Assessment of Fatty Acid and Oxylipin Profile of Resprouting Olive Trees Positive to Xylella fastidiosa subsp. pauca in Salento (Apulia, Italy)
Abstract
:1. Introduction
2. Results
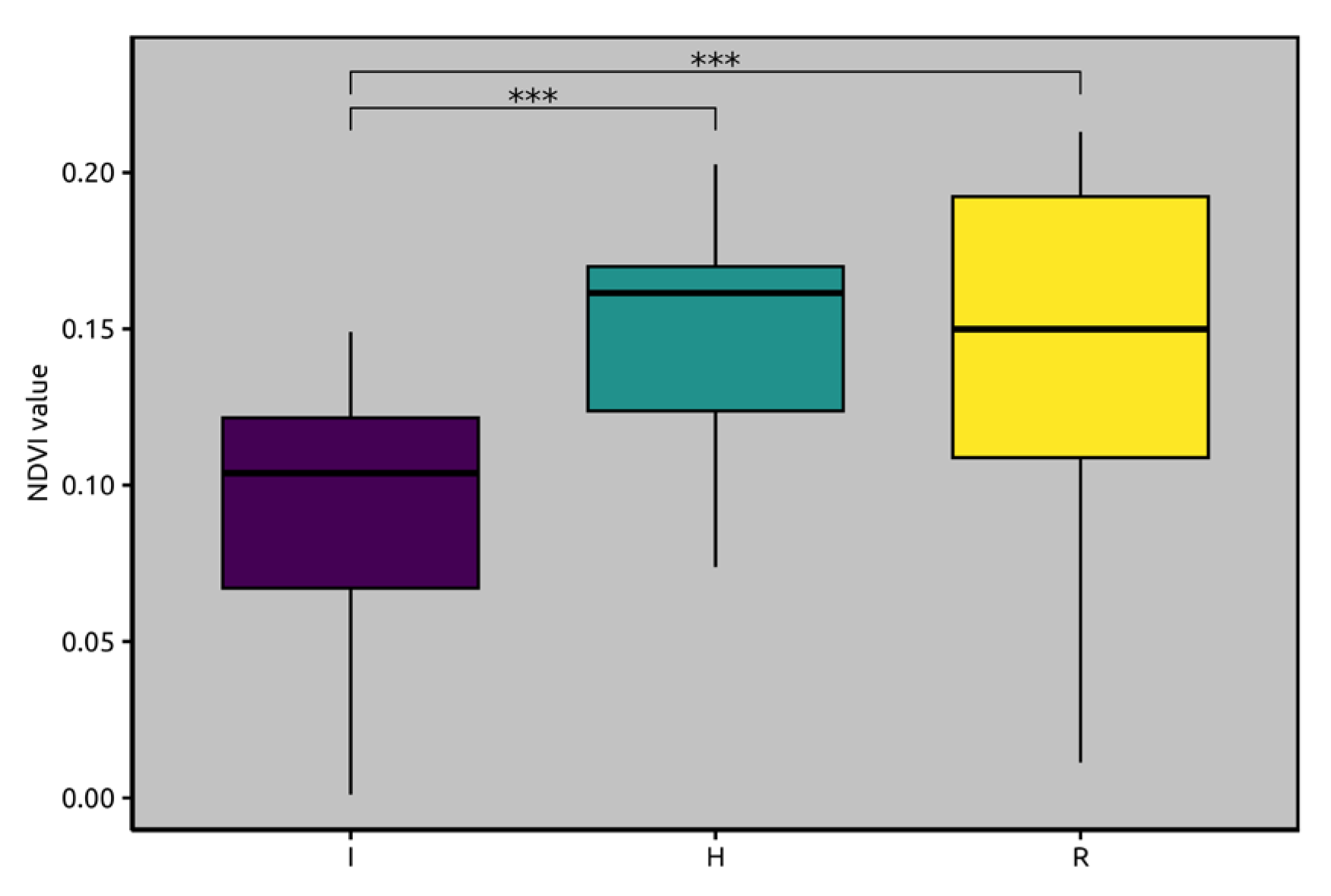
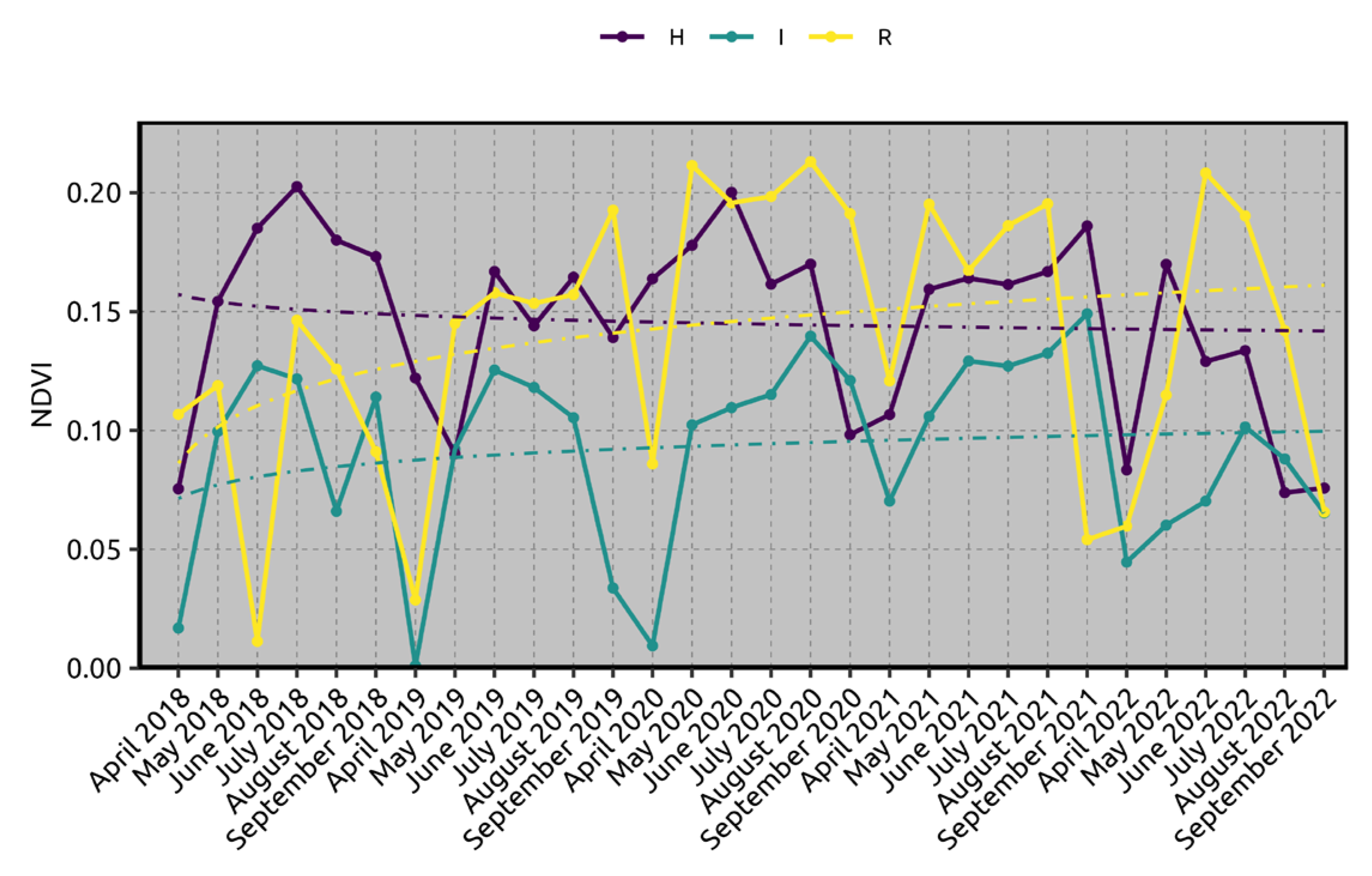
