Green Lacewing Chrysoperla externa Is Attracted to Volatile Organic Compounds and Essential Oils Extracted from Eucalyptus urograndis Leaves
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study System
2.2. Plant Culture
2.3. Simulated Herbivory Treatment
2.4. EO Extraction
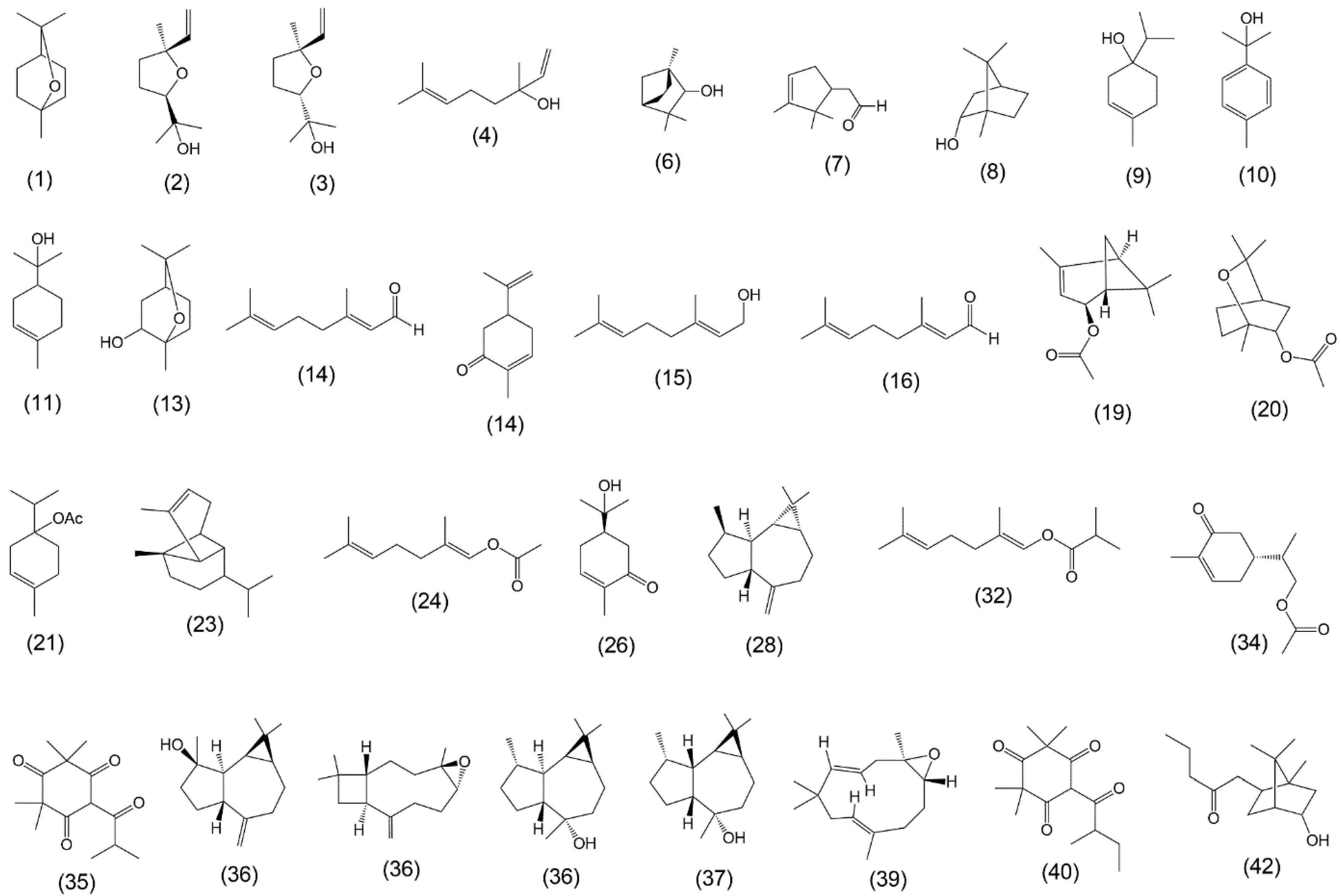
2.5. EO Chemical Composition
2.6. Insect Culture
2.7. Behavioral Evaluation Using Y-Tube Olfactometer System
2.8. Data Analysis
3. Results
3.1. Essential Oil Extraction
3.2. Essential Oil Chemical Composition
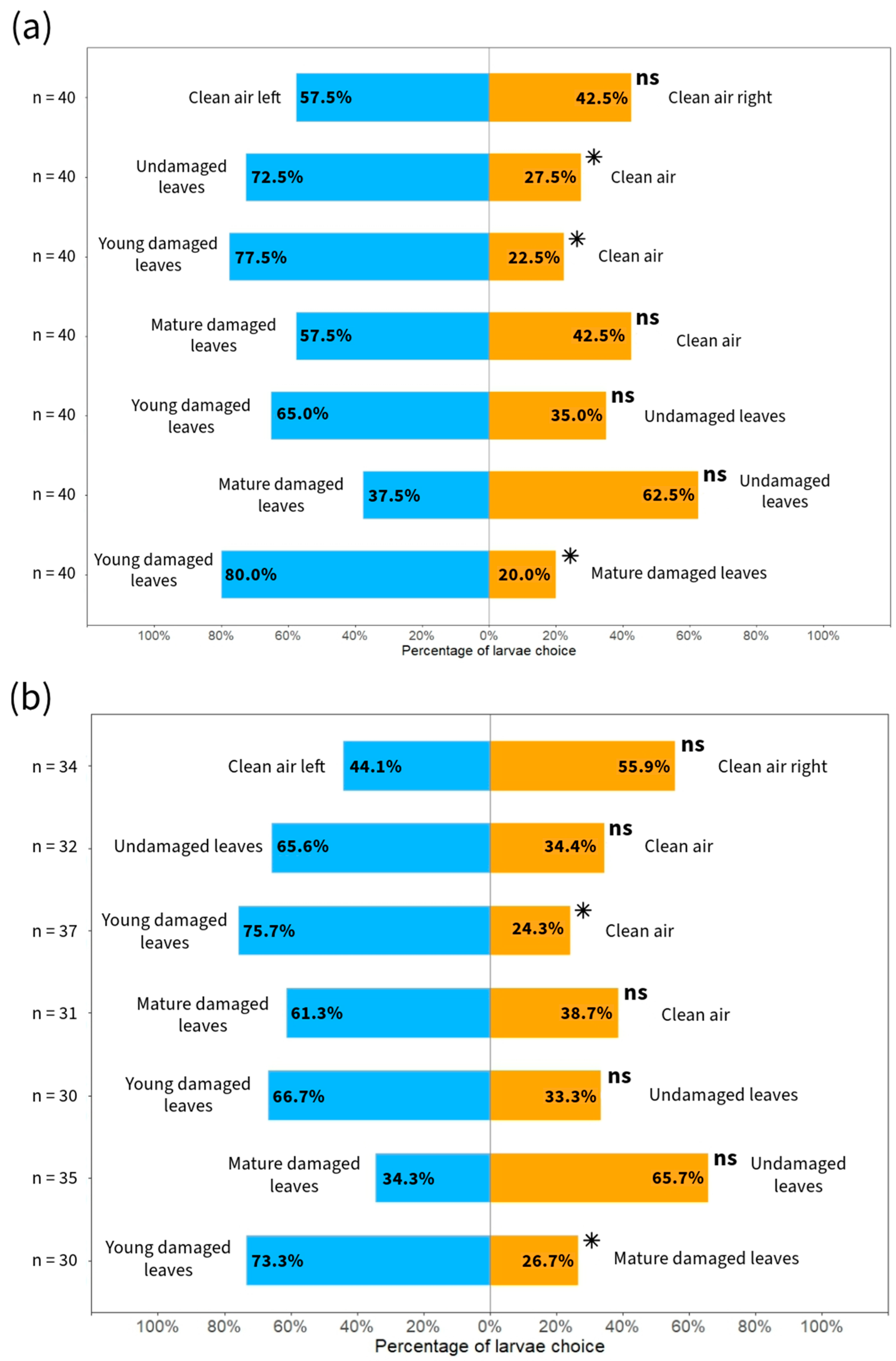
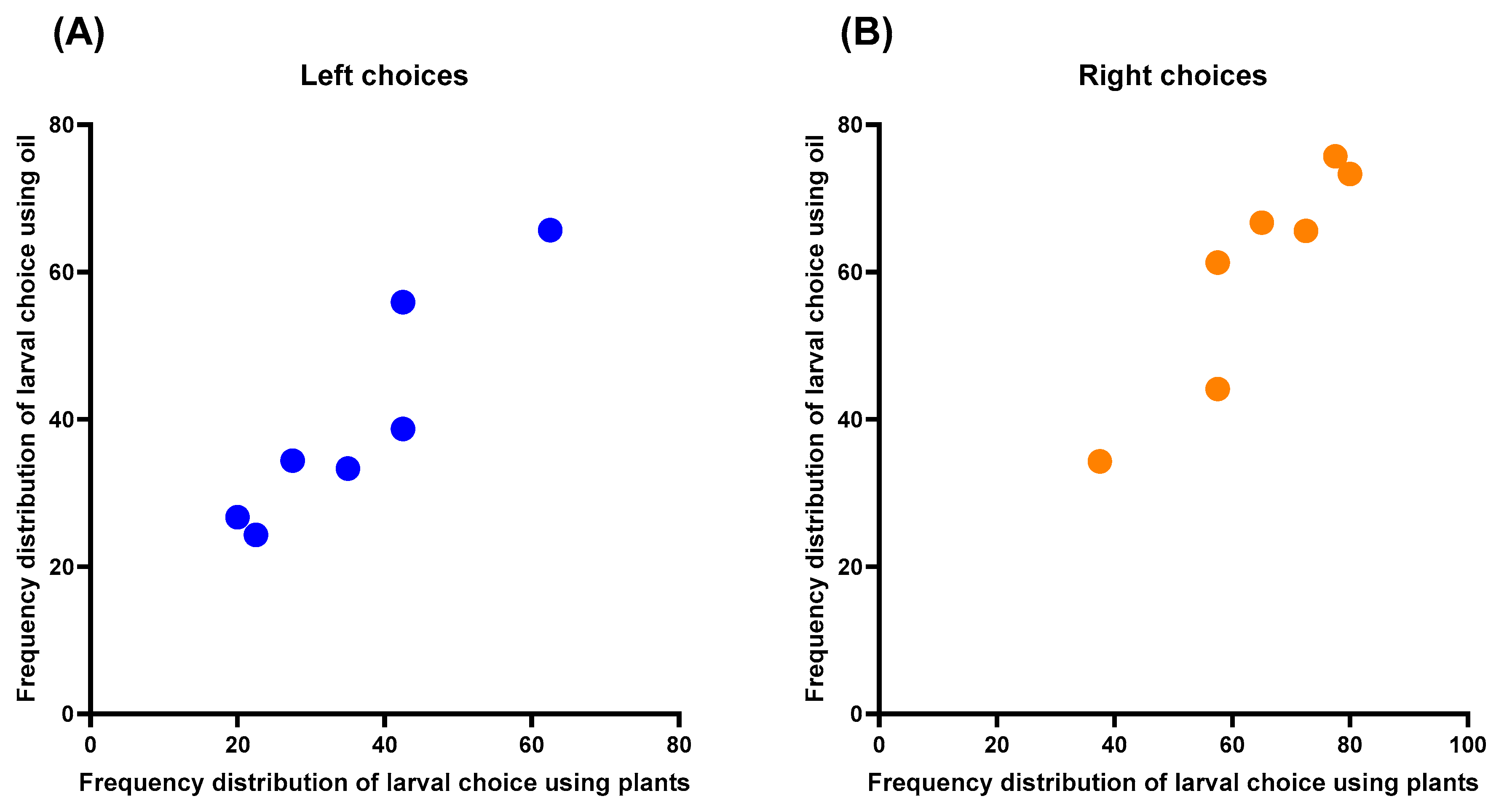
3.3. Olfactometry Tests
4. Discussion
4.1. EO Extraction
4.2. EO Chemical Composition
4.3. Simulated Herbivory and Olfactometry Tests
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef] [PubMed]
- Holopainen, J.K. Multiple functions of inducible plant volatiles. Trends Plant Sci. 2004, 9, 529–533. [Google Scholar] [CrossRef]
- Heil, M. Herbivore-induced plant volatiles: Targets, perception and unanswered questions. New Phytol. 2014, 204, 297–306. [Google Scholar] [CrossRef]
- Kessler, A.; Mueller, M.B.; Kalske, A.; Chautá, A. Volatile-mediated plant–plant communication and higher-level ecological dynamics. Curr. Biol. 2023, 33, R519–R529. [Google Scholar] [CrossRef] [PubMed]
- Bouwmeester, H.; Schuurink, R.C.; Bleeker, P.M.; Schiestl, F. The role of volatiles in plant communication. Plant J. 2019, 100, 892–907. [Google Scholar] [CrossRef] [PubMed]
- Barbero, F.; Maffei, M.E. Recent advances in plant–insect interactions. Int. J. Mol. Sci. 2023, 24, 11338. [Google Scholar] [CrossRef] [PubMed]
- Adler, F.R. Plant signalling: The opportunities and dangers of chemical communication. Biol. Lett. 2011, 7, 161–162. [Google Scholar] [CrossRef] [PubMed]
- Schiestl, F.P. The evolution of floral scent and insect chemical communication. Ecol. Lett. 2010, 13, 643–656. [Google Scholar] [CrossRef] [PubMed]
- Halitschke, R.; Stenberg, J.A.; Kessler, D.; Kessler, A.; Baldwin, I.T. Shared signals–‘alarm calls’ from plants increase apparency to herbivores and their enemies in nature. Ecol. Lett. 2008, 11, 24–34. [Google Scholar] [CrossRef]
