Molecular Investigations to Improve Fusarium Head Blight Resistance in Wheat: An Update Focusing on Multi-Omics Approaches
Abstract
1. Introduction
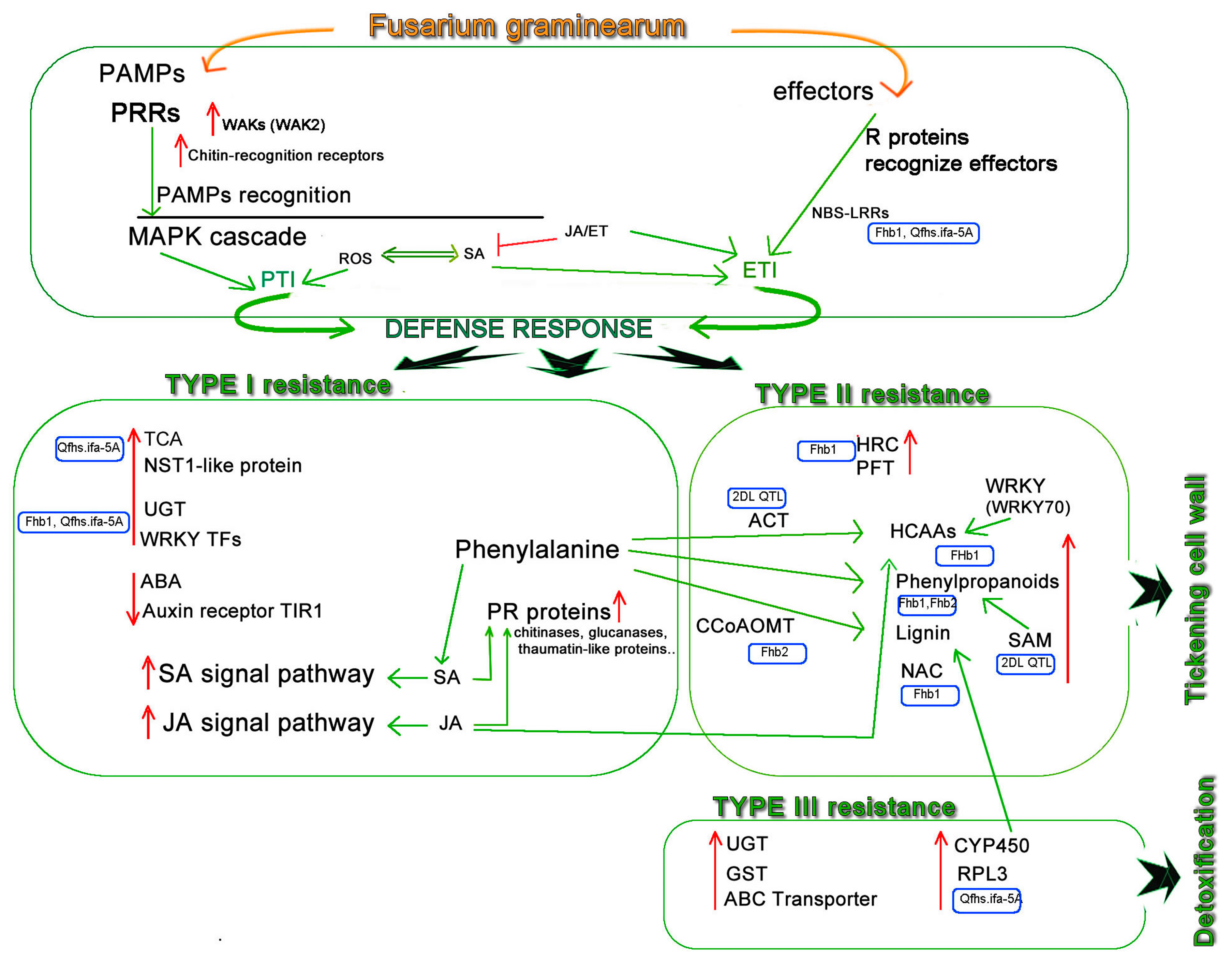
2. Wheat Immune Response to Fg
3. Type I Resistance
4. Type II Resistance
5. Type III Resistance
6. Type IV Resistance
7. Type V Resistance
8. Quantitative Traits Loci Associated with Fg Resistance: Other Relevant Analysis Approaches
9. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Definition |
| ABA | Abscisic Acid |
| ABC transporters | ATP-Binding Cassette Transporters |
| ACT | Agmatine Coumaroyl Transferase |
| CS | Callose Synthase |
| CCoAOMT | Caffeoyl-Coenzyme A O-Methyltransferase |
| CAD | Cinnamyl Alcohol Dehydrogenase |
| CYP450 | Cytochromes P450 |
| DEGs | Differentially Expressed Genes |
| DON | Deoxynivalenol |
| ET | Ethylene |
| ETI | Effector-Triggered Immunity |
| GS | Genomic Selection |
| GST | Glutathione S-Transferase |
| GWAS | Genome-Wide Association Studies |
| HCAA | Hydroxycinnamic Acid Amide |
| HRC | Histidine-Rich Calcium-binding protein |
| JA | Jasmonic Acid |
| LEA | Late Embryogenesis Abundant proteins |
| LRR | Leucine-Rich Repeat |
| LTP | Lipid Transfer Protein |
| MAPK | Mitogen-Activated Protein Kinase |
| MTAs | Marker-Trait Associations |
| NBS | Nucleotide Binding Site |
| NILs | Near-Isogenic Lines |
| NIV | Nivalenol |
| PAL | Phenylalanine Ammonia Lyase |
| PAMPs | Pathogen-Associated Molecular Patterns |
| PCR | Polymerase Chain Reaction |
| Phe | Phenylalanine |
| PFT | Pore-Forming Toxin-like |
| PR | Pathogenesis-Related protein |
| PRRs | Pattern Recognition Receptors |
| PTI | PAMP-Triggered Immunity |
| QTL | Quantitative Trait Loci |
| RIL | Recombinant Inbred Line |
| RLKs | Receptor-Like Kinases |
| ROS | Reactive Oxygen Species |
| RPL3 | Ribosomal Protein L3 |
| SA | Salicylic Acid |
| SAM | S-Adenosyl Methionine |
| SNP | Single-Nucleotide Polymorphism |
| TCA | Tricarboxylic Acid Cycle |
| UGT | UDP-Glucuronosyltransferase |
| WAKs | Wall-Associated Kinases |
References
- Zhang, Y.; Ma, L.J. Chapter Five—Deciphering pathogenicity of Fusarium oxysporum from a phylogenomics perspective. In Advances in Genetics; Jeffrey, P.T., Zheng, W., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 179–209. [Google Scholar]
- Tassone, M.R.; Bagnaresi, P.; Desiderio, F.; Bassolino, L.; Barchi, L.; Florio, F.E.; Sunseri, F.; Sirangelo, T.M.; Rotino, G.L.; Toppino, L. A Genomic BSAseq approach for the characterization of QTLs underlying resistance to Fusarium oxysporum in eggplant. Cells 2022, 11, 2548. [Google Scholar] [CrossRef] [PubMed]
- Sirangelo, T.M.; Ludlow, R.A.; Spadafora, N.D. Molecular mechanisms underlying potential pathogen resistance in Cannabis sativa. Plants 2023, 12, 2764. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, Z.; Lu, P.; Li, R.; Ma, P.; Wu, J.; Li, T.; Zhang, H. Increasing Fusarium verticillioides resistance in maize by genomics-assisted breeding: Methods, progress, and prospects. Crop J. 2023, 11, 1626–1641. [Google Scholar] [CrossRef]
- Yao, G.; Chen, W.; Sun, J.; Wang, X.; Wang, H.; Meng, T.; Zhang, L.; Guo, L. Gapless genome assembly of Fusarium verticillioides, a filamentous fungus threatening plant and human health. Sci. Data 2023, 10, 229. [Google Scholar] [CrossRef] [PubMed]
- Del Ponte, E.M.; Moreira, G.M.; Ward, T.J.; O’Donnell, K.; Nicolli, C.P.; Machado, F.J.; Duffeck, M.R.; Alves, K.S.; Tessmann, D.J.; Waalwijk, C.; et al. Fusarium graminearum species complex: A bibliographic analysis and web-accessible database for global mapping of species and trichothecene toxin chemotypes. Phytopathology 2022, 112, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wang, Q.; Wang, G.; Zhang, X.; Liu, H.; Jiang, C. Combatting Fusarium head blight: Advances in molecular interactions between Fusarium graminearum and wheat. Phytopathol. Res. 2022, 4, 37. [Google Scholar] [CrossRef]
- Ma, H.; Liu, Y.; Zhao, X.; Zhang, S.; Ma, H. Exploring and applying genes to enhance the resistance to Fusarium head blight in wheat. Front. Plant Sci. 2022, 13, 1026611. [Google Scholar] [CrossRef] [PubMed]
- Mawcha, K.T.; Zhang, N.; Wang, Y.A.; Yang, W.X. Advances in wheat breeding for resistance to Fusarium head blight. Czech J. Genet. Plant Breed. 2022, 58, 167–188. [Google Scholar] [CrossRef]
- Bechtel, D.; Kaleikau, L.; Gaines, R.; Seitz, L. The effects of Fusarium graminearum infection on wheat kernels. Cereal Chem. 1985, 62, 191–197. [Google Scholar]
- Desjardins, A.E. Fusarium mycotoxins. In Chemistry, Genetics, and Biology; American Phytopathological Society Press: St. Paul, MN, USA, 2006. [Google Scholar]
- Bai, G.H.; Shaner, G.E. Scab of wheat: Prospects for control. Plant Dis. 1994, 78, 760–766. [Google Scholar]
- Ma, Z.; Xie, Q.; Li, G.; Jia, H.; Zhou, J.; Kong, Z.; Li, N.; Yuan, Y. Germplasms, genetics and genomics for better control of disastrous wheat Fusarium head blight. Theor. Appl. Genet. 2020, 133, 1541–1568. [Google Scholar] [CrossRef] [PubMed]
- Dweba, C.C.; Figlan, S.; Shimelis, H.A.; Motaung, T.E.; Sydenham, S.; Mwadzingeni, L.; Tsilo, T.J. Fusarium head blight of wheat: Pathogenesis and control strategies. Crop Prot. 2017, 91, 114–122. [Google Scholar] [CrossRef]