2.1. Satellite Imagery and Normalized Difference Vegetation Index (NDVI) Analyses
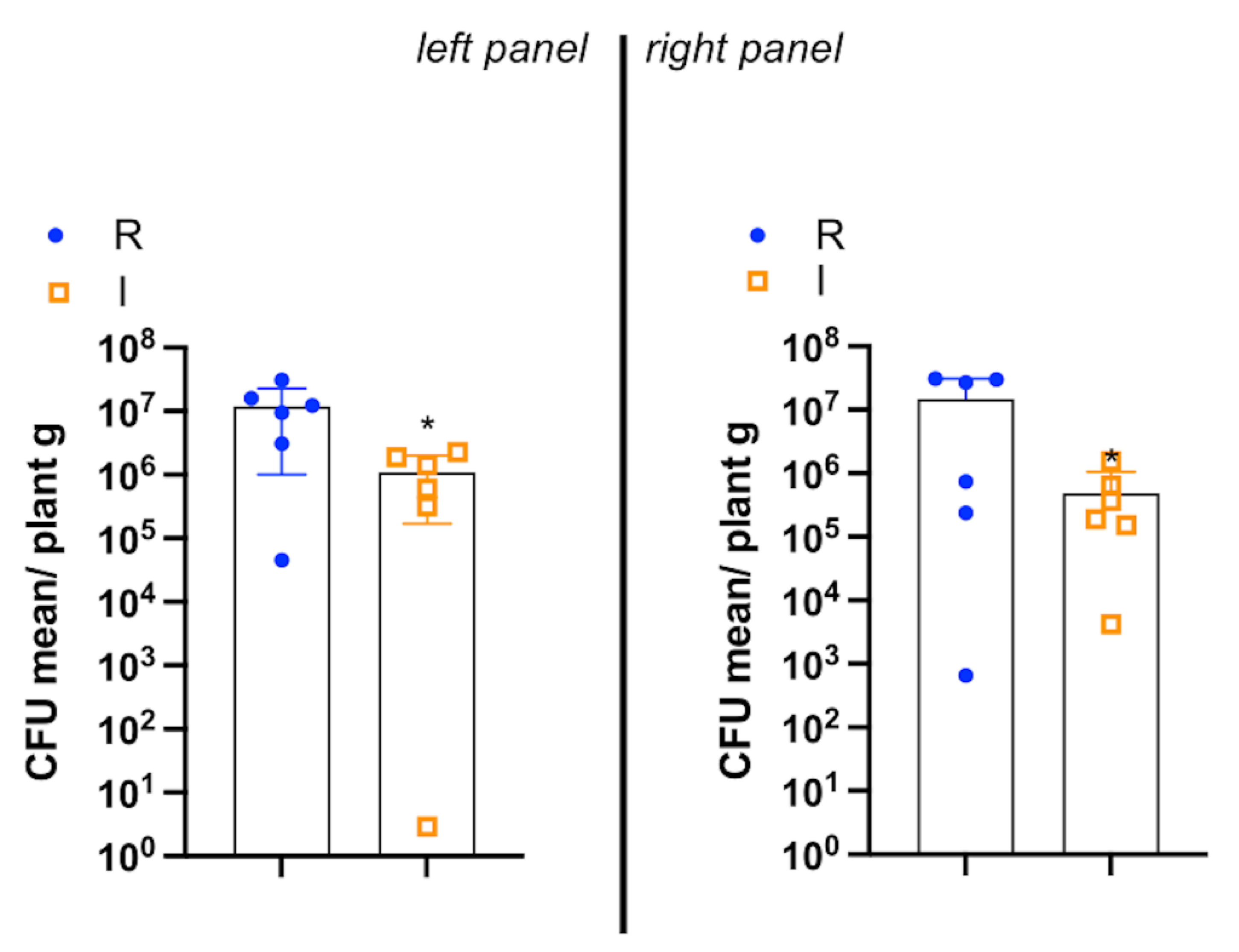
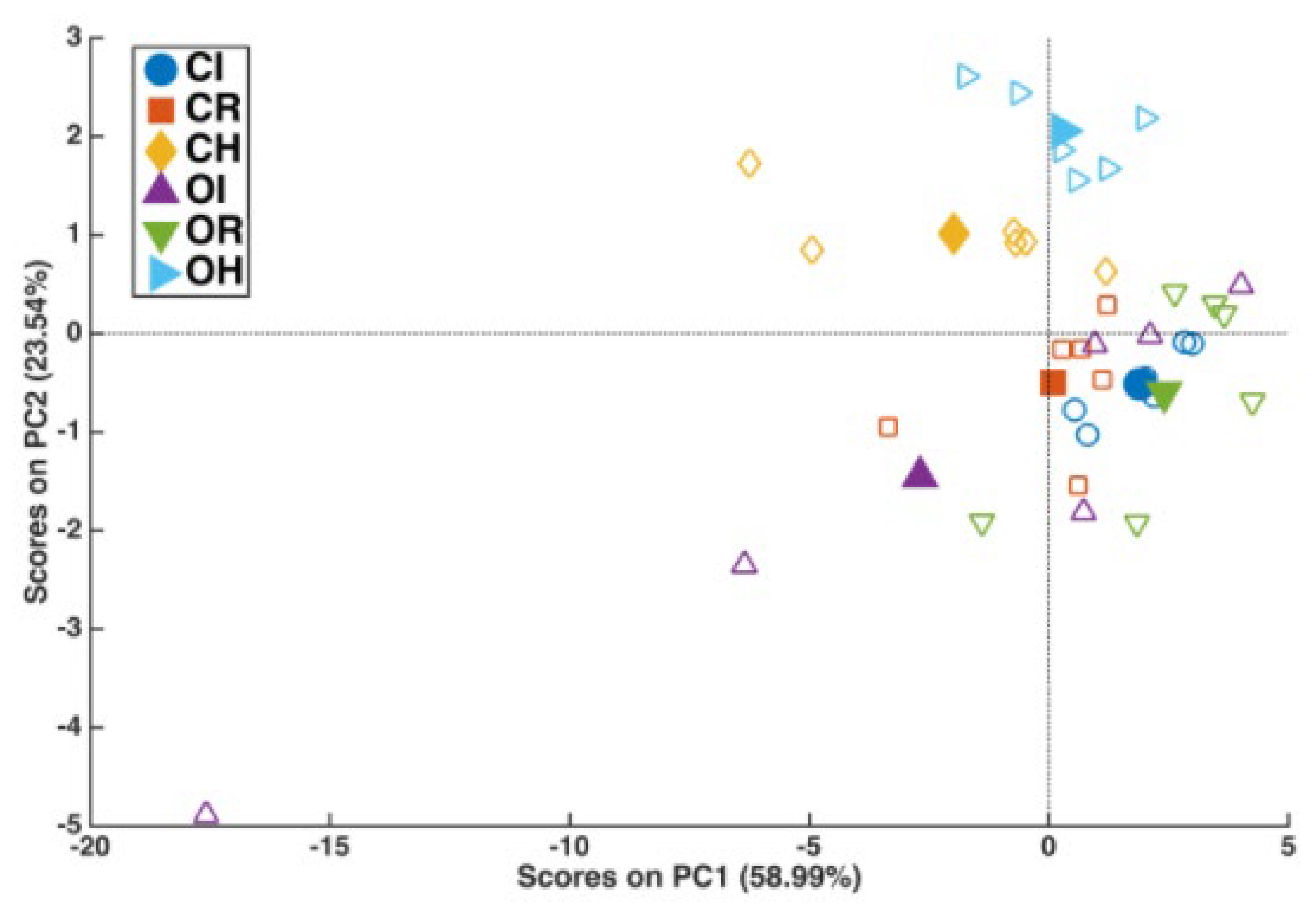
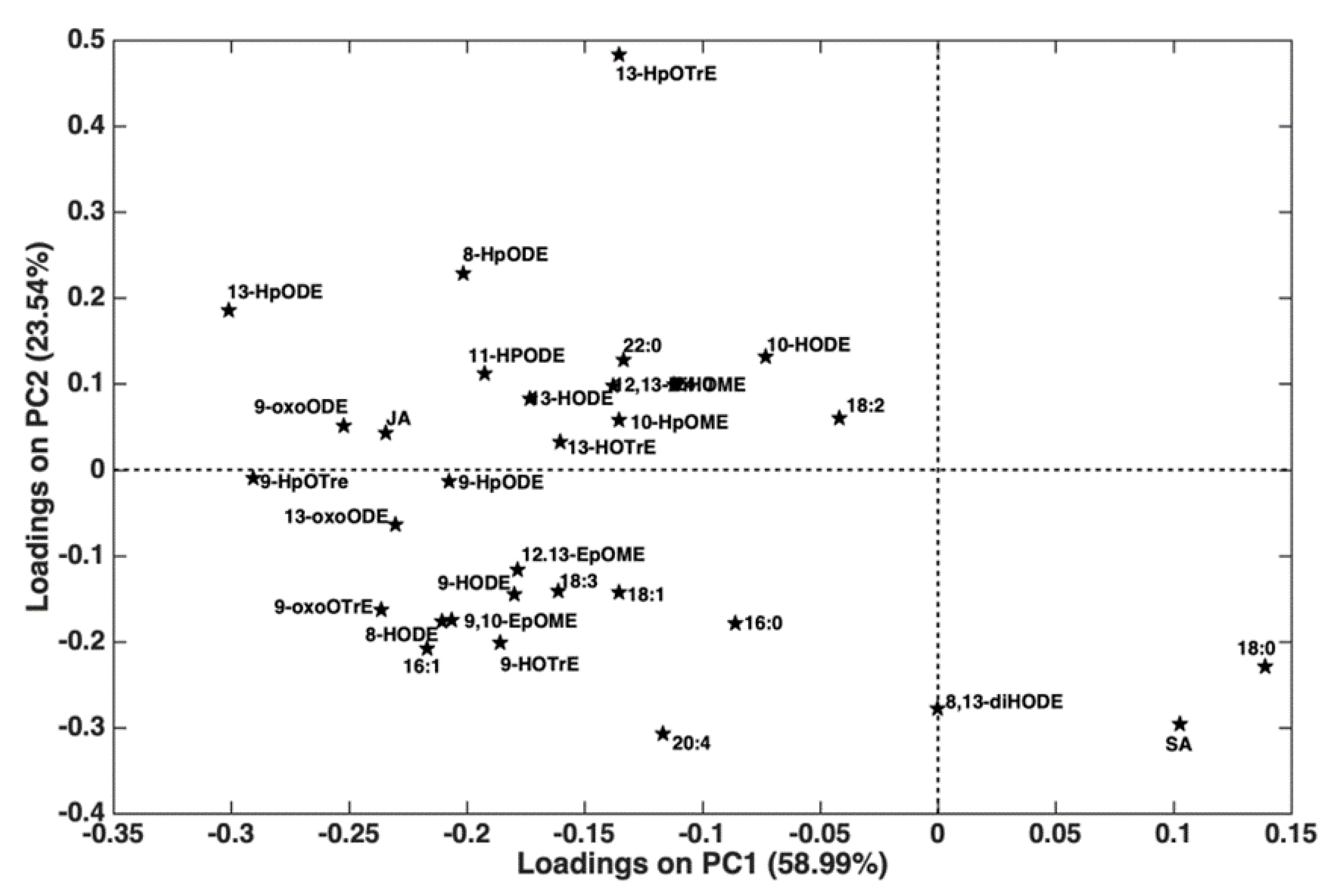
2.2. Xylella fastidiosa subsp. pauca PCR Quantification and Lipidomic Analyses
3. Discussion
4. Materials and Methods
4.1. Study Site and Sampling Procedures
4.2. Satellite Imagery and NDVI Analyses
4.3. DNA, Fatty Acid, and Oxylipins Analyses
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Schneider, K.; Van der Werf, W.; Cendoya, M.; Mourits, M.; Navas-Cortés, J.A.; Vicent, A.; Oude Lansink, A.O. Impact of Xylella fastidiosa subsp. pauca in European olives. Proc. Natl. Acad. Sci. USA 2020, 117, 9250–9259. [Google Scholar] [CrossRef] [PubMed]
- Scholten, R.; Martinez Sanchez, L.; Hornero, A.; Navas-Cortes, J.A.; Zarco-Tejada, P.J.; Beck, P.S.A. Monitoring the impact of Xylella on Apulia’s olive orchards using Sentinel-2 satellite data and aerial data. In Proceedings of the 2nd European Conference on Xylella fastidiosa, Ajaccio, France, 29–30 October 2019; Available online: https://www.efsa.europa.eu/sites/default/files/event/191029-xylella/S6.P1_BECK.pdf (accessed on 24 June 2024).
