Comparison of Festuca glauca ‘Uchte’ and Festuca amethystina ‘Walberla’ Varieties in a Simulated Extensive Roof Garden Environment
Abstract
:1. Introduction
1.1. The Importance of Ornamental Grasses in Settlements
1.1.1. The Poaceae Family and the Festuca Genus
1.1.2. Festuca amethystina ‘Walberla’
1.1.3. Festuca glauca ‘Uchte’
1.1.4. A Comparison of the Two Species Based on the Ellenberg Index Values
1.2. Plant Application on Green Roofs
1.3. The Responses of Ornamental Grass Species to Abiotic Stress
1.3.1. The General Environmental Characteristics of Green Roofs
1.3.2. The Stress Management of Grasses
1.3.3. The Role of POD and Proline Levels in Plant Stress Management
1.4. The Effect of the Planting Medium
2. Results
2.1. Morphological Studies
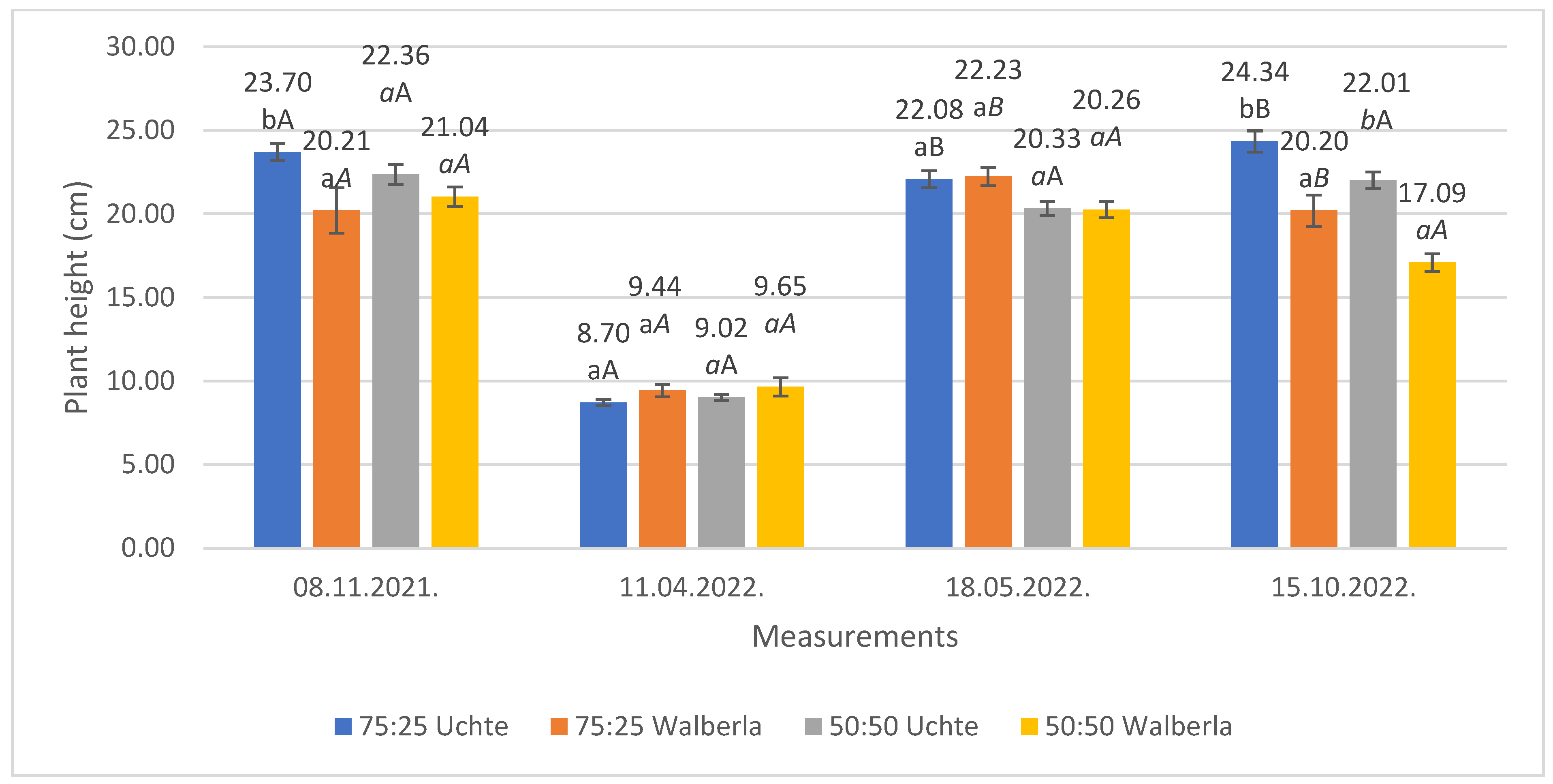
2.1.1. Plant Height
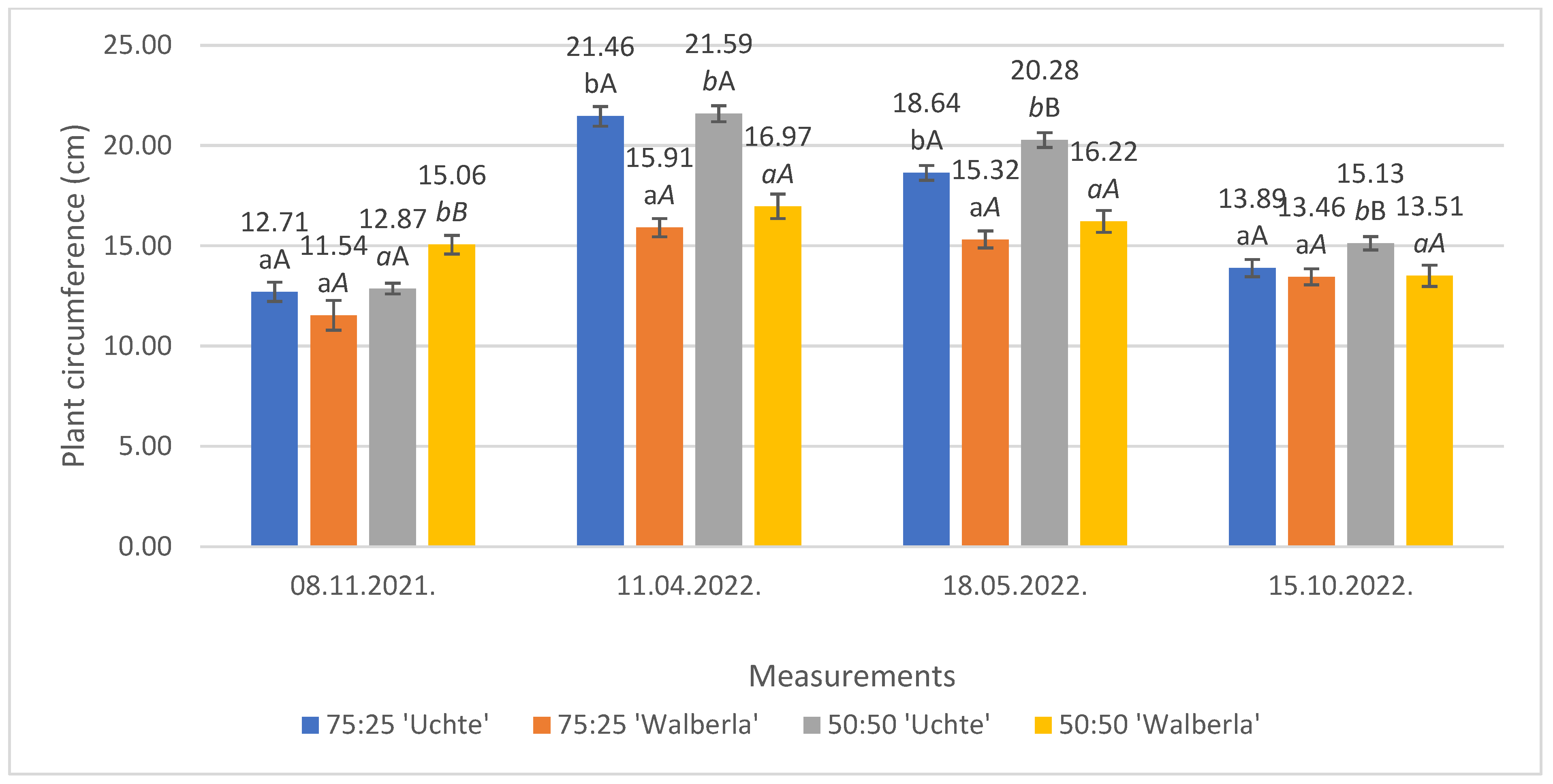
