Anti-Skin Aging and Cytotoxic Effects of Methanol-Extracted Solanum betaceum Red Fruit Seed Extract on Ca9-22 Gingival Carcinoma Cells
Abstract
:1. Introduction
2. Results
2.1. Total Phenolic Content (TPC)
2.2. Total Flavonoid Content (TFC)
2.3. Anti-Skin Aging Potential
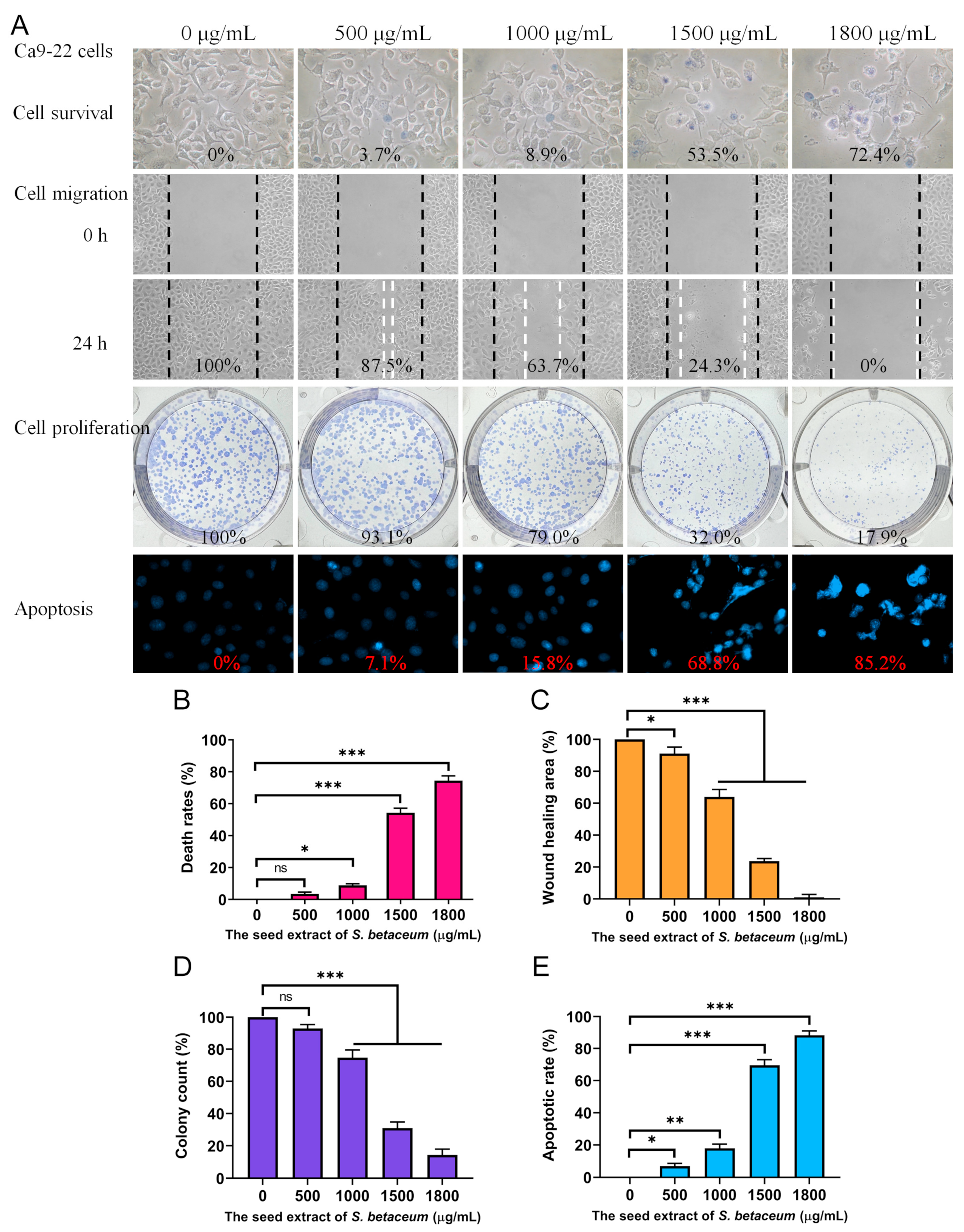
2.4. Cytotoxic Effects of Methanol-Extracted Red Fruit Seed Extract
2.5. Methanol-Extracted Red Fruit Seed Extract Inhibited the Migration of Ca9-22 Cells
2.6. Methanol-Extracted Red Fruit Seed Extract Inhibited the Proliferation of Ca9-22 Cells
2.7. Methanol-Extracted Red Fruit Seed Extract Induced Apoptosis in Ca9-22 Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Plant Materials and Extract Preparations
4.3. Determination of TPC
4.4. Determination of TFC
4.5. Tyrosinase Inhibition
4.6. Hyaluronidase Inhibition
4.7. Elastase Inhibition
4.8. Trypan Blue Cytotoxicity Assay
4.9. Chromatin Condensation Assay
4.10. Clonogenic Formation Assay
4.11. Wound-Healing Assay
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Jakobušić Brala, C.; Karković Marković, A.; Kugić, A.; Torić, J.; Barbarić, M. Combination Chemotherapy with Selected Polyphenols in Preclinical and Clinical Studies-An Update Overview. Molecules 2023, 28, 3746. [Google Scholar] [CrossRef]
- Toma, L.; Deleanu, M.; Sanda, G.M.; Barbălată, T.; Niculescu, L.; Sima, A.V.; Stancu, C.S. Bioactive Compounds Formulated in Phytosomes Administered as Complementary Therapy for Metabolic Disorders. Int. J. Mol. Sci. 2024, 25, 4162. [Google Scholar] [CrossRef] [PubMed]
- Konstantinou, E.K.; Gioxari, A.; Dimitriou, M.; Panoutsopoulos, G.I.; Panagiotopoulos, A.A. Molecular Pathways of Genistein Activity in Breast Cancer Cells. Int. J. Mol. Sci. 2024, 25, 5556. [Google Scholar] [CrossRef] [PubMed]
- Joma, N.; Bielawski, P.B.; Saini, A.; Kakkar, A.; Maysinger, D. Nanocarriers for natural polyphenol senotherapeutics. Aging Cell 2024, 23, e14178. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, F.K.F.; Júnior, A.; Filho, A.L.N.; Fonseca, C.J.N.; Isidorio, D.K.M.; Araújo, F.A.; Oliveira, P.H.A.; Veiga Júnior, V.F.D. Graphene and Natural Products: A Review of Antioxidant Properties in Graphene Oxide Reduction. Int. J. Mol. Sci. 2024, 25, 5182. [Google Scholar] [CrossRef] [PubMed]
