Abstract
Chagas disease, caused by the protozoan Trypanosoma cruzi, represents an important and worldwide public health issue, particularly in Latin America. Limitations of conventional treatment with benznidazole and nifurtimox underscore the urgent need for new therapeutic strategies for this disease. Schinus molle, a tree used in traditional medicine for various ailments, has demonstrated promising antiparasitic activity. The in vitro anti-T. cruzi activity of Schinus molle crude methanol extract, partitions, and fractions, as well as their cytotoxicity in Vero cells and Artemia salina, and hemolytic activity in human erythrocytes were assessed. Most of the extracts possessed anti-T. cruzi effects, with Sm-CF3 being the fraction with the highest activity (IC50 = 19 µg/mL; SI = 6.8). Gas chromatography–mass spectrometry analysis identified 20 compounds, with fatty acyls comprising the predominant chemical class (55%). We also identified the antiparasitic compounds cis-5,8,11,14,17-eicosapentaenoic acid and trans-Z-α-bisabolene epoxide, suggesting their potential contribution to the observed anti-T. cruzi activity. In conclusion, our findings support the therapeutic potential of S. molle as a source of novel antiparasitic agents against T. cruzi.
1. Introduction
Chagas disease, also known as American trypanosomiasis, is a parasitic zoonotic disease caused by the hemoflagellate protozoan Trypanosoma cruzi (Chagas, 1909) [1]. It is the most important parasitic disease in Latin America and is classified among the 20 Neglected Tropical Diseases (NTDs), with an estimated 6 to 8 million people infected worldwide, mostly in Latin America [2,3]. However, the distribution of the disease is changing due to the relocation of individuals from endemic countries. It is also estimated that more than 12,000 people die each year from this disease, and more than 75 million individuals are at risk of contracting it [2]. In Latin America, T. cruzi infection mainly occurs through contact with contaminated feces of hematophagous triatomine bugs, which are the vectors of this parasite [4]. In addition, the transmission may occur congenitally or by blood transfusions and organ transplants, representing the main modes of infection in urban areas and non-endemic countries. Moreover, T. cruzi may be transmitted through the consumption of food contaminated with the parasite [5].
Once the parasite is acquired, Chagas disease involves acute and chronic clinical phases. The acute phase usually goes unnoticed due to non-specific symptoms and generally lasts 4 to 8 weeks [6]. However, during the chronic phase, which may develop 10 to 30 years after infection, an estimated 30% to 40% of infected individuals develop potentially lethal cardiac or gastrointestinal disease [6,7].
Chagas disease becomes chronic without treatment, and only the drugs nifurtimox and benznidazole, developed more than 50 years ago, are commercially available for its treatment [3]. These drugs show more than 80% efficacy during the acute phase [5]. Nevertheless, they have limitations. They are marginally effective during the chronic phase, require long treatment periods, and produce side effects [8]. Benznidazole commonly generates dermatitis, peripheral neuropathy, anorexia, bone marrow suppression, nausea, and vomiting, whereas nifurtimox causes anorexia, nausea, vomiting, abdominal pain, headache, dizziness, and neuropathy. As a result, many patients discontinue treatment [1]. Furthermore, these drugs are cytotoxic and genotoxic [9]. In addition, some T. cruzi strains have been reported to be naturally resistant to both drugs, making them ineffective [10]. Therefore, it is of utmost importance to seek alternative treatments.
The development of new antiparasitic drugs has not been a priority for the pharmaceutical industry because many parasitic diseases occur in poor countries, where people cannot afford high-priced medications [11]. An alternative approach involves exploring plant extracts or their secondary metabolites for their antiparasitic properties. Plants are a source of a wide range of natural products that possess various therapeutic properties and are continuously explored to develop novel drugs [12]. Nowadays, more than 25% of drugs used during the last 20 years are directly derived from plants or are chemically altered molecules. However, only 5% to 15% of the approximately 260,000 higher plants have been investigated for bioactive compounds [13].
Schinus molle L. (1753) commonly known as pepper tree or pirul, is an evergreen tree from the Anacardiaceae family that originates in South America and is widespread around the globe, including the Mediterranean, tropical, and subtropical regions, as well as South Africa [14]. Traditionally, this plant has been used to treat cough, tuberculosis, bronchitis, fever, eye infection, allergy, hemorrhoids, respiratory infections, jaundice, diarrhea, and tonsillitis. In Ethiopia, this plant is also used to treat malaria [15]. Furthermore, its crude methanol extract has been shown to possess anti-T. cruzi activity [16,17]. However, its active compounds have not been identified yet. Therefore, we aimed to evaluate the anti-T. cruzi potential of different S. molle extracts, partitions, and fractions and identify their active compounds.
