Abstract
To explore and utilize the abundant soil microorganisms and their beneficial functions, high-throughput sequencing technology was used to analyze soil microbial compositions in the rhizosphere of red and green amaranth varieties. The results showed that significant differences in soil microbial composition could be found in the rhizosphere of amaranth plants with different color phenotypes. Firstly, soil bacterial compositions in the rhizosphere were significantly different between red and green amaranths. Among them, Streptomyces, Pseudonocardia, Pseudolabrys, Acidibacter, norank_ f_ Micropepsaceae, Bradyrhizobium, and Nocardioides were the unique dominant soil bacterial genera in the rhizosphere of red amaranth. In contrast, Conexibacter, norank_f_norank_o_norank_c_TK10, and norank_f_ norank_o_ norank_ c_AD3 were the special dominant soil bacterial genera in the rhizosphere of green amaranth. Additionally, even though the soil fungal compositions in the rhizosphere were not significantly different between red and green amaranths, the abundance of the dominant soil fungal genera in the rhizosphere showed significant differences between red and green amaranths. For example, unclassified_k__Fungi, Fusarium, Cladophialophora, unclassified_c__Sordariomycetes and unclassified_p__Chytridiomycota significantly enriched as the dominant soil fungal genera in the rhizosphere of the red amaranth. In contrast, Aspergillues only significantly enriched as the dominant soil fungal genus in the rhizosphere of green amaranth. All of the above results indicated that amaranth with various color phenotypes exactly recruited different microorganisms in rhizosphere, and the enrichments of soil microorganisms in the rhizosphere could be speculated in contributing to amaranth color formations.
1. Introduction
Amaranth (Amaranthus tricolor L.) is an annual herbaceous plant of the Amaranthaceae family that originated in the Americas and Asia, and now grows widely in Asia, the Americas, and Europe [1,2]. It is rich in carotenoids, vitamins, and minerals as well as many other substances beneficial to human health [3,4]. Amaranth plants exhibit a variety of colors, which is one of their most important commercial qualities. Different based on color classification, amaranth can be classified as red or green [5,6]. Different colored amaranth species have different nutritional values. Among them, red amaranth plants contain more nutrients than green amaranth plants [7]. Therefore, it is of great significance to explore the formation mechanism of amaranth color.
Generally speaking, the color of plants mainly depends on the pigment species and content in the plant body. These pigments mainly include chlorophyll, carotenoids, and flavonoids (such as anthocyanins) [8,9,10]. Among them, the different colors of red amaranth and green amaranth are mainly due to the different betalain content in plants. In comparison with green amaranth, the content of betalain in the leaves of beet and red amaranth was significantly higher. Therefore, it can make amaranth appear red [11,12,13]. Betaine is a water-soluble pigment, mainly divided into betacyanin and betaxanthin [14,15], which is widely found in the roots, stems, and leaves of plants [16,17,18]. However, the biosynthesis of betalain as a secondary metabolite is affected by many factors, such as light, temperature, humidity, and hormone levels, especially phytohormones [19,20,21]. For example, salicylic and jasmonic acids significantly promote betaine synthesis [22]. In contrast, gibberellin inhibits amaranth pigment biosynthesis [23]. Moreover, betaine can also be produced by microorganisms, such as the fungal lineage and some diazotrophic gluconic isolate bacteria [24].
Soil microorganisms in the rhizosphere are the most active component of the soil-plant interaction system and are considered the second most common type of genome in plants [25,26]. A large number of studies have shown that rhizospheric soil microorganisms participate not only in material circulation but also in energy metabolism, which plays an important role in maintaining soil fertility, health, and sustainable agricultural production [27,28,29]. For example, Abinandan et al. [30] found that soil microorganisms play an important role in maintaining nutrient balances, carbon sinks, and soil health. Meanwhile, Priya et al. [31] also found that the state of soil health and its nutrient pools are dependent on soil microbial community structure and function. In addition, rhizospheric microorganisms can also directly or indirectly affect the growth and development of crops [32,33]. The direct mechanism involved microbes in the rhizosphere producing plant hormones or enzymes, which regulate the growth and development of plants [34]. For example, auxin [35], gibberellin (GA) [36], cytokinin [37], and ethylene [38] can be produced by microbes in the rhizosphere to improve their adaptability. An indirect mechanism involves the participation of soil microbes in defensive metabolic processes, plant pathogen inhibition, and environmental stresses in the rhizosphere [39].
Like all higher organisms, plants have evolved in the context of the microbial world. For instance, microorganisms co-evolved with their hosts and co-existing microbial consortia to expand the host’s genetic pool, known as “extended genotypes” [40]. The hosts can integrate the expanded microbial genomes into their phenotype, resulting in profound changes in phenotypic characteristics, the “extended phenotype” [41]. However, whether different genotypes of amaranth recruit different rhizosphere microorganisms by controlling the secretion of certain substances (enzymes, hormones, etc.) to promote the formation of their own colors has not been explored before. Therefore, we hypothesized that amaranth species with different color phenotypes would recruit different microbes in synthesizing their color. To test this hypothesis, the soil microbial compositions in the rhizospheres of red and green amaranths were analyzed using a high-throughput sequencing technique.
2. Methods
2.1. Field Site Description and Experimental Design
Two different colored amaranths were used in this study. First, red amaranth varieties (R), which included “a little red” (Qingxian Xingyun Vegetable Breeding Co., Ltd., Cangzhou, China) and “bonus Garden Leaf” (Qiangkun Vegetable Seed Co., Ltd., Qinzhou, China), were used. Green amaranth varieties (G), including “green amaranth” (Jiuquan Changfeng Agricultural Development Co., Ltd., Jiuquan, China) and “Qingyuan Leaf” (Guangzhou Hongye Seed Technology Co., Ltd., Guangzhou, China), were also used for comparative study.
The experiment was carried out in pots with a diameter of 32 cm and a height of 20 cm from December 2021 to June 2022 at the vegetable base of the College of Agriculture, Guangxi University (108°17′15″ E, 22°51′02″ N); 30 pots were planted for each amaranth variety. The soil type was red loam, and all the amaranth varieties were identically treated. The study area is a typical subtropical monsoon climate, with abundant precipitation, long summer, and short winter. The average annual air temperature and rainfall were 21.8 °C and 1286 mm, respectively. The physicochemical properties of the soil were as follows: pH 5.71; organic matter content, 8.42 g·kg−1; and total nitrogen, phosphorus, and potassium, 0.51 g·kg−1, 0.67 g·kg−1, and 7.21 g·kg−1, respectively. Moreover, the available phosphorus, potassium, and alkaline nitrogen contents were 0.59 mg·kg−1, 51.01 mg·kg−1, and 13.17 mg·kg−1, respectively.
2.2. Soil Sampling
After the amaranth had reached maturity, three uniformly growing amaranth plants were selected for each variety, and rhizospheric soil samples of the amaranth were collected using the root-shaking method [42]. Specifically, the shovel was disinfected with alcohol and used to loosen the soil around the amaranth plant; then, the whole amaranth plant was poured out and shaken, and the soil adhering to the root of the plant was collected as rhizospheric soil samples. The plants were put into sterile bags, brought back to the laboratory, screened through 10 mesh, and subjected to microbial community analysis. In addition, soil without any plants was also collected and used as a bulk (CK) soil.
2.3. Soil Microbial DNA Extraction and Illumina Sequencing
The soil microbial community structures in the rhizosphere of amaranths of various color phenotypes were sequenced by Shanghai Meiji Biomedical Technology Co.(Shanghai, China) Total DNA was extracted according to the instructions of the E.Z.N.A.A. Soil DNA kit (Omega, Norcross, GA, USA), DNA concentration and purity were detected using a NanoDrop2000 spectrophotometer (Thermo, Wilmington, DE, USA), and extracted genomic DNA was detected using 1% agarose gel electrophoresis. The extracted interrhizosphere soil microbial DNA was used as a template. The primers 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) were selected for PCR amplification of the 16S rRNA V5-V7 region of interrhizosphere soil bacteria, and the primer ITS1F (5′-CTTGGTCATTTAGAGGA) was selected for PCR amplification of the 16S rRNA V5-V7 region of interrhizosphere soil bacteria. CTTGGTCATTTAGAGGAAGTAA-3′) and ITS2F (5′-GCTGCGTTCTTCATCATCGATFC-3′) were selected for PCR amplification of the 18S rRNA ITS region of the interrhizosphere soil fungus, and the PCR instrument used was an ABI GeneAmp® 9700 model. The PCR products were recovered by 2% agarose gel electrophoresis, purified using an Axy PrepDNA Gel Recovery Kit (Axygen, San Francisco, CA, USA), and eluted with Tris_HCl. The PCR products were detected and quantified using a QuantiFluor™-ST Blue Fluorescence Quantification System (Promega, Madison, WI, USA). The purified amplified fragments were used to construct libraries according to the standard protocols of the Illumina MiSeq platform.
2.4. Data Analysis
The experimental data were statistically analyzed using Excel 2019 and SPSS 221.0, and Duncan’s multiple range test was used to compare the means. The alpha diversity of the bacterial and fungal communities was calculated using Mothur (version v.1.30.2; https://mothur.org/wiki/calculators/, accessed on 9 April 2022). Non-metric multidimensional scaling (NMDS) and partial least squares discriminant analysis (PLS-DA) were used for statistical analysis and mapping, respectively, using R language (version 3.3.1) tools. For microbial community composition and Venn diagram analysis, Operational taxonomic units (OTU) tables with 97% similarity were selected and used for statistical and mapping purposes via the R language (version 3.3.1) tool. The linear discriminant analysis effect size (LEfSe) method was used to identify the soil microbial community structures in the rhizosphere. Network correlation analysis of rhizospheric soils between red and green amaranths was carried out using correlation coefficient Spearman [43]. Second, PICRUSt, the Kyoto Encyclopedia of Genes and Genomes (KEGG) dataset, was used to estimate the functional composition of the bacterial community. Additionally, the functional prediction of fungal communities was also evaluated using the Fungi Functional Association (FUNGuild) tool. All the online data analyses were conducted using the free online platform Majorbio Cloud Platform (www.majorbio.com, accessed on 8 August 2024) through Majorbio Biopharm Technology Co., Ltd., (Shanghai, China). The data were visualized by ImageGP (https://onlinelibrary.wiley.com/doi/10.1002/imt2.5, accessed on 9 April 2022).
3. Results
3.1. Analysis of Soil Bacterial and Fungi Alpha Diversity in the Rhizosphere of Red and Green Amaranth Plants
As shown in Table 1, the soil bacterial diversity (Shannon and Simpson) and richness (Ace and Chao1) in the rhizospheres of red and green amaranths were all not significantly different between each other. Also, there were no significant differences with bulk soil. Additionally, soil fungi diversity (Shannon and Simpson) and richness (Ace and Chao1) in the rhizospheres of red and green amaranths were also not significantly different between each other, and there were no significant differences between bulk soil either.