- Wari, D.; Aboshi, T.; Shinya, T.; Galis, I. Integrated view of plant metabolic defense with particular focus on chewing herbivores. J. Integr. Plant Biol. 2022, 64, 449–475. [Google Scholar] [CrossRef]
- War, A.R.; Sharma, H.C.; Paulraj, M.G.; War, M.Y.; Ignacimuthu, S. Herbivore induced plant volatiles: Their role in plant defense for pest management. Plant Signal Behav. 2011, 6, 1973–1978. [Google Scholar] [CrossRef]
- Arimura, G.I.; Kost, C.; Boland, W. Herbivore-induced, indirect plant defences. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2005, 1734, 91–111. [Google Scholar] [CrossRef] [PubMed]
- Dahlin, I.; Rubene, D.; Glinwood, R.; Ninkovic, V. Pest suppression in cultivar mixtures is influenced by neighbor-specific plant–plant communication. Ecol. Appl. 2018, 28, 2187–2196. [Google Scholar] [CrossRef] [PubMed]
- Degen, T.; Dillmann, C.; Marion-Poll, F.; Turlings, T.C. High genetic variability of herbivore-induced volatile emission within a broad range of maize inbred lines. Plant Physiol. 2004, 135, 1928–1938. [Google Scholar] [CrossRef] [PubMed]
- Sedio, B.E.; Archibold, A.D.; Echeverri, J.C.R.; Debyser, C.; Boya P, C.A.; Wright, S.J. A comparison of inducible, ontogenetic, and interspecific sources of variation in the foliar metabolome in tropical trees. PeerJ 2019, 7, 7536. [Google Scholar] [CrossRef] [PubMed]
- Rhoades, D.F. Evolution of plant chemical defense against herbivores. In Herbivores: Their Interaction with Secondary Plant Metabolites, 1st ed.; Rosenthal, G.A., Janzen, D.H., Eds.; Academic Press: New York, NY, USA, 1979; pp. 5–54. [Google Scholar]
- Ohnmeiss, T.E.; Baldwin, I.T. Optimal defense theory predicts the ontogeny of an induced nicotine defense. Ecology 2000, 81, 1765–1783. [Google Scholar] [CrossRef]
- Pichersky, E.; Noel, J.P.; Dudareva, N. Biosynthesis of plant volatiles: Nature’s diversity and ingenuity. Science 2006, 311, 808–811. [Google Scholar] [CrossRef]
- Ponzio, C.; Gols, R.; Weldergergis, B.T.; DICKE, M. Plant volatiles and parasitoid foraging behaviour. Plant Cell Environ. 2014, 37, 1924–1935. [Google Scholar] [CrossRef]
- Dudareva, N.; Pichersky, E.; Gershenzon, J. Biochemistry of plant volatiles. Plant Physiol. 2004, 135, 1893–1902. [Google Scholar] [CrossRef]