2. Results
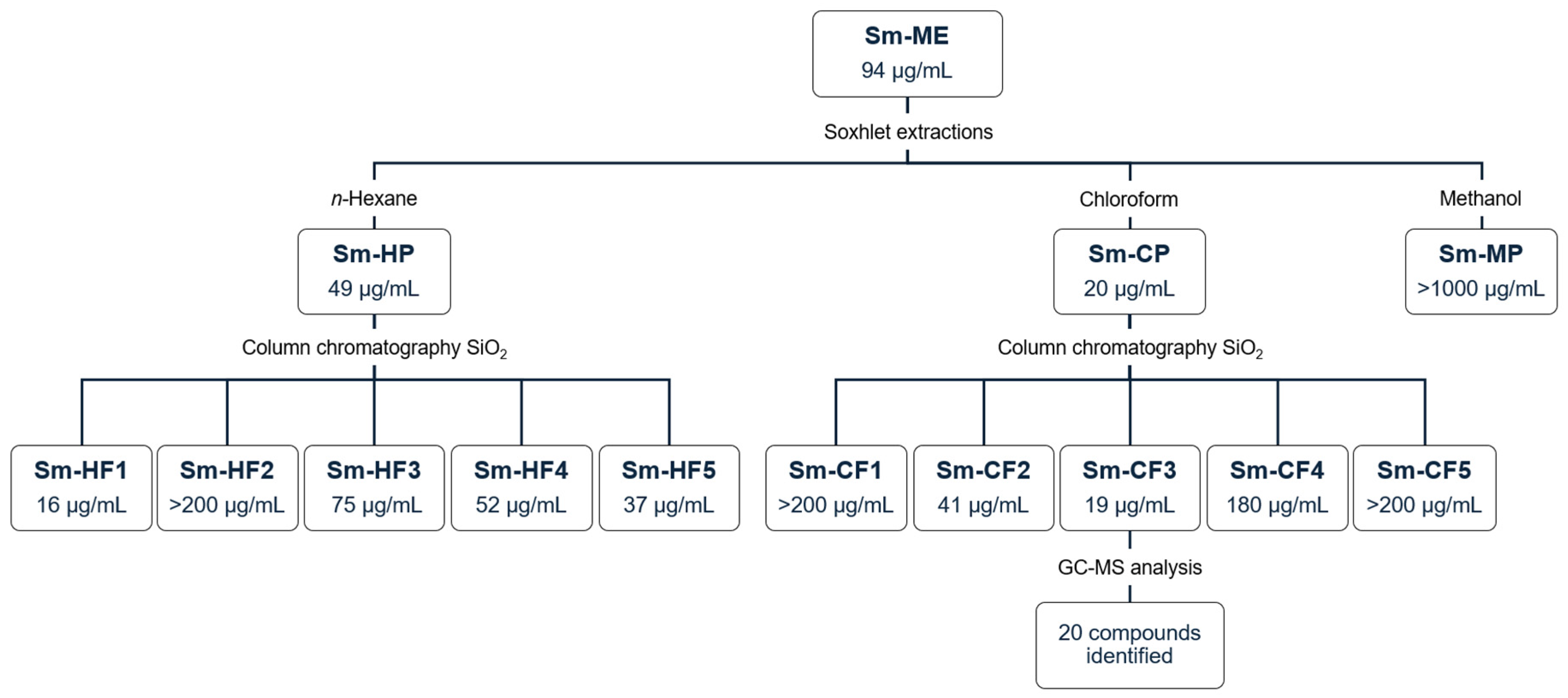
Figure 1 shows the general experimental protocol implemented during the bioguided fractionation of S. molle crude methanol extract, as well as the IC50 against T. cruzi obtained for each extract.
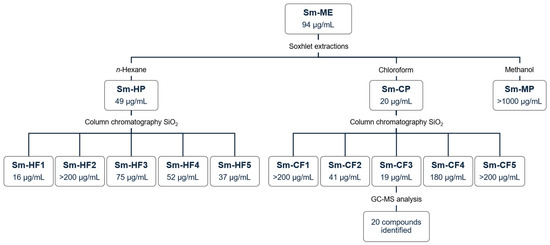
Figure 1.
Bioguided fractionation of S. molle. Data represent IC50 against T. cruzi. Sm-ME: S. molle crude methanol extract; Sm-HP: S. molle n-hexane partition; Sm-CP: S. molle chloroform partition; Sm-MP: S. molle methanol partition; Sm-HF: S. molle hexane fraction; Sm-CF: S. molle chlorofom fraction.
2.1. Biological Activity of S. molle Crude Methanol Extract and Partitions
We evaluated S. molle crude methanol extract (Sm-ME) and partitions obtained with solvents of increasing polarity (n-hexane, chloroform, and methanol). The yield and biological activity of these extracts are shown in Table 1. Sm-ME showed a yield of 21.5%, with an anti-T. cruzi IC50 of 94 µg/mL and an SI of 2.3. The partition with the highest yield was Sm-MP (42.4%, 2.4 g), followed by Sm-HP (24.8%, 1.4 g) and Sm-CP (8.3%, 0.5 g). It is important to mention that after continuous Soxhlet extractions, an insoluble fraction was obtained with a significant yield (19.9%, 1.1 g), which was not evaluated because of its insolubility. Furthermore, despite presenting the lowest yield, Sm-CP was the one that showed the highest anti-T. cruzi activity with an IC50 of 20 µg/mL and an SI of 5.6, whereas the partition with the highest yield (Sm-MP) did not show biological activity. We observed that Sm-CP showed better activity than nifurtimox, which exhibited an IC50 of 32 µg/mL. Based on their IC50 values, Sm-ME and Sm-CP were moderately cytotoxic, whereas Sm-HP was cytotoxic on Vero cells; Sm-HP and Sm-CP were moderately toxic, whereas Sm-ME was not toxic to A. salina, and all extracts were non-hemolytic (Table 1).

Table 1.
Yield, anti-Trypanosoma cruzi, cytotoxic, toxic, and hemolytic activity of S. molle crude methanol extract and partitions.
2.2. Activity of S. molle Hexane and Chloroform Fractions
Five collective fractions of Sm-HP (Sm-HF1 to Sm-HF5) and Sm-CP (Sm-CF1 to Sm-CF5) were obtained, showing yields from 2.7% to 66.9%. The yield and biological activity of the fractions are shown in Table 2. SmHF1 presented the highest anti-T. cruzi activity with an IC50 of 16 µg/mL and an SI of 5.8 and Sm-CF3 showed the highest activity with an IC50 of 21 µg/mL and an SI of 6.8, having the highest SI of the ten fractions. Based on their IC50 values, all fractions evaluated were classified as moderately cytotoxic except for Sm-HF1, which was cytotoxic on Vero cells. All fractions were moderately toxic except for Sm-HF2 and Sm-CF4, which were not toxic on A. salina, and all were non-hemolytic (Table 2).

Table 2.
Yield, anti-Trypanosoma cruzi, cytotoxic, toxic, and hemolytic activity of S. molle hexane and chloroform fractions.