- Bai, G.; Su, Z.; Cai, J. Wheat resistance to Fusarium head blight. Can. J. Plant Pathol. 2018, 40, 336–346. [Google Scholar]
- Buerstmayr, M.; Steiner, B.; Buerstmayr, H. Breeding for Fusarium head blight resistance in wheat—Progress and challenges. Plant Breed. 2020, 139, 429–454. [Google Scholar] [CrossRef]
- Berraies, S.; Cuthbert, R.; Knox, R.; Singh, A.; DePauw, R.; Ruan, Y.; Bokore, F.; Henriquez, M.A.; Kumar, S.; Burt, A.; et al. High-density genetic mapping of Fusarium head blight resistance and agronomic traits in spring wheat. Front. Plant Sci. 2023, 14, 1134132. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, H.W.; Christensen, J.J. Factors affecting resistance of wheat to scab by Gibberella zeae. Phytopathology 1963, 53, 831–838. [Google Scholar]
- Mesterhazy, A. Types and components of resistance to Fusarium head blight of wheat. Plant Breed. 1995, 114, 377–386. [Google Scholar] [CrossRef]
- Mesterhazy, A. Updating the breeding philosophy of wheat to Fusarium head blight (FHB): Resistance components, QTL identification, and phenotyping—A review. Plants 2020, 9, 1702. [Google Scholar] [CrossRef]
- Venske, E.; dos Santos, R.S.; Farias, D.D.R.; Rother, V.; Da Maia, L.C.; Pegoraro, C.; Costa de Oliveira, A. Meta-analysis of the QTLome of Fusarium head blight resistance in bread wheat: Refining the current puzzle. Front. Plant Sci. 2019, 10, 727. [Google Scholar] [CrossRef]
- Zheng, T.; Hua, C.; Li, L.; Sun, Z.; Yuan, M.; Bai, G.; Humphreys, G.; Li, T. Integration of meta-QTL discovery with omics: Towards a molecular breeding platform for improving wheat resistance to fusarium head blight. Crop J. 2021, 9, 739–749. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, X.; Pumphrey, M.O.; Stack, R.W.; Gill, B.S.; Anderson, J.A. Complex microcolinearity among wheat, rice, and barley revealed by fine mapping of the genomic region harboring a major QTL for resistance to Fusarium head blight in wheat. Funct. Integr. Genom. 2006, 6, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Cuthbert, P.A.; Somers, D.J.; Brulé-Babel, A. Mapping of Fhb2 on chromosome 6BS: A gene controlling Fusarium head blight field resistance in bread wheat (Triticum aestivum L.). Theor. Appl. Genet. 2007, 114, 429–437. [Google Scholar] [CrossRef]
- Xue, S.; Li, G.; Jia, H.; Xu, F.; Lin, F.; Tang, M.; Wang, Y.; An, X.; Xu, H.; Zhang, L.; et al. Fine mapping Fhb4, a major QTL conditioning resistance to Fusarium infection in bread wheat (Triticum aestivum L.). Theor. Appl. Genet. 2010, 121, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Steiner, B.; Buerstmayr, M.; Wagner, C.; Danler, A.; Eshonkulov, B.; Ehn, M.; Buerstmayr, H. Fine-mapping of the Fusarium head blight resistance QTL Qfhs.ifa-5A identifies two resistance QTL associated with anther extrusion. Theor. Appl. Genet. 2019, 132, 2039–2053. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.L.; Pumphrey, M.O.; Friebe, B.; Chen, P.D.; Gill, B.S. Molecular cytogenetic characterization of alien introgressions with gene Fhb3 for resistance to Fusarium head blight disease of wheat. Theor. Appl. Genet. 2008, 117, 1155–1166. [Google Scholar] [CrossRef] [PubMed]
- Cainong, J.C.; Bockus, W.W.; Feng, Y.; Chen, P.; Qi, L.; Sehgal, S.K.; Danilova, T.V.; Koo, D.-H.; Friebe, B.; Gill, B.S. Chromosome engineering, mapping, and transferring of resistance to Fusarium head blight disease from Elymus tsukushiensis into wheat. Theor. Appl. Genet. 2015, 128, 1019–1027. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, X.; Hou, Y.; Cai, J.; Shen, X.; Zhou, T.; Xu, H.; Ohm, H.W.; Wang, H.; Li, A.; et al. High-density mapping of the major FHB resistance gene Fhb7 derived from Thinopyrum ponticum and its pyramiding with Fhb1 by marker-assisted selection. Theor. Appl. Genet. 2015, 128, 2301–2316. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Dreisigacker, S.; Singh, R.P.; Singh, P.K. Genetics for low correlation between Fusarium head blight disease and deoxynivalenol (DON) content in a bread wheat mapping population. Theor. Appl. Genet. 2019, 132, 2401–2411. [Google Scholar] [CrossRef] [PubMed]
- Shude, S.P.N.; Yobo, K.S.; Mbili, N.C. Progress in the management of fusarium head blight of wheat: An overview. S. Afr. J. Sci. 2020, 116, 7854. [Google Scholar] [CrossRef]
- Wu, L.; He, X.; He, Y.; Jiang, P.; Xu, K.; Zhang, X.; Singh, P.K. Genetic sources and loci for Fusarium head blight resistance in bread wheat. Front. Genet. 2022, 13, 988264. [Google Scholar] [CrossRef]
- Khan, M.M.; Ernst, O.; Manes, N.P.; Oyler, B.L.; Fraser, I.D.C.; Goodlett, D.R.; Nita-Lazar, A. Multi-Omics Strategies Uncover Host-Pathogen Interactions. ACS Infect. Dis. 2019, 5, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Sirangelo, T.M.; Rogers, H.J.; Spadafora, N.D. Multi-omic approaches to investigate molecular mechanisms in peach post-harvest ripening. Agriculture 2022, 12, 553. [Google Scholar] [CrossRef]
- Sirangelo, T.M.; Ludlow, R.A.; Spadafora, N.D. Multi-omics approaches to study molecular mechanisms in Cannabis sativa. Plants 2022, 11, 2182. [Google Scholar] [CrossRef] [PubMed]