Table 1.
Soil bacterial and fungi diversity and richness in the rhizosphere of red and green amaranths.
3.2. Analysis of Soil Bacterial and Fungi Beta Diversity in the Rhizospheres of Red and Green Amaranth Plants
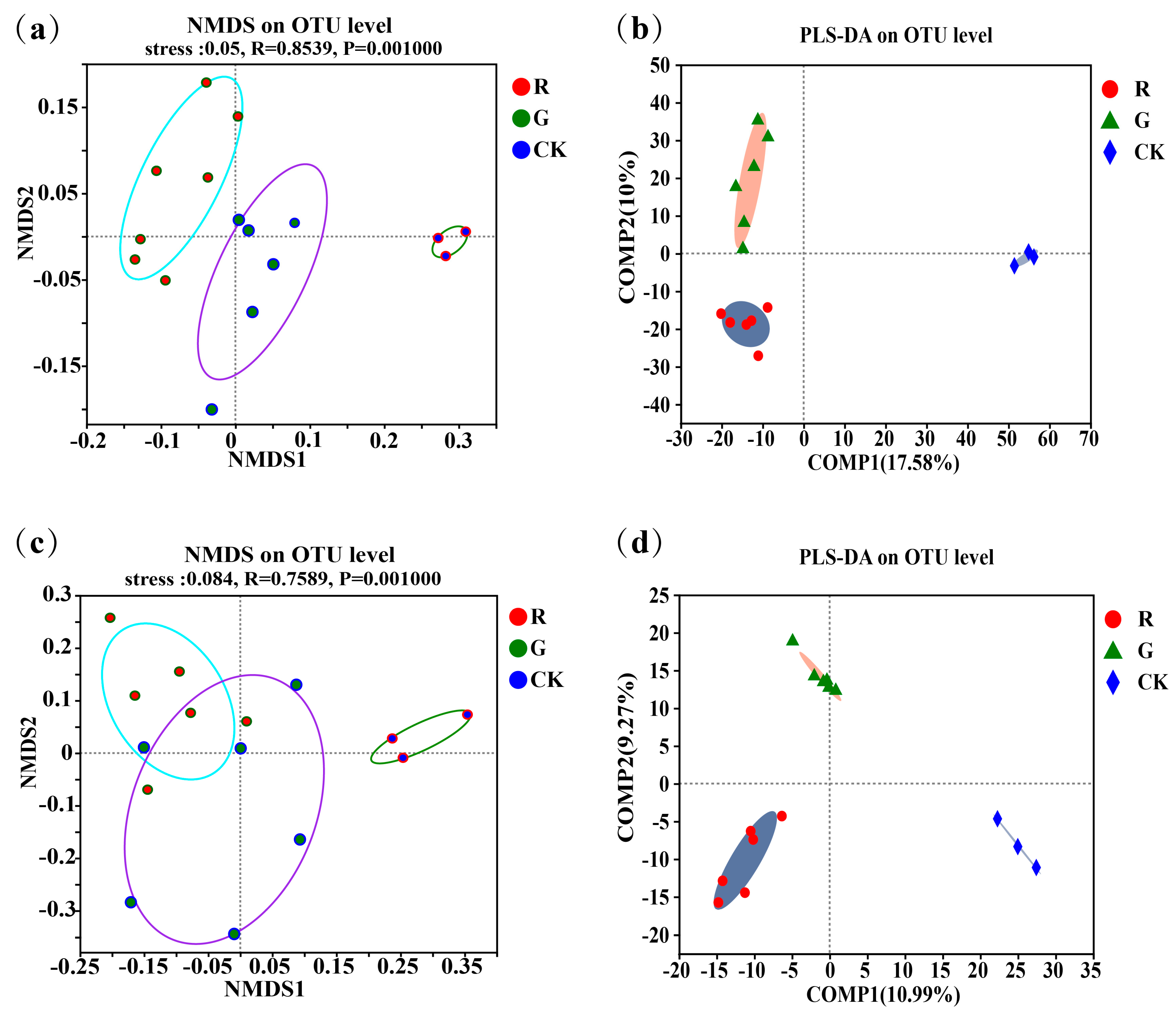
Non-metric multidimensional scaling (NMDS) analyses of the soil microbial community structures in the rhizospheres of red and green amaranths were carried out at the operational taxonomic unit (OTU) level (Figure 1a,c). The results showed that not only soil bacterial (R = 0.8539, p = 0.001) but also soil fungal (R = 0.7589, p = 0.001) community structures in the rhizospheres were all significantly different in the rhizospheres between red and green amaranths. In addition, we also performed partial least squares discriminant analysis (PLS-DA) at the operational taxonomic unit (OTU) level (Figure 1b). The results also showed that soil bacteria and fungi clustered separately in the rhizosphere between red and green amaranths, respectively. All of the above results indicated that different soil microbes were exactly recruited in the rhizospheres of red and green amaranths even though they were grown in the same field under identical management.
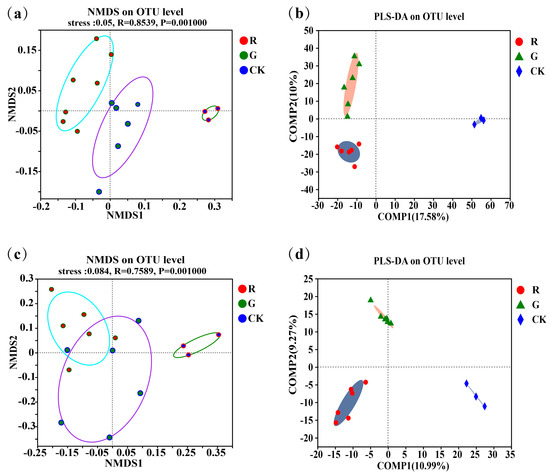
Figure 1.
Comparison of soil bacterial communities in the rhizospheres of red and green amaranth plants; (a) the non-metric multidimensional scaling (NMDS) of soil bacteria in the rhizospheres of red and green amaranth plants at the operational taxonomic unit (OTU) level; (b) the partial least squares discriminant analysis (PLS-DA) of soil bacteria in the rhizospheres of red and green amaranth plants at the operational taxonomic unit (OTU) level; (c) the non-metric multidimensional scaling (NMDS) of soil fungi in the rhizospheres of red and green amaranth plants at the operational taxonomic unit (OTU) level; (d) the partial least squares discriminant analysis (PLS-DA) of soil fungi in the rhizospheres of red and green amaranth plants at the operational taxonomic unit (OTU) level.
3.3. Community Composition Analysis of Soil Bacteria and Fungi in the Rhizospheres of Red and Green Amaranth Plants
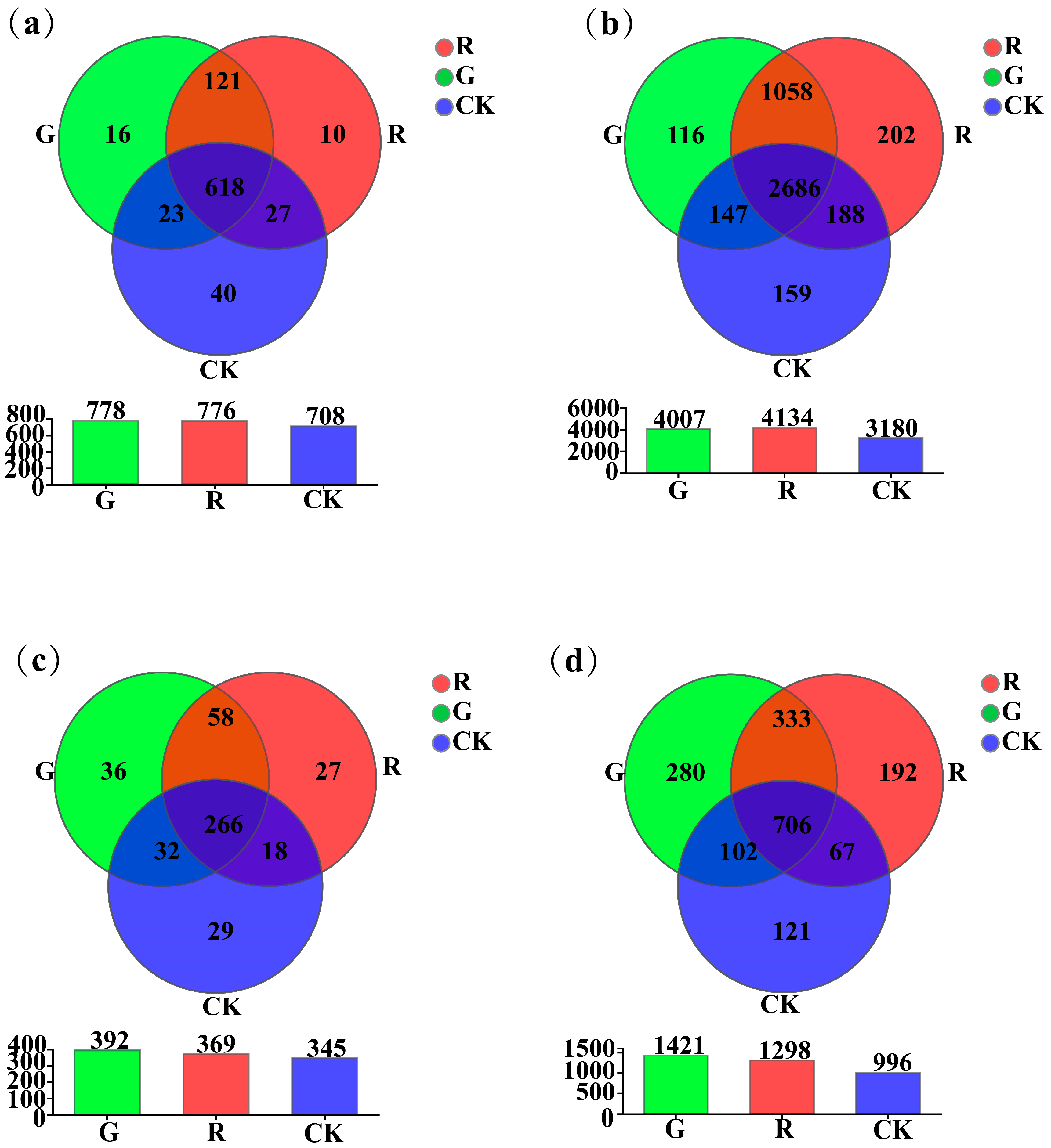
As shown in Figure 2, at the genus level, the results of Venn diagram analyses showed that the numbers of unique soil dominant bacterial genera in the rhizospheres of red and green amaranths, and the bulk soil were 10, 16, and 40, respectively (Figure 2a). However, at the operational taxonomic unit (OTU) level, they were 202, 116, and 159, respectively (Figure 2b). For soil fungi, at the genus level, the results of Venn diagram analyses also showed that the numbers of unique soil dominant fungal genera in the rhizosphere of red amaranth, green amaranth, and bulk soil (CK) were 27, 36, and 27, respectively (Figure 2c). Moreover, at the operational taxonomic unit (OTU) level, they were 192, 280, and 121, respectively (Figure 2d).