- Cecerska-Heryć, E.; Wiśniewska, Z.; Serwin, N.; Polikowska, A.; Goszka, M.; Engwert, W.; Michałów, J.; Pękała, M.; Budkowska, M.; Michalczyk, A.; et al. Can Compounds of Natural Origin Be Important in Chemoprevention? Anticancer Properties of Quercetin, Resveratrol, and Curcumin-A Comprehensive Review. Int. J. Mol. Sci. 2024, 25, 4505. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, G. Molecular mechanisms of skin ageing. Mech. Ageing Dev. 2002, 123, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Dańczak-Pazdrowska, A.; Gornowicz-Porowska, J.; Polańska, A.; Krajka-Kuźniak, V.; Stawny, M.; Gostyńska, A.; Rubiś, B.; Nourredine, S.; Ashiqueali, S.; Schneider, A.; et al. Cellular senescence in skin-related research: Targeted signaling pathways and naturally occurring therapeutic agents. Aging Cell 2023, 22, e13845. [Google Scholar] [CrossRef]
- Rathore, G.; Das, K.; Landau, M.; Verner, I.; Kassir, M.; Galadari, H.I.; Gold, M.H.; Babaei, M.; Goldust, M. Clinical Assessment, Diagnosis, and Management of Infraorbital Wrinkles and Pigmentation. Dermatol. Clin. 2024, 42, 79–88. [Google Scholar] [CrossRef]
- Goh, C.F. Diversity of Asian skin: A review on skin biophysical properties. Exp. Dermatol. 2024, 33, e14959. [Google Scholar] [CrossRef] [PubMed]
- Michalak, M. Plant Extracts as Skin Care and Therapeutic Agents. Int. J. Mol. Sci. 2023, 24, 15444. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Duan, E. Fighting against Skin Aging: The Way from Bench to Bedside. Cell Transplant. 2018, 27, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Orqueda, M.E.; Zampini, I.C.; Bravo, K.; Osorio, E.; Isla, M.I. Potential use of native fruits waste from Argentina as nonconventional sources of cosmetic ingredients. J. Cosmet. Dermatol. 2022, 21, 5058–5065. [Google Scholar] [CrossRef] [PubMed]
- Isla, M.I.; Orqueda, M.E.; Moreno, M.A.; Torres, S.; Zampini, I.C. Solanum betaceum Fruits Waste: A Valuable Source of Bioactive Compounds to Be Used in Foods and Non-Foods Applications. Foods 2022, 11, 3363. [Google Scholar] [CrossRef] [PubMed]
- Diep, T.T.; Rush, E.C.; Yoo, M.J.Y. Tamarillo (Solanum betaceum Cav.): A Review of Physicochemical and Bioactive Properties and Potential Applications. Food Rev. Int. 2022, 38, 1343–1367. [Google Scholar] [CrossRef]
- Diep, T.; Pook, C.; Yoo, M. Phenolic and Anthocyanin Compounds and Antioxidant Activity of Tamarillo (Solanum betaceum Cav.). Antioxidants 2020, 9, 169. [Google Scholar] [CrossRef] [PubMed]
