A Rapid Method for Screening Pathogen-Associated Molecular Pattern-Triggered Immunity-Intensifying Microbes
Abstract
1. Introduction
2. Materials and Methods
2.1. Growth Conditions of Arabidopsis thaliana Plants and Bacteria
2.2. Analysis of GSL5 Gene Expression
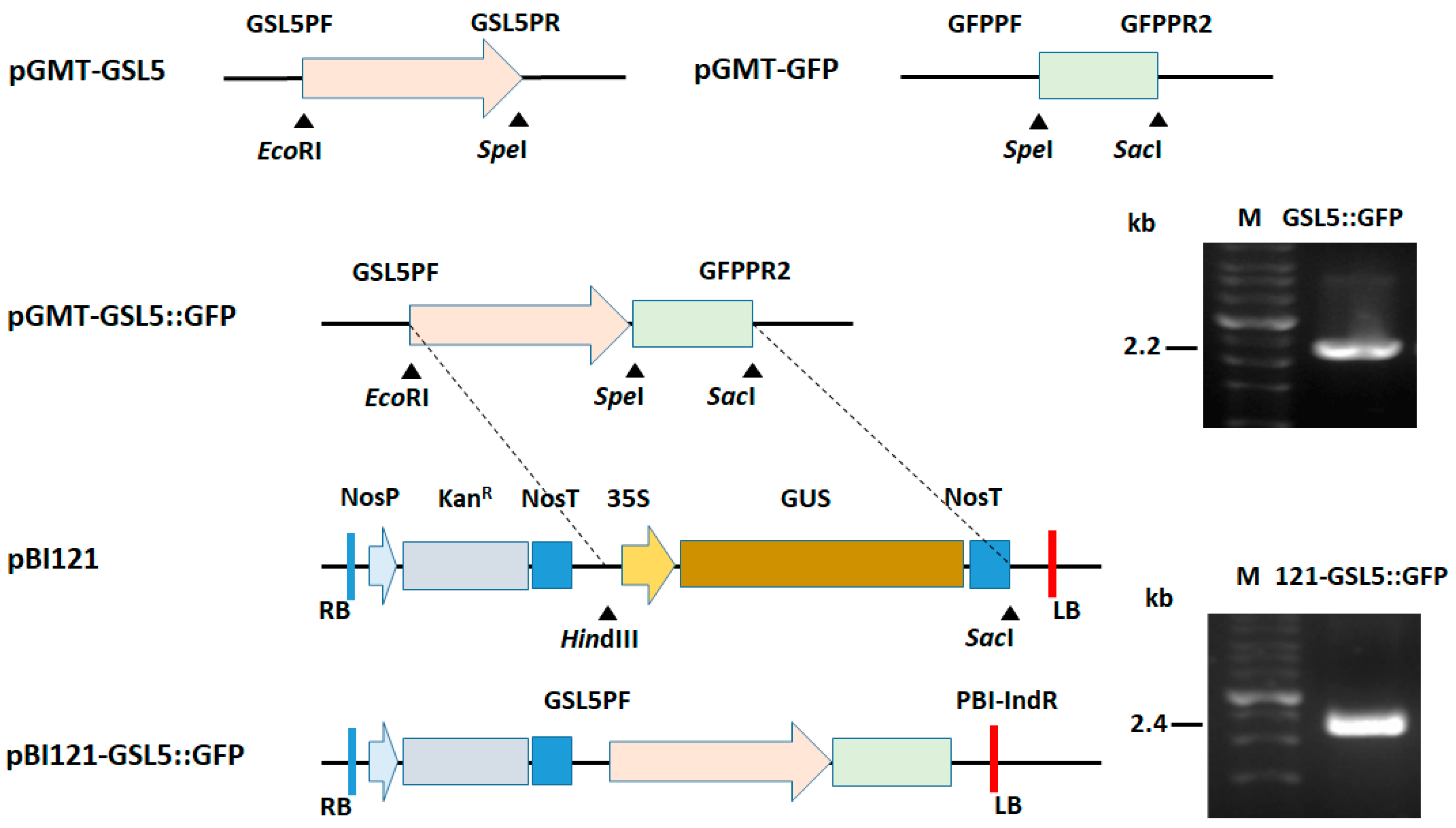
2.3. Construction of GSL5::GFP
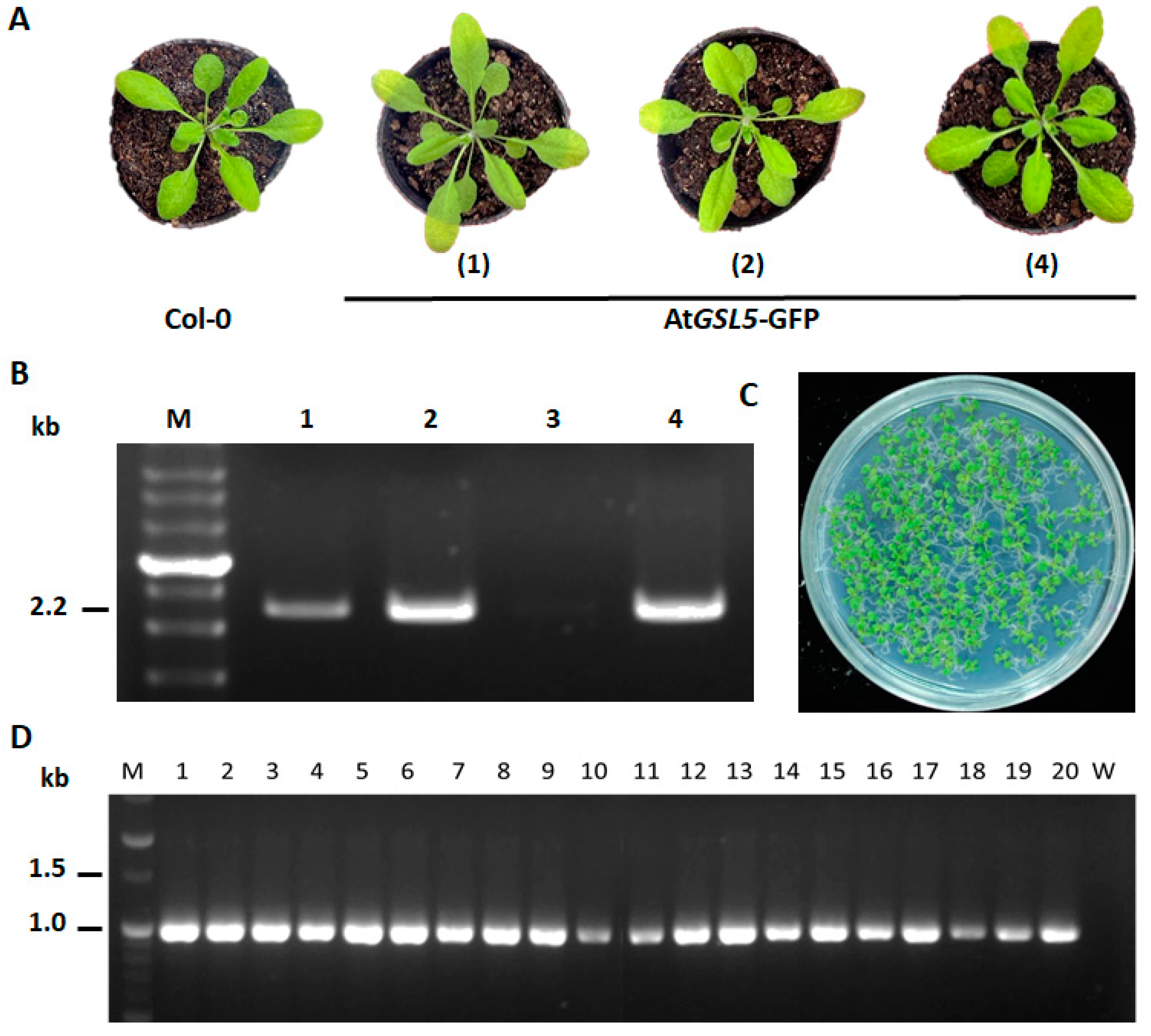
2.4. Plant Transformation and Transgenic Plant Screening
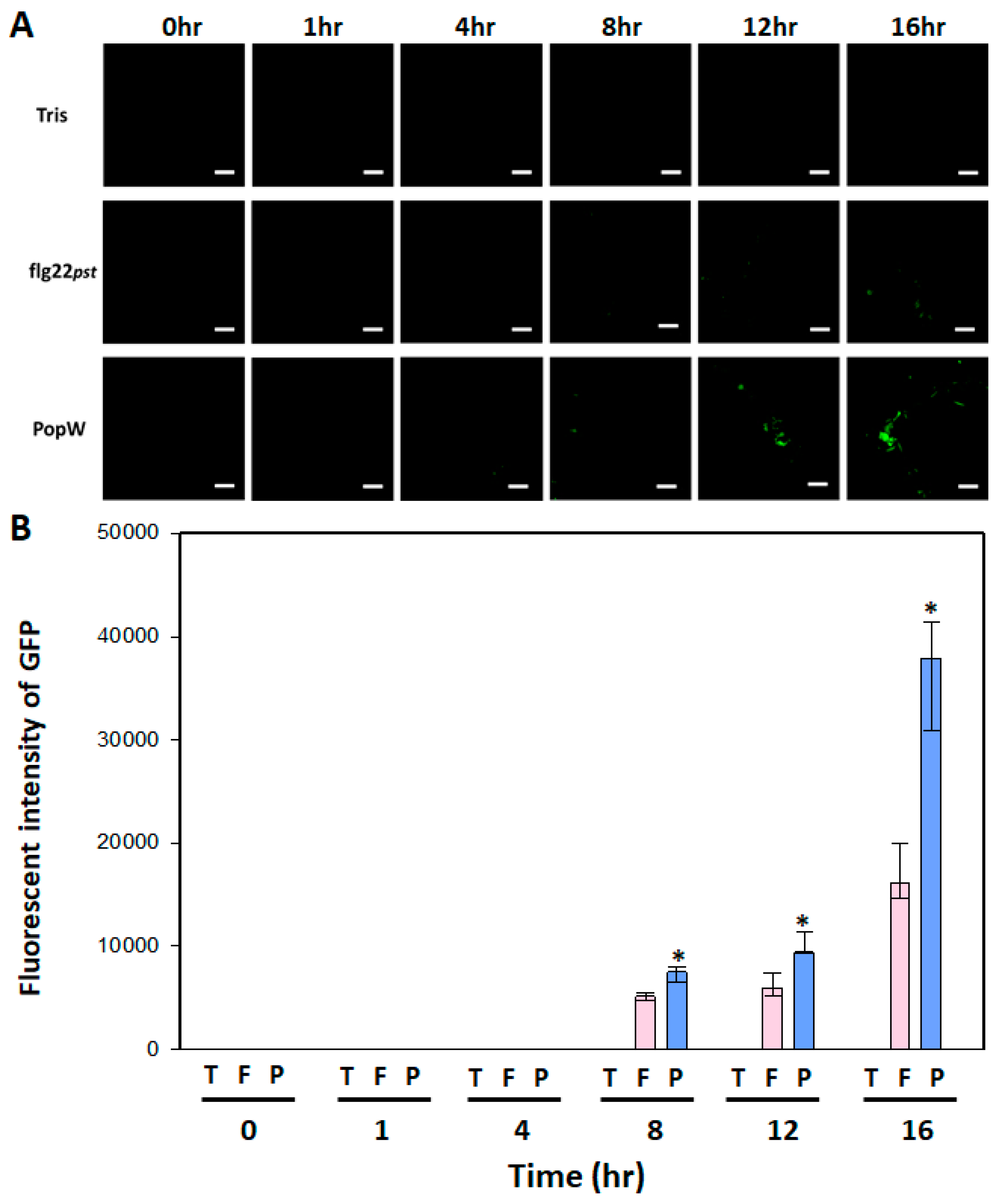
2.5. Leaf Fluorescence Image Analysis of AtGSL5-GFP
2.6. Observation of Callose Deposition
2.7. Disease Severity Assay
2.8. Statistical Analysis
3. Results
3.1. Gene Expression Changes in GSL5 Gene in Response to flg22Pst Activation
3.2. Plasmid Construction for Plant Transformation
3.3. Confirmation of AtGSL5-GFP Transgenic Line
3.4. Leaf Fluorescence Performance AtGSL5-GFP upon PAMP Activation
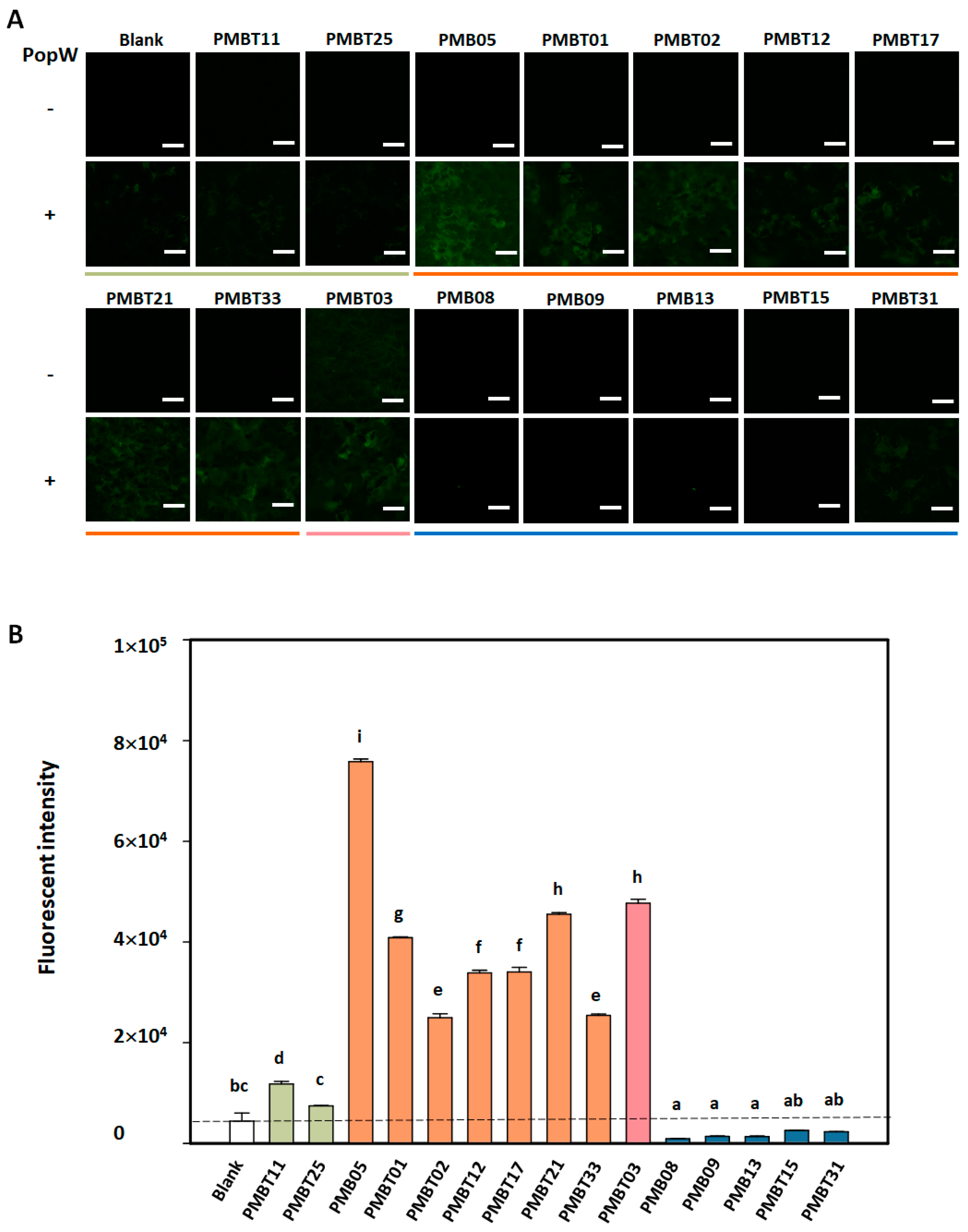
3.5. Regulation of PopW-Induced GFP Fluorescence by Bacillus spp. Strains on AtGSL5-GFP
3.6. Regulation of PopW-Mediated Callose Deposition by Bacillus spp. Strains
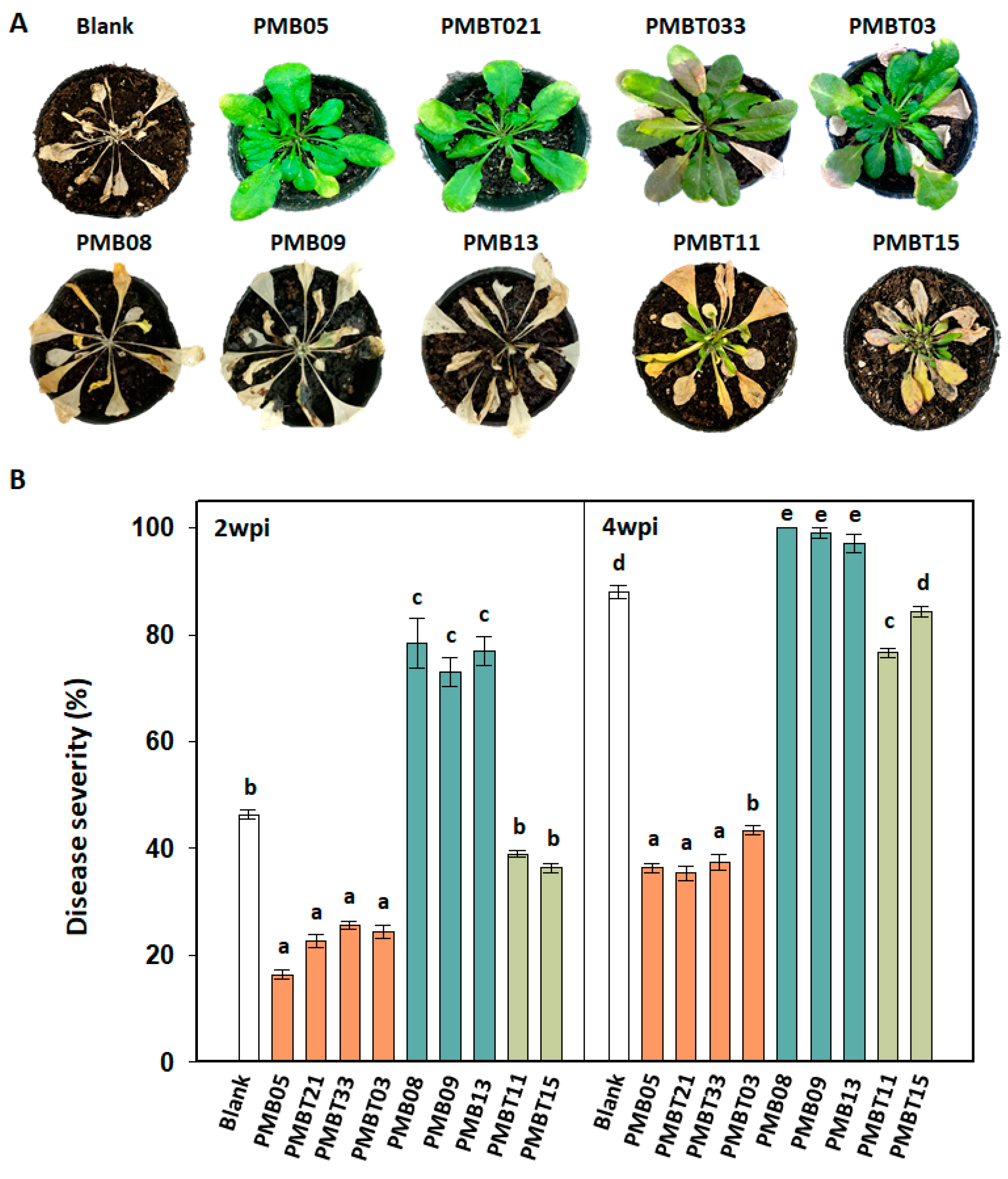
3.7. Disease Resistance Affected by Bacillus spp. Strains