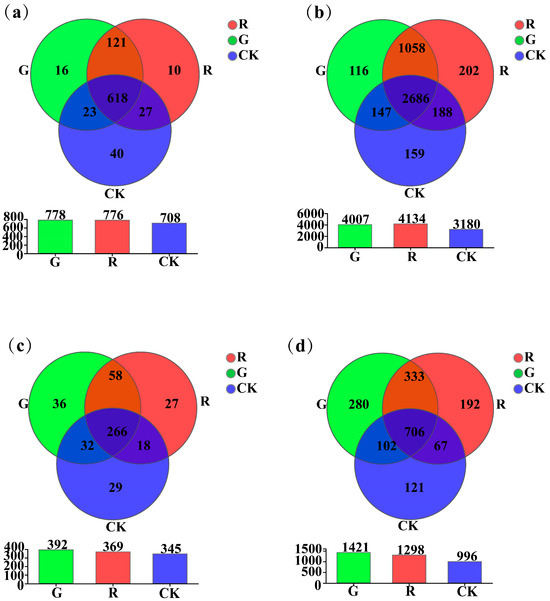
Figure 2.
Venn plot analysis of soil bacteria (a,b) and fungi (c,d) in the rhizosphere of red and green amaranths at the genus and operational taxonomic unit (OTU) levels.
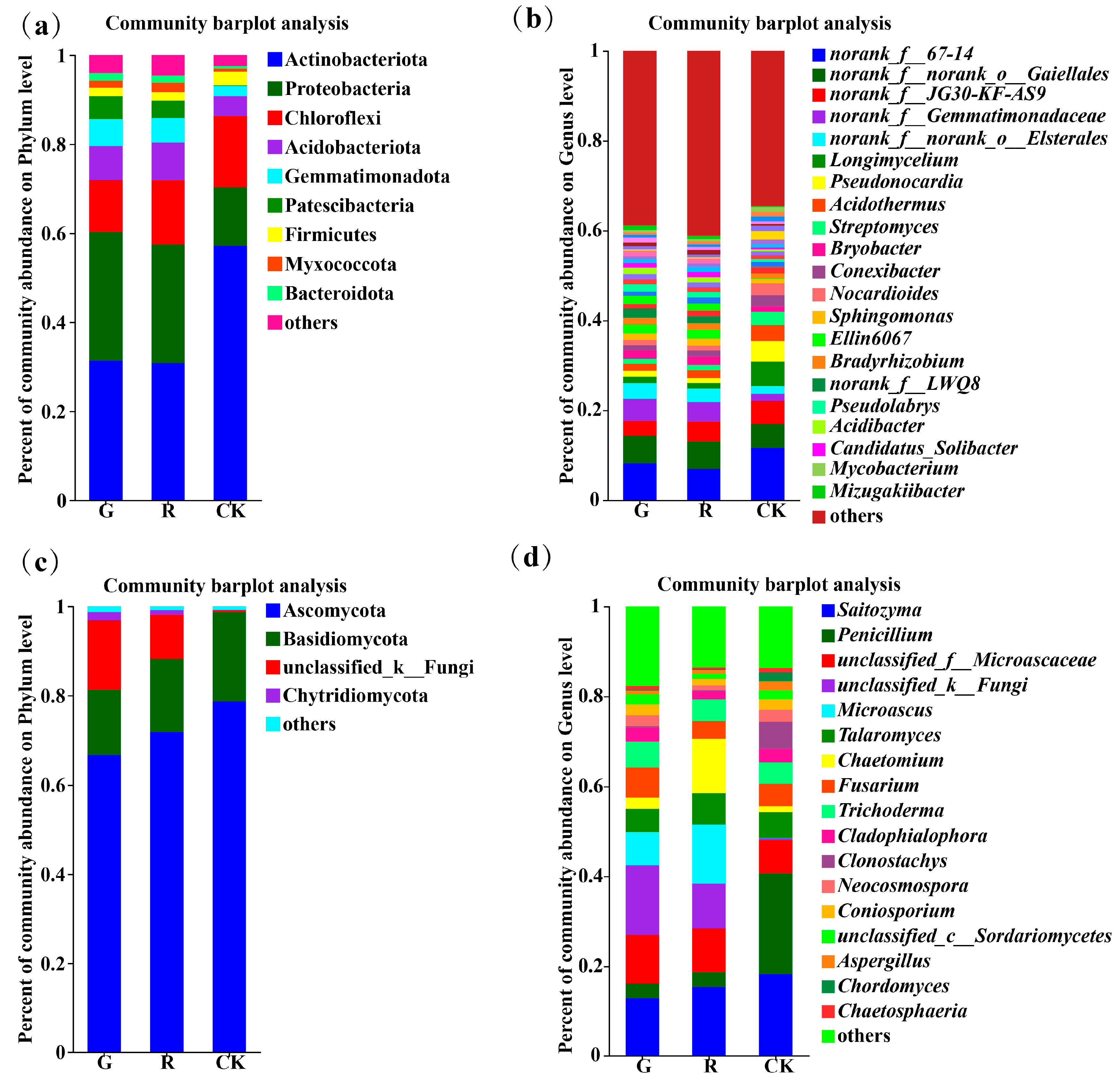
At the phylum level, there were 9, 9, and 6 dominant soil bacterial phyla (with an abundance greater than 1%) in the rhizosphere of red and green amaranths and bulk soil, respectively (Figure 3a). Among them, the dominant bacterial phyla in the rhizosphere of red amaranth were Actinobacteriota (31.37%), Proteobacteria (28.91%), Chloroflexi (11.65%), Acidobacteriota (7.62%), Gemmatimonadota (6.09%), Patescibacteria (5.17%), Firmicutes (1.92%), Myxococcota (1.59%), and Bacteroidota (1.79%). In contrast, the dominant soil bacterial phyla in the rhizosphere of green amaranth were Actinobacteriota (30.87%), Proteobacteria (26.54%), Chloroflexi (14.5%), Acidobacteriota (8.46%), Gemmatimonadota (5.52%), Patescibacteria (3.92%), Myxococcota (2. 05%), Firmicutes (1.85%), and Bacteroidota (1.64%). Moreover, the dominant bacterial phyla of the bulk soil were Actinobacteria (57.14%), Proteobacteria (13.19%), Chloroflexi (11.65%), Acidobacteriota (4.50%), Gemmatimonadota (2.27%), and Firmicutes (3.07%).
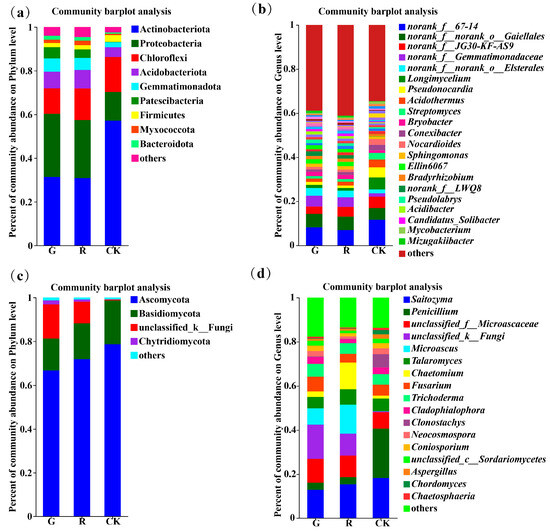
Figure 3.
Composition of soil bacterial communities in the rhizosphere of red and green amaranth plants at the phylum (a) and genus (b) levels. Composition of soil fungi communities in the rhizosphere of red and green amaranth plants at the phylum (c) and genus (d) levels.
At the genus level, the numbers of dominant soil bacterial genera (those with an abundance greater than 1%) in the rhizosphere of red and green amaranths and bulk soil were 18, 17, and 18, respectively (Figure 3b).
First, norank_f_67-14 (8.25%), norank_f_norank_o_Gaiellales (6.06%), norank_f_ Gemmatimonadaceae (4.93%), norank_f_norank_o_Elsterales (3.47%), norank_f_JG30-KF-AS9 (3.32%), Streptomyces (2.91%), Ellin6067 (2.00%), norank_LWQ8 (2.11%), Bryobucter (1.94%), unclassified_o_Saccharimonadales (1.88%), Pseudolabrps (1.59%), Sphingomons (1.51%), Acidothermus (1.50%), Longimycelium (1.47%), Acidibacter (1.46%), Bradyrhizobium (1.45%), Pseudonocardia (1.33%), norank_f_Micropepsaceae (1.28%), and Nocardioides (1.28%) were the dominant soil bacterial genera in the rhizosphere of red amaranth.
In contrast, norank_f_67-14 (6.94%), norank_f_ norank_o_Gaiellales (6.14%), norank_f_JG30-KF-AS9 (4.41%), norank_f_ Gemmatimonadaceae (4.35%), norank_f_ norank_o_Elsterales (3.01%), Ellin6067 (1.92%), Bryobacter (1.89%), Acidothermus (1.75%), Sphingomonas (1.62%), norank_f_LWQ8 (1.53%), unclassified_o_Saccharimonadales (1.50%), norank_f_ norank_o_ norank_ c_ AD3 (1.35%), norank_f_norank_o _norank _c_ TK10 (1.34%), Comexibacter (1.30%), Longimyelium (1.22%), and Pseudolabrys (1.21%) were the dominant soil bacterial genera in the rhizosphere of green amaranth.
Moreover, norank_f_67-14 (11.67%), Longimycelium (5.44%), norank_f_ norank_o_Gaiellales (5.33%), norank_f_JG30-KF-AS9 (5.14%), Pseudonocardia (4.55%), Acidothermus (3.60%), Nocardioides (2.59%), Conexibacter (2.46%), Actinoallomurus (1.82%), norank_f_norank_o_ Elsterales (1.79%), norank_f_ Gemmatimonadaceae (1.51%), norank_f_norank_ o_norank_c_TK10 (1.38%), Bryobucter (1.26%), Actinospica (1.20%), norank_f_ norank_ o_ norank_ c_ AD3 (1.10%), Jatrophihabitans (1.10%), and Mycobacterium (1.09%) were the dominant soil bacterial genera of the bulk soil.
All these results indicated that the soil bacterial community composition in the rhizosphere varies significantly between red amaranth and green amaranth. Among them, Streptomyces, Pseudonocardia, Pseudolabrys, Acidibacter, norank_ f_ Micropepsaceae, Bradyrhizobium, and Nocardioides were the unique dominant soil bacterial genera in the rhizosphere of red amaranth. In contrast, Conexibacter, norank_f_norank_o_norank_c_TK10 and norank_f_ norank_o_ norank_ c_AD3 were the unique dominant soil bacterial genera in the rhizosphere of green amaranth.