- Kou, M.C.; Yen, J.H.; Hong, J.T.; Wang, C.L.; Lin, C.W.; Wu, M.J. Cyphomandra betacea Sendt. phenolics protect LDL from oxidation and PC12 cells from oxidative stress. LWT—Food Sci. Technol. 2009, 42, 458–463. [Google Scholar] [CrossRef]
- Orqueda, M.E.; Rivas, M.; Zampini, I.C.; Alberto, M.R.; Torres, S.; Cuello, S.; Sayago, J.; Thomas-Valdes, S.; Jiménez-Aspee, F.; Schmeda-Hirschmann, G.; et al. Chemical and functional characterization of seed, pulp and skin powder from chilto (Solanum betaceum), an Argentine native fruit. Phenolic fractions affect key enzymes involved in metabolic syndrome and oxidative stress. Food Chem. 2017, 216, 70–79. [Google Scholar] [CrossRef]
- Gil, G.F.; Anderson, J.A.; Aravkin, A.; Bhangdia, K.; Carr, S.; Dai, X.; Flor, L.S.; Hay, S.I.; Malloy, M.J.; McLaughlin, S.A.; et al. Health effects associated with chewing tobacco: A Burden of Proof study. Nat. Commun. 2024, 15, 1082. [Google Scholar] [CrossRef]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef]
- Cramer, J.D.; Burtness, B.; Le, Q.T.; Ferris, R.L. The changing therapeutic landscape of head and neck cancer. Nat. Rev. Clin. Oncol. 2019, 16, 669–683. [Google Scholar] [CrossRef]
- Panarese, I.; Aquino, G.; Ronchi, A.; Longo, F.; Montella, M.; Cozzolino, I.; Roccuzzo, G.; Colella, G.; Caraglia, M.; Franco, R. Oral and Oropharyngeal squamous cell carcinoma: Prognostic and predictive parameters in the etiopathogenetic route. Expert Rev. Anticancer Ther. 2019, 19, 105–119. [Google Scholar] [CrossRef]
- Contrera, K.J.; Zafereo, M.E.; Yaniv, D.; Roberts, D.B.; Gillenwater, A.M.; Hanna, E.Y.; Weber, R.S.; Myers, J.N.; Chang, E.I.; Garvey, P.B.; et al. Outcomes for recurrent oral cavity squamous cell carcinoma. Oral Oncol. 2022, 134, 106127. [Google Scholar] [CrossRef] [PubMed]
- Nandini, D.B.; Rao, R.S.; Hosmani, J.; Khan, S.; Patil, S.; Awan, K.H. Novel therapies in the management of oral cancer: An update. Dis. Mon. 2020, 66, 101036. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomas-Barberan, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef] [PubMed]
- Flieger, J.; Raszewska-Famielec, M.; Radzikowska-Büchner, E.; Flieger, W. Skin Protection by Carotenoid Pigments. Int. J. Mol. Sci. 2024, 25, 1431. [Google Scholar] [CrossRef]
- Đurović, S.; Kojić, I.; Radić, D.; Smyatskaya, Y.A.; Bazarnova, J.G.; Filip, S.; Tosti, T. Chemical Constituents of Stinging Nettle (Urtica dioica L.): A Comprehensive Review on Phenolic and Polyphenolic Compounds and Their Bioactivity. Int. J. Mol. Sci. 2024, 25, 3430. [Google Scholar] [CrossRef]