2.1.2. Plant Circumference
2.1.3. Root Length
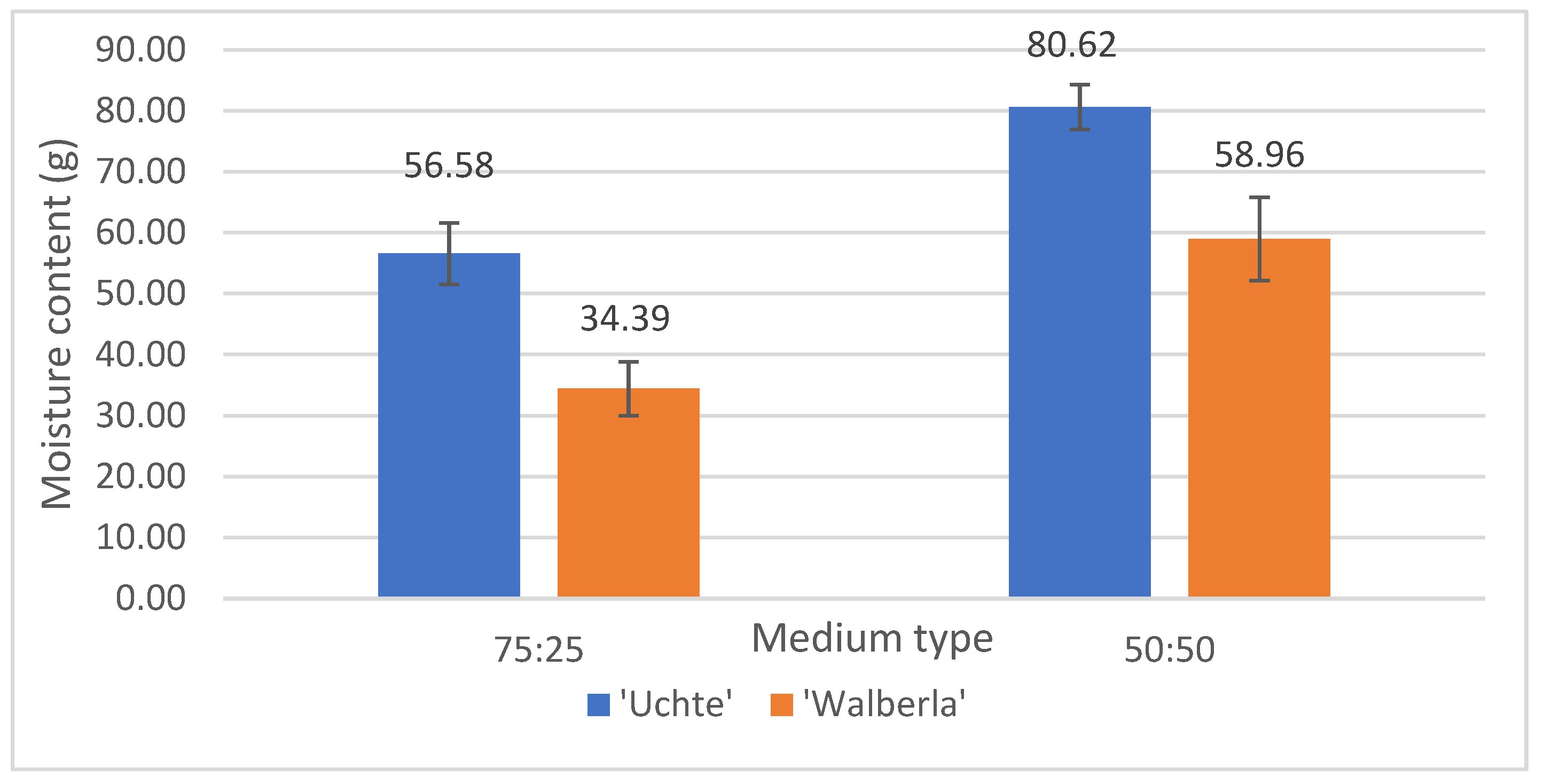
2.1.4. Fresh and Dry Plant Weight and Moisture Content
2.2. Physiological Measurements
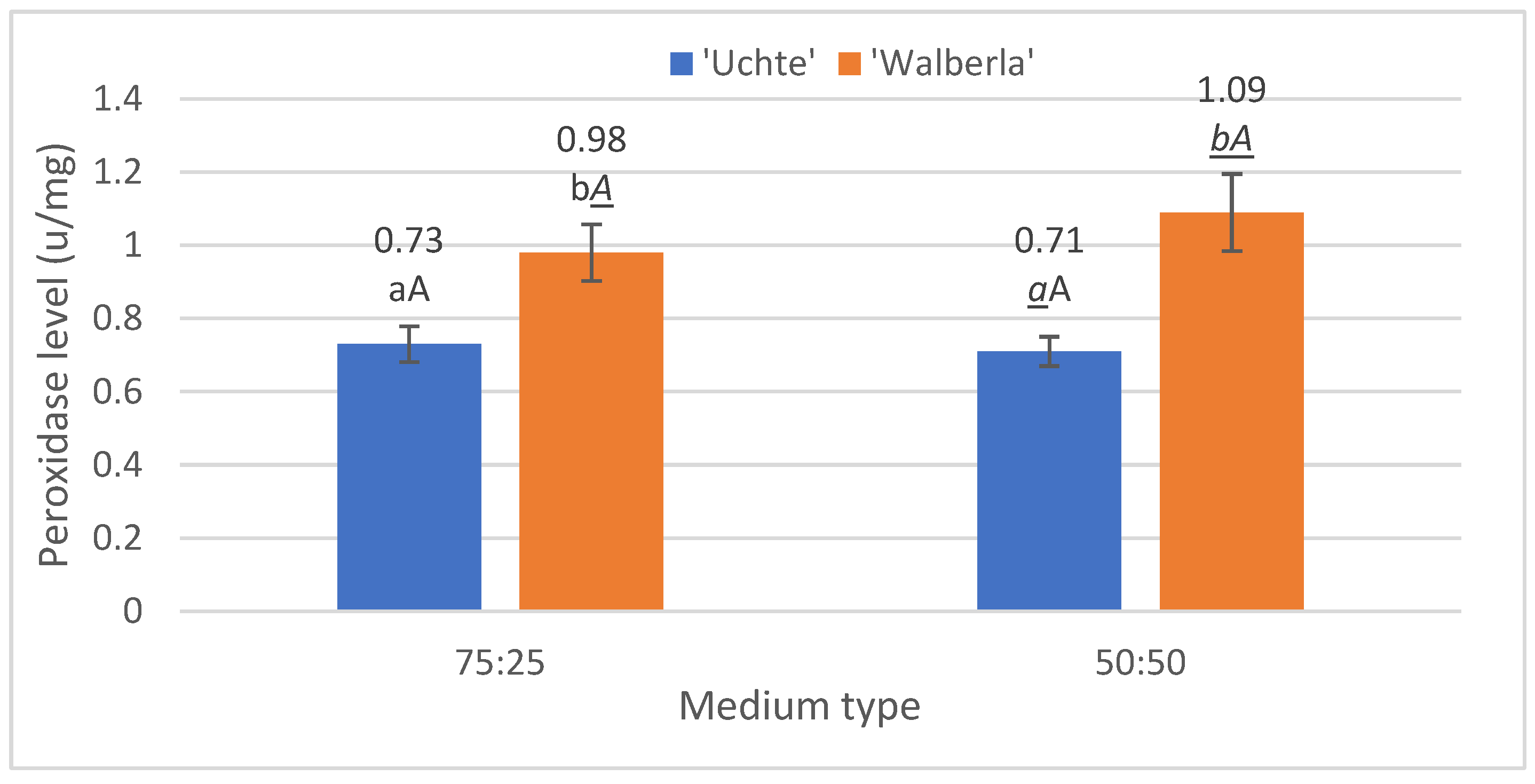
2.2.1. Peroxidase
2.2.2. Proline
3. Discussion