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Adhikari, M.; Yadav, D.R.; Kim, S.W.; Um, Y.H.; Kim, H.S.; Lee, S.C.; Song, J.Y.; Kim, H.G.; Lee, Y.S. Biological control of bacterial fruit blotch of watermelon pathogen (Acidovorax citrulli) with rhizosphere associated bacteria. Plant Pathol. J. 2017, 33, 170–183. [Google Scholar] [CrossRef]
- Ahmed, W.; Dai, Z.; Zhang, J.; Li, S.; Ahmed, A.; Munir, S.; Liu, Q.; Tan, Y.; Ji, G.; Zhao, Z. Plant-Microbe Interaction: Mining the Impact of Native Bacillus amyloliquefaciens WS-10 on Tobacco Bacterial Wilt Disease and Rhizosphere Microbial Communities. Microbiol. Spectr. 2022, 10, e01471-22. [Google Scholar] [CrossRef]
- Compant, S.; Duffy, B.; Nowak, J.; Clément, C.; Barka, E.A. Use of plant growth-promoting bacteria for biocontrol of plant diseases: Principles, mechanisms of action, and future prospects. Appl. Environ. Microbiol. 2005, 71, 4951–4959. [Google Scholar] [CrossRef]
- Huang, H.-E.; Liu, C.-A.; Lee, M.-J.; Kuo, C.-G.; Chen, H.-M.; Ger, M.-J.; Tsai, Y.-C.; Chen, Y.-R.; Lin, M.-K.; Feng, T.-Y. Resistance enhancement of transgenic tomato to bacterial pathogens by the heterologous expression of sweet pepper ferredoxin-I protein. Phytopathology 2007, 97, 900–906. [Google Scholar] [CrossRef]