2.3. Identified Compounds in the S. molle Sm-CF3 Fraction
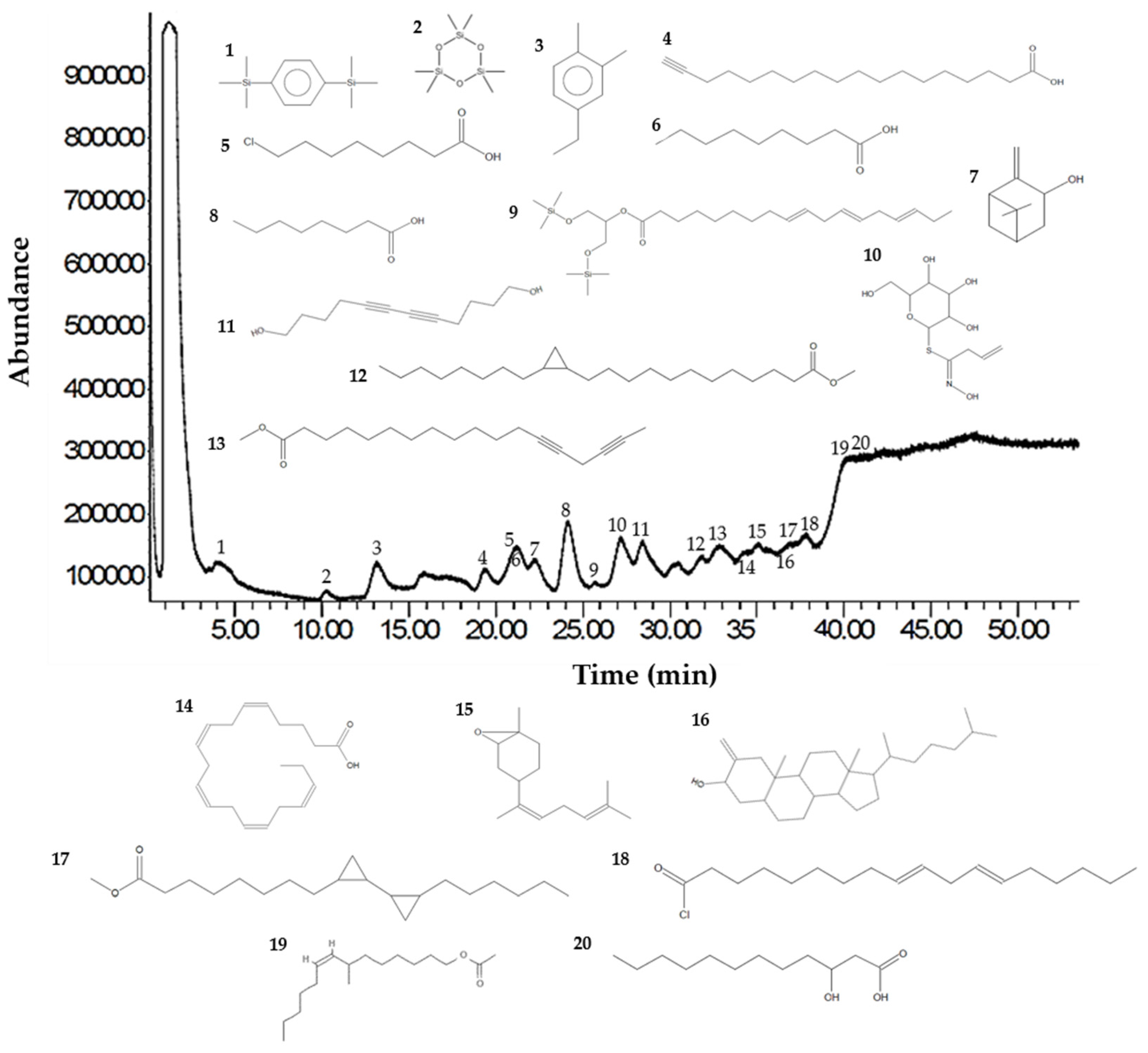
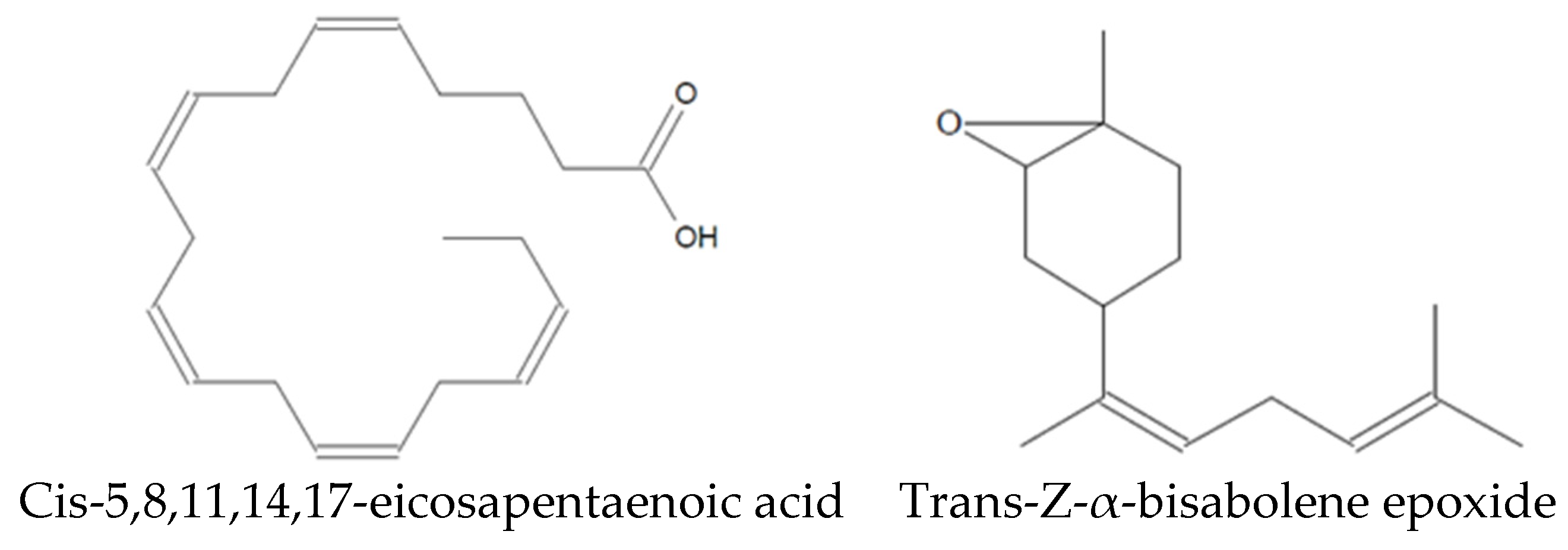
Analysis of the Sm-CF3 fraction with GC-MS revealed the presence of 20 compounds (Table 3, Figure 2, and Supplementary Figure S1A–D). Most of them belong to the chemical class of fatty acyls (11/20 = 55%), followed by organometalloid compounds (2/20 = 10%), prenol lipids (2/20 = 10%), and benzene and substituted derivatives, organooxygen compounds, steroids and steroid derivatives, acyl halides, and hydroxy acids and derivatives (1/20 = 10% each one). These compounds have different pharmacological effects but most of these reports evaluated antimicrobial activity, followed by antiparasitic, and antifungal activity. Compounds with antiparasitic activity reported were cis-5,8,11,14,17-eicosapentaenoic acid and trans-Z-α-bisabolene epoxide (Figure 3).

Table 3.
GC-MS results of the Sm-CF3 fraction.