- Picciotti, U.; Lahbib, N.; Sefa, V.; Porcelli, F.; Garganese, F. Aphrophoridae role in Xylella fastidiosa subsp. pauca ST53 invasion in Southern Italy. Pathogens 2021, 10, 1035. [Google Scholar]
- Kottelenberg, D.; Hemerick, L.; Saponari, M.; Van der Werf, W. Shape and rate of movement of the invasion front of Xylella fastidiosa spp. pauca in Puglia. Sci. Rep. 2021, 11, 1061. [Google Scholar] [CrossRef] [PubMed]
- White, S.M.; Bullock, J.M.; Hooftman, D.A.P.; Chapman, D.S. Modelling the spread and control of Xylella fastidiosa in the early stages of invasion in Apulia, Italy. Biol. Invasions 2017, 19, 1825–1837. [Google Scholar] [CrossRef]
- White, S.M.; Navas-Cortes, J.A.; Bullock, J.M.; Boscia, D.; Chapman, D.S. Estimating the epidemiology of Xylella fastidiosa outbreaks in olives. Plant Pathol. 2020, 69, 1403–1413. [Google Scholar] [CrossRef]
- Dongiovanni, C.; Cavalieri, V.; Bodino, N.; Tauro, T.; Di Carolo, M.; Fumarola, G.; Altamura, G.; Lasorella, C.; Bosco, D. Plant selection and population trend of spittlebug immatures (Hemiptera: Aphrophoridae) in olive groves of the Apulia region of Italy. J. Econ. Entomol. 2018, 112, 67–74. [Google Scholar] [CrossRef]
- Strona, G.; Carstens, C.J.; Beck, P.S.A. Network analysis reveal why Xylella fastidiosa will persist in Europe. Sci. Rep. 2017, 7, 71. [Google Scholar] [CrossRef]
- Martelli, G.P. The current status of the quick decline syndrome of olive in southern Italy. Phytoparasitica 2016, 44, 1–10. [Google Scholar] [CrossRef]
- Semeraro, T.; Buccolieri, R.; Vergine, M.; De Bellis, L.; Luvisi, A.; Emmanuel, R.; Marwan, R. Analysis of olive grove destruction by Xylella fastidiosa bacterium on the land surface temperature in Salento detected using satellite images. Forests 2021, 12, 1266. [Google Scholar] [CrossRef]
- Camposeo, S.; Vivaldi, G.A.; Saponari, M. Attempts to reduce the systemic spread of Xylella fastidiosa in olive trees by pruning. Agronomy 2022, 12, 2917. [Google Scholar] [CrossRef]
- Scortichini, M. The epidemiology and control of “Olive Quick Decline Syndrome” in Salento (Apulia, Italy). Agronomy 2022, 12, 2475. [Google Scholar] [CrossRef]
- Loreti, S.; Scala, V.; Pucci, N.; L’Aurora, A.; Tatulli, G.; Fiorani, R.; Scortichini, M. Preliminary observation on “resilient” olive trees infected by Xylella fastidiosa in the Salento area (Apulia, Italy). J. Plant Pathol. 2023, 105, 1237–1323. [Google Scholar] [CrossRef]
- Clarke, P.J.; Lawes, M.J.; Midgley, J.J.; Ojeda, F.; Burrows, G.E.; Enright, N.J.; Knox, K.J.E. Resprouting as a key functional trait: How buds, protection and resources drive persistence after fire. New Phytol. 2013, 197, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Bendall, E.R.; Bedward, M.; Boer, M.; Clarke, H.; Collins, L.; Leigh, A.; Bradstock, R.A. Changes in the resilience of resprouting juvenile tree populations in temperate forests due to coupled severe drought and fire. Plant Ecol. 2022, 223, 907–923. [Google Scholar] [CrossRef]
- Bussotti, F.; Pollastrini, M. Revisiting the concept of stress in forest trees at the time of global change and issues for stress monitoring. Plant Stress 2021, 2, 100013. [Google Scholar] [CrossRef]
- Simler, A.B.; Metz, M.R.; Frangioso, K.M.; Meentemeyer, R.K.; Rizzo, D.M. Novel disturbance interactions between fire and an emerging disease impact survival and growth of resprouting trees. Ecology 2018, 99, 2217–2229. [Google Scholar] [CrossRef] [PubMed]
- Zeppel, M.J.B.; Harrison, S.P.; Adams, H.D.; Kelley, D.I.; Li, G.; Tissue, D.T.; Dawson, T.E.; Fensham, R.; Medlyin, B.E.; Palmer, A.; et al. Drought and resprouting plants. New Phytol. 2014, 206, 583–589. [Google Scholar] [CrossRef]