- Kaari, M.; Joseph, J.; Manikkam, R.; Sreenivasan, A.; Venugopal, G.; Alexander, B.; Krishnan, S. Biocontrol Streptomyces Induces Resistance to Bacterial Wilt by Increasing Defense-Related Enzyme Activity in Solanum melongena L. Curr. Microbiol. 2022, 79, 146. [Google Scholar] [CrossRef]
- Merga, I.F.; Tripathi, L.; Hvoslef-Eide, A.K.; Gebre, E. Application of Genetic Engineering for Control of Bacterial Wilt Disease of Enset, Ethiopia’s Sustainability Crop. Front. Plant Sci. 2019, 10, 133. [Google Scholar] [CrossRef]
- Chen, M.; Wang, J.; Liu, B.; Zhu, Y.; Xiao, R.; Yang, W.; Ge, C.; Chen, Z. Biocontrol of tomato bacterial wilt by the new strain Bacillus velezensis FJAT-46737 and its lipopeptides. BMC Microbiol. 2020, 20, 160. [Google Scholar] [CrossRef]
- Jinal, N.H.; Amaresan, N. Evaluation of biocontrol Bacillus species on plant growth promotion and systemic-induced resistant potential against bacterial and fungal wilt-causing pathogens. Arch. Microbiol. 2020, 202, 1785–1794. [Google Scholar] [CrossRef]
- Moussa, Z.; Rashad, E.M.; Elsherbiny, E.A.; Al-Askar, A.A.; Arishi, A.A.; Al-Otibi, F.O.; Saber, W.I.A. New Strategy for Inducing Resistance against Bacterial Wilt Disease Using an Avirulent Strain of Ralstonia solanacearum. Microorganisms 2022, 10, 1814. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Dong, Y.; Liao, H.; Huang, J.; Song, S.; Xu, Y.; Shen, Q. Antagonistic bacterium Bacillus amyloliquefaciens induces resistance and controls the bacterial wilt of tomato. Pest Manag. Sci. 2013, 69, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.-Y.; Lin, S.-T.; Li, A.-T.; Li, S.-H.; Hsiao, C.-Y.; Lin, Y.-H. Bacillus amyloliquefaciens PMB05 Increases Resistance to Bacterial Wilt by Activating Mitogen-Activated Protein Kinase and Reactive Oxygen Species Pathway Crosstalk in Arabidopsis thaliana. Phytopathology 2022, 112, 2495–2502. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.-H.; Chuang, C.-Y.; Zheng, J.-L.; Chen, H.-H.; Liang, Y.-S.; Huang, T.-P.; Lin, Y.-H. Bacillus amyloliquefaciens strain PMB05 intensifies plant immune responses to confer resistance against bacterial wilt of tomato. Phytopathology 2020, 110, 1877–1885. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Lai, I.-L.; Zheng, J.-L.; Lin, Y.-H. Using dynamic changes of chlorophyll fluorescence in Arabidopsis thaliana to evaluate plant immunity-intensifying Bacillus spp. strains. Phytopathology 2019, 109, 1566–1576. [Google Scholar] [CrossRef]
