-H.; Wen, H.-M.; Chern, J.-C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 3. [Google Scholar] [CrossRef]
- Osman, H.S.; Salim, B.B.M. Enhancing antioxidants defense system of snap bean under NaCl salinity using foliar application of salicylic acid, spermidine and glycine betaine. Am.-Eurasian J. Agric. Environ. Sci. 2016, 16, 1200–1210. [Google Scholar]
- Osman, H.S. Enhancing antioxidant–yield relationship of pea plant under drought at different growth stages by exogenously applied glycine betaine and proline. Ann. Agric. Sci. 2015, 60, 389–402. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Analyt. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Jebara, S.; Jebara, M.; Limam, F.; Aouani, M.E. Changes in ascorbate peroxidase, catalase, guaiacol peroxidase and superoxide dismutase activities in common bean (Phaseolus vulgaris) nodules under salt stress. J. Plant Physiol. 2005, 162, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Raymond, J.; Rakariyatham, N.; Azanza, J.L. Purification and some properties of polyphenoloxidase from sunflower seeds. Phytochemistry 1993, 34, 927–931. [Google Scholar] [CrossRef]
- Grossman, S.; Zakut, R. Determination of the Activity of Lipoxygenase (Lipoxidase). In Methods of Biochemical Analysis; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1979; pp. 303–329. [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC International, 18th ed.; Association of Official Analytical Chemists: Washengton, DC, USA, 2005. [Google Scholar]




| Year Month | 2021/2022 | 2022/2023 | ||||||
|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | Rainfall (mm) | Relative Humidity (%) | Temperature (°C) | Rainfall (mm) | Relative Humidity (%) | |||
| Max | Min | Max | Min | |||||
| November | 25.52 | 16.44 | 1.02 | 34.56 | 26.06 | 16.2 | 1.48 | 32.14 |
| December | 24.42 | 14.33 | 1.15 | 36.22 | 24.96 | 14.3 | 1.36 | 33.74 |
| January | 23.32 | 12.76 | 1.25 | 37.14 | 23.86 | 12.4 | 1.08 | 33.24 |
| February | 21.74 | 9.44 | 2.48 | 47.36 | 22.28 | 11.1 | 3.86 | 42.94 |
| March | 22.36 | 10.67 | 1.48 | 46.47 | 22.9 | 10.7 | 7.39 | 43.64 |
| April | 23.14 | 11.69 | 0.89 | 45.65 | 23.68 | 12.5 | 1.17 | 45.34 |
| May | 24.36 | 12.48 | 0.75 | 44.25 | 24.9 | 16.2 | 0.54 | 44.54 |
| Cations (meq L−1) | Anions (meq L−1) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Season | OM (%) | Soil Texture | EC (dS m−1) | FC (%) | pH | Na+ | K+ | Mg+2 | Ca+2 | Cl− | HCO3− | SO4−2 | |
| Soil | 2021/2022 | 1.24 | Clayey | 8.33 | 32.56 | 8.99 | 18.01 | 10.96 | 14.99 | 16.77 | 23.79 | 17.45 | 19.26 |
| 2022/2023 | 1.38 | Clayey | 7.87 | 33.23 | 8.67 | 18.88 | 12.09 | 16.46 | 18.52 | 24.44 | 19.9 | 21.38 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hafez, E.M.; Gao, Y.; Alharbi, K.; Chen, W.; Elhawat, N.; Alshaal, T.; Osman, H.S. Antioxidative and Metabolic Responses in Canola: Strategies with Wood Distillate and Sugarcane Bagasse Ash for Improved Growth under Abiotic Stress. Plants 2024, 13, 2152. https://doi.org/10.3390/plants13152152
Hafez EM, Gao Y, Alharbi K, Chen W, Elhawat N, Alshaal T, Osman HS. Antioxidative and Metabolic Responses in Canola: Strategies with Wood Distillate and Sugarcane Bagasse Ash for Improved Growth under Abiotic Stress. Plants. 2024; 13(15):2152. https://doi.org/10.3390/plants13152152
Chicago/Turabian StyleHafez, Emad M., Yan Gao, Khadiga Alharbi, Wei Chen, Nevien Elhawat, Tarek Alshaal, and Hany S. Osman. 2024. "Antioxidative and Metabolic Responses in Canola: Strategies with Wood Distillate and Sugarcane Bagasse Ash for Improved Growth under Abiotic Stress" Plants 13, no. 15: 2152. https://doi.org/10.3390/plants13152152
APA StyleHafez, E. M., Gao, Y., Alharbi, K., Chen, W., Elhawat, N., Alshaal, T., & Osman, H. S. (2024). Antioxidative and Metabolic Responses in Canola: Strategies with Wood Distillate and Sugarcane Bagasse Ash for Improved Growth under Abiotic Stress. Plants, 13(15), 2152. https://doi.org/10.3390/plants13152152