- Cavaco, A.R.; Matos, A.R.; Figueiredo, A. Speaking the language of lipids: The cross-talk between plants and pathogens in defence and disease. Cell. Mol. Life Sci. 2021, 78, 4399–4415. [Google Scholar] [CrossRef]
- Scala, V.; Salustri, M.; Loreti, S.; Pucci, N.; Cacciotti, A.; Tatulli, G.; Scortichini, M.; Reverberi, M. Mass spectrometry-based targeted lipidomics and supervised machine learning algorithms in detecting disease, cultivar, and treatment biomarkers in Xylella fastidiosa subsp. pauca-infected olive trees. Front. Plant Sci. 2022, 13, 833245. [Google Scholar] [CrossRef]
- Dyall, S.C.; Balas, L.; Bazan, N.G.; Brenna, J.T.; Chiang, N.; da Costa Souza, F.; Dalli, J.; Durand, T.; Galano, J.-M.; Lein, P.J.; et al. Polyunsaturated fatty acids and fatty acid-derived lipid mediators: Recent advances in the understanding of their biosynthesis, structures, and functions. Prog. Lipid Res. 2022, 86, 101165. [Google Scholar] [CrossRef]
- Muzzalupo, I.; Lombardo, N.; Musacchio, A.; Noce, M.E.; Pellegrino, G.; Perri, E.; Sajjad, A. DNAs Sequence analysis of microsatellite markers enhances their efficiency for germplasm management in an Italian olive collection. J. Am. Soc. Hortic. Sci. 2006, 131, 352–359. [Google Scholar] [CrossRef]
- Girelli, C.R.; Del Coco, L.; Angilè, F.; Scortichini, M.; Fanizzi, F.P. Olive cultivars susceptible or tolerant to Xylella fastidiosa subsp. pauca exhibit mid-term different metabolomes upon natural infection or a curative treatment. Plants 2021, 10, 772. [Google Scholar] [CrossRef]
- Blonda, P.; Tarantino, C.; Scortichini, M.; Maggi, S.; Tarantino, M.; Adamo, A. Satellite monitoring of bio-fertilizer restoration in olive groves affetced by Xylella fastidiosa subsp. pauca. Sci. Rep. 2023, 13, 5695. [Google Scholar] [CrossRef] [PubMed]
- Siebers, M.; Brands, M.; Wewer, V.; Duan, Y.; Hölzl, G.; Dörmann, P. Lipids in plant-microbe interactions. Biochim. Biophys. Acta 2016, 1861, 1379–1395. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Castroverde, C.D.M.; Huang, S.; Li, C.; Hilleary, R.; Seroka, A.; Sohrabi, R.; Medina-Yerena, D.; Huot, B.; Nomura, K.; et al. Increasing the resilience of plant immunity to a warming climate. Nature 2022, 607, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Scala, V.; Reverberi, M.; Salustri, M.; Pucci, N.; Modesti, V.; Lucchesi, S.; Loreti, S. Lipid profile of Xylella fastidiosa subsp. pauca associated with the Olive Quick Decline Syndrome. Front. Microbiol. 2018, 9, 1839. [Google Scholar] [CrossRef]
- Nascimento, R.; Gouran, H.; Chakraborty, S.; Gillespie, H.W.; Almeida-Souza, H.O.; Tu, A.; Rao, B.J.; Feldstein, P.A.; Bruening, G.; Goulart, L.R.; et al. The type II secreted lipase/esterase LesA is a key virulence factor required for Xylella fastidiosa pathogenesis in grapevines. Sci. Rep. 2016, 6, 18598. [Google Scholar]
- Martínez, E.; Campos-Gómez, J. Oxylipins produced by Pseudomonas aeruginosa promote biofilm formation and virulence. Nat. Commun. 2016, 7, 13823. [Google Scholar] [CrossRef]
- Martínez, E.; Cosnahan, R.K.; Wu, M.; Gadila, S.K.; Quick, E.B.; Mobley, J.A.; Campos-Gomez, J. Oxylipins mediate cell-to-cell communication in Pseudomonas aeruginosa. Commun. Biol. 2019, 2, 66. [Google Scholar] [CrossRef]
- Blèe, E. Impact of phyto-oxylipins in plant defense. Trends Plant Sci. 2002, 7, 315–321. [Google Scholar] [CrossRef]
- Wang, K.D.; Borrego, E.J.; Kenerley, C.M.; Kolomiets, M.V. Oxylipins other than jasmonic acid are xylem-resident signals regulating systemic resistance induced by Trichoderma virens in maize. Plant Cell 2020, 32, 166–185. [Google Scholar] [CrossRef] [PubMed]