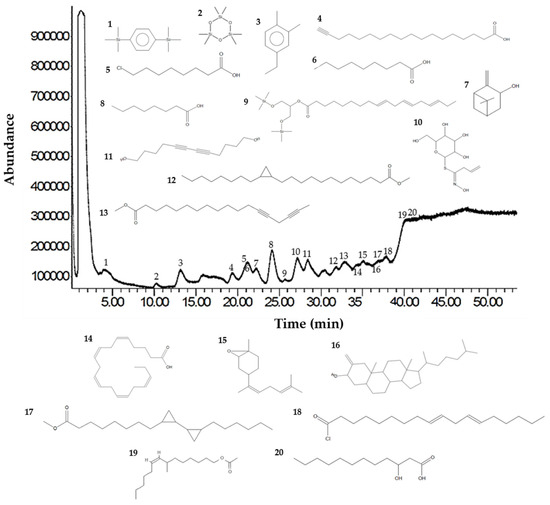
Figure 2.
GC-MS chromatogram of the Sm-CF3 fraction. The UPAC names, synonym, formula, mass, m/z, and class of the compounds are explained in Table 3.
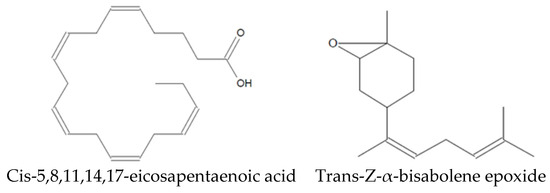
Figure 3.
Compounds identified in Sm-CF3 with reported antiparasitic activity.
3. Discussion
In this study, the pharmacological potential of S. molle against T. cruzi was evaluated. Although there are reports of its anti-T. cruzi activity [16,17], only the crude methanol extract has been investigated. However, identification of anti-T. cruzi compounds and their toxicity and SI remain to be elucidated. According to Osorio et al. classification [18], most evaluated extracts fall into the category of active (IC50 between 10 µg/mL to 50 µg/mL), with Sm-HF1 being the most potent with an IC50 of 16 µg/mL, as compared with nifurtimox activity (IC50 = 32 µg/mL). Ten-fold differences among the IC50 values of Sm-ME and SM-MP were obtained. This may be due to the crude methanol extract (Sm-ME), which contains all non-polar, moderately polar, and polar compounds that were extracted from the plant. Furthermore, in the methanol partition (Sm-MP), only polar compounds of the plant are present, since non-polar and moderately polar compounds were extracted in the hexane and chloroform partitions, respectively. This indicates that the compounds with activity against T. cruzi are mainly non-polar and moderately polar compounds, as confirmed by their identification. This confirms the previously reported anti-T. cruzi activity of the plant. Furthermore, this plant has shown activity against other protozoa such as Leishmania amazonensis [19] and Plasmodium berghei [14], as well as helminthicide activity against Haemonchus contortus [20]. This underscores its broad-spectrum antiparasitic potential, which makes this plant an ideal candidate to identify compounds with antiparasitic activity.
Moreover, the T. cruzi strain used in this study belongs to the discrete typing unit TcI, which is the most prevalent in Latin America and known for its resistance to conventional antichagasic drugs [21,22]. Our findings indicate significant activity of S. molle against this strain, suggesting its potential as an alternative or adjunct therapy for Chagas disease, particularly in regions with prevalent drug-resistant strains.
In addition, Vero cells were selected to evaluate cytotoxicity and determine the SIs of the extracts, as they are commonly used for such evaluations in studies investigating extracts of plants for antiparasitic activity [23]. According to Osorio et al.’s classification [18], most of the extracts were classified as moderately cytotoxic (IC50 between 100 µg/mL to 1000 µg/mL), as compared with the well-known cytotoxicity of nifurtimox and benznidazole [9]. For parasites, a plant extract may be assumed bioactive and non-toxic if SI > 1, indicating differing toxic and parasitic components [24]. The higher the SI value, the safer the extract. Most evaluated extracts in this study have SIs > 2, indicating optimal antiparasitic activity, with Sm-CF3 showing the highest SI of 6.8, suggesting promising activity.
Furthermore, the Artemia salina assay serves as a preliminary toxicity assessment tool [25] and correlates strongly with acute oral toxicity results in mice, and according to Fernández-Calienes et al. classification [26], most of the extracts are moderately toxic (IC50 between 100 µg/mL to 1000 µg/mL), indicating promising results, as they demonstrated adequate anti-T. cruzi activity and low toxicity [26].
Since Sm-CF3 showed the highest SI, we identified its constituent compounds. Most of the identified compounds belong to the chemical class of fatty acyls (55%). It has been previously reported that fatty acids possess important biological properties such as antibacterial, antifungal, and antiparasitic activity [27]. Therefore, this group may be responsible for the anti-T. cruzi activity of the fraction.
Among the 20 compounds identified, two of them have been reported as antiparasitic. However, they have not been assessed in their pure form. Cholestan-3-ol, 2-methylene- (3β,5α) has been identified in Achillea wilhelmsii extract, which shows activity against Leishmania major [28], whereas isopinocarveol has been identified in Melaleuca styphelioides extract, showing activity against Acanthamoeba castellanii [29]. In addition, other compounds have been evaluated in their pure form, demonstrating antiparasitic activity. Cis-5,8,11,14,17-eicosapentaenoic acid has activity against Trichomonas vaginalis [30], whereas trans-Z-α-bisabolene epoxide has activity against Leishmania sp. [31]. As Leishmania and Trypanosoma are very similar protozoa, with them being hemoflagellates and belonging to the Trypanosomatidae family [32], trans-Z-α-bisabolene epoxide may have anti-T. cruzi potential.
Nevertheless, further biotargeted fractionation is required to isolate and identify the bioactive compounds with antiparasitic activity. Moreover, it is essential to evaluate these compounds in an in vivo murine model to validate their antiparasitic efficacy and to exclude any potential toxicity to humans.
4. Materials and Methods
4.1. Ethical Statement
The procedures used in this study were approved by the Ethics Committee of the Facultad de Ciencias Biológicas (FCB) at the Universidad Autónoma de Nuevo León (UANL), registration no. CI-08-2020. Experiments involving human erythrocytes were conducted with the informed consent of a healthy donor in compliance with the Official Mexican Technical Standard NOM-253-SSA1-2012 [33].
4.2. Plant Material
Schinus molle L. was collected in June 2020 in Cadereyta Jiménez, Nuevo León, México (N 25°32′17.079″; W 99°56′52.457″). Taxonomic identification of the plant was performed at the herbarium of the FCB-UANL, with a voucher number 030594. The taxonomic validation of the name and family of the plant was performed using the International Plant Names Index (https://www.ipni.org/; accessed on 20 April 2024).