- Chang, J.-J.; Wu, P.-Y.; Lin, Y.-N.; Deng, W.-L.; Lin, Y.-H. Intensification of PAMP-triggered immunity in watermelon by Bacillus spp. strains as a strategy for controlling bacterial fruit blotch disease. J. Plant Med. 2019, 61, 39–48. [Google Scholar]
- Hsiao, C.-Y.; Yan, J.-C.; Peng, A.-L.; Chen, B.-W.; Li, J.-R.; Lin, Y.-H. Seed treatment of Bacillus amyloliquefaciens PMB05 powder for control black rot disease of cabbage. J. Plant Med. 2022, 64, 149–158. [Google Scholar]
- Li’aini, A.S.; Lin, Y.-H.; Huang, T.-C.; Sulistyowati, L. Application of Bacillus amyloliquefaciens to control black rot disease on cabbage caused by Xanthomonas campestris pv. campestris. J. Plant Med. 2017, 59, 39–44. [Google Scholar] [CrossRef]
- Lin, K.-W.; Liang, Y.-S.; Hsiao, C.-Y.; Wang, F.; Huang, T.-P.; Lin, Y.-H. Application of fermentation broth of Bacillus amyloliquefaciens PMB05 to control bacterial canker disease on lemon. J. Plant Med. 2021, 63, 17–26. [Google Scholar]
- Wu, Y.-M.; Chen, X.; Wang, F.; Hsiao, C.-Y.; Yang, C.-Y.; Lin, S.-T.; Wu, L.-H.; Chen, Y.-K.; Liang, Y.-S.; Lin, Y.-H. Bacillus amyloliquefaciens strains control strawberry anthracnose through antagonistic activity and plant immune response intensification. Biol. Control 2021, 157, 104592. [Google Scholar] [CrossRef]