- Vellosillo, T.; Martínez, M.; Lopez, M.A.; Vicente, J.; Cascon, T.; Dolan, L.; Hamberg, M.; Castresana, C. Oxylipins produced by the 9-lipoxygenase pathway in Arabidopsis regulate lateral root development and defense responses through a specific signaling cascade. Plant Cell 2007, 19, 831–846. [Google Scholar] [CrossRef] [PubMed]
- Vicente, J.; Cascón, T.; Vicedo, B.; García-Agustín, P.; Hamberg, M.; Castresana, C. Role of 9-lipoxygenase and α-dioxygenase oxylipin pathways as modulators of local and systemic defense. Mol. Plant 2012, 5, 914–928. [Google Scholar] [CrossRef] [PubMed]
- Rapicavoli, J.N.; Blanco-Ulate, B.; Muszyński, A.; Figueroa-Balderas, R.; Morales-Cruz, A.; Azadi, P.; Dobruchowska, J.M.; Castro, C.; Cantu, D.; Roper, M.C. Lipopolysaccharide O-antigen delays plant innate immune recognition of Xylella fastidiosa. Nat. Commun. 2018, 9, 390. [Google Scholar] [CrossRef] [PubMed]
- Troha, K.; Ayres, J.S. Cooperative defenses during enteropathogenic infection. Curr. Opin. Microbiol. 2022, 65, 123–130. [Google Scholar] [CrossRef]
- Gorelick, N.; Hancher, M.; Dixon, M.; Ilyushchenko, S.; Thau, D.; Moore, R. Google earth engine: Planetary-scale geospatial analysis for everyone. Remote Sens. Environ. 2017, 202, 18–27. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. 2022. Available online: https://www.r-project.org/ (accessed on 25 June 2024).
- European and Mediterranean Plant Protection Organization. PM 7/24 (5) Xylella fastidiosa. EPPO Bull. 2023, 53, 205–276. [Google Scholar] [CrossRef]
- Harper, S.J.; Ward, L.I.; Clover, G.R.G. Development of LAMP and Real-Time PCR methods for the rapid detection of Xylella fastidiosa for quarantine and field applications. Phytopathology 2010, 100, 1282–1288. [Google Scholar] [CrossRef]
- Smilde, A.K.; Jansen, J.J.; Hoefsloot, H.C.J.; Lamers, R.-J.A.N.; van der Greef, J.; Timmerman, M.E. ANOVA-simultaneous component analysis (ASCA): A new tool for analyzing designed metabolomics data. Bioinformatics 2005, 21, 3043. [Google Scholar] [CrossRef]
- Türker-Kaya, S.; Huck, C.W. A Review of Mid-Infrared and Near-Infrared Imaging: Principles, Concepts and Applications in Plant Tissue Analysis. Molecules 2017, 22, 168. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Zhang, Y.; Wang, D.; Wang, X.; Yu, L.; Zhang, W.; Li, P. Review of NIR spectroscopy methods for nondestructive quality analysis of oilseeds and edible oils. Trends Food Sci. Technol. 2020, 101, 172–181. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scala, V.; Scortichini, M.; Marini, F.; La Montagna, D.; Beccaccioli, M.; Micalizzi, K.; Cacciotti, A.; Pucci, N.; Tatulli, G.; Fiorani, R.; et al. Assessment of Fatty Acid and Oxylipin Profile of Resprouting Olive Trees Positive to Xylella fastidiosa subsp. pauca in Salento (Apulia, Italy). Plants 2024, 13, 2186. https://doi.org/10.3390/plants13162186
Scala V, Scortichini M, Marini F, La Montagna D, Beccaccioli M, Micalizzi K, Cacciotti A, Pucci N, Tatulli G, Fiorani R, et al. Assessment of Fatty Acid and Oxylipin Profile of Resprouting Olive Trees Positive to Xylella fastidiosa subsp. pauca in Salento (Apulia, Italy). Plants. 2024; 13(16):2186. https://doi.org/10.3390/plants13162186
Chicago/Turabian StyleScala, Valeria, Marco Scortichini, Federico Marini, Dario La Montagna, Marzia Beccaccioli, Kristina Micalizzi, Andrea Cacciotti, Nicoletta Pucci, Giuseppe Tatulli, Riccardo Fiorani, and et al. 2024. "Assessment of Fatty Acid and Oxylipin Profile of Resprouting Olive Trees Positive to Xylella fastidiosa subsp. pauca in Salento (Apulia, Italy)" Plants 13, no. 16: 2186. https://doi.org/10.3390/plants13162186