- Li, J.W.; Vederas, J.C. Drug discovery and natural products: End of an era or an endless frontier? Science 2009, 325, 161–165. [Google Scholar] [CrossRef]
- Baur, J.A.; Sinclair, D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef]
- Ross, J.A.; Kasum, C.M. Dietary flavonoids: Bioavailability, metabolic effects, and safety. Annu. Rev. Nutr. 2002, 22, 19–34. [Google Scholar] [CrossRef]
- Chang, S.K.; Alasalvar, C.; Shahidi, F. Superfruits: Phytochemicals, antioxidant efficacies, and health effects—A comprehensive review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1580–1604. [Google Scholar] [CrossRef]
- Lee, K.W.; Bode, A.M.; Dong, Z. Molecular targets of phytochemicals for cancer prevention. Nat. Rev. Cancer 2011, 11, 211–218. [Google Scholar] [CrossRef]
- Papaemmanouil, C.D.; Peña-García, J.; Banegas-Luna, A.J.; Kostagianni, A.D.; Gerothanassis, I.P.; Pérez-Sánchez, H.; Tzakos, A.G. ANTIAGE-DB: A Database and Server for the Prediction of Anti-Aging Compounds Targeting Elastase, Hyaluronidase, and Tyrosinase. Antioxidants 2022, 11, 2268. [Google Scholar] [CrossRef] [PubMed]
- Dzialo, M.; Mierziak, J.; Korzun, U.; Preisner, M.; Szopa, J.; Kulma, A. The Potential of Plant Phenolics in Prevention and Therapy of Skin Disorders. Int. J. Mol. Sci. 2016, 17, 160. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Uyama, H.; Kobayashi, S. Inhibition effects of (+)-catechin-aldehyde polycondensates on proteinases causing proteolytic degradation of extracellular matrix. Biochem. Biophys. Res. Commun. 2004, 320, 256–261. [Google Scholar] [CrossRef]
- Chen, Q.X.; Kubo, I. Kinetics of mushroom tyrosinase inhibition by quercetin. J. Agric. Food Chem. 2002, 50, 4108–4112. [Google Scholar] [CrossRef]
- Orhan, I.E.; Deniz, F.S.S. Inhibition of Melanogenesis by Some Well-Known Polyphenolics: A Review. Curr. Pharm. Biotechnol. 2021, 22, 1412–1423. [Google Scholar] [CrossRef]
- Jakimiuk, K.; Sari, S.; Milewski, R.; Supuran, C.T.; Şöhretoğlu, D.; Tomczyk, M. Flavonoids as tyrosinase inhibitors in in silico and in vitro models: Basic framework of SAR using a statistical modelling approach. J. Enzym. Inhib. Med. Chem. 2022, 37, 421–430. [Google Scholar] [CrossRef]