- Tholl, D.; Boland, W.; Hansel, A.; Loreto, F.; Röse, U.S.R.; Schnitzler, J.-P. Practical approaches to plant volatile analysis. Plant J. 2006, 45, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Martínez, L.C.; Plata-Rueda, A.; Zanuncio, J.C.; Serrao, J.E. Bioactivity of six plant extracts on adults of Demotispa neivai (Coleoptera: Chrysomelidae). J. Insect Sci. 2015, 15, 34. [Google Scholar] [CrossRef]
- Denoirjean, T.; Riviere, M.; Doury, G.; Le Goff, G.J.; Ameline, A. Behavioral disruption of two orchard hemipteran pests by garlic essential oil. Entomol. Exp. Appl. 2022, 170, 782–791. [Google Scholar] [CrossRef]
- Bruce, T.J.; Birkett, M.A.; Blande, J.; Hooper, A.M.; Martin, J.L.; Khambay, B.; Prosser, I.; Smart, L.E.; Wadhams, L.J. Response of economically important aphids to components of Hemizygia petiolata essential oil. Pest Manag. Sci. 2005, 61, 1115–1121. [Google Scholar] [CrossRef] [PubMed]
- Mithöfer, A.; Boland, W. Plant defense against herbivores: Chemical aspects. Ann. Rev. Plant Biol. 2012, 63, 431–450. [Google Scholar] [CrossRef] [PubMed]
- Zitzelsberger, C.; Buchbauer, G. Essential oils as “a cry for help”. A Review. Nat. Prod. Commun. 2015, 10, 1127–1138. [Google Scholar] [CrossRef] [PubMed]
- Ormeño, E.; Goldstein, A.; Niinemets, Ü. Extracting and trapping biogenic volatile organic compounds stored in plant species. Trends Anal. Chem. 2011, 30, 978–989. [Google Scholar] [CrossRef]
- Divekar, P.A.; Narayana, S.; Divekar, B.A.; Kumar, R.; Gadratagi, B.G.; Ray, A.; Singh, A.K.; Rani, V.; Singh, V.; Singh, A.K.; et al. Plant secondary metabolites as defense tools against herbivores for sustainable crop protection. Int. J. Mol. Sci. 2022, 23, 2690. [Google Scholar] [CrossRef] [PubMed]
- Peñaflor, M.F.G.V.; Bento, J.M.S. Herbivore-induced plant volatiles to enhance biological control in agriculture. Neotrop. Entomol. 2013, 42, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Nerio, L.S.; Olivero-Verbel, J.; Stashenko, E. Repellent activity of essential oils: A review. Bioresour. Technol. 2010, 101, 372–378. [Google Scholar] [CrossRef] [PubMed]
- IBÁ Annual Report. 2023. Available online: https://iba.org/eng/datafiles/publicacoes/relatorios/iba-annual-report-2023.pdf (accessed on 17 June 2024).