In addition, as shown in Figure 3c, the numbers of dominant soil fungal phyla (those with an abundance greater than 1%) in the rhizosphere of red and green amaranths and bulk soil were 4, 4, and 2, respectively. First, the dominant soil fungal phyla in the rhizosphere of red amaranth were Ascomycota (66.68%), Basidiomycota (16.35%), unclassified _ k _ Fungi (9.94%), and Chytridiomycota (1.84%). In contrast, the main dominant soil fungal phyla in the green amaranth treatment were Ascomycota (71.85%), Basidiomycota (14.64%), unclassified_k_Fungi (15.55%), and Chytridiomycota (1.03%). The main dominant soil fungal phyla in the bulk soil treatment were Ascomycota (78.63%) and Basidiomycota (20.08%).
At the genus level, the numbers of dominant soil fungal genera (with an abundance greater than 1%) in the rhizosphere of red and green amaranths and bulk soil were 13, 13, and 15, respectively (Figure 3d).
First, unclassified_ k_ Fungi (15.55%), Saitozyma (12.90%), unclassified_f_Microascaceae (10.79%), Microuscus (7.39%), Fusarium (6. 66%), Trichoderma (5.77%), Talaromyces (5.19%), Penicillium (2.24%), Cadophialophora (3.09%), Neocosmospora (2.48%), Chaetomium (2.46%), Coniosporium (2.44%), unclassified_c_Sordariomycetes (2.22%), and Aspergillues (1.02%) were the dominant soil fungal genera in the rhizosphere of the red amaranth.
In contrast, Saitozyma (15.36%), Microuscus (13.17%), Chaetonium (12.06%), unclassified_f_Microascaceae (9.73%), unclassified_K Fungi (9.94%), Talaromyces (6.96%), Fusarium (3.92%), Penicillium (3.35%), Aspergillues (2.96%), Trichoderma (2.86%), Cladophialophora (1.85%), Coniosporium (1.40%), Urificified_ c_ Sordariomycetes (1.17%), and Neocosmospora (1.05%) were the dominant soil fungal genera in the rhizosphere of green amaranth.
Additionally, Penicillium (22.40%), Saitozyma (18.26%), unclassified_f_Microascaceae (7.43%), Clonostachys (6.04%), Talaromyees (5.74%), Talaromyces (5.74%), Fusarium (5.01%), Trichoderma (4.73%), Cludophialophora (2.98%), Neocosmospora (2.70%), Coniosporium (2.31%), unclassified_c Sordariomycetes (2.05%), Chordomyces (1.87%), Chaetomium (1.29%), and Chaetosplaeria (1.03%) were the dominant soil fungal genera in the bulk soil.
All of the above results suggested that the composition of the dominant soil fungi at the genus level did not change in the rhizosphere of red or green amaranths. However, the abundances of the dominant soil fungal genera in the rhizosphere differed between red and green amaranths. In comparison with those of green amaranth, the abundances of unclassified_f_Microascaceae, Fusarium, Trichoderma, Cladophialophora, Neocosmospora, Coniosporium, and unclassified_c_Sordariomycetes were greater, and the abundances of Saitozyma, Penicillium, Microascus, Aspergillues, Talaromyces, and Chaetomium were lower in the rhizosphere of red amaranth.
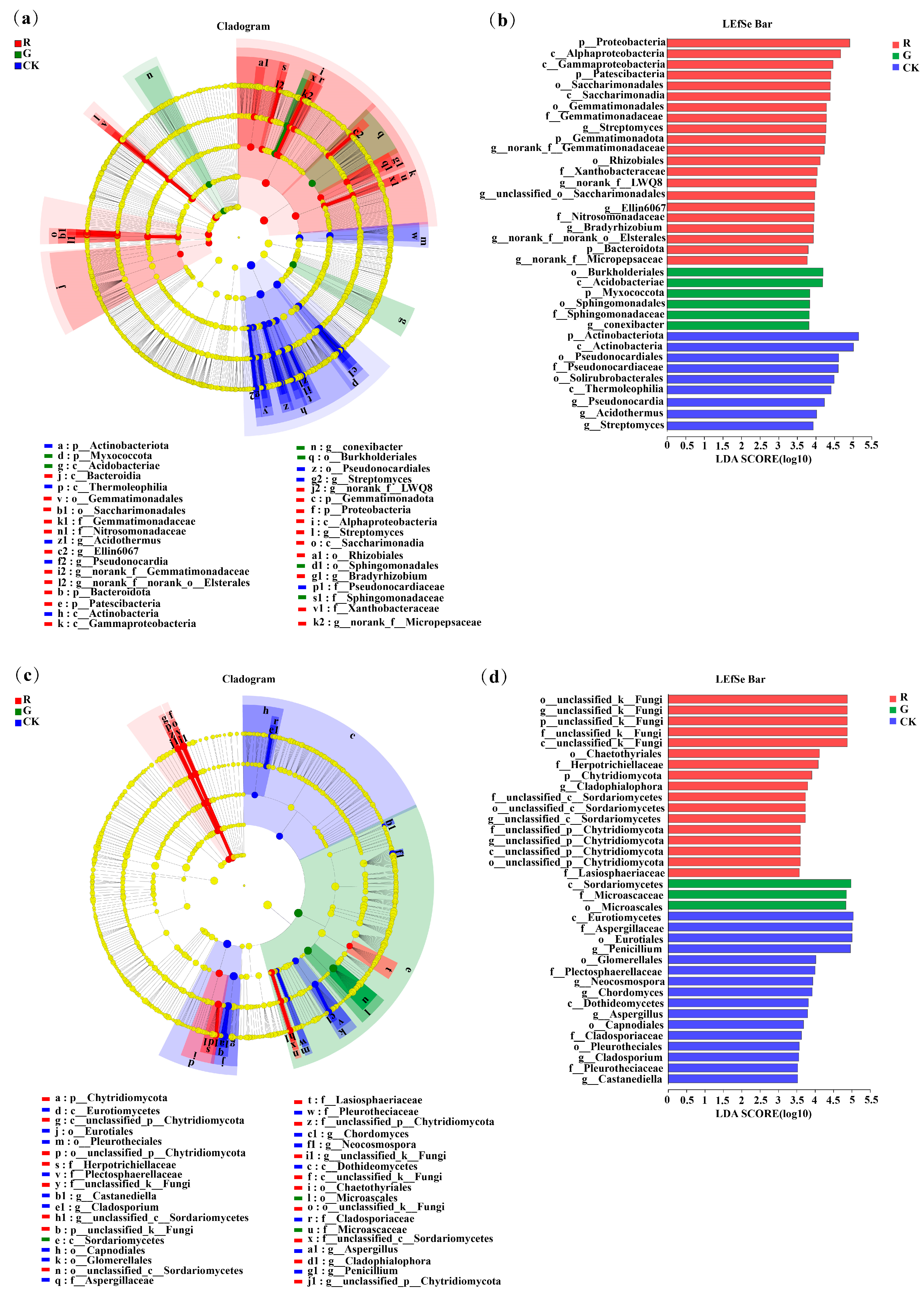
3.4. LEfSe Analysis of Soil Bacterial Communities in the Rhizospheres of Red and Green Amaranth Plants
As shown in Figure 4a, LEfSe analysis at the phylum-to-genus level was also conducted to identify bacteria with significant differences in abundance in the rhizospheres of red and green amaranths and in the bulk soil. The results showed that 35 bacterial branches were significantly different (LDA > 3.5, p < 0.05). In particular, at the phylum level, Proteobacteria, Patescibacteria, Gemmatimonadota, and Bacteroidota were significantly enriched as the dominant soil bacterial phyla in the rhizosphere of red amaranth. In contrast, Myxococcota was significantly enriched as the dominant soil bacterial phylum in the rhizosphere of green amaranth. In addition, at the genus level, Streptomyces, norank_f__Gemmatimonadaceae, Bradyrhizobium, norank_f__LWQ8, unclassified_o__Saccharimonadales, Ellin6067, norank_f__Micropepsaceae and norank_f __norank_o__ElsteralesH were significantly enriched as the dominant soil bacterial genera in the rhizosphere of red amaranth; in contrast, Conexibacter was significantly enriched as the dominant soil bacterial genus in the rhizosphere of green amaranth.
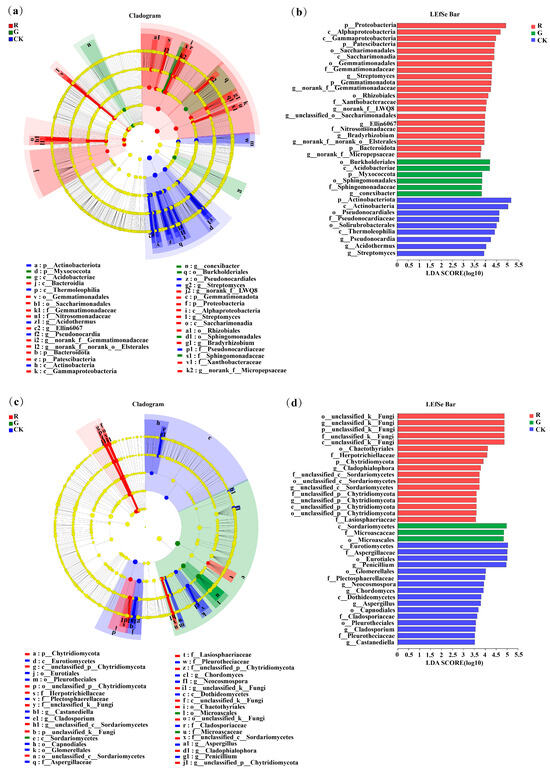
Figure 4.
LEfSe analysis of soil bacterial (a) and fungi (b) communities in the rhizospheres of red and green amaranth plants.Score plots of bacterial (c) and fungi (d) communities in the rhizospheres of red and green amaranth plants.Pathologically, nodes indicate microbial taxa that are significantly enriched in the corresponding group and have a significant effect on the differences between groups (p, phylum; C, class; 0, order; f, family; and g, genus). (p < 0.05, LDA scores ≥ 3.5).
Moreover, 35 fungal branches were also significantly different according to LEfSe analysis (Figure 4c,d). At the phylum level, unclassified_k__Fungi and Chytridiomycota were significantly enriched as the dominant soil fungal phyla in the rhizosphere of red amaranth. Additionally, at the genus level, unclassified_k__Fungi, Fusarium, Cladophialophora, unclassified_c__Sordariomycetes and unclassified_p__Chytridiomycota were significantly enriched as the dominant soil fungal genera in the rhizosphere of red amaranth. In contrast, Aspergillues was significantly enriched as the dominant soil fungal genera in the rhizosphere of green amaranth.