- Ayari-Guentri, S.; Saad, S.; Ait Kettout, T.; Gaceb-Terrak, R.; Djemouai, N. Seeds of Hyoscyamus muticus L. subsp. falezlez: Morpho-anatomical Features, Phytochemical Investigation and Evidence for Antioxidant Activities. Chem. Biodivers. 2024, e202401026. [Google Scholar] [CrossRef] [PubMed]
- Zolghadri, S.; Beygi, M.; Mohammad, T.F.; Alijanianzadeh, M.; Pillaiyar, T.; Garcia-Molina, P.; Garcia-Canovas, F.; Munoz-Munoz, J.; Saboury, A.A. Targeting tyrosinase in hyperpigmentation: Current status, limitations and future promises. Biochem. Pharmacol. 2023, 212, 115574. [Google Scholar] [CrossRef] [PubMed]
- Nagatsu, T.; Nakashima, A.; Watanabe, H.; Ito, S.; Wakamatsu, K. Neuromelanin in Parkinson’s Disease: Tyrosine Hydroxylase and Tyrosinase. Int. J. Mol. Sci. 2022, 23, 4176. [Google Scholar] [CrossRef]
- Bose, A.; Petsko, G.A.; Eliezer, D. Parkinson’s Disease and Melanoma: Co-Occurrence and Mechanisms. J. Park. Dis. 2018, 8, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Lleó, A.; Greenberg, S.M.; Growdon, J.H. Current pharmacotherapy for Alzheimer’s disease. Annu. Rev. Med. 2006, 57, 513–533. [Google Scholar] [CrossRef] [PubMed]
- Bottiglieri, T.; Arning, E.; Wasek, B.; Nunbhakdi-Craig, V.; Sontag, J.M.; Sontag, E. Acute administration of L-DOPA induces changes in methylation metabolites, reduced protein phosphatase 2A methylation, and hyperphosphorylation of Tau protein in mouse brain. J. Neurosci. 2012, 32, 9173–9181. [Google Scholar] [CrossRef] [PubMed]
- Iannitelli, A.F.; Weinshenker, D. Riddles in the dark: Decoding the relationship between neuromelanin and neurodegeneration in locus coeruleus neurons. Neurosci. Biobehav. Rev. 2023, 152, 105287. [Google Scholar] [CrossRef] [PubMed]
- Lin, E.S.; Huang, Y.H.; Chung, J.C.; Su, H.H.; Huang, C.Y. The Inhibitory Effects and Cytotoxic Activities of the Stem Extract of Nepenthes miranda against Single-Stranded DNA-Binding Protein and Oral Carcinoma Cells. Plants 2023, 12, 2188. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.H.; Liou, C.Y.; Liu, S.C.; Ou, C.H. Cyphomandra betacea (Cav.) Sendt. (Solanaceae), a newly naturalized plant in Taiwan. Quart. J. Chin. For. 2008, 41, 425–429. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Huang, C.Y. Inhibition of a putative dihydropyrimidinase from Pseudomonas aeruginosa PAO1 by flavonoids and substrates of cyclic amidohydrolases. PLoS ONE 2015, 10, e0127634. [Google Scholar] [CrossRef]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Peng, W.F.; Huang, C.Y. Allantoinase and dihydroorotase binding and inhibition by flavonols and the substrates of cyclic amidohydrolases. Biochimie 2014, 101, 113–122. [Google Scholar] [CrossRef]