- Santarosa, E.; Junior, J.E.P.; Goulart, I.C.G.R.; Penteado-Junior, J.F. Importância socioeconômica e principais usos do eucalipto. In Transferência de Tecnologia Florestal: Cultivo de Eucalipto em Propriedades Rurais: Diversificação da Produção e Renda, 1st ed.; Santarosa, E., Junior, J.E.P., Goulart, I.C.G.R., Penteado-Junior, J.F., Eds.; Embrapa: Brasília, Brazil, 2014; pp. 13–22. [Google Scholar]
- Queiroz, D.L.; Barbosa, L.R.; Iede, E.T. Principais pragas e seu controle. In Transferência de Tecnologia Florestal: Cultivo de Eucalipto em Propriedades Rurais: Diversificação da Produção e Renda, 1st ed.; Santarosa, E., Junior, J.F.P., Goulart, I.C.G.D.R., Eds.; Embrapa: Brasília, Brazil, 2014; pp. 87–102. [Google Scholar]
- Machado, D.; Costa, E.; Garlet, J.; Boscardin, J.; Pedron, L.; Perini, C.; Bolzan, L. Avaliação de inseticidas no controle de Thaumastocoris peregrinus (Hemiptera: Thaumastocoridae) percevejo-bronzeado em condições de laboratório. Floresta Ambiente 2016, 32, 245–260. [Google Scholar] [CrossRef]
- Cuello, E.M.; Andorno, A.V.; Hernandez, C.M.; Lopez, S.N. Prey consumption and development of the indigenous lacewing Chrysoperla externa feeding on two exotic Eucalyptus pests. Biocontrol Sci. Technol. 2019, 29, 1159–1171. [Google Scholar] [CrossRef]
- Albuquerque, G.; Tauber, C.; Tauber, M. Chrysoperla externa (Neuroptera: Chrysopidae): Life history and potential for biological control in Central and South America. Biol. Control 1994, 4, 8–13. [Google Scholar] [CrossRef]
- Resende, A.L.S.; Souza, B.; Ferreira, R.B.; Aguiar-Menezes, E.L. Flowers of Apiaceous species as sources of pollen for adults of Chrysoperla externa (Hagen) (Neuroptera). Biol. Control 2017, 106, 40–44. [Google Scholar] [CrossRef]
- Loney, P.E.; McArthur, C.; Potts, B.M.; Jordan, G.J. How Does ontogeny in a Eucalyptus species affect patterns of herbivory by brushtail possums? Funct. Ecol. 2006, 20, 982–988. Available online: http://www.jstor.org/stable/4139335 (accessed on 20 October 2020). [CrossRef]
- Goodger, J.Q.D.; Heskes, A.M.; Woodrow, I.E. Contrasting ontogenetic trajectories for phenolic and terpenoid defences in Eucalyptus froggattii. Ann. Bot. 2013, 112, 651–659. [Google Scholar] [CrossRef]
- Júnior, J.E.P.; Santarosa, E.; Goulart, I.C.G.R. Histórico do cultivo do eucalipto. In Transferência de Tecnologia Florestal: Cultivo de Eucalipto em Propriedades Rurais: Diversificação da Produção e Renda, 1st ed.; Júnior, J.E.P., Santarosa, E., Goulart, I.C.G.R., Eds.; Embrapa: Brasília, Brazil, 2014; pp. 11–12. [Google Scholar]
- Paludzyszyn-Filho, E.; Pacheco, A.R.; Dittmar, H.; Cordeiro, C.A. Estratégias Para o Melhoramento de Eucaliptos Tropicais na Embrapa; Embrapa Florestas: Colombo, Brazil, 2004; 27p. [Google Scholar]
- Valeri, S.V.; Ferreira, M.E.; Martins, M.I.E.G.; Banzatto, D.A.; Alvarenga, S.F.; Corradini, L.; do Valle, C.F. Recovery of a Eucalyptus urophylla plantation with nitrogen, potassium and dolomitic lime applications. Sci. For. 2001, 60, 53–71. [Google Scholar]
- Brooks, S.J.; Barnard, P.C. The green lacewings of the world: A generic review (Neuroptera: Chrysopidae). Bull. Br. Mus. Nat. Hist. Entomol. 1990, 59, 117–286. [Google Scholar]
- Albuquerque, G.S. Crisopídeos (Neuroptera: Chrysopidae). In Bioecologia e Nutrição de Insetos: Base Para o Manejo Integrado de Pragas; Panizzi, A.R., Parra, J.R.P., Eds.; Embrapa Informação Tecnológica: Brasília, Brazil, 2013; pp. 969–1022. [Google Scholar]
- Soares, J.J.; do Nascimento, A.R.B.; da Silva, M.V. Informações sobre Chrysoperla externa. Embrapa Algodão. 2007. Available online: https://ainfo.cnptia.embrapa.br/digital/bitstream/CNPA/21100/1/DOC175.PDF (accessed on 20 October 2020).