- Sirangelo, T.M.; Forgione, I.; Zelasco, S.; Benincasa, C.; Perri, E.; Vendramin, E.; Angilè, F.; Fanizzi, F.P.; Sunseri, F.; Salimonti, A.; et al. Combined transcriptomic and metabolomic approach revealed a relationship between light control, photoprotective pigments, and lipid biosynthesis in olives. Int. J. Mol. Sci. 2023, 24, 14448. [Google Scholar] [CrossRef] [PubMed]
- Larsen, P.; Sreedasyam, A.; Trivedi, G.; Desai, S.; Dai, Y.; Cseke, L.J.; Collart, F.R. Multi-Omics Approach Identifies Molecular Mechanisms of Plant-Fungus Mycorrhizal Interaction. Front. Plant Sci. 2016, 6, 1061. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, Q.; Liu, Z.; Surendra, A.; Pan, Y.; Li, Y.; Zaharia, L.I.; Ouellet, T.; Fobert, P.R. Integrated transcriptome and hormone profiling highlight the role of multiple phytohormone pathways in wheat resistance against fusarium head blight. PLoS ONE 2018, 13, e0207036. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, D.; Dhakate, P.; Ambreen, H.; Shaik, K.H.B.; Rathan, N.D.; Anusha, N.M.; Deshmukh, R.; Vikram, P. Wheat Omics: Advancements and opportunities. Plants 2023, 12, 426. [Google Scholar] [CrossRef] [PubMed]
- Teli, B.; Purohit, J.; Rashid, M.M.; Jailani, A.A.K.; Chattopadhyay, A. Omics insight on fusarium head blight of wheat for translational research perspective. Curr. Genom. 2020, 21, 411–428. [Google Scholar] [CrossRef] [PubMed]
- Hao, G.; Tiley, H.; McCormick, S. Chitin triggers tissue-specific immunity in wheat associated with Fusarium head blight. Front. Plant Sci. 2022, 13, 832502. [Google Scholar] [CrossRef]
- Grant, J.J.; Loake, G.J. Role of reactive oxygen intermediates and cognate redox signaling in disease resistance. Plant Physiol. 2000, 124, 21–29. [Google Scholar] [CrossRef]
- Buttar, Z.A.; Cheng, M.; Wei, P.; Zhang, Z.; Lv, C.; Zhu, C.; Ali, N.F.; Kang, G.; Wang, D.; Zhang, K. Update on the Basic Understanding of Fusarium graminearum Virulence Factors in Common Wheat Research. Plants 2024, 13, 1159. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Xu, H.; Yi, H.; Yang, L.; Kong, Z.; Zhang, L.; Xue, S.; Jia, H.; Ma, Z. Resistance to hemi-biotrophic F. graminearum infection is associated with coordinated and ordered expression of diverse defense signaling pathways. PLoS ONE 2011, 6, e19008. [Google Scholar] [CrossRef]
- Benjamin, G.; Pandharikar, G.; Frendo, P. Salicylic acid in plant symbioses: Beyond plant pathogen interactions. Biology 2022, 11, 861. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.I.; Leon, J.; Raskin, I. Biosynthesis and metabolism of salicylic acid. Proc. Natl. Acad. Sci. USA 1995, 92, 4076–4079. [Google Scholar] [CrossRef] [PubMed]
- Hao, G.; McCormick, S.; Usgaard, T.; Tiley, H.; Vaughan, M.M. Characterization of three Fusarium graminearum effectors and their roles during Fusarium head blight. Front. Plant Sci. 2020, 11, 579553. [Google Scholar] [CrossRef]
- Ding, L.N.; Li, Y.T.; Wu, Y.Z.; Li, T.; Geng, R.; Cao, J.; Zhang, W.; Tan, X.-L. Plant disease resistance-related signaling pathways: Recent progress and future prospects. Int. J. Mol. Sci. 2022, 23, 16200. [Google Scholar] [CrossRef]
- Boba, A.; Kostyn, K.; Kozak, B.; Zalewski, I.; Szopa, J.; Kulma, A. Transcriptomic profiling of susceptible and resistant flax seedlings after Fusarium oxysporum lini infection. PLoS ONE. 2021, 16, e0246052. [Google Scholar] [CrossRef] [PubMed]
- Kugler, K.G.; Siegwart, G.; Nussbaumer, T.; Ametz, C.; Spannagl, M.; Steiner, B.; Lemmens, M.; Mayer, K.F.X.; Buerstmayr, H.; Schweiger, W. Quantitative trait loci-dependent analysis of a gene co-expression network associated with Fusarium head blight resistance in bread wheat (Triticum aestivum L.). BMC Genom. 2013, 14, 728. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Ngou, B.P.M.; Ding, P.; Xin, X.-F. PTI-ETI crosstalk: An integrative view of plant immunity. Curr. Opin. Plant Biol. 2021, 62, 102030. [Google Scholar] [CrossRef]
- Fan, A.; Wei, L.; Zhang, X.; Liu, J.; Sun, L.; Xiao, J.; Wang, Y.; Wang, H.; Hua, J.; Singh, R.P.; et al. Heterologous expression of the Haynaldia villosa pattern-recognition receptor CERK1-V in wheat increases resistance to three fungal diseases. Crop J. 2022, 10, 1733–1745. [Google Scholar] [CrossRef]
- Guo, F.; Wu, T.; Xu, G.; Qi, H.; Zhu, X.; Zhang, Z. TaWAK2A-800, a wall-associated kinase, participates positively in resistance to fusarium head blight and sharp eyespot in wheat. Int. J. Mol. Sci. 2021, 22, 11493. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zhou, Y.; Shen, Y.; Sun, Z.; Li, L.; Li, T. Linking multi-omics to wheat resistance types to fusarium head blight to reveal the underlying mechanisms. Int. J. Mol. Sci. 2022, 23, 2280. [Google Scholar] [CrossRef] [PubMed]
- Ren, R.; Zhou, X.; Zhang, X.; Li, X.; Zhang, P.; He, Y. Genome-wide identification and characterization of thaumatin-like protein family genes in wheat and analysis of their responses to Fusarium head blight infection. Food Prod. Process Nutr. 2022, 4, 24. [Google Scholar] [CrossRef]
- Walker, P.L.; Belmonte, M.F.; McCallum, B.D.; McCartney, C.A.; Randhawa, H.S.; Henriquez, M.A. Dual RNA-sequencing of Fusarium head blight resistance in winter wheat. Front. Plant Sci. 2024, 14, 1299461. [Google Scholar] [CrossRef] [PubMed]
- Foroud, N.A.; Ouellet, T.; Laroche, A.; Oosterveen, B.; Jordan, M.C.; Ellis, B.E.; Eudes, F. Differential transcriptome analyses of three wheat genotypes reveal different host response pathways associated with Fusarium head blight and trichothecene resistance. Plant Pathol. 2012, 61, 296–314. [Google Scholar] [CrossRef]