- Lee, C.Y.; Chen, Y.C.; Huang, Y.H.; Lien, Y.; Huang, C.Y. Cytotoxicity and Multi-Enzyme Inhibition of Nepenthes miranda Stem Extract on H838 Human Non-Small Cell Lung Cancer Cells and RPA32, Elastase, Tyrosinase, and Hyaluronidase Proteins. Plants 2024, 13, 797. [Google Scholar] [CrossRef]
- Shimizu, K.; Kondo, R.; Sakai, K.; Lee, S.H.; Sato, H. The inhibitory components from Artocarpus incisus on melanin biosynthesis. Planta Med. 1998, 64, 408–412. [Google Scholar] [CrossRef]
- Tu, P.T.; Tawata, S. Anti-Oxidant, Anti-Aging, and Anti-Melanogenic Properties of the Essential Oils from Two Varieties of Alpinia zerumbet. Molecules 2015, 20, 16723–16740. [Google Scholar] [CrossRef]
- Guan, H.H.; Huang, Y.H.; Lin, E.S.; Chen, C.J.; Huang, C.Y. Plumbagin, a Natural Product with Potent Anticancer Activities, Binds to and Inhibits Dihydroorotase, a Key Enzyme in Pyrimidine Biosynthesis. Int. J. Mol. Sci. 2021, 22, 6861. [Google Scholar] [CrossRef]
- Strober, W. Trypan blue exclusion test of cell viability. Curr. Protoc. Immunol. 2001. [Google Scholar] [CrossRef]
- Liu, H.W.; Chiang, W.Y.; Huang, Y.H.; Huang, C.Y. The Inhibitory Effects and Cytotoxic Activities of the Stem Extract of Sarracenia purpurea against Melanoma Cells and the SsbA Protein. Plants 2022, 11, 3164. [Google Scholar] [CrossRef]
- Larsson, R.; Nygren, P. A rapid fluorometric method for semiautomated determination of cytotoxicity and cellular proliferation of human tumor cell lines in microculture. Anticancer Res. 1989, 9, 1111–1119. [Google Scholar]
- Chen, M.H.; Yang, W.L.; Lin, K.T.; Liu, C.H.; Liu, Y.W.; Huang, K.W.; Chang, P.M.; Lai, J.M.; Hsu, C.N.; Chao, K.M.; et al. Gene expression-based chemical genomics identifies potential therapeutic drugs in hepatocellular carcinoma. PLoS ONE 2011, 6, e27186. [Google Scholar] [CrossRef]
- Liang, C.C.; Park, A.Y.; Guan, J.L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef]
| Methanol | Ethanol | Acetone | |
|---|---|---|---|
| Red fruit | |||
| Seeds | 9.89 ± 0.22 | 7.08 ± 0.11 | 1.92 ± 0.03 |
| Pulp | 5.65 ± 0.09 | 2.96 ± 0.04 | 0.97 ± 0.02 |
| Peel | 7.92 ± 0.13 | 3.61 ± 0.05 | 2.92 ± 0.05 |
| Yellow fruit | |||
| Seeds | 3.61 ± 0.06 | 3.60 ± 0.06 | 1.98 ± 0.04 |