- Duelli, P. Lacewings in field crops. In Lacewings in the Crop Environment; McEwen, P.K., New, T.R., Whittington, A.E., Eds.; Cambridge University Press: Cambridge, UK, 2001; pp. 158–164. [Google Scholar]
- Souza, B.; Carvalho, C.F. Population dynamics and seasonal occurrence of adults of Chrysoperla externa (Hagen, 1861) (Neuroptera: Chrysopidae) in a citrus orchard in Southern Brazil. Acta Zool. Acad. Sci. Hung. 2002, 48, 301–310. [Google Scholar]
- Carvalho, C.F.; Souza, B. Métodos de criação e produção de crisopídeos. In Controle Biológico de Pragas: Produção Massal e Controle de Qualidade; Bueno, V.H.P., Ed.; UFLA: Lavras, Brazil, 2009; pp. 77–115. [Google Scholar]
- Salamanca, J.; Varón, D.E.H.; Santos, A.O. Breeding and test of the predatory capacity of Chrysoperla externa on Neohydatothrips signifer, a pestiferous trips of the passion fruit crop. Corp. Cien Tecnol. Agropecu. 2010, 11, 31–40. [Google Scholar] [CrossRef]
- Salamanca, J.; Pareja, M.; Rodriguez-Saona, C.; Resende, A.L.S.; Souza, B. Behavioral responses of adult lacewings, Chrysoperla externa, to a rose–aphid–coriander complex. Biol. Control 2015, 80, 103–112. [Google Scholar] [CrossRef]
- Resende, A.L.S.; Ferreira, R.B.; Souza, B.G. Attractiveness of Chrysoperla externa (Hagen, 1861) adults to volatile compounds of coriander, dill and fennel (Apiaceae) in laboratory conditions. Rev. Ceres. 2015, 62, 37–43. [Google Scholar] [CrossRef]
- Pareja, M.; Mohib, A.; Birkett, M.A.; Dufour, S.; Glinwood, R.T. Multivariate statistics coupled to generalized linear models reveal complex use of chemical cues by a parasitoid. Anim. Behav. 2009, 77, 901–909. [Google Scholar] [CrossRef]
- Blassioli-Moraes, M.C.; Laumann, R.; Sujii, E.; Pires, C.; Borges, M. Induced volatiles in soybean and pigeon pea plants artificially infested with the Neotropical brown stink bug, Euschistus heros, and their effect on the egg parasitoid, Telenomus podisi. Entomol. Exp. Appl. 2005, 115, 227–237. [Google Scholar] [CrossRef]
- Saad, K.; Mohamad Roff, M.; Hallett, R.; Idris, A.B. Aphid-induced defences in chilli affect preferences of the whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae). Sci. Rep. 2015, 5, 13697. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.V.S.G.; Silva, S.A.; Teixeira, T.L.; de Oliveira, A.; Morais, S.A.I.; da Silva, C.V.; Espindola, L.S.; Sousa, R.M.F. Essential oil from leaves of Eugenia calycina Cambes: Natural larvicidal against Aedes aegypti. J. Sci. Food Agric. 2020, 101, 1202–1208. [Google Scholar] [CrossRef] [PubMed]
- Wallace, W.E. Retention Indices. In NIST Mass Spectrometry Data Center; Linstrom, P.J., Mallard, W.G., Eds.; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2018; Volume 17. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy, 4th ed.; Allured Bussiness Media: Carol Stream, IL, USA, 2007; 804p. [Google Scholar]
- Macedo, L.P.M.; Soares, J.J. Criação de Chrysoperla externa para o controle biológico de pragas do algodoeiro. EMBRAPA-CNPA Circ. Técnica 2000, 36, 1–9. [Google Scholar]
- Akol, A.M.; Njagi, P.G.N.; Sithanantham, S.; Mueke, J.M. Effects of two neem insecticide formulations on the attractiveness, acceptability and suitability of diamondback moth larvae to the parasitoid, Diadegma mollipla (Holmgren) (Hym., Ichneumonidae). J. Appl. Entomol. 2003, 127, 325–331. [Google Scholar] [CrossRef]
- Du, Y.J.; Poppy, G.M.; Powell, W. Relative importance of semiochemicals from first and second trophic levels in host foraging behavior of Aphidius ervi. J. Chem. Ecol. 1996, 22, 1591–1605. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Chen, Z. Behavioral and electrophysiological responses of natural enemies to synomones from tea shoots and kairomones from tea aphids, Toxoptera aurantii. J. Chem. Ecol. 2022, 28, 2203–2219. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Zevallos, D.M.; Hellén, H.; Hakola, H.; van Nouhuys, S.; Holopainen, J.K. Induced defenses of Veronica spicata: Variability in herbivore-induced volatile organic compounds. Phytochem. Lett. 2013, 6, 653–656. [Google Scholar] [CrossRef]
- Pinto-Zevallos, D.M.; Martins, C.B.C.; Pellegrino, A.C.; Zarbin, P.H.G. Volatile organic compounds in induced plant defense against herbivorous insects. Quim. Nova 2013, 36, 1395–1405. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Regnault-Roger, C.; Vincent, C.; Arnason, J.T. Essential oils in insect control: Low-risk products in a high-stakes world. Annu. Rev. Entomol. 2012, 57, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Pino, J.A.; Marbot, R.; Quert, R.; García, H. Study of essential oils of Eucalyptus resinifera Smith, E. tereticornis Smith and Corymbia maculata (Hook.) KD Hill & LAS Johnson, grown in Cuba. Flavour. Fragr. 2002, J17, 1–4. [Google Scholar] [CrossRef]
- Vitti, A.M.S.; Brito, O.J. Óleo essencial de eucalipto. Doc. Florestais 2003, 17, 1–30. Available online: https://www.ipef.br/publicacoes/acervohistoricoexterno/DocumentosFlorestaisNumero17.pdf (accessed on 6 June 2024).