- Klessig, D.F.; Choi, H.W.; Dempsey, D.A. Systemic Acquired Resistance and Salicylic Acid: Past, Present, and Future. Mol. Plant-Microbe Interact. 2018, 31, 871–888. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.Q.; Lin, H.X. Contribution of phenylpropanoid metabolism to plant development and plant–environment interactions. J. Integr. Plant Biol. 2021, 63, 180–209. [Google Scholar] [CrossRef] [PubMed]
- Qi, P.F.; Jiang, Y.F.; Guo, Z.R.; Chen, Q.; Ouellet, T.; Zong, L.J.; Wei, Z.Z.; Wang, Y.; Zhang, Y.Z.; Xu, B.J.; et al. Transcriptional reference map of hormone responses in wheat spikes. BMC Genom. 2019, 20, 390. [Google Scholar] [CrossRef]
- Gunnaiah, R.; Kushalappa, A.C.; Duggavathi, R.; Fox, S.; Somers, D.J. Integrated metabolo-proteomic approach to decipher the mechanisms by which wheat QTL (Fhb1) contributes to resistance against Fusarium graminearum. PLoS ONE 2012, 7, e40695. [Google Scholar] [CrossRef]
- Sunic, K.; Brkljacic, L.; Vukovic, R.; Katanic, Z.; Salopek-Sondi, B.; Spanic, V. Fusarium head blight infection induced responses of six winter wheat varieties in ascorbate–glutathione pathway, photosynthetic efficiency and stress hormones. Plants 2023, 12, 3720. [Google Scholar] [CrossRef]
- Su, P.; Zhao, L.; Li, W.; Zhao, J.; Yan, J.; Ma, X.; Li, A.; Wang, H.; Kong, L. Integrated metabolo-transcriptomics and functional characterization reveals that the wheat auxin receptor TIR1 negatively regulates defense against Fusarium graminearum. J. Integr. Plant Biol. 2021, 63, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Buerstmayr, H.; Steiner, B.; Hartl, L.; Griesser, M.; Angerer, N.; Lengauer, D.; Miedaner, T.; Schneider, B.; Lemmens, M. Molecular mapping of QTLs for fusarium head blight resistance in spring wheat. II. Resistance to fungal penetration and spread. Theor. Appl. Genet. 2003, 14, 503–508. [Google Scholar] [CrossRef]
- Schweiger, W.; Steiner, B.; Ametz, C.; Siegwart, G.; Wiesenberger, G.; Berthiller, F.; Lemmens, M.; Jia, H.; Adam, G.; Muehlbauer, G.J.; et al. Transcriptomic characterization of two major Fusarium resistance quantitative trait loci (QTLs), Fhb1 and Qfhs. ifa-5A, identifies novel candidate genes. Mol. Plant Pathol. 2013, 14, 772–785. [Google Scholar] [CrossRef]
- Nussbaumer, T.; Warth, B.; Sharma, S.; Ametz, C.; Bueschl, C.; Parich, A.; Pfeifer, M.; Siegwart, G.; Steiner, B.; Lemmens, M.; et al. Joint Transcriptomic and Metabolomic Analyses Reveal Changes in the Primary Metabolism and Imbalances in the Subgenome Orchestration in the Bread Wheat Molecular Response to Fusarium graminearum. G3 Genes Genomes Genet. 2015, 5, 2579–2592. [Google Scholar] [CrossRef]
- Buerstmayr, M.; Wagner, C.; Nosenko, T.; Omony, J.; Steiner, B.; Nussbaumer, T.; Mayer, K.F.X.; Buerstmayr, H. Fusarium head blight resistance in European winter wheat: Insights from genome-wide transcriptome analysis. BMC Genom. 2021, 22, 470. [Google Scholar] [CrossRef]
- Yan, H.; Li, G.; Shi, J.; Tian, S.; Zhang, X.; Cheng, R.; Wang, X.; Yuan, Y.; Cao, S.; Zhou, J.; et al. Genetic control of Fusarium head blight resistance in two Yangmai 158-derived recombinant inbred line populations. Appl. Genet. 2021, 134, 3037–3049. [Google Scholar] [CrossRef] [PubMed]
- Bai, G.; Shaner, G. Management and resistance in wheat and barley to Fusarium head blight. Annu. Rev. Phytopathol. 2004, 42, 135–161. [Google Scholar] [CrossRef]
- Rawat, N.; Pumphrey, M.O.; Liu, S.; Zhang, X.; Tiwari, V.K.; Ando, K.; Trick, H.N.; Bockus, W.W.; Akhunov, E.; Anderson, J.A.; et al. Wheat Fhb1 encodes a chimeric lectin with agglutinin domains and a pore-forming toxin-like domain conferring resistance to Fusarium head blight. Nat. Genet. 2016, 48, 1576–1580. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Luo, Y.; Zhao, P.; Jia, H.; Ma, Z. Overexpression of TaJRL53 enhances the Fusarium head blight resistance in wheat. Acta Agron. Sin. 2021, 47, 19–29. [Google Scholar] [CrossRef]
- Su, Z.; Bernardo, A.; Tian, B.; Chen, H.; Wang, S.; Ma, H.; Cai, S.; Liu, D.; Zhang, D.; Li, T.; et al. A deletion mutation in TaHRC confers Fhb1 resistance to fusarium head blight in wheat. Nat. Genet. 2019, 51, 1099–1105. [Google Scholar] [CrossRef]
- Li, G.; Zhou, J.; Jia, H.; Gao, Z.; Fan, M.; Luo, Y.; Panting Zhao, P.; Xue, S.; Li, N.; Yuan, Y.; et al. Mutation of a histidine-rich calcium-binding-protein gene in wheat confers resistance to fusarium head blight. Nat. Genet. 2019, 51, 1106–1112. [Google Scholar] [CrossRef] [PubMed]
- Lagudah, E.S.; Krattinger, S.G. A new player contributing to durable Fusarium resistance. Nat. Genet. 2019, 51, 1070–1071. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yang, X.; Xia, X.; Wang, Y.; Dong, Y.; Wu, L.; Jiang, P.; Zhang, X.; Jiang, C.; Ma, H.; et al. A phase-separated protein hub modulates resistance to Fusarium head blight in wheat. Cell Host Microbe 2024, 32, 710–726. [Google Scholar] [CrossRef] [PubMed]