3.5. Analysis of the Collinearity of Soil Bacterial and Fungi Communities in the Rhizosphere of Red and Green Amaranth Plants
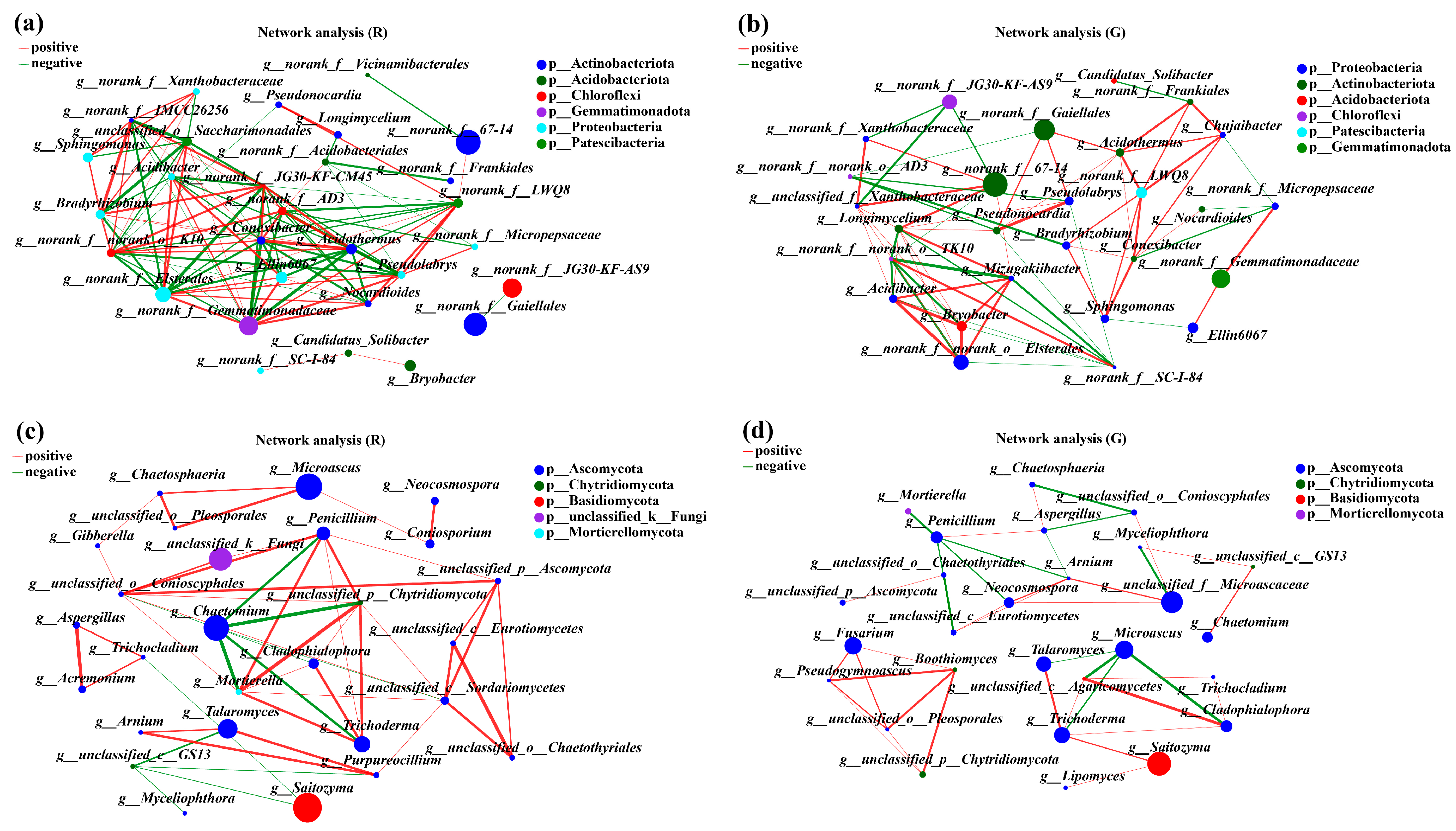
To describe the potential relationships of soil microbial communities in the rhizospheres of red and green amaranths, network analysis was constructed based on Spearman correlation coefficients. The topological characteristics of the soil bacteria in the rhizospheres of red and green amaranths were shown in Table 2. In comparison with green amaranth, the Average Degree (avgk) and Average Path Distance (GD) in rhizospheres of red amaranth were all increased. However, the Average Clustering Coefficient (avgCC) and Modularity were decreased. Meanwhile, a total of 108 edges were detected in rhizospheres of red amaranth; 53 edges were positively correlated and 55 edges were negatively correlated. Among them, norank_f__norank_o__norank_c__TK10, norank_f__Gemmatimonadaceae, norank_f__norank_o__Elsterales, Acidothermus, and Conexibacter showed the strongest correlations between each other. In green amaranth, a total of 90 edges were detected, and 45 edges showed positive correlations and 25 edges revealed negative correlations. Meanwhile, Longimycelium, norank_f__SC-I-84, Mizugakiibacter, norank_f__norank_o__Elsterales, and Acidibacter were the five bacteria most strongly correlated with other bacteria in rhizospheres of green amaranth (Figure 5a,b).

Table 2.
Topological characteristics of the red and green amaranth network analysis.
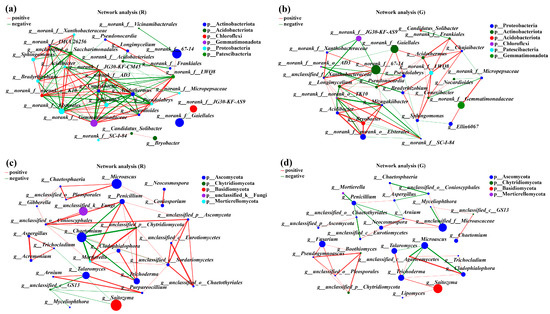
Figure 5.
Collinear network analysis of the soil bacterial (a,b) and fungi (c,d) communities in the rhizospheres of red and green amaranths. Different levels are indicated by different prefixes (p, phylum; g, genus). The size of the nodes in the graph indicates the size of the species abundance, and different colors indicate different species; the colors of the connecting lines indicate positive and negative correlations, with red indicating positive correlation and green indicating negative correlation (p < 0.05); the thickness of the lines indicates the size of the correlation coefficient; the coarser the line is, the greater the correlation between the species; and the greater the number of lines is, the closer the connection between the species and other species.
For soil fungi, in comparison with green amaranth, the Average Degree (avgk), Average Clustering Coefficient (avgCC), and Average Path Distance (GD) were all increased in rhizospheres of red amaranth. Also, a total of 52 edges were detected in rhizospheres of red amaranth, of which 40 were positively correlated, and 12 were negatively correlated. The five fungal groups most strongly associated with other soil fungi in rhizospheres of red amaranth were unclassified_c__Sordariomycetes, unclassified_o__Conioscyphales, Penicillium, Mortierella, and unclassified_p__Chytridiomycota. By contrast, 41 edges were detected in rhizospheres of green amaranth. Among them, 28 and 13 edges showed positive and negative correlations, respectively. Moreover, the five fungal groups most strongly associated with other soil fungi in rhizospheres of green amaranth were Trichoderma, Penicillium, Arnium, unclassified_f__Microascaceae, and unclassified_c__Eurotiomycetes (Figure 5c,d).
3.6. Functional Analysis of Soil Bacterial and Fungi Communities in the Rhizospheres of Red and Green Amaranth Plants
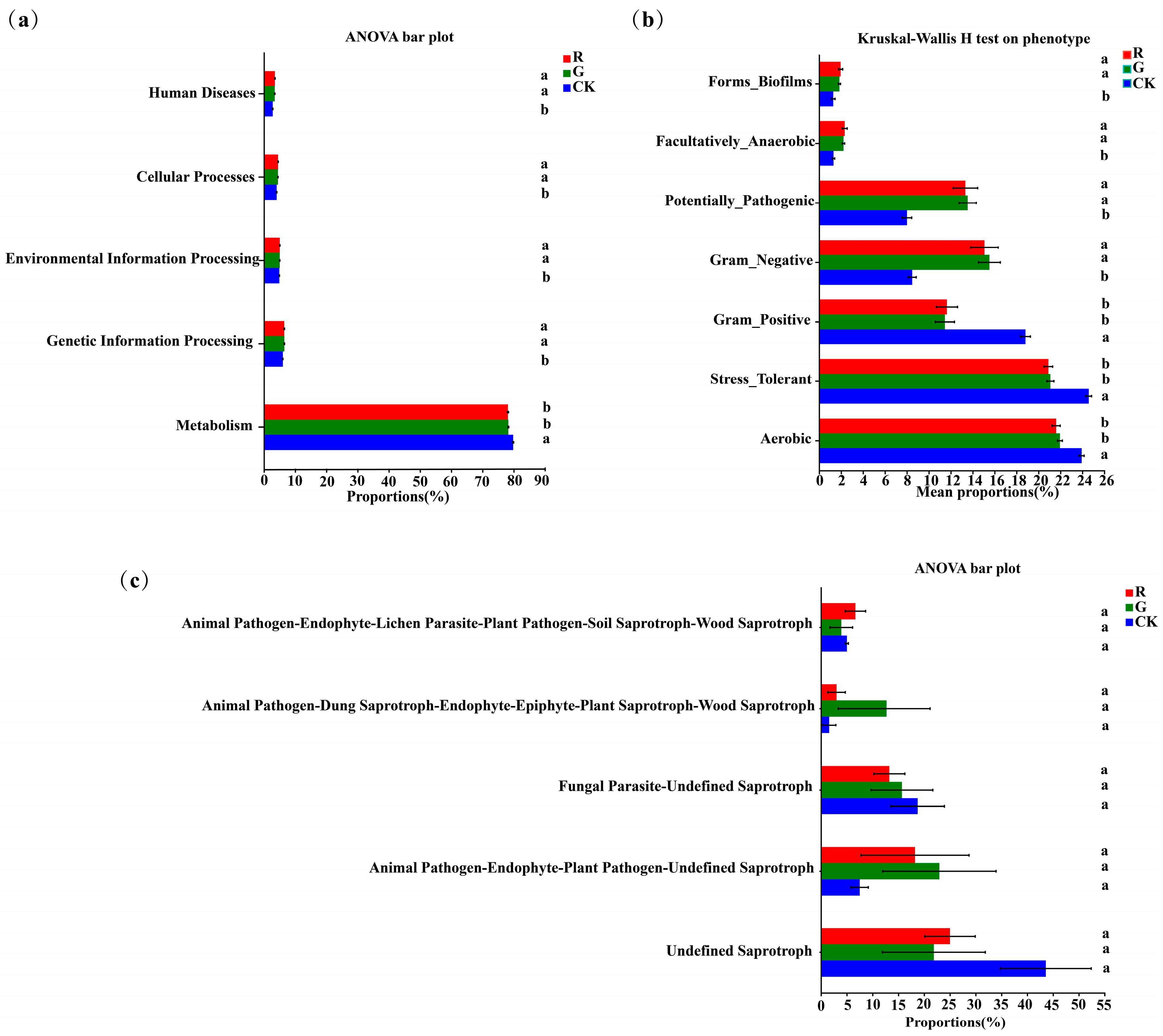
PICRUSt analysis was also carried out to predict bacterial functions in the rhizospheres of red and green amaranths. At the operational taxonomic unit (OTU) level, no significant changes were detected in functional genes between red and green amaranths (Figure 6a). Furthermore, significant differences of the bacterial community phenotypes were also not found between red and green amaranths. However, the Aerobic, gram_positive and stress_tolerant functions of soil bacteria were significantly higher in bulk soil than those in the rhizospheric soil of red and green amaranths. Additionally, gram_negative, forms_biofilms, facultatively_anaerobic, and potentially_pathogenic were significantly higher in the rhizospheric soils of red and green amaranths than those of bulk soil (Figure 6b).