- Lu, H.; Shao, X.; Cao, J.; Ou, C.; Pan, D. Antimicrobial activity of eucalyptus essential oil against Pseudomonas in vitro and potential application in refrigerated storage of pork meat. Int. J. Food Sci. Technol. 2016, 51, 994–1001. [Google Scholar] [CrossRef]
- Pereira, J.L. Composição química dos óleos essenciais de espécies de Eucalyptus L. Herit (Myrtaceae). Master’s Thesis, Universidade Federal de Viçosa, Viçosa, Brazil, 2019. [Google Scholar]
- Goldbeck, J.; Nascimento, J.; Jacob, R.; Fiorentini, A.; Silva, W. Bioactivity of essential oils from Eucalyptus globulus and Eucalyptus urograndis against planktonic cells and biofilms of Streptococcus mutans. Ind. Crops Prod. 2014, 60, 304–309. [Google Scholar] [CrossRef]
- Bonora, F.S. Prospecção de Compostos Químicos Presentes nos Óleos Essenciais das Folhas e Flores de Eucalipto. Master’s Thesis, Universidade de São Paulo, Piracicaba, Brazil, 2016. [Google Scholar]
- Silva, P.H.M.D.; Brito, J.O.; Silva-Junior, F.G.D. Potential of eleven Eucalyptus species for the production of essential oils. Sci. Agric. 2006, 63, 85–89. [Google Scholar] [CrossRef]
- Silvestre, A.J.D.; Cavaleiro, J.S.; Delmond, B.; Filliatre, C. Analysis of the variation of the essential oil composition of Eucalyptus globulus Labill. from Portugal using multivariate statistical analysis. Ind. Crops Prod. 1997, 6, 27–33. [Google Scholar] [CrossRef]
- Li, H.; Madden, J.L.; Davies, N.W. Variation in leaf oils of Eucalyptus nitens and E. denticulata. Biochem. Syst. Ecol. 1994, 22, 631–640. [Google Scholar] [CrossRef]
- Araújo, F.O.L.; Rietzler, A.C.; Duarte, L.P.; Silva, G.D.F.; Carazza, F.; Filho, S.A.V. Chemical constituents and ecotoxicological effect of the volatile oil from leaves of Eucalyptus urograndis (Mirtaceae). Quim. Nova 2010, 33, 1510–1513. [Google Scholar] [CrossRef]
- Darrow, K.; Bowers, M.D. Phenological and population variation in iridoid glycosides of Plantago lanceolata (Plantaginaceae). Biochem. Syst. Ecol. 1997, 25, 1–11. [Google Scholar] [CrossRef]
- Harborne, J.B. Recent advances in the ecological chemistry of plant terpenoids. In Ecological Chemistry and Biochemistry of Plant Terpenoids; Harborne, J.B., Tomas-Barberan, F.A., Eds.; University Press: Oxford, UK, 1991; pp. 399–426. [Google Scholar]
- Andrew, R.L.; Keszei, A.; Foley, W.J. Intensive sampling identifies previously unknown chemotypes, population divergence and biosynthetic connections among terpenoids in Eucalyptus tricarpa. Phytochemistry 2013, 94, 48–158. [Google Scholar] [CrossRef] [PubMed]
- Lawler, I.R.; Stapley, J.; Foley, W.J.; Eschler, B.M. Ecological example of conditioned flavor aversion in plant–herbivore interactions: Effect of terpenes of Eucalyptus leaves on feeding by common ringtail and brushtail possums. J. Chem. Ecol. 1999, 25, 401–415. [Google Scholar] [CrossRef]
- Marsh, K.J.; Wallis, I.R.; McLean, S.; Sorensen, J.S.; Foley, W.J. Conflicting demands on detoxification pathways influence how common brushtail possums choose their diets. Ecology 2006, 87, 2103–2112. [Google Scholar] [CrossRef] [PubMed]
- Dellacassa, E.; Moyna, P. Eucalyptus Leaf Oils, Use, Chemistry, Distillation and Marketing; Inkata Press: Melbourne, Australia, 1991; 252p. [Google Scholar]
- Turlings, T.; Tumlinson, J.; Lewis, W. Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science 1990, 250, 1251–1253. [Google Scholar] [CrossRef] [PubMed]
- Adams, B.; Yusuf, A.A.; Torto, B.; Khamis, F.M. Tritrophic interactions mediated by zoophytophagous predator-induced host plant volatiles. J. Chem. Ecol. 2024, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Turlings, T.C.J.; Wackers, F. Recruitment of predators and parasitoids by herbivore-injured plants. In Advances in Insect Chemical Ecology, 1st ed.; Cardé, R.T., Millar, J.G., Eds.; Cambridge University Press: Cambridge, UK, 2004; pp. 21–75. [Google Scholar] [CrossRef]
- Bell, K.; Naranjo-Guevara, N.; Santos, R.C.D.; Meadow, R.; Bento, J.M. Predatory earwigs are attracted by herbivore-induced plant volatiles linked with plant growth-promoting rhizobacteria. Insects 2020, 11, 271. [Google Scholar] [CrossRef]
- Lee, B.W.; Basu, S.; Bera, S.; Casteel, C.L.; Crowder, D.W. Responses to predation risk cues and alarm pheromones affect plant virus transmission by an aphid vector. Oecologia 2021, 196, 1005–1015. [Google Scholar] [CrossRef] [PubMed]