4.3. Preparation of Extracts
Leaves and stems of S. molle were dried at room temperature, after which they were powdered with a manual grinder. The crude methanol extract (Sm-ME) was obtained via Soxhlet extraction. For this, 25 g of the plant was placed in a Soxhlet extractor with 500 mL of absolute methanol (CTR Scientific, Monterrey, NL, Mexico). Extraction was sustained for 48 h, after which the extract was filtered and concentrated under reduced pressure at 40 °C with a rotary evaporator (Yamato Digital Rotary Evaporator, RE301, Santa Clara, CA, USA). The residual solvent was evaporated at room temperature with a vacuum desiccator [34]. Next, hexane (Sm-HP), chloroform Sm-CP), and methanol (Sm-MP) partitions were obtained via continuous Soxhlet extractions of the Sm-ME. For this, we used five grams of the Sm-ME and 250 mL of n-hexane, chloroform, and methanol as extraction solvents. Each extraction was maintained for 48 h, after which fractions were filtered and concentrated as performed for the Sm-ME [35].
Extract yield was calculated as previously reported [36]. Next, 25 mg of each extract was solubilized in one milliliter of dimethyl sulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO, USA) and stored at 4 °C until use. The final concentration of DMSO used in the experiments was less than 1% (v/v), which did not alter cell and parasite viability.
4.4. Anti-Trypanosoma Cruzi Activity
The T. cruzi NL strain was originally isolated from Triatoma gerstaeckeri collected in Nuevo León, México [17]. T. cruzi epimastigotes were cultured in Liver Infusion Tryptose (LIT) medium supplemented with 10% fetal bovine serum (FBS; Gibco, Grand Island, NY, USA) at 28 °C and harvested during the exponential growth phase, with a cell density of approximately 1 × 106 parasites/mL [16].
We placed 1 × 106 parasites/well in round-bottomed 96-well microplates (Corning Incorporated, Corning, NY, USA) in LIT medium. Parasites were treated with 10 μg/mL to 1000 μg/mL of extracts. We used 35 µg/mL nifurtimox (Sigma-Aldrich) as a positive control and untreated culture medium with parasites as a negative control. We also used as a control 1% DMSO. Microplates were incubated for 72 h at 28 °C, and parasite viability was determined using the 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT; Affymetrix, Cleveland, OH, USA) colorimetric assay, as previously reported [37]. T. cruzi percentage growth inhibition was calculated as previously reported [36]. Extracts were classified as highly active (IC50 < 10 µg/mL), active (IC50 > 10 < 50 µg/mL), moderately active (IC50 > 50 < 100 µg/mL), and non-active (IC50 > 100 µg/mL) [18].
4.5. Cytotoxic Activity in Vero Cells
African green monkey kidney epithelial cells (Vero; ATCC CCL-81) were grown in RPMI-1640 culture medium (Gibco) supplemented with 10% heat-inactivated FBS (Gibco), 2 g/L sodium bicarbonate (NaHCO3), and 1% penicillin/streptomycin solution (Gibco) (referred to as complete RPMI medium). Cells were cultured at 37 °C in an atmosphere of 5% CO2 in air [38].
Vero cells were seeded at a concentration of 2.2 × 104 cells/well in flat-bottomed 96-well microplates (Corning Incorporated) in complete RPMI medium and incubated 24 h before treatment. Next, the cells were exposed to 10 μg/mL to 1000 μg/mL of extracts for 24 h. The controls were culture medium alone and 1% DMSO. Cell viability was determined with the MTT colorimetric assay by adding 20 μL of MTT (5 mg/mL) to each well and incubating them for one hour. The plates were then decanted and formazan crystals were dissolved with 100 µL of DMSO. Optical densities (ODs) were determined at 570 nm using a microplate reader (ELISA Variouskan LUX multimode; Thermo Fisher Scientific, Waltham, MA, USA) [39]. Percentage growth inhibition was calculated as previously reported [20] and extracts were classified as highly cytotoxic (IC50 < 10 µg/mL), cytotoxic (IC50 > 10 < 100 µg/mL), moderately cytotoxic (IC50 > 100 < 1000 µg/mL), and potentially non-cytotoxic (IC50 > 1000 µg/mL) [18].
4.6. Determination of Selectivity Indices of Extracts
In this study, Vero cells were used as a mammalian cell model for testing the unspecific cytotoxicity of the extracts. Extract selectivity indices (SIs) were calculated by dividing the IC50 of Vero cells by the IC50 of T. cruzi using the following formula [38]:
4.7. Toxic Activity in Artemia Salina
Artemia salina eggs (INVE Aquaculture NV, Basrode, Belgium) were hatched in a microaquarium with 4 L of 3.7% saline solution at pH 8, suitably oxygenated, and at 25 °C to 30 °C under continuous light. Water was supplemented with 0.75 g/L of yeast extract. Eggs hatched in 24 h and we obtained larvae (nauplii). At 48 h after hatching, the larvae were transferred to flat-bottomed 24-well microplates at 10 larvae/well, and 10 μg/mL to 1000 μg/mL of the extracts was evaluated for 24 h at 25 °C to 30 °C under continuous light. We used 100 μg/mL potassium dichromate (K2Cr2O7) as a positive control and saline solution as a negative control; we also used as a control 1% DMSO. Larvae survival was evaluated as previously reported [25]. Results were considered valid if the percentage of mortality in the negative controls did not exceed 10% [40].
Extracts were classified as highly toxic (IC50 < 10 µg/mL), toxic (IC50 > 10 < 100 µg/mL), moderately toxic (IC50 > 100 < 1000 µg/mL), and non-toxic (IC50 > 1000 µg/mL) [26].
4.8. Human Erythrocyte Hemolytic Activity Assay
Blood from healthy donors (20 mL) was deposited in tubes containing the anticoagulant EDTA (BD Diagnostics, Franklin Lakes, NJ, USA). Red blood cells were washed three times with phosphate-buffered saline solution (PBS; pH 7.2) and a 5% erythrocytes suspension was prepared in sterile PBS. Next, 200 µg/mL to 1000 µg/mL extracts and 5% erythrocytes suspension were incubated for 30 min at 37 °C. Distilled water was used as a positive control for hemolysis and PBS as a negative control; we also used as a control 1% DMSO. After incubation, the samples were centrifuged at 13,000 for five minutes at 4 °C, after which 200 µL of the supernatant from each tube was transferred to a flat-bottomed 96-well microplate to measure the OD at 540 nm of the released hemoglobin in a microplate reader (ASYS UVM; Biochrom Ltd., Cambridge, UK). The percentage of hemolysis for each sample was calculated as previously reported [34].