- Gunnaiah, R.; Kushalappa, A.C. Metabolomics deciphers the host resistance mechanisms in wheat cultivar Sumai-3, against trichothecene producing and non-producing isolates of Fusarium graminearum. Plant Physiol. Biochem. 2014, 83, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Hofstad, A.N.; Nussbaumer, T.; Akhunov, E.; Shin, S.; Kugler, K.G.; Kistler, H.C.; Mayer, K.F.X.; Muehlbauer, G.J. Examining the transcriptional response in wheat Fhb1 near-isogenic lines to Fusarium graminearum infection and deoxynivalenol treatment. Plant Genome 2016, 9, plantgenome2015-05. [Google Scholar] [CrossRef] [PubMed]
- Soni, N.; Hegde, N.; Dhariwal, A.; Kushalappa, A.C. Role of laccase gene in wheat NILs differing at QTL-Fhb1 for resistance against fusarium head blight. Plant Sci. 2020, 298, 110574. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Li, F.; Sun, Y.; Tian, J.J.X.; Li, Q.; Duan, K.; Lin, J.; Liu, H.; Wang, Q. Basal defense is enhanced in a wheat cultivar resistant to Fusarium head blight. J. Integr. Agric. 2024, 23, 1238–1258. [Google Scholar] [CrossRef]
- Yang, G.; Pan, W.; Zhang, R.; Pan, Y.; Guo, Q.; Song, W.; Zheng, W.; Nie, X. Genome-wide identification and characterization of caffeoyl-coenzyme A O-methyltransferase genes related to the Fusarium head blight response in wheat. BMC Genom. 2021, 22, 504. [Google Scholar] [CrossRef] [PubMed]
- Soni, N.; Altartouri, B.; Hegde, N.; Duggavathi, R.; Nazarian-Firouzabadi, F.; Kushalappa, A.C. TaNAC032 transcription factor regulates lignin-biosynthetic genes to combat fusarium head blight in wheat. Plant Sci. 2021, 304, 110820. [Google Scholar] [CrossRef]
- Zhong, R.; Demura, T.; Ye, Z.H. SND1, a NAC domain transcription factor, is a key regulator of secondary wall synthesis in fibers of Arabidopsis. Plant Cell 2006, 18, 3158–3170. [Google Scholar] [CrossRef]
- Mitsuda, N.; Seki, M.; Shinozaki, K.; Ohme-Takagi, M. The NAC Transcription Factors NST1 and NST2 of Arabidopsis regulate secondary wall thickenings and are required for anther dehiscence. Plant Cell 2005, 17, 2993–3006. [Google Scholar] [CrossRef] [PubMed]
- Perochon, A.; Kahla, A.; Vranić, M.; Jia, J.; Malla, K.B.; Craze, M.; Wallington, E.; Doohan, F.M. A wheat NAC interacts with an orphan protein and enhances resistance to fusarium head blight disease. Plant Biotechnol. J. 2019, 17, 1892–1904. [Google Scholar] [CrossRef] [PubMed]
- Dhokane, D.; Karre, S.; Kushalappa, A.C.; McCartney, C. Integrated metabolo-transcriptomics reveals Fusarium head blight candidate resistance genes in wheat Qtl-fhb2. PLoS ONE 2016, 11, e0155851. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Balcerzak, M.; Gulden, S.; Cao, W.; Fedak, G.; Wei, Y.M.; Zheng, Y.L.; Somers, D.; Ouellet, T. Expression profiling identifies differentially expressed genes associated with the fusarium head blight resistance QTL 2DL from the wheat variety Wuhan-1. Physiol. Mol. Plant Pathol. 2015, 90, 1–11. [Google Scholar] [CrossRef]
- Biselli, C.; Bagnaresi, P.; Faccioli, P.; Hu, X.; Balcerzak, M.; Mattera, M.G.; Yan, Z.; Ouellet, T.; Cattivelli, L.; Valè, G. Comprehensive transcriptome profiles of near-isogenic hexaploid wheat lines differing for effective alleles at the 2DL FHB resistance QTL. Front. Plant Sci. 2018, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Rocheleau, H.; McCartney, C.; Biselli, C.; Bagnaresi, P.; Balcerzak, M.; Fedak, G.; Yan, Z.; Valè, G.; Khanizadeh, S.; et al. Identification and mapping of expressed genes associated with the 2DL QTL for fusarium head blight resistance in the wheat line Wuhan 1. BMC Genet. 2019, 20, 47. [Google Scholar]
- Kage, U.; Karre, S.; Kushalappa, A.C.; McCartney, C. Identification and characterization of a fusarium head blight resistance gene TaACT in wheat QTL-2DL. Plant Biotechnol. J. 2017, 15, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Kage, U.; Yogendra, K.N.; Kushalappa, A.C. TaWRKY70 transcription factor in wheat QTL-2DL regulates downstream metabolite biosynthetic genes to resist Fusarium graminearum infection spread within spike. Sci. Rep. 2017, 7, 42596. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sun, S.; Ge, W.; Zhao, L.; Hou, B.; Wang, K.; Lyu, Z.; Chen, L.; Xu, S.; Guo, J.; et al. Horizontal gene transfer of Fhb7 from fungus underlies fusarium head blight resistance in wheat. Science 2020, 368, eaba5435. [Google Scholar] [CrossRef]
- Miller, J.D.; Young, J.C.; Sampson, D.R. Deoxynivalenol and Fusarium head blight resistance in spring cereals. J. Phytopathol. 1985, 113, 359–367. [Google Scholar] [CrossRef]
- Mahmood, K.; Orabi, J.; Kristensen, P.S.; Sarup, P.; Jorgensen, L.N.; Jahoor, A. A comparative transcriptome analysis, conserved regulatory elements and associated transcription factors related to accumulation of fusariotoxins in grain of rye (Secale cereale L.) Hybrids. Int. J. Mol. Sci. 2020, 21, 7418. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; Guo, J.; He, D.; Li, G.; Ouellet, T. Deoxynivalenol accumulation and detoxification in cereals and its potential role in wheat-Fusarium graminearum interactions. aBIOTECH 2023, 4, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Mesterhazy, A. What Is Fusarium Head Blight (FHB) Resistance and What Are Its Food Safety Risks in Wheat? Problems and Solutions—A Review. Toxins 2024, 16, 31. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Guo, Q.; He, Q.; Tian, Z.; Hao, W.; Shan, X.; Lu, J.; Barkla, B.J.; Ma, C.; Si, H. Comparative transcriptomic analysis of wheat cultivars differing in their resistance to Fusarium head blight infection during grain-filling stages reveals unique defense mechanisms at play. BMC Plant Biol. 2023, 23, 433. [Google Scholar] [CrossRef] [PubMed]