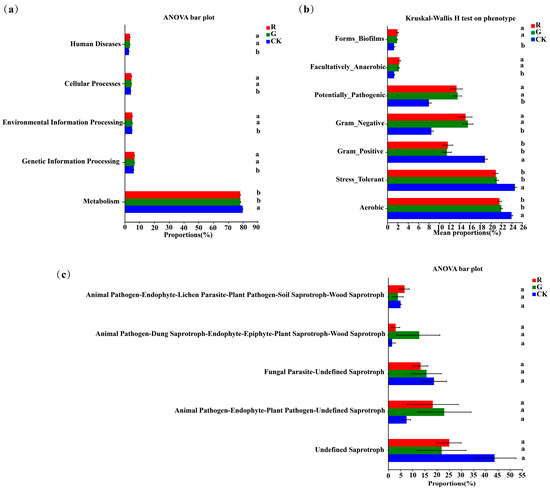
Figure 6.
Functional analysis of soil bacteria (a,b) and fungi (c) in the rhizospheres of red and green amaranths. Different lowercase letters indicate significant differences between soil microbes of different amaranth color varieties (p < 0.05).
FUNGuild (Fungi Functional Guild) fungal communities can be classified by a microecological guild (Figure 6c). The Undefined Saprotroph, Animal Pathogen-Endophyte—Plant Pathogen-Undefined Saprotroph, Fungal Parasite-Undefined Saprotroph, Animal Pathogen-Dung Saprotroph-Endophyte-Epiphyte-Plant Saprotroph-Wood Saprotroph, and Animal Pathogen-Endophyte-Lichen Parasite-Plant Pathogen-Soil Saprotroph-Wood Saprotroph were the dominant fungal functional groups in the rhizosphere of red and green amaranths and bulk soil. However, for soil fungal functions, there was no significant difference in the rhizosphere between red and green amaranths.
4. Discussion
Plant traits are tightly coupled with soil bacterial composition [44]. Previous research on how plant sex influences the microbiome assembly in dioecious plants has found that male and female plants have different root exudates, which recruit different rhizosphere bacteria. This may ultimately reflect differences in their root morphology and physiological characteristics [45,46]. For example, male plants typically secreted higher levels of specific compounds, such as certain phenolics or growth hormones, which could enrich specific bacterial communities [47]. In contrast, female plants tended to secrete higher levels of organic acids or amino acids, which might be more suitable for the growth and reproduction of certain growth-promoting bacteria [48,49]. Additionally, soil microorganisms affected the plant traits through the production of phytohormones, which could enhance plant antioxidant capacity, and could improve plant uptake of certain types of micronutrients [50,51,52]. For instance, phosphorus solubilizing bacteria (e.g., Bacillus megateriumand Pseudomonas fluorescens) could improve phosphorus uptake by plants, which could indirectly affect carotenoid synthesis and change the leaves’ color [53]. Meanwhile, rhizobacteria (e.g., Trichoderma and Pseudomonas) could promote chlorophyll synthesis and improve the leaves’ green color by increasing nitrogen levels in legumes [54]. Moreover, microorganisms could enhance the antioxidant capacity of plants and reduce the damage of leaf pigmentation under stresses (e.g., drought, salinity, high temperature) [55]. In our study, although significant differences of soil microbial diversity and richness in rhizospheres could not be detected, the soil bacterial and fungal compositions significant altered in the rhizospheres of red and green amaranths. This indicates that different color amaranths exactly recruited different soil bacteria and fungi in rhizospheres.
A higher degree and lower modularity of the soil microbial symbiotic network tended to be a whole function interacting with microorganisms complexly [56,57]. Meanwhile, the complexity of the network is often positively correlated with community stability [58]. Our network analysis also indicated that a higher degree and clustering coefficient could be found in the rhizospheres of red amaranth compared to those of green amaranth. It also indicated that higher stability and more functions of soil microbial community rhizospheres of red amaranth could be detected compared to those of green amaranth.
At the phylum level, LEfSe analysis found that Proteobacteria, Patescibacteria, Gemmatimonadota, and Bacteroidota were significantly enriched in red amaranth rhizosphere soil (p < 0.05); Myxococcota was significantly enriched in green amaranth soil. Proteobacteria are oligotrophic denitrifying bacteria that utilize a variety of carbon sources, promote plant growth, and are found in a variety of ecosystems and biological microcosms [59]. Chen et al. [60] have shown a significant negative correlation between Proteobacteria and abscisic acid. In addition, Ascomycota and Basidiomycota were the dominant fungi phyla in the rhizospheres of red and green amaranth, and the proportion of Basidiomycota in the rhizosphere of red amaranth increased. Interestingly, Timoneda et al. [24] found that Basidiomycota can produce betalains.
At the genus level, Streptomyces, Pseudonocardia, Pseudolabrys, Acidibacter, norank_ f_ Micropepsaceae, and Bradyrhizobium were the unique dominant soil bacterial genera in the rhizosphere of red amaranth. In contrast, Conexibacter, norank_f_norank_o_norank_c_TK10 and norank_f_ norank_o_ norank_ c_AD3 were the unique dominant soil bacterial genera in the rhizosphere of green amaranth. The physiological and molecular genetic analysis of ethylene biodegradation by Nocardia species conducted by previous researchers has confirmed that Nocardia can produce ethylene [61]. Cytokinins could also promote the accumulation of betatin pigments in plants under either dark or light conditions [62]. Bradyrhizobium can not only produce indium-3-acetic acid (IAA) but also induce an increase in the intracellular Ca2+ concentration in plant cells [63,64]. It is reported that auxin (IAA) can increase the level of a plant’s free tyrosine and strongly promote the accumulation of betatin [65]. Wang et al. [66] reported that Ca2+ can be directly or indirectly involved in betalain biosynthesis. In addition, in comparison with green amaranth, we also found a significant increase in the proportion of streptomyces in red amaranth (P < 0.05). Streptomyces gene sequencing identified a large number of cytochrome P450 genes, which are involved in the biosynthesis of secondary metabolites [20]. Previous research on the catalytic products of cytochrome P450 enzymes in Streptomyces has found that cytochrome P450 genes are key genes in the betaine metabolic pathway and play an important role in the biosynthesis of betalains [67,68].
Meanwhile, unclassified_k__Fungi, Fusarium, Cladophialophora, unclassified_c__Sordariomycetes, and unclassified_p__Chytridiomycota were significantly enriched as the dominant soil fungal genera in the rhizosphere of red amaranth. In contrast, Aspergillues was significantly enriched as the dominant soil fungal genera in the rhizosphere of green amaranth. Warhade et al. [69] reported a positive effect of Fusarium on the biosynthesis of betalain pigments. Abdul et al. [70] found that gibberellins can be produced by Aspergillues and Penicillium. However, gibberellic acid (GA3) inhibits the synthesis of betalain [71]. In this experiment, we found that Penicillium decreased in the rhizosphere of red amaranth compared to green amaranth. In addition, the abundance of Trichoderma in the rhizosphere soil of red amaranth was about twice as high as that of green amaranth. Segarra et al. [72] reported that plant defense-protective hormones, such as jasmonic acid, are widely present in Trichoderma. Previous research on the synthesis of betalains in Portulaca oleracea stimulated by exogenous methyl jasmonate and other inducers has shown that jasmonic acid and methyl jasmonate can effectively promote the accumulation of betalains in the plant [73]. These results suggest that Fusarium and Trichoderma are enriched in the rhizosphere of red amaranth and are most likely involved in the synthesis and accumulation of pigments in red amaranth. In contrast, Aspergillues and Penicillium were enriched in the rhizosphere soil of green amaranth, probably inhibiting betatin synthesis.
All of the above results suggested that different color amaranths exactly recruited different soil microbes. Among them, soil microorganisms, in association with betain synthesis, were significantly enriched in the rhizospheres of red amaranth. By contrast, soil microorganisms, in relation to betain synthesis inhibition, were also significantly enriched in the rhizospheres of green amaranth.
5. Conclusions
Soil microorganisms in the rhizosphere of red amaranth and green amaranth were significant. Among them, soil microorganisms promoting betatin synthesis were enriched in the rhizosphere of red amaranth. For example, Nocardioides, Bradyrhizobium, Streptomyces, Fusarium, and Trichoderma microorganisms closely related to betatin synthesis or accumulation were enriched in the rhizosphere of red amaranth, which may promote color formation. On the contrary, soil microorganisms that inhibit betatin synthesis are enriched in the rhizosphere of green amaranth, such as Aspergillues and Penicillium inhibiting the synthesis of betatain enrichment in the rhizosphere of green amaranth. The above results suggest that different genotypes of amaranth species, especially red amaranth, need to recruit some unique rhizosphere bacteria and fungi to promote their own color formation. However, the mechanism of association between microorganisms and the host is complex. The function of microorganisms involved in amaranth color formation is still unknown, and further studies are needed to elucidate this mechanism of action.
Author Contributions
X.-R.L.: conceptualization, methodology, software, formal analysis, data curation, writing—original draft, writing—review & editing, and visualization. D.Y.: Software, Data curation. Y.-F.W.: Software, Formal analysis. D.-C.D.: Software, Formal analysis, Data curation. H.-P.O.: conceptualization, resources, supervision, funding acquisition. S.-D.Y.: Conceptualization, Methodology, Resources, Writing—review & editing, Supervision, Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Agricultural Resources and Environmental Research Institute, Guangxi Academy of Agricultural Sciences/Guangxi Key Laboratory of Arable Land Conservation Fund (23-026-12-23KF03).