4.9. Column Chromatography Fractionation
As Sm-HP and Sm-CP partitions showed the highest anti-T. cruzi activity, they were fractionated using column chromatography. For this, open glass chromatographic columns (330 mm × 28 mm) were prepared with 20 g of 0.063 to 0.200 mm particle size silica gel 60 G (Merck, Darmstadt, Germany) [41]. For Sm-HP, 500 mg of the partition was added to the column and eluted with stepwise gradients of 20 mL of n-hexane-chloroform, chloroform-ethyl acetate, and ethyl acetate-methanol at 100:0, 90:10, 80:20, 70:30, 60:40, 50:50, 40:60, 30:70, 20:80, 10:90, and 0:100 v/v stepwise gradient proportions. We then obtained 124 fractions of five milliliters and pooled them based on their thin layer chromatography (TLC) profile (hexane-chloroform 1:1) to yield five collective fractions (Sm-HF1 to Sm-HF5). For Sm-CP, 500 mg of the partition was added to the column and eluted with stepwise gradients of chloroform–ethyl acetate and ethyl acetate–methanol as performed by Sm-HP. We then obtained 84 fractions of five milliliters and pooled them based on their TLC profile (chloroform-ethyl acetate 1:1) to yield five collective fractions (Sm-CF1 to Sm-CF5). The anti-T. cruzi, cytotoxic, toxic, and hemolytic activity of the fractions were evaluated using the methods mentioned above.
4.10. Identification of Bioactive Compound Using Gas Chromatography–Mass Spectrometry (GC-MS)
We selected the fraction with the highest anti-T. cruzi activity (Sm-CF3) to identify its constituent compounds using GC-MS in the external services laboratory of the Instituto Politécnico Nacional (IPN) in México City.
For the analysis, we used a gas chromatograph (Agilent Technologies 7890A; Santa Clara, CA, USA) equipped with an Agilent Technologies 5975C triple mass detector and an Agilent 123-2332DB-23 column (60 m × 320 µm × 0.25 µm). The operating conditions were as follows: the initial oven temperature was set at 170 °C for two minutes and then increased by 5 °C/min until reaching 250 °C, where it was kept for 15 min. The injector temperature was maintained at 250 °C. Helium (99.999%) was used as the carrier gas with a flow rate of one milliliter per minute, and the injection volume was two microliters. The GC–MS mass spectrum data were analyzed and identified using the National Institute Standard and Technology (NIST) database to determine their m/z values according to their 100% abundance [42]. The chemical classes of the identified metabolites were automatically determined using the Classyfire web-based application (http://classyfire.wishartlab.com, accessed on 10 March 2023) [43].
4.11. Statistical Analysis
Data represent the mean ± SD of at least triplicate determinations and three independent experiments, with a confidence level of 95%. The Probit test was used to calculate the IC50 (half maximal inhibitory concentration) values. A one-way analysis of variance was used to determine the significant difference between the tested extracts and Tukey’s post hoc test was used to determine the difference between the treatment means. Statistical analyses were performed using IBM SPSS Statistics v25.0 software (IBM Corp., Armonk, NY, USA).
5. Conclusions
This study demonstrates that the crude methanol extract, partitions, and fractions of S. molle exhibit anti-T. cruzi activity against the NL strain. In particular, Sm-CF3 showed promising activity (IC50 = 19 µg/mL; SI 6.8). In this fraction, we identified 20 compounds via GC-MS, among which the antiparasitic activity of cis-5,8,11,14,17-eicosapentaenoic acid and trans-Z-α-bisabolene epoxide has been reported, suggesting their potential role in the observed anti-T. cruzi activity.
Our findings support the therapeutic potential of S. molle as a source of novel antiparasitic agents against T. cruzi. However, further research is warranted to validate the efficacy of identified compounds in preclinical models and ultimately advance them toward clinical trials for the treatment of Chagas disease.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants13162177/s1.
Author Contributions
Conceptualization, Z.J.M.-G.; methodology, Z.J.M.-G. and R.Q.-L.; software, N.E.R.-G. and R.G.-F.; validation, L.G.-S.; formal analysis, N.E.R.-G.; investigation, N.E.R.-G., R.Q.-L. and Z.J.M.-G.; resources, L.G.-S., R.Q.-L. and Z.J.M.-G.; data curation, R.Q.-L. and R.G.-F.; writing—original draft preparation, N.E.R.-G. and Z.J.M.-G.; writing—review and editing, R.G.-F., L.G.-S. and R.Q.-L.; visualization, Z.J.M.-G. and R.Q.-L.; supervision, Z.J.M.-G. and R.Q.-L.; project administration, L.G.-S.; funding acquisition, L.G.-S. and Z.J.M.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Programa de Apoyo a la Investigación Científica y Tecnológica (PAICYT) of the Universidad Autónoma de Nuevo León, grant CN1618-21 to Z.J.M.G. and the Consejo Nacional de Humanidades, Ciencia y Tecnología (CONAHCYT), grant 808132 (CVU: 1006989) to N.E.R.-G.
Data Availability Statement
Data are contained within the article.
Acknowledgments
We are grateful to Rosa Ma. Hernandez Izaguirre, Manager of the Biotechnology Department, Centro de Biotecnología Genómica, IPN, Reynosa, Tamaulipas, Mexico, for her support with the GC-MS.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hochberg, N.S.; Montgomery, S.P. Chagas Disease. Ann. Intern. Med. 2023, 176, ITC17–ITC32. [Google Scholar] [CrossRef] [PubMed]
- WHO. Chagas Disease (Also Known as American Trypanosomiasis). Available online: https://www.who.int/news-room/questions-and-answers/item/chagas-disease (accessed on 8 March 2024).