- Jing, T.; Qian, X.; Du, W.; Gao, T.; Li, D.; Guo, D.; He, F.; Yu, G.; Li, S.; Schwab, W.; et al. Herbivore-induced volatiles influence moth preference by increasing the β-ocimene emission of neighbouring tea plants. Plant Cell Environ. 2021, 44, 3667–3680. [Google Scholar] [CrossRef] [PubMed]
- Delaquis, P.J.; Stanich, K.; Girard, B.; Mazza, G. Antimicrobial activity of individual and mixed fractions of dill, cilantro, coriander and eucalyptus essential oils. Int. J. Food Microbiol. 2002, 74, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Poppy, G.M.; Powell, W.; Pickett, J.A.; Wadhams, L.J.; Woodcock, C.M. Identification of semiochemicals released during aphid feeding that attract parasitoid Aphidius ervi. J. Chem. Ecol. 1998, 24, 1355–1368. [Google Scholar] [CrossRef]
- Giacomuzzi, V.; Mattheis, J.; Basoalto, E.; Angeli, S.; Knight, A.L. Survey of conspecific herbivore-induced volatiles from apple as possible attractants for Pandemis pyrusana (Lepidoptera: Tortricidae). Pest Manag. Sci. 2017, 73, 1837–1845. [Google Scholar] [CrossRef]
- Zhu, J.; Obrycki, J.; Ochieng, S.; Baker, T.; Pickett, J.; Smiley, D. Attraction of two lacewing species to volatiles produced by host plants and aphid prey. Naturwissenschaften 2005, 92, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Reddy, G. Plant volatiles mediate orientation and plant preference by the predator Chrysoperla carnea Stephens (Neuroptera: Chrysopidae). Biol. Control 2002, 25, 49–55. [Google Scholar] [CrossRef]
- Ananthakrishnan, T.N. Chemical ecology in biological control. In Emerging Trends in Biological Control of Phytophagous Insects; Ananthakrishnan, T.N., Ed.; Oxford and IBH Publishing: New Delhi, India, 1992; pp. 59–67. [Google Scholar]
- Hogervorst, P.A.M.; Wäckers, F.L.; Carette, A.-C.; Romeis, J. The importance of honeydew as food for larvae of Chrysoperla carnea in the presence of aphids. J. Appl. Entomol. 2008, 132, 18–25. [Google Scholar] [CrossRef]
- Limburg, D.; Rosenheim, J. Extrafloral nectar consumption and its influence on survival and development of an omnivorous predator, larval Chrysoperla plorabunda (Neuroptera: Chrysopidae). Environ. Entomol. 2001, 30, 595–604. [Google Scholar] [CrossRef]
- Takabayashi, J.; Shiojiri, K. Multifunctionality of herbivory-induced plant volatiles in chemical communication in tritrophic interactions. Curr. Opin. Insect Sci. 2019, 32, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Boege, K.; Marquis, R.J. Facing herbivory as you grow up: The ontogeny of resistance in plants. Trends Ecol. Evol. 2005, 20, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Bracho-Nunez, A.; Welter, S.; Staudt, M.; Kesselmeier, J. Plant-specific volatile organic compound emission rates from young and mature leaves of Mediterranean vegetation. J. Geophys. Res. 2011, 116, 1–13. [Google Scholar] [CrossRef]
- Cole, R.A. Volatile components produced during ontogeny of some cultivated crucifers. J. Sci. Food Agric. 1980, 31, 549–557. [Google Scholar] [CrossRef]
- Li, H.; Madden, J.L.; Potts, B.M. Variation in volatile leaf oils of the Tasmanian Eucalyptus species II. Subgenus Symphyomyrtus. Biochem. Syst. Ecol. 1996, 24, 547–569. [Google Scholar] [CrossRef]
- Maatallah, S.; Dabbou, S.; Castagna, A.; Guizani, M.; Hajlaoui, H.; Ranieri, A.M.; Flamini, G. Prunus persica by-products: A source of minerals, phenols and volatile compounds. Sci. Hortic. 2020, 261, 109016. [Google Scholar] [CrossRef]
- Nahrung, H.F.; Allen, G.R. Intra-plant host selection, oviposition preference and larval survival of Chrysophtharta agricola (Chapuis) (Coleoptera: Chrysomelidae: Paropsini) between foliage types of a heterophyllous host. Agric. For. Entomol. 2003, 5, 155–162. [Google Scholar] [CrossRef]
- Tanaka, M.; Nakasuji, F. Dynamic interaction between a leaf beetle, Galerucella nipponensis, and an aquatic plant, Trapa japonica. II. Dispersal behavior of larvae. Popul. Ecol. 2002, 44, 1–6. [Google Scholar] [CrossRef]
- Parizad, S.; Bera, S. The effect of organic farming on water reusability, sustainable ecosystem, and food toxicity. Environ. Sci. Pollut. Res. 2023, 30, 71665–71676. [Google Scholar] [CrossRef] [PubMed]

| Samples | Mass of Leaves (g) | Moisture Content (%) | Mass of EO (mg) | Yield (%) |
|---|---|---|---|---|
| Young leaves without damage | 50.0 ± 0.2 | 60.7 ± 2.42 | 99.5 ± 0.3 | 0.50 ± 0.02 |
| Young leaves with damage | 50.0 ± 0.2 | 60.7 ± 2.42 | 100.4 ± 0.6 | 0.50 ± 0.03 |
| Mature leaves without damage | 50.0 ± 0.2 | 55.37 ± 3.16 | 82.0 ± 0.1 | 0.37 ± 0.05 |
| Mature leaves with damage | 50.0 ± 0.2 | 55.37 ± 3.16 | 65.4 ± 0.7 | 0.29 ± 0.03 |