- Eggert, D.; Naumann, M.; Reimer, R.; Voigt, C.A. Nanoscale glucan polymer network causes pathogen resistance. Sci. Rep. 2014, 4, 4159. [Google Scholar] [CrossRef]
- Ellinger, D.; Naumann, M.; Falter, C.; Zwikowics, C.; Jamrow, T.; Manisseri, C.; Somerville, S.C.; Voigt, C.A. Elevated early callose deposition results in complete penetration resistance to powdery mildew in Arabidopsis. Plant Physiol. 2013, 161, 1433–1444. [Google Scholar] [CrossRef]
- Voigt, C.A. Callose-mediated resistance to pathogenic intruders in plant defense-related papillae. Front. Plant Sci. 2014, 5, 168. [Google Scholar] [CrossRef]
- Hong, C.-Y.; Zheng, J.-L.; Chen, T.-Y.; Chao, H.-R.; Lin, Y.-H. PFLP-intensified disease resistance against bacterial soft rot through MAPK pathway in PAMP-triggered immunity. Phytopathology 2018, 108, 1467–1474. [Google Scholar] [CrossRef]
- Hutter, H. Fluorescent reporter methods. Methods Mol. Biol. 2006, 351, 155–173. [Google Scholar] [CrossRef]
- Kanno, T.; Lin, W.-D.; Fu, J.L.; Wu, M.-T.; Yang, H.-W.; Lin, S.-S.; Matzke, A.J.M.; Matzke, M. Identification of Coilin Mutants in a Screen for Enhanced Expression of an Alternatively Spliced GFP Reporter Gene in Arabidopsis thaliana. Genetics 2016, 203, 1709–1720. [Google Scholar] [CrossRef]