- Martín-Escolano, J.; Marín, C.; Rosales, M.J.; Tsaousis, A.D.; Medina-Carmona, E.; Martín-Escolano, R. An updated view of the Trypanosoma cruzi life cycle: Intervention points for an effective treatment. ACS Infect. Dis. 2022, 8, 1107–1115. [Google Scholar] [CrossRef]
- Rodríguez, M.S.; Nitahara, Y.; Cornejo, M.; Siliezar, K.; Grande, R.; González, A.; Tasaki, K.; Nakagama, Y.; Michimuko, Y.; Onizuka, Y.; et al. Re-emerging threat of Trypanosoma cruzi vector transmission in El Salvador, update from 2018 to 2020. Infect. Dis. Poverty 2022, 11, 89. [Google Scholar] [CrossRef] [PubMed]
- Bern, C.; Messenger, L.A.; Whitman, J.D.; Maguire, J.H. Chagas disease in the United States: A public health approach. Clin. Microbiol. Rev. 2019, 33, 10–1128. [Google Scholar] [CrossRef]
- de Sousa, A.S.; Vermeij, D.; Ramos, A.N.; Luquetti, A.O. Chagas disease. Lancet 2024, 403, 203–218. [Google Scholar] [CrossRef]
- Benziger, C.P.; do Carmo, G.A.L.; Ribeiro, A.L.P. Chagas Cardiomyopathy. Cardiol. Clin. 2017, 35, 31–47. [Google Scholar] [CrossRef]
- Nguyen, T.; Waseem, M. Chagas Disease; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar] [PubMed]
- Buschini, A.; Ferrarini, L.; Franzoni, S.; Galati, S.; Lazzaretti, M.; Mussi, F.; Northfleet de Albuquerque, C.; Araújo, T.M.; Poli, P. Genotoxicity revaluation of three commercial nitroheterocyclic drugs: Nifurtimox, benznidazole, and metronidazole. J. Parasitol. Res. 2009, 2009, 463575. [Google Scholar] [CrossRef] [PubMed]
- Lima, D.A.; Gonçalves, L.O.; Reis-Cunha, J.L.; Guimarães, P.A.S.; Ruiz, J.C.; Liarte, D.B.; Murta, S.M.F. Transcriptomic analysis of benznidazole-resistant and susceptible Trypanosoma cruzi populations. Parasites Vectors 2023, 16, 167. [Google Scholar] [CrossRef]
- Daley, S.; Cordell, G.A. Alkaloids in contemporary drug discovery to meet global disease Needs. Molecules 2021, 26, 3800. [Google Scholar] [CrossRef]
- Nasim, N.; Sandeep, I.S.; Mohanty, S. Plant-derived natural products for drug discovery: Current approaches and prospects. Nucleus 2022, 65, 399–411. [Google Scholar] [CrossRef]
- Aryan, H. The role of herbal medicine as anti-cancer medicine: From the claim to truth. Galen Med. J. 2018, 7, e1179. [Google Scholar] [CrossRef] [PubMed]
- Bvenura, C.; Kambizi, L. Composition of phenolic compounds in South African Schinus molle L. berries. Foods 2022, 11, 1376. [Google Scholar] [CrossRef] [PubMed]
- Mekuria, A.B.; Geta, M.; Birru, E.M.; Gelayee, D.A. Antimalarial activity of seed extracts of Schinus molle against Plasmodium berghei in mice. J. Evid. Based Integr. Med. 2021, 26, 2515690X20984287. [Google Scholar] [CrossRef] [PubMed]
- Molina-Garza, Z.J.; Bazaldúa-Rodríguez, A.F.; Quintanilla-Licea, R.; Galaviz-Silva, L. Anti-Trypanosoma cruzi activity of 10 medicinal plants used in Northeast Mexico. Acta Trop. 2014, 136, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Treviño, K.C.; Galaviz, L.; Iracheta-Villarreal, J.M.; Lucero-Velasco, E.A.; Molina-Garza, Z.J. Actividad contra Trypanosoma cruzi (Kinetoplastida: Trypanosomatidae) de extractos metanólicos de plantas de uso medicinal en México. Rev. Biol. Trop. 2017, 65, 1459. [Google Scholar] [CrossRef]
- Osorio, E.; Arango, G.J.; Jiménez, N.; Alzate, F.; Ruiz, G.; Gutiérrez, D.; Paco, M.A.; Giménez, A.; Robledo, S. Antiprotozoal and cytotoxic activities in vitro of colombian Annonaceae. J. Ethnopharmacol. 2007, 111, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Altamirano, R.; Monzote, L.; Piñón-Tápanes, A.; Vibrans, H.; Rivero-Cruz, J.F.; Ibarra-Alvarado, C.; Rojas-Molina, A. In vitro antileishmanial activity of mexican medicinal plants. Heliyon 2017, 3, e00394. [Google Scholar] [CrossRef] [PubMed]
- Zenebe, S.; Feyera, T.; Assefa, S. In vitro anthelmintic activity of crude extracts of aerial parts of Cissus quadrangularis L. and leaves of Schinus molle L. against Haemonchus contortus. Biomed. Res. Int. 2017, 2017, 1905987. [Google Scholar] [CrossRef] [PubMed]
- Revollo, S.; Oury, B.; Vela, A.; Tibayrenc, M.; Sereno, D. In vitro benznidazole and nifurtimox susceptibility profile of Trypanosoma cruzi strains belonging to discrete typing units TcI, TcII, and TcV. Pathogens 2019, 8, 197. [Google Scholar] [CrossRef] [PubMed]
- Vela, A.; Coral-Almeida, M.; Sereno, D.; Costales, J.A.; Barnabé, C.; Brenière, S.F. In vitro susceptibility of Trypanosoma cruzi discrete typing units (DTUs) to benznidazole: A systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2021, 15, e0009269. [Google Scholar] [CrossRef]
- Santos, F.O.; de Lima, H.G.; de Souza Santos, N.S.; Serra, T.M.; Uzeda, R.S.; Reis, I.M.A.; Botura, M.B.; Branco, A.; Batatinha, M.J.M. In vitro anthelmintic and cytotoxicity activities the Digitaria insularis (Poaceae). Vet. Parasitol. 2017, 245, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Cho-Ngwa, F.; Abongwa, M.; Ngemenya, M.N.; Nyongbela, K.D. Selective activity of extracts of Margaritaria discoidea and Homalium africanum on Onchocerca ochengi. BMC Complement. Altern. Med. 2010, 10, 62. [Google Scholar] [CrossRef] [PubMed]