Data Availability Statement
The original reads were stored in the NCBI Sequence Read Archive (SRA) database (accession number: SUB14314150).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bang, J.-H.; Lee, K.J.; Jeong, W.T.; Han, S.; Jo, I.-H.; Choi, S.H.; Cho, H.; Hyun, T.K.; Sung, J.; Lee, J.; et al. Antioxidant Activity and Phytochemical Content of Nine Amaranthus Species. Agronomy 2021, 11, 1032. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Nutraceuticals, antioxidant pigments, and phytochemicals in the leaves of Amaranthus spinosus and Amaranthus viridis weedy species. Sci. Rep. 2019, 9, 20413. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Mahato, A.K.; Maurya, A.; Rajkumar, S.; Singh, A.K.; Bhardwaj, R.; Kaushik, S.K.; Kumar, S.; Gupta, V.; Singh, K.; et al. Amaranth Genomic Resource Database: An integrated database resource of Amaranth genes and genomics. Front. Plant Sci. 2023, 14, 1203855. [Google Scholar] [CrossRef] [PubMed]
- Zuo, R.; Kong, X.; Wang, Y.; He, Y.; Deng, S.; Zhuang, X.; Qiu, D. Isolation and characterization of natural nano starch from amaranth starch. Int. J. Biol. Macromol. 2024, 260, 129525. [Google Scholar] [CrossRef] [PubMed]
- Naik, R.R.; Ye, Q.Y.; Wang, Y.; Selomulya, C. Assessing the effect of Maillard reaction products on the functionality and antioxidant properties of Amaranth-red seaweed blends. Food Res. Int. 2023, 175, 113759. [Google Scholar] [CrossRef] [PubMed]
- Managa, G.M.; Nemadodzi, L.E. Comparison of Agronomic Parameters and Nutritional Composition on Red and Green Amaranth Species Grown in Open Field Versus Greenhouse Environment. Agriculture 2023, 13, 685. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Color attributes, betacyanin, and carotenoid profiles, bioactive components, and radical quenching capacity in selected Amaranthus gangeticus leafy vegetables. Sci. Rep. 2021, 11, 11559. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Xu, H.; Xiao, Q.; Li, X.; Shao, Q. Combined analysis of metabolome and transcriptome provides insights into metabolisms of chlorophylls, carotenoids, and flavonoids in the yellowing leaves of ‘HAES344’ macadamia. Sci. Hortic. 2023, 308, 111600. [Google Scholar] [CrossRef]
- Rocha, F.; Marques, C.S.; de Sousa, L.S.; Minim, V.P.R.; Pires, A.C.D.S.; Minim, L.A.; Stringheta, P.C.; Jones, O.G.; Vidigal, M.C.T.R. Betalains nanodispersions: Effects on betalains stability and on rheological properties of Greek yogurt. Food Res. Int. 2022, 159, 111583. [Google Scholar] [CrossRef]
- Madadi, E.; Mazloum-Ravasan, S.; Yu, J.S.; Ha, J.W.; Hamishehkar, H.; Kim, K.H. Therapeutic Application of Betalains: A Review. Plants 2020, 9, 1219. [Google Scholar] [CrossRef]
- Li, H.; Deng, Z.; Liu, R.; Zhu, H.; Draves, J.; Marcone, M.; Sun, Y.; Tsao, R. Characterization of phenolics, betacyanins and antioxidant activities of the seed, leaf, sprout, flower and stalk extracts of three Amaranthus species. J. Food Compos. Anal. 2015, 37, 75–81. [Google Scholar] [CrossRef]
- Jahan, F.; Bhuiyan, M.N.H.; Islam, M.J.; Ahmed, S.; Hasan, M.S. Amaranthus tricolor (red amaranth), an indigenous source of nutrients, minerals, amino acids, phytochemicals, and assessment of its antibacterial activity. J. Agric. Food Res. 2022, 10, 100419. [Google Scholar] [CrossRef]
- Khandaker, L.; Akond, A.M.; Ali, M.B.; Oba, S. Biomass yield and accumulations of bioactive compounds in red amaranth (Amaranthus tricolor L.) grown under different colored shade polyethylene in spring season. Sci. Hortic. 2010, 123, 289–294. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, Y.; Long, T.; Wang, S.; Yang, J. Regulation Mechanism of Plant Pigments Biosynthesis: Anthocyanins, Carotenoids, and Betalains. Metabolites 2022, 12, 871. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Rubio, M.A.; Walker-Hale, N.; Guo, R.; Sheehan, H.; Timoneda, A.; Gandia-Herrero, F.; Brockington, S.F. Are seven amino acid substitutions sufficient to explain the evolution of high l-DOPA 4,5-dioxygenase activity leading to betalain pigmentation? Revisiting the gain-of-function mutants of Bean. New Phytol. 2023, 239, 2265–2276. [Google Scholar] [CrossRef] [PubMed]
- Yasmeen, T.; Arif, M.S.; Tariq, M.; Akhtar, S.; Syrish, A.; Haidar, W.; Rizwan, M.; Hussain, M.I.; Ahmad, A.; Ali, S. Biofilm producing plant growth promoting bacteria in combination with glycine betaine uplift drought stress tolerance of maize plant. Front. Plant Sci. 2024, 15, 1327552. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, P.T.; Meramo, S.; Ninivaggi, L.; Pasutto, E.; Babaei, M.; Avila-Neto, P.M.; Pastor, M.C.; Sabri, P.; Rago, D.; Parekh, T.U.; et al. Beet red food colourant can be produced more sustainably with engineered Yarrowia lipolytica. Nat Microbiol. 2023, 8, 2290–2303. [Google Scholar] [CrossRef] [PubMed]
- Winkler, T.S.; Vollmer, S.K.; Dyballa-Rukes, N.; Metzger, S.; Stetter, M.G. Isoform-resolved genome annotation enables mapping of tissue-specific betalain regulation in amaranth. New Phytol. 2024, 243, 1082–1100. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; He, P.; Zhao, Z.; Liu, J.; Liu, H.; Ma, S.; Shen, Y.; Li, B. Effect of temperature on betacyanins synthesis and the transcriptome of Suaeda salsa. Front. Plant Sci. 2023, 14, 1203089. [Google Scholar] [CrossRef]
- Liu, S.; Zheng, X.; Pan, J.; Peng, L.; Cheng, C.; Wang, X.; Zhao, C.; Zhang, Z.; Lin, Y.; XuHan, X.; et al. RNA-sequencing analysis reveals betalains metabolism in the leaf of Amaranthus tricolor L. PLoS ONE 2019, 14, e0216001. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, S.; Cheng, C.; Guo, R.; Chen, Y.; Xie, L.; Mao, Y.; Lin, Y.; Zhang, Z.; Lai, Z. Cloning and expression analysis of betalain biosynthesis genes in Amaranthus tricolor. Biotechnol. Lett. 2016, 38, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Tossi, V.E.; Tosar, L.M.; Pitta-Álvarez, S.I.; Causin, H.F. Casting light on the pathway to betalain biosynthesis: A review. Environ. Exp. Bot. 2021, 186, 104464. [Google Scholar] [CrossRef]
- Yang, D.; Lin, X.; Zhou, X.; Li, Z.; Kurokawa, H.; Matsui, H.; Fujita, T.; Yang, S.-D. Differences in endophytic bacterial and fungal compositions in roots between red and green Amaranthus sp. S. Afr. J. Bot. 2023, 163, 275–284. [Google Scholar] [CrossRef]
- Timoneda, A.; Feng, T.; Sheehan, H.; Walker-Hale, N.; Pucker, B.; Lopez-Nieves, S.; Guo, R.; Brockington, S. The evolution of betalain biosynthesis in Caryophyllales. New Phytol. 2019, 224, 71–85. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, J.; Liu, Y.; Shi, P.; Wei, G. Co-occurrence patterns of soybean rhizosphere microbiome at a continental scale. Soil Biol. Biochem. 2018, 118, 178–186. [Google Scholar] [CrossRef]
- Compant, S.; Samad, A.; Faist, H.; Sessitsch, A. A review on the plant microbiome: Ecology, functions, and emerging trends in microbial application. J. Adv. Res. 2019, 19, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.; Kruijt, M.; de Bruijn, I.; Dekkers, E.; Van Der Voort, M.; Schneider, J.H.; Piceno, Y.M.; DeSantis, T.Z.; Andersen, G.L.; Bakker, P.A.; et al. Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science 2011, 332, 1097–1110. [Google Scholar] [CrossRef]
- Palberg, D.; Kisiała, A.; Jorge, G.L.; Emery, R.J.N. A survey of Methylobacterium species and strains reveals widespread production and varying profiles of cytokinin phytohormones. BMC Microbiol. 2022, 22, 49. [Google Scholar] [CrossRef]
- Abinandan, S.; Subashchandrabose, S.R.; Venkateswarlu, K.; Megharaj, M. Soil microalgae and cyanobacteria: The biotechnological potential in the maintenance of soil fertility and health. Crit. Rev. Biotechnol. 2019, 39, 981–998. [Google Scholar] [CrossRef]
- Fuke, P.; Kumar, M.; Sawarkar, A.D.; Pandey, A.; Singh, L. Role of microbial diversity to influence the growth and environmental remediation capacity of bamboo: A review. Ind. Crops Prod. 2021, 167, 113567. [Google Scholar] [CrossRef]
- Ali, S.; Moon, Y.-S.; Hamayun, M.; Khan, M.A.; Bibi, K.; Lee, I.-J. Pragmatic role of microbial plant biostimulants in abiotic stress relief in crop plants. J. Plant Interact. 2022, 17, 705–718. [Google Scholar] [CrossRef]
- Che, J.; Wu, Y.; Yang, H.; Chang, Y.; Wu, W.; Lyu, L.; Wang, X.; Cao, F.; Li, W. Metabolites of blueberry roots at different developmental stages strongly shape microbial community structure and intra-kingdom interactions at the root-soil interface. Sci. Total Environ. 2024, 947, 174333. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Bonkowski, M.; Shen, Y.; Griffiths, B.S.; Jiang, Y.; Wang, X.; Sun, B. Root ethylene mediates rhizosphere microbial community reconstruction when chemically detecting cyanide produced by neighbouring plants. Microbiome 2020, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Dhar, N.; Gopalan, N.S.R.; Nikhil, P.T.; Mohapatra, S. Role of Phytohormones in Plant-Microbial Interaction. In Auxins, Cytokinins and Gibberellins Signaling in Plants. Signaling and Communication in Plants; Aftab, T., Ed.; Springer: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Mathur, P.; Roy, S. Insights into the plant responses to drought and decoding the potential of root associated microbiome for inducing drought tolerance. Physiol. Plant 2021, 172, 1016–1029. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jousset, A.; de Boer, W.; Carrión, V.J.; Zhang, T.; Wang, X.; E Kuramae, E. Legacy of land use history determines reprogramming of plant physiology by soil microbiome. ISME J. 2019, 13, 738–751. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Elkabetz, D.; Leibman-Markus, M.; Jami, E.; Bar, M. Cytokinin-microbiome interactions regulate developmental functions. Environ. Microbiome 2022, 17, 2. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Pei, J.; Li, H.; Zhu, X.; Zhang, Y.; Wang, Y.; Li, W.; Wang, Z.; Liu, K.; Du, B.; et al. Mechanisms on salt tolerant of Paenibacillus polymyxa SC2 and its growth-promoting effects on maize seedlings under saline conditions. Microbiol. Res. 2024, 282, 127639. [Google Scholar] [CrossRef]