- Miedaner, T.; Longin, C.F.H. Genetic variation for resistance to Fusarium head blight in winter durum material. Crop Pasture Sci. 2014, 65, 46–51. [Google Scholar] [CrossRef]
- Goddard, R.; Steed, A.; Scheeren, P.L.; Maciel, J.L.N.; Caierão, E.; Torres, G.A.M.; Consoli, L.; Santana, F.M.; Fernandes, J.M.C.; Simmonds, J.; et al. Identification of Fusarium head blight resistance loci in two Brazilian wheat mapping populations. PLoS ONE 2021, 16, e0248184. [Google Scholar] [CrossRef] [PubMed]
- Lucyshyn, D.; Busch, B.L.; Abolmaali, S.; Steiner, B.; Chandler, E.; Sanjarian, F.; Mousavi, A.; Nicholson, P.; Buerstmayr, H.; Adam, G. Cloning and characterization of the ribosomal protein L3 (RPL3) gene family from Triticum aestivum. Mol. Genet. Genom. 2007, 277, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Walter, S.; Kahla, A.; Arunachalam, C.; Perochon, A.; Khan, M.R.; Scofield, S.R.; Doohan, F.M. A wheat ABC transporter contributes to both grain formation and mycotoxin tolerance. J. Exp. Bot. 2015, 66, 2583–2593. [Google Scholar] [CrossRef] [PubMed]
- Gunupuru, L.R.; Arunachalam, C.; Malla, K.B.; Kahla, A.; Perochon, A.; Jia, J.; Thapa, G.; Doohan, F.M. A wheat cytochrome P450 enhances both resistance to deoxynivalenol and grain yield. PLoS ONE 2018, 13, e0204992. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Ma, X.; Su, P.; Ge, W.; Wu, H.; Guo, X.; Li, A.; Wang, H.; Kong, L. Cloning and characterization of a specific UDP-glycosyltransferase gene induced by DON and Fusarium graminearum. Plant Cell Rep. 2018, 37, 641–652. [Google Scholar] [CrossRef]
- He, Y.; Wu, L.; Liu, X.; Jiang, P.; Yu, L.; Qiu, J.; Wang, G.; Zhang, X.; Zhang, X.; Ma, H. TaUGT6, a novel UDP-glycosyltransferase gene enhances the resistance to FHB and DON accumulation in wheat. Front. Plant Sci. 2020, 11, 574775. [Google Scholar] [CrossRef] [PubMed]
- Mandalà, G.; Tundo, S.; Francesconi, S.; Gevi, F.; Zolla, L.; Ceoloni, C.; D’Ovidio, R. Deoxynivalenol detoxification in transgenic wheat confers resistance to fusarium head blight and crown rot diseases. Mol. Plant Microbe Interact. 2019, 32, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Gatti, M.; Cambon, F.; Tassy, C.; Macadre, C.; Guerard, F.; Langin, T.; Dufresne, M. The Brachypodium distachyon UGT Bradi5gUGT03300 confers type II fusarium head blight resistance in wheat. Plant Pathol. 2019, 68, 334–343. [Google Scholar] [CrossRef]
- Gallé, Á.; Pelsőczi, A.; Benyó, D.; Podmaniczki, A.; Szabó-Hevér, A.; Poór, P.; Tóth, B.; Horváth, E.; Erdei, L.; Csiszár, J. Systemic response to Fusarium graminearum and culmorum inoculations: Changes in detoxification of flag leaves in wheat. Cereal Res. Commun. 2022, 50, 1055–1063. [Google Scholar] [CrossRef]
- Ackerman, A.J.; Holmes, R.; Gaskins, E.; Jordan, K.E.; Hicks, D.S.; Fitzgerald, J.; Griffey, C.A.; Mason, R.E.; Harrison, S.A.; Murphy, J.P.; et al. Evaluation of Methods for Measuring Fusarium-Damaged Kernels of Wheat. Agronomy 2022, 12, 532. [Google Scholar] [CrossRef]
- Buerstmayr, H.; Ban, T.; Anderson, J.A. QTL mapping and marker-assisted selection for Fusarium head blight resistance in wheat: A review. Plant Breed. 2009, 128, 1–26. [Google Scholar] [CrossRef]
- Sneller, C.; Guttieri, M.; Paul, P.; Costa, J.; Jackwood, R. Variation for resistance to kernel infection and toxin accumulation in winter wheat infected with Fusarium graminearum. Phytopathology 2012, 102, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Mesterházy, A. Role of deoxynivalenol in aggressiveness of Fusarium graminearum and F. culmorum and in resistance to Fusarium head blight. Eur. J. Plant Pathol. 2002, 108, 675–684. [Google Scholar] [CrossRef]
- Lamb, K.E.; Gonzalez-Hernandez, J.; Zhang, B.; Green, M.; Neate, S.M.; Schwarz, P.B.; Horsley, R.D. Identification of QTL conferring resistance to Fusarium head blight resistance in the breeding line C93-3230-24. Crop Sci. 2009, 49, 1675–1680. [Google Scholar] [CrossRef]
- Loffler, M.; Schon, C.C.; Miedaner, T. Revealing the genetic architecture of FHB resistance in hexaploid wheat (Triticum aestivum L.) by QTL meta-analysis. Mol. Breed. 2009, 23, 473–488. [Google Scholar] [CrossRef]
- Góral, T.; Wiśniewska, H.; Ochodzki, P.; Nielsen, L.K.; Walentyn-Góral, D.; Stępień, Ł. Relationship between Fusarium Head Blight, Kernel Damage, Concentration of Fusarium Biomass, and Fusarium Toxins in Grain of Winter Wheat Inoculated with Fusarium culmorum. Toxins 2019, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Balut, A.L.; Clark, A.J.; Brown-Guedira, G.; Souza, E.; Sanford, D.A.V. Validation of Fhb1 and QFhs.Nau-2DL in Several Soft Red Winter Wheat Populations. Crop Sci. 2013, 53, 934–945. [Google Scholar] [CrossRef]
- Gaire, R.; Arruda, M.P.; Mohammadi, M.; Brown-Guedira, G.; Kolb, F.L.; Rutkoski, J. Multi-trait genomic selection can increase selection accuracy for deoxynivalenol accumulation resulting from Fusarium head blight in wheat. Plant Genome 2022, 15, e20188. [Google Scholar] [CrossRef] [PubMed]