- Prasher, D.C. Using GFP to see the light. Trends Genet. 1995, 11, 320–323. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Huang, H.-E.; Wu, F.-S.; Ger, M.-J.; Liao, P.-L.; Chen, Y.-R.; Tzeng, K.-C.; Feng, T.-Y. Plant ferredoxin-like protein (PFLP) outside chloroplast in Arabidopsis enhances disease resistance against bacterial pathogens. Plant Sci. 2010, 179, 450–458. [Google Scholar] [CrossRef]
- Boudsocq, M.; Willmann, M.R.; McCormack, M.; Lee, H.; Shan, L.; He, P.; Bush, J.; Cheng, S.H.; Sheen, J. Differential innate immune signalling via Ca2+ sensor protein kinases. Nature 2010, 464, 418–423. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.J.; Mott, E.K.; Parsley, K.; Aspinall, S.; Gray, J.C.; Cottage, A. A rapid and robust method of identifying transformed Arabidopsis thaliana seedlings following floral dip transformation. Plant Methods 2006, 2, 19. [Google Scholar] [CrossRef] [PubMed]
- Rasband, W.S. ImageJ; U. S. National Institutes of Health: Bethesda, MD, USA, 2015. Available online: https://imagej.nih.gov/ij/ (accessed on 1 May 2024).