- Lin, X.; Yang, D.; Zhu, Y.; Qin, Y.; Liang, T.; Yang, S.; Tan, H. Changes in root metabolites and soil microbial community structures in rhizosphere of sugarcanes under different propagation methods. Microb. Biotechnol. 2024, 17, e14372. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Guo, B.; Zhang, H.; Zhang, H.; Liu, Y.; Liu, Y.; Chen, J.; Chen, J.; Li, J.; Li, J. Drought-resistant trait of different crop genotypes determines assembly patterns of soil and phyllosphere microbial communities. Microbiol. Spectr. 2023, 11, e00068-23. [Google Scholar] [CrossRef]
- Henry, L.P.; Bruijning, M.; Forsberg, S.K.G.; Ayroles, J.F. The microbiome extends host evolutionary potential. Nat. Commun. 2021, 12, 5141. [Google Scholar] [CrossRef]
- Yang, S.; Xiao, J.; Liang, T.; He, W.; Tan, H. Response of soil biological properties and bacterial diversity to different levels of nitrogen application in sugarcane fields. AMB Expr. 2021, 11, 172. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Liang, T.; Yang, S.; Tan, H. Can Sugarcane Yield and Health Be Altered with Fully Mechanized Management? Agronomy 2023, 13, 153. [Google Scholar] [CrossRef]
- Colin, Y.; Goberna, M.; Verdú, M.; Navarro-Cano, J.A. Successional trajectories of soil bacterial communities in mine tailings: The role of plant functional traits. J. Environ. Manag. 2019, 241, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, J.H.; Zhao, W.T.; Korpelainen, H.; Li, C.Y. Females face more positive plant-soil feedback and intersexual competition under adequate nitrogen conditions compared to males in Populus cathayana. Sci. Total Environ. 2023, 874, 162479. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.X.; Zhu, Y.; Korpelainen, H.; Niinemets, Ü.; Li, C. How does plant sex alter microbiota assembly in dioecious plants? Trends Microbiol. 2023, 31, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Lan, Q.; Liu, G.; Song, H.; Liu, G.; Xu, X. Plant sex alters rhizosphere microorganisms assembly of Salix gordejevii across diverse sandy habitats. Plant Soil 2024. [Google Scholar] [CrossRef]
- Guo, Q.; Liu, L.; Liu, J.; Korpelainen, H.; Li, C. Plant sex affects plant-microbiome assemblies of dioecious Populus cathayana trees under different soil nitrogen conditions. Microbiome 2022, 10, 191. [Google Scholar] [CrossRef] [PubMed]
- Doniger, T.; Kerfahi, D.; Wachtel, C.; Marais, E.; Maggs-Kölling, G.; Sherman, C.; Adams, J.M.; Steinberger, Y. Plant Gender Affects Soil Fungal Microbiota Associated with Welwitschia mirabilis, an Unusual Desert Gymnosperm. Microb. Ecol. 2023, 86, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Wissuwa, M.; Gonzalez, D.; Watts-Williams, S.J. The contribution of plant traits and soil microbes to phosphorus uptake from low-phosphorus soil in upland rice varieties. Plant Soil 2020, 448, 523–537. [Google Scholar] [CrossRef]
- Chaudhary, S.; Sindhu, S.S.; Dhanker, R.; Kumari, A. Microbes-mediated sulphur cycling in soil: Impact on soil fertility, crop production and environmental sustainability. Microbiol. Res. 2023, 271, 127340. [Google Scholar] [CrossRef]
- Zhao, J.; Yu, X.; Zhang, C.; Hou, L.; Wu, N.; Zhang, W.; Wang, Y.; Yao, B.; Delaplace, P.; Tian, J. Harnessing microbial interactions with rice: Strategies for abiotic stress alleviation in the face of environmental challenges and climate change. Sci. Total Environ. 2024, 912, 168847. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, P.; Sharma, P.; Chouhan, R.; Mir, B.A.; Gandhi, S.G.; Bhardwaj, R.; Alam, P.; Ahmad, P. Interactive effect of 24-epibrassinolide and plant growth promoting rhizobacteria inoculation restores photosynthetic attributes in Brassica juncea L. under chlorpyrifos toxicity. Environ. Pollut. 2023, 320, 120760. [Google Scholar] [CrossRef] [PubMed]
- Kshetri, L.; Kotoky, R.; Debnath, S.; Maheshwari, D.K.; Pandey, P. Shift in the soil rhizobacterial community for enhanced solubilization and bioavailability of phosphorus in the rhizosphere of Allium hookeri Thwaites, through bioaugmentation of phosphate-solubilizing bacteria. 3 Biotech. 2024, 14, 185. [Google Scholar] [CrossRef] [PubMed]
- Ben Laouane, R.; Meddich, A.; Bechtaoui, N.; Oufdou, K.; Wahbi, S. Effects of Arbuscular Mycorrhizal Fungi and Rhizobia Symbiosis on the Tolerance of Medicago Sativa to Salt Stress. Gesunde Pflanz 2019, 71, 135–146. [Google Scholar] [CrossRef]
- Li, J.; Li, C.; Kou, Y.; Yao, M.; He, Z.; Li, X. Distinct mechanisms shape soil bacterial and fungal co-occurrence networks in a mountain ecosystem. FEMS Microbiol. Ecol. 2020, 96, fiaa030. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chai, Y.; Xie, H.; Zhang, L.; Zhang, Z.; Yang, X.; Hao, S.; Gai, J.; Chen, Y. Responses of soil microbial diversity, network complexity and multifunctionality to three land-use changes. Sci. Total Environ. 2023, 859 Pt 1, 160255. [Google Scholar] [CrossRef] [PubMed]
- Gui, H.; Breed, M.; Li, Y.; Xu, Q.; Yang, J.; Wanasinghe, D.N.; Li, Y.; Xu, J.; Mortimer, P. Continental-scale insights into the soil microbial co-occurrence networks of Australia and their environmental drivers. Soil Biol. Biochem. 2023, 186, 109177. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, G.B.; Xue, S.; Wang, G.L. Soil bacterial community dynamics reflect changes in plant community and soil properties during the secondary succession of abandoned farmland in the Loess Plateau. Soil Biol. Biochem. 2016, 97, 40–49. [Google Scholar] [CrossRef]
- Chen, S.; Qin, R.; Yang, D.; Liu, W.; Yang, S.A. Comparison of Rhizospheric and Endophytic Bacteria in Early and Late-Maturing Pumpkin Varieties. Microorganisms 2022, 10, 1667. [Google Scholar] [CrossRef]
- Mattes, T.E.; Coleman, N.V.; Spain, J.C.; Gossett, J.M. Physiological and molecular genetic analyses of vinyl chloride and ethene biodegradation in Nocardioides sp. strain JS614. Arch. Microbiol. 2005, 183, 95–106. [Google Scholar] [CrossRef]
- Khan, M.I.; Giridhar, P. Plant betalains: Chemistry and biochemistry. Phytochemistry 2015, 117, 267–295. [Google Scholar] [CrossRef] [PubMed]
- Torres, D.; Mongiardini, E.; Donadío, F.; Donoso, R.; Recabarren-Gajardo, G.; Gualpa, J.; Spaepen, S.; Defez, R.; Lopez, G.; Bianco, C.; et al. Molecular and physiological analysis of indole-3-acetic acid degradation in Bradyrhizobium japonicum E109. Res. Microbiol. 2021, 172, 103814. [Google Scholar] [CrossRef] [PubMed]
- Torres, D.; Benavidez, I.; Donadio, F.; Mongiardini, E.; Rosas, S.; Spaepen, S.; Vanderleyden, J.; Pěnčík, A.; Novák, O.; Strnad, M.; et al. New insights into auxin metabolism in Bradyrhizobium japonicum. Res. Microbiol. 2018, 169, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, T.; Kobayashi, N.; Kouchi, H.; Minamisawa, K.; Kaku, H.; Tsuchiya, K. A lipochito-oligosaccharide, Nod factor, induces transient calcium influx in soybean suspension-cultured cells. Plant J. 2000, 22, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Q.; Wang, B.-S. Ca2+-Calmodulin is Involved in Betacyanin Accumulation Induced by Dark in C3 Halophyte Suaeda salsa. J. Integr. Plant Biol. 2007, 49, 1378–1385. [Google Scholar] [CrossRef]
- Yu, H.L.; Li, S.M. Two Cytochrome P450 Enzymes from Streptomyces sp. NRRL S-1868 Catalyze Distinct Dimerization of Tryptophan-Containing Cyclodipeptides. Org. Lett. 2019, 21, 7094–7098. [Google Scholar] [CrossRef] [PubMed]
- Boukaew, S.; Cheirsilp, B.; Prasertsan, P.; Yossan, S. Anti-fungal effect of volatile organic compounds produced by Streptomyces salmonis PSRDC -09 against anthracnose pathogen Colle totric hum gloeos porioides PSU-03 in posthar-vest chili fruit. J. Appl. Microbiol. 2021, 131, 1452–1463. [Google Scholar] [CrossRef] [PubMed]
- Warhade, M.I.; Badere, R.S. Fusarium oxysporum cell elicitor enhances betalain content in the cell suspension culture of Celosia cristata. Physiol. Mol. Biol. Plants 2018, 24, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.L.; Hamayun, M.; Kim, Y.-H.; Kang, S.-M.; Lee, J.-H.; Lee, I.-J. Gibberellins producing endophytic Aspergillus fumigatus sp. LH02 influenced endogenous phytohormonal levels, isoflavonoids production and plant growth in salinity stress. Process Biochem. 2010, 46, 440–447. [Google Scholar] [CrossRef]
- Stobart, A.K.; Kinsman, L.T. The hormonal control of betacyanin synthesis in Amaranthus caudatus. Phytochemistry 1977, 16, 1139–1142. [Google Scholar] [CrossRef]
- Segarra, G.; Casanova, E.; Bellido, D.; Odena, M.A.; Oliveira, E.; Trillas, I. Proteome, salicylic acid and jasmonic acid changes in cucumber plants inoculated with Trichoderma asperellum strain T34. Proteomics 2007, 7, 3943–3952. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, M.N.H.; Adachi, T. Stimulation of betacyanin synthesis through exogenous methyl jasmonate and other elicitors in suspension-cultured cells ofPortulaca. J. Plant Physiol. 2003, 160, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).