- Berraies, S.; Ruan, Y.; Knox, R.; DePauw, R.; Bokore, F.; Cuthbert, R.; Blackwell, B.; Henriquez, M.A.; Konkin, D.; Yu, B.; et al. Genetic mapping of deoxynivalenol and Fusarium damaged kernel resistance in an adapted durum wheat population. BMC Plant Biol. 2024, 24, 183. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; He, X.; Zhang, Y.; Li, L.; Sun, Z.; Bai, G.; Singh, P.K.; Li, T. Development of an evaluation system for Fusarium resistance in wheat grains and its application in assessment of the corresponding effects of Fhb1. Plant Dis. 2020, 104, 2210–2216. [Google Scholar] [CrossRef] [PubMed]
- Maričević, M.; Španić, V.; Bukan, M.; Rajković, B.; Šarčević, H. Diallel Analysis of Wheat Resistance to Fusarium Head Blight and Mycotoxin Accumulation under Conditions of Artificial Inoculation and Natural Infection. Plants 2024, 13, 1022. [Google Scholar] [CrossRef] [PubMed]
- Serajazari, M.; Torkamaneh, D.; Gordon, E.; Lee, E.; Booker, H.; Pauls, K.P.; Navabi, A. Identification of fusarium head blight resistance markers in a genome-wide association study of CIMMYT spring synthetic hexaploid derived wheat lines. BMC Plant Biol. 2023, 23, 290. [Google Scholar] [CrossRef] [PubMed]
- Goffinet, B.; Gerber, S. Quantitative trait loci: A meta-analysis. Genetics 2000, 155, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Maccaferri, M.; Ricci, A.; Silvio Salvi, S.; Milner, S.G.; Noli, E.; Martelli, P.L.; Casadio, R.; Akhunov, E.; Scalabrin, S.; Vendramin, V.; et al. A high-density, SNP-based consensus map of tetraploid wheat as a bridge to integrate durum and bread wheat genomics and breeding. Plant Biotechnol. J. 2015, 13, 648–663. [Google Scholar] [CrossRef]
- Soriano, J.M.; Colasuonno, P.; Marcotuli, I.; Gadaleta, A. Meta-QTL analysis and identification of candidate genes for quality, abiotic and biotic stress in durum wheat. Sci. Rep. 2021, 11, 11877. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Z.; Ma, H.; Huang, L.; Ding, F.; Du, Y.; Jia, H.; Li, G.; Kong, Z.; Ran, C.; et al. Pyramiding of Fusarium Head Blight Resistance Quantitative Trait Loci, Fhb1, Fhb4, and Fhb5, in Modern Chinese Wheat Cultivars. Front. Plant Sci. 2021, 12, 694023. [Google Scholar] [CrossRef]
- Dai, X.; Huang, Y.; Xue, X.; Yu, S.; Li, T.; Liu, H.; Yang, L.; Zhou, Y.; Li, H.; Zhang, H. Effects of Fhb1, Fhb2 and Fhb5 on Fusarium head blight resistance and the development of promising lines in winter wheat. Int. J. Mol. Sci. 2022, 23, 15047. [Google Scholar] [CrossRef] [PubMed]
- Kirana, R.P.; Michel, S.; Moreno-Amores, J.; Prat, N.; Lemmens, M.; Buerstmayr, M.; Buerstmayr, H.; Steiner, B. Pyramiding Fusarium head blight resistance QTL from T. aestivum, T. dicoccum and T. dicoccoides in durum wheat. Theor. Appl. Genet. 2023, 136, 201. [Google Scholar] [CrossRef]
- Voss-Fels, K.P.; Cooper, M.; Hayes, B.J. Accelerating crop genetic gains with Genomic Selection. Theor. Appl. Genet. 2019, 132, 669–686. [Google Scholar] [CrossRef]
- Liu, H.J.; Yan, J. Crop Genome-wide Association study: A harvest of biological relevance. Plant J. 2019, 97, 8–18. [Google Scholar] [CrossRef]
- Larkin, D.L.; Mason, R.E.; Moon, D.E.; Holder, A.L.; Ward, B.P.; Brown-Guedira, G. Predicting Fusarium head blight resistance for advanced trials in a soft red winter wheat breeding program with Genomic Selection. Front. Plant Sci. 2021, 12, 715314. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, B.; Mergoum, M.; Martinez-Espinoza, A.D.; Sapkota, S.; Pradhan, S.; Babar, M.A.; Bai, G.; Dong, Y.; Buck, J.W. Genetics of Fusarium head blight resistance in soft red winter wheat using a genome-wide association study. Plant Genome 2022, 15, 20222. [Google Scholar] [CrossRef]
- Shi, C.; Chao, H.; Sun, X.; Suo, Y.; Chen, Z.; Li, Z.; Ma, L.; Li, J.; Ren, Y.; Hua, W.; et al. Genome-Wide Association Study for Fusarium head blight resistance in common wheat from China. Agronomy 2023, 13, 1712. [Google Scholar] [CrossRef]
- Michel, S.; Wagner, C.; Nosenko, T.; Steiner, B.; Samad-Zamini, M.; Buerstmayr, M.; Mayer, K.; Buerstmayr, H. Merging genomics and transcriptomics for predicting fusarium head blight resistance in wheat. Genes 2021, 12, 114. [Google Scholar] [CrossRef]
| Type I Resistance | Omics Sciences | Wheat Cultivars/Species | QTL Analysis | Short Description | References |
|---|---|---|---|---|---|
| Genomics, transcriptomics | CM-82036 and Remus | Fhb1, Qfhs.ifa-5A | The predominant role of glucanases, NBS-LRR, WRKY transcription factors and UDP-glycosyltransferases in pathogen response was underlined | [50] | |
| Genomics, transcriptomics | CM-82036 and Remus | Fhb1, Qfhs.ifa-5A | After inoculation with Fg spores, LTP and UDP genes were detected | [65] | |
| Genomics, transcriptomics, metabolomics | Bread wheat | Fhb1, Qfhs.ifa-5A | Results showed glutamate metabolism changes in lines hosting Qfhs.ifa-5A. TCA genes showed greater expression levels, playing important roles in the early stage of Fg infection. | [66] | |
| Transcriptomics, metabolomics | Sumai3 and three regionally adapted Canadian cultivars | Investigation of the role of plant hormones during the interaction of wheat with Fg | [38] | ||