| Pulp | 5.09 ± 0.10 | 3.58 ± 0.09 | 0.34 ± 0.03 |
| Peel | 8.37 ± 0.15 | 3.42 ± 0.07 | 2.42 ± 0.05 |
| Methanol | Ethanol | Acetone | |
|---|---|---|---|
| Red fruit | |||
| Seeds | 2.05 ± 0.06 | 1.68 ± 0.04 | 0.69 ± 0.02 |
| Pulp | 1.07 ± 0.02 | 0.78 ± 0.02 | 0.79 ± 0.03 |
| Peel | 2.85 ± 0.09 | 1.92 ± 0.08 | 1.14 ± 0.06 |
| Yellow fruit | |||
| Seeds | 1.96 ± 0.04 | 1.56 ± 0.05 | 0.73 ± 0.05 |
| Pulp | 1.43 ± 0.01 | 0.94 ± 0.02 | 0.65 ± 0.04 |
| Peel | 3.02 ± 0.10 | 2.11 ± 0.09 | 1.58 ± 0.07 |
| Extract (100 μg/mL) | Inhibition% | ||
|---|---|---|---|
| Tyrosinase | Elastase | Hyaluronidase | |
| Red fruit | |||
| Seeds, methanol | 50.4 ± 1.6 | 0.0 ± 0.0 | 14.1 ± 0.5 |
| Seeds, ethanol | 24.7 ± 1.0 | 0.0 ± 0.0 | 7.1 ± 0.2 |
| Seeds, acetone | 4.4 ± 0.5 | 0.0 ± 0.0 | 4.5 ± 0.3 |
| Pulp, methanol | 3.6 ± 0.5 | 0.0 ± 0.0 | 6.8 ± 0.4 |
| Pulp, ethanol | 3.0 ± 0.2 | 0.0 ± 0.0 | 6.6 ± 0.2 |
| Pulp, acetone | 1.4 ± 0.1 | 0.0 ± 0.0 | 3.4 ± 0.3 |
| Peel, methanol | 32.7 ± 1.5 | 3.2 ± 0.4 | 9.8 ± 0.6 |
| Peel, ethanol | 30.4 ± 0.8 | 3.6 ± 0.3 | 9.9 ± 0.8 |
| Peel, acetone | 6.1 ± 0.4 | 1.0 ± 0.2 | 4.7 ± 0.3 |
| Yellow fruit | |||
| Seeds, methanol | 3.2 ± 0.2 | 8.4 ± 0.9 | 20.2 ± 0.7 |
| Seeds, ethanol | 1.1 ± 0.1 | 8.7 ± 0.6 | 17.4 ± 0.5 |
| Seeds, acetone | 10.8 ± 0.7 | 1.5 ± 0.2 | 1.5 ± 0.1 |
| Pulp, methanol | 2.3 ± 0.2 | 26.4 ± 1.2 | 13.5 ± 0.5 |
| Pulp, ethanol | 2.4 ± 0.3 | 28.1 ± 0.9 | 14.7 ± 0.4 |
| Pulp, acetone | 4.5 ± 0.5 | 7.4 ± 0.7 | 15.0 ± 0.3 |
| Peel, methanol | 30.7 ± 1.2 | 13.4 ± 0.8 | 14.5 ± 0.4 |
| Peel, ethanol | 32.4 ± 1.0 | 14.5 ± 0.6 | 13.7 ± 0.3 |
| Peel, acetone | 11.5 ± 0.4 | 5.8 ± 0.3 | 15.5 ± 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.-H.; Huang, C.-Y. Anti-Skin Aging and Cytotoxic Effects of Methanol-Extracted Solanum betaceum Red Fruit Seed Extract on Ca9-22 Gingival Carcinoma Cells. Plants 2024, 13, 2215. https://doi.org/10.3390/plants13162215
Huang Y-H, Huang C-Y. Anti-Skin Aging and Cytotoxic Effects of Methanol-Extracted Solanum betaceum Red Fruit Seed Extract on Ca9-22 Gingival Carcinoma Cells. Plants. 2024; 13(16):2215. https://doi.org/10.3390/plants13162215
Chicago/Turabian StyleHuang, Yen-Hua, and Cheng-Yang Huang. 2024. "Anti-Skin Aging and Cytotoxic Effects of Methanol-Extracted Solanum betaceum Red Fruit Seed Extract on Ca9-22 Gingival Carcinoma Cells" Plants 13, no. 16: 2215. https://doi.org/10.3390/plants13162215