- Winstead, N.N.; Kelman, A. Inoculation techniques for evaluating resistance to Pseudomonas solanacearum. Phytopathology 1952, 42, 628–634. [Google Scholar]
- Ayaz, M.; Li, C.-H.; Ali, Q.; Zhao, W.; Chi, Y.-K.; Shafiq, M.; Ali, F.; Yu, X.-Y.; Yu, Q.; Zhao, J.-T.; et al. Bacterial and Fungal Biocontrol Agents for Plant Disease Protection: Journey from Lab to Field, Current Status, Challenges, and Global Perspectives. Molecules 2023, 28, 6735. [Google Scholar] [CrossRef]
- Ganeshan, G.; Kumar, A.M. Pseudomonas fluorescence, a potential bacterial antagonist to control plant disease. J. Plant Interact. 2005, 1, 123–134. [Google Scholar] [CrossRef]
- Pal, K.K.; Gardener, B.M. Biological Control of Plant Pathogens. Plant Health Instr. 2006, 6, 1117–1142. [Google Scholar] [CrossRef]
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef]
- Hsiao, C.-Y.; Blanco, S.D.; Peng, A.-L.; Fu, J.-Y.; Chen, B.-W.; Luo, M.-C.; Xie, X.-Y.; Lin, Y.-H. Seed Treatment with Calcium Carbonate Containing Bacillus amyloliquefaciens PMB05 Powder Is an Efficient Way to Control Black Rot Disease of Cabbage. Agriculture 2023, 13, 926. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Fan, B.; Zhu, C.; Chen, Z. Regulation and Function of Defense-Related Callose Deposition in Plants. Int. J. Mol. Sci. 2021, 22, 2393. [Google Scholar] [CrossRef]
- Ellinger, D.; Voigt, C.A. Callose biosynthesis in arabidopsis with a focus on pathogen response: What we have learned within the last decade. Ann. Bot. 2014, 114, 1349–1358. [Google Scholar] [CrossRef]
- Chou, H.-P.; Huang, Y.-C.; Lin, Y.-H.; Deng, W.-L. Selection, Formulation, and Field Evaluation of Bacillus amyloliquefaciens PMB01 for Its Application to Manage Tomato Bacterial Wilt Disease. Agriculture 2022, 12, 1714. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, J.-L.; Li, J.-R.; Li, A.-T.; Li, S.-H.; Blanco, S.D.; Chen, S.-Y.; Lai, Y.-R.; Shi, M.-Q.; Lin, T.-C.; Su, J.-F.; et al. A Rapid Method for Screening Pathogen-Associated Molecular Pattern-Triggered Immunity-Intensifying Microbes. Plants 2024, 13, 2185. https://doi.org/10.3390/plants13162185
Zheng J-L, Li J-R, Li A-T, Li S-H, Blanco SD, Chen S-Y, Lai Y-R, Shi M-Q, Lin T-C, Su J-F, et al. A Rapid Method for Screening Pathogen-Associated Molecular Pattern-Triggered Immunity-Intensifying Microbes. Plants. 2024; 13(16):2185. https://doi.org/10.3390/plants13162185
Chicago/Turabian StyleZheng, Jing-Lin, Jia-Rong Li, Ai-Ting Li, Sin-Hua Li, Sabrina Diana Blanco, Si-Yan Chen, Yun-Ru Lai, Ming-Qiao Shi, Tsung-Chun Lin, Jiunn-Feng Su, and et al. 2024. "A Rapid Method for Screening Pathogen-Associated Molecular Pattern-Triggered Immunity-Intensifying Microbes" Plants 13, no. 16: 2185. https://doi.org/10.3390/plants13162185
APA StyleZheng, J.-L., Li, J.-R., Li, A.-T., Li, S.-H., Blanco, S. D., Chen, S.-Y., Lai, Y.-R., Shi, M.-Q., Lin, T.-C., Su, J.-F., & Lin, Y.-H. (2024). A Rapid Method for Screening Pathogen-Associated Molecular Pattern-Triggered Immunity-Intensifying Microbes. Plants, 13(16), 2185. https://doi.org/10.3390/plants13162185