| Genomics, transcriptomics | European winter wheat genotypes (including Sumai3) | Fhb1, Qfhs.ifa-5A | The Sumai3-derivative lines showed higher expression of genes associated with cell wall and terpene metabolism. In Qfhs.ifa-5A, a gene encoding a stress response NST1-like protein was identified | [67] | |
| Transcriptomics, metabolomics | Sumai3, Taimai198, Huaimai33 and JWI | Wheat auxin receptor TIR1 negatively regulates defense against Fg | [63] |
| Type II Resistance | Omics Sciences | Wheat Cultivars/Species | QTL Analysis | Short Description | References |
|---|---|---|---|---|---|
| Genomics, transcriptomics | Chinese Spring | Fhb1 | A TaHRC/His gene, encoding histidine-rich calcium-binding protein, was identified in Fhb1 | [72,73,74] | |
| Metabolomics, proteomics | Nyubai genotype | Fhb1 | Hydroxycinnamic acid amides and flavonoids, played an important role in FHB resistance | [76] | |
| Genomics, transcriptomics, metabolomics | Sumai 3, Stoa | Fhb1 | Results showed that the rachis is a crucial location for Type II resistance | [77] | |
| Genomics, transcriptomics | Sumai3*5, Thatcher | FHb1 | The role of laccase gene for FHB resistance is underlined | [78] | |
| Genomics, transcriptomics | Chinese Spring, Sumai3, Thatcher | Fhb1 | The role of NAC transcription factor, regulating the biosynthesis of lignin, is relevant in resistance to FHB infestation | [81] | |
| Transcriptomics proteomics | CM82036, Fielder | The gene TaNACL-D1 interacts with an orphan protein and enhances resistance to FHB | [84] | ||
| Genomics, transcriptomics | Fhb2 | The lignin and CCoAOMT role in FHB resistance was investigated | [80] | ||
| Transcriptomics, metabolomics | BW-278, AC Foremost | Fhb2 | Phenylpropanoids, lignin, flavonoids, mycotoxin detoxification proteins are involved in FHB response | [85] | |
| Genomics, transcriptomics | HC374, CDC Alsask | 2DL | ~25 DEG located on chromosome arm 2DL were selected. SAM-dependent methyltransferase genes were identified | [88] | |
| Genomics, transcriptomics, metabolomics | BW301, HC374 | 2DL | Several genes conferring resistance to FHB, including TaACT encoding agmatine coumaroyl transferase were identified | [89] | |
| Transcriptomics, metabolomics | BW301, HC374 | 2DL | HCAAs were identified as resistance metabolites in rachis. TaWRKY70 transcription factor regulates the biosynthetic of these genes | [90] | |
| Genomics, transcriptomics | Th. elongatum | FHb7 | Fhb7 was transferred from Thinopyrum and was cloned | [91] |
| Type III Resistance | Omics Sciences | Wheat Cultivars/Species | QTL Analysis | Short Description | References |
|---|---|---|---|---|---|
| Genomics, transcriptomics | CM82036, Remus | The TaABCC3.1 gene, associated with DON resistance in wheat, was characterized | [100] | ||
| Genomics, transcriptomics | CM82036, Remus | The TaCYP72A gene was found to be activated by DON treatment and Fg infection | [101] | ||
| Genomics, transcriptomics | Sumai3, Ning7840, Apogee73S2, Shannong22, Jimai22, Apogee, Liangxing66, Kenong199, Jiyin1, Chinese Spring | The TaUGT5 gene was characterized and reported to be effective in reducing DON content | [102] | ||
| Genomics, transcriptomics, metabolomics | Sumai 3, Annong 8455, Fielder | The TaUGT6 gene was characterized and its positive role in reducing DON content in wheat was confirmed | [103] | ||
| Genomics, transcriptomics, metabolomics | Transgenic durum and bread wheat plants | FHB symptoms were reduced in two transgenic wheat plants, obtained introducing the barley HvUGT13248 | [104] | ||
| Transcriptomics, metabolomics | Apogee | The Brachypodium distachyon UGT Bradi5gUGT03300 confers FHB resistance in wheat | [105] | ||
| Transcriptomics, proteomics | Suma3 and a crossing inbred population of GK Mini Manó/Nobeokabozu | Systemic changes in many elements of the antioxidant/detoxification defense system are detected, and the positive role of GSTs in FHB resistance was underlined | [106] |
| Type IV Resistance | Omics Sciences | Wheat Cultivars/Species | Short Description | References |
|---|---|---|---|---|
| Transcriptomics, metabolomics | winter wheat | Fusarium biomass was analyzed. Significant correlation was found between head infection symptoms and FDK | [113] | |
| Genomics | soft red winter wheat (SRWW) | Results showed that FDK is the most important secondary trait to predict DON | [115] | |
| Genomics | durum wheat | DON accumulation resistance QTLs and FDK resistance QTLs were identified | [116] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sirangelo, T.M. Molecular Investigations to Improve Fusarium Head Blight Resistance in Wheat: An Update Focusing on Multi-Omics Approaches. Plants 2024, 13, 2179. https://doi.org/10.3390/plants13162179
Sirangelo TM. Molecular Investigations to Improve Fusarium Head Blight Resistance in Wheat: An Update Focusing on Multi-Omics Approaches. Plants. 2024; 13(16):2179. https://doi.org/10.3390/plants13162179
Chicago/Turabian StyleSirangelo, Tiziana M. 2024. "Molecular Investigations to Improve Fusarium Head Blight Resistance in Wheat: An Update Focusing on Multi-Omics Approaches" Plants 13, no. 16: 2179. https://doi.org/10.3390/plants13162179
APA StyleSirangelo, T. M. (2024). Molecular Investigations to Improve Fusarium Head Blight Resistance in Wheat: An Update Focusing on Multi-Omics Approaches. Plants, 13(16), 2179. https://doi.org/10.3390/plants13162179