- Santos Filipe, M.; Isca, V.M.S.; Ntungwe, E.; Princiotto, S.; Díaz-Lanza, A.M.; Rijo, P. Lethality bioassay using Artemia salina L. J. Vis. Exp. 2022, 188, e64472. [Google Scholar] [CrossRef]
- Fernández-Calienes, A.; Mendiola, J.; Monzote, L.; García, M.; Sariego, I.; Acuña, D.; Scull, R.; Gutiérrez, Y. Evaluación de la toxicidad de extractos de plantas cubanas con posible acción antiparasitaria utilizando larvas de Artemia salina L. Rev. Cubana Med. Trop. 2009, 61, 254–258. [Google Scholar]
- Carballeira, N.M. New advances in fatty acids as antimalarial, antimycobacterial and antifungal agents. Prog. Lipid Res. 2008, 47, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Achakzai, J.K.; Anwar Panezai, M.; Kakar, A.M.; Akhtar, B.; Akbar, A.; Kakar, S.; Khan, J.; Khan, N.Y.; Khan, G.M.; Baloch, N.; et al. In vitro antileishmanial activity and GC-MS analysis of whole plant hexane fraction of Achillea wilhelmsii (WHFAW). J. Chem. 2019, 2019, 5734257. [Google Scholar] [CrossRef]
- Albouchi, F.; Sifaoui, I.; Reyes-Batlle, M.; López-Arencibia, A.; Piñero, J.E.; Lorenzo-Morales, J.; Abderrabba, M. Chemical composition and Anti-Acanthamoeba activity of Melaleuca styphelioides essential oil. Exp. Parasitol. 2017, 183, 104–108. [Google Scholar] [CrossRef]
- Korosh, T.; Jordan, K.D.; Wu, J.; Yarlett, N.; Upmacis, R.K. Eicosapentaenoic acid modulates Trichomonas vaginalis activity. J. Eukaryot. Microbiol. 2016, 63, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Chaves, D.; Bagnarello-Madrigal, V.; Alpizar-Cordero, J.; Calvo-Vargas, A.; Cordero-Villalobos, M.; Chinchilla-Carmona, M.; Valerio-Campos, I.; Sánchez Porras, R. Actividad in vitro anti-Leishmania (Trypanosomatidae) del epóxido trans-Z-α-bisaboleno y del safrol, en frutos de Piper auritum (Piperaceae). Rev. Biol. Trop. 2018, 66, 826. [Google Scholar] [CrossRef]
- Vermelho, A.B.; Capaci, G.R.; Rodrigues, I.A.; Cardoso, V.S.; Mazotto, A.M.; Supuran, C.T. Carbonic anhydrases from Trypanosoma and Leishmania as anti-protozoan drug targets. Bioorg. Med. Chem. 2017, 25, 1543–1555. [Google Scholar] [CrossRef]
- NOM-253-SSA1-2012; Para la Disposición de Sangre Humana y sus Componentes con Fines Terapéuticos. Diario Oficial de la Federación: Ciudad de México, Mexico, 2012.
- Rodríguez-Garza, N.E.; Quintanilla-Licea, R.; Romo-Sáenz, C.I.; Elizondo-Luevano, J.H.; Tamez-Guerra, P.; Rodríguez-Padilla, C.; Gomez-Flores, R. In vitro biological activity and lymphoma cell growth inhibition by selected mexican medicinal plants. Life 2023, 13, 958. [Google Scholar] [CrossRef] [PubMed]
- Elizondo-Luévano, J.H.; Rodríguez-Garza, N.E.; Bazaldúa-Rodríguez, A.F.; Romo-Sáenz, C.I.; Tamez-Guerra, P.; Verde-Star, M.J.; Gomez-Flores, R.; Quintanilla-Licea, R. Cytotoxic, anti-hemolytic, and antioxidant activities of Ruta chalepensis L. (Rutaceae) extract, fractions, and isolated compounds. Plants 2023, 12, 2203. [Google Scholar] [CrossRef] [PubMed]
- Revathy, S.; Elumalai, S.; Benny, M.; Antony, B. Isolation, purification, and identification of curcuminoids from turmeric Curcuma longa L. by column chromatography. J. Exp. Sci. 2011, 27, 21–25. [Google Scholar]
- Muelas-Serrano, S.; Nogal, J.J.; Martínez-Díaz, R.A.; Escario, J.A.; Martínez-Fernández, A.R.; Gómez-Barrio, A. In vitro screening of american plant extracts on Trypanosoma cruzi and Trichomonas vaginalis. J. Ethnopharmacol. 2000, 71, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Lima, H.; Gomes, D.; Santos, N.; Dias, Ê.; Botura, M.; Batatinha, M.; Branco, A. Prosopis juliflora pods alkaloid-rich fraction: In vitro anthelmintic activity on goat gastrointestinal parasites and its cytotoxicity on Vero cells. Pharmacogn. Mag. 2017, 13, 684–687. [Google Scholar] [CrossRef]
- Elizondo-Luévano, J.H.; Gomez-Flores, R.; Verde-Star, M.J.; Tamez-Guerra, P.; Romo-Sáenz, C.I.; Chávez-Montes, A.; Rodríguez-Garza, N.E.; Quintanilla-Licea, R. In vitro cytotoxic activity of methanol extracts of selected medicinal plants traditionally used in Mexico against human hepatocellular carcinoma. Plants 2022, 11, 2862. [Google Scholar] [CrossRef] [PubMed]
- Righi, N.; Boumerfeg, S.; Deghima, A.; Fernandes, P.A.R.; Coelho, E.; Baali, F.; Cardoso, S.M.; Coimbra, M.A.; Baghiani, A. Phenolic profile, safety assessment, and anti-inflammatory activity of Salvia verbenaca L. J. Ethnopharmacol. 2021, 272, 113940. [Google Scholar] [CrossRef]
- Bazaldúa-Rodríguez, A.F.; Quintanilla-Licea, R.; Verde-Star, M.J.; Hernández-García, M.E.; Vargas-Villarreal, J.; Garza-González, J.N. Furanocoumarins from Ruta chalepensis with amebicide activity. Molecules 2021, 26, 3684. [Google Scholar] [CrossRef] [PubMed]
- Ajilogba, C.F.; Babalola, O.O. GC–MS analysis of volatile organic compounds from Bambara groundnut rhizobacteria and their antibacterial properties. World J. Microbiol. Biotechnol. 2019, 35, 83. [Google Scholar] [CrossRef]
- Contreras-Angulo, L.A.; Moreno-Ulloa, A.; Carballo-Castañeda, R.A.; León-Felix, J.; Romero-Quintana, J.G.; Aguilar-Medina, M.; Ramos-Payán, R.; Heredia, J.B. Metabolomic analysis of phytochemical compounds from agricultural residues of eggplant (Solanum melongena L.). Molecules 2022, 27, 7013. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).