| Peak | Compound | TIC (%) | |||
|---|---|---|---|---|---|
| Young Leaves without Damage | Young Leaves with Damage | Mature Leaves without Damage | Mature Leaves with Damage | ||
| 01 | Eucalyptol | 28.16 | 10.36 | 20.33 | 7.59 |
| 02 | Linalool oxide <cis-> (furanoid) | 0.25 | 0.20 | 0.19 | 0.13 |
| 03 | Linalool oxide <trans-> (furanoid) | 0.29 | 0.19 | 0.22 | 0.16 |
| 04 | Linalool | 0.60 | 0.57 | 0.57 | 0.49 |
| 05 | NI | 0.40 | 0.06 | 0.1 | 0.05 |
| 06 | Fenchol<endo-> | 0.63 | 0.54 | 0.69 | 0.56 |
| 07 | Campholenal<alpha-> | 0.26 | 0.06 | 0.18 | 0.16 |
| 08 | Borneol | 1.31 | 1.80 | 2.08 | 2.38 |
| 09 | Terpinen-4-ol | 0.99 | 1.18 | 1.43 | 1.38 |
| 10 | ρ-Cymen-8-ol | 1.17 | 0.85 | 0.55 | 0.50 |
| 11 | α-Terpineol | 10.85 | 11.90 | 11.49 | 13.73 |
| 12 | NI | 1.48 | 0.55 | 0.58 | 0.48 |
| 13 | 2-Hydroxy-1,8-cineole | 3.08 | 1.68 | 1.59 | 1.46 |
| 14 | Neral Carvone * | 0.48 | 0.27 | 0.27 | 0.23 |
| 15 | Geraniol | 0.55 | 0.76 | 0.90 | 1.04 |
| 16 | Geranial | 1.14 | 0.55 | 0.52 | 0.58 |
| 17 | NI | 1.48 | 1.02 | 0.94 | 0.92 |
| 18 | NI | 1.52 | 0.74 | 1.12 | 1.13 |
| 19 | Verbenyl acetate <trans-> | 0.18 | 0.21 | 0.17 | 0.19 |
| 20 | Exo-2-hydroxycineole acetate | 0.53 | 0.61 | 0.48 | 0.56 |
| 21 | α-Terpinyl acetate | 14.24 | 21.06 | 17.62 | 21.96 |
| 22 | NI | 0.35 | 0.33 | 0.34 | 0.37 |
| 23 | Alpha-copaene | 0.27 | 0.40 | 0.30 | 0.38 |
| 24 | Geranyl acetate | 1.67 | 2.26 | 1.65 | 1.89 |
| 25 | NI | 0.82 | 0.33 | 0.32 | 0.48 |
| 26 | Carvone hydrate | 1.27 | 1.53 | 1.49 | 1.55 |
| 27 | NI | 1.02 | 1.15 | 1.20 | 0.91 |
| 28 | Aromadendrene | 0.70 | 1.05 | 0.49 | 0.71 |
| 29 | NI | 1.10 | 1.11 | 1.08 | 1.38 |
| 30 | NI | 1.70 | 2.02 | 2.40 | 2.61 |
| 31 | NI | 0.59 | 0.73 | 0.59 | 0.65 |
| 32 | Geranyl isobutyrate | 2.26 | 3.60 | 3.22 | 4.44 |
| 33 | NI | 1.13 | 1.59 | 1.25 | 1.27 |
| 34 | Flavesone | 0.39 | 0.71 | 0.49 | 0.67 |
| 35 | 8-Acetoxy-carvotanacetone | 2.84 | 3.99 | 3.49 | 3.61 |
| 36 | Spathulenol ** Caryophyllene oxide ** Globulol ** | 7.25 | 12.20 | 10.75 | 12.37 |
| 37 | Viridiflorol | 0.90 | 1.55 | 2.14 | 2.82 |
| 38 | NI | 0.49 | 0.83 | 0.77 | 0.94 |
| 39 | Humulene epoxide II | 0.28 | 0.48 | 0.35 | 0.55 |
| 40 | Isoleptospermone | 1.58 | 2.64 | 2.39 | 3.21 |
| 41 | NI | 2.52 | 4.64 | 1.88 | 2.43 |
| 42 | 5-Hydroxy-isobornyl-isobutanoate | 1.16 | 1.62 | 1.29 | 0.97 |
| Total identified (%) | 85.28 | 84.82 | 87.33 | 86.27 | |
| Peak | Compounds | TIC (%) Average | |||||
|---|---|---|---|---|---|---|---|
| Young Leaves without Damage | Young Leaves with Damage | Difference (%) | Mature Leaves without Damage | Mature Leaves with Damage | Difference (%) | ||
| 01 | Eucalyptol | 28.16 | 10.36 | −63.2 | 20.33 | 7.59 | −62.7 |
| 11 | α-Terpineol | 10.85 | 11.90 | 9.6 | 11.49 | 13.73 | 19.5 |
| 21 | α-Terpinyl acetate | 14.24 | 21.06 | 47.9 | 17.60 | 22.00 | 25.0 |
| 28 | Aromadendrene | 0.70 | 1.05 | 50.0 | 0.49 | 0.71 | 44.9 |
| 42 | 5-Hydroxy-isobornyl isobutanoate | 1.16 | 1.62 | 39.7 | 1.29 | 0.97 | −24.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borges, D.J.V.; Souza, R.A.C.; de Oliveira, A.; de Sousa, R.M.F.; Venâncio, H.; Demetrio, G.R.; Ambrogi, B.G.; Santos, J.C. Green Lacewing Chrysoperla externa Is Attracted to Volatile Organic Compounds and Essential Oils Extracted from Eucalyptus urograndis Leaves. Plants 2024, 13, 2192. https://doi.org/10.3390/plants13162192
Borges DJV, Souza RAC, de Oliveira A, de Sousa RMF, Venâncio H, Demetrio GR, Ambrogi BG, Santos JC. Green Lacewing Chrysoperla externa Is Attracted to Volatile Organic Compounds and Essential Oils Extracted from Eucalyptus urograndis Leaves. Plants. 2024; 13(16):2192. https://doi.org/10.3390/plants13162192
Chicago/Turabian StyleBorges, David Jackson Vieira, Rafael Aparecido Carvalho Souza, Alberto de Oliveira, Raquel Maria Ferreira de Sousa, Henrique Venâncio, Guilherme Ramos Demetrio, Bianca Giuliano Ambrogi, and Jean Carlos Santos. 2024. "Green Lacewing Chrysoperla externa Is Attracted to Volatile Organic Compounds and Essential Oils Extracted from Eucalyptus urograndis Leaves" Plants 13, no. 16: 2192. https://doi.org/10.3390/plants13162192
APA StyleBorges, D. J. V., Souza, R. A. C., de Oliveira, A., de Sousa, R. M. F., Venâncio, H., Demetrio, G. R., Ambrogi, B. G., & Santos, J. C. (2024). Green Lacewing Chrysoperla externa Is Attracted to Volatile Organic Compounds and Essential Oils Extracted from Eucalyptus urograndis Leaves. Plants, 13(16), 2192. https://doi.org/10.3390/plants13162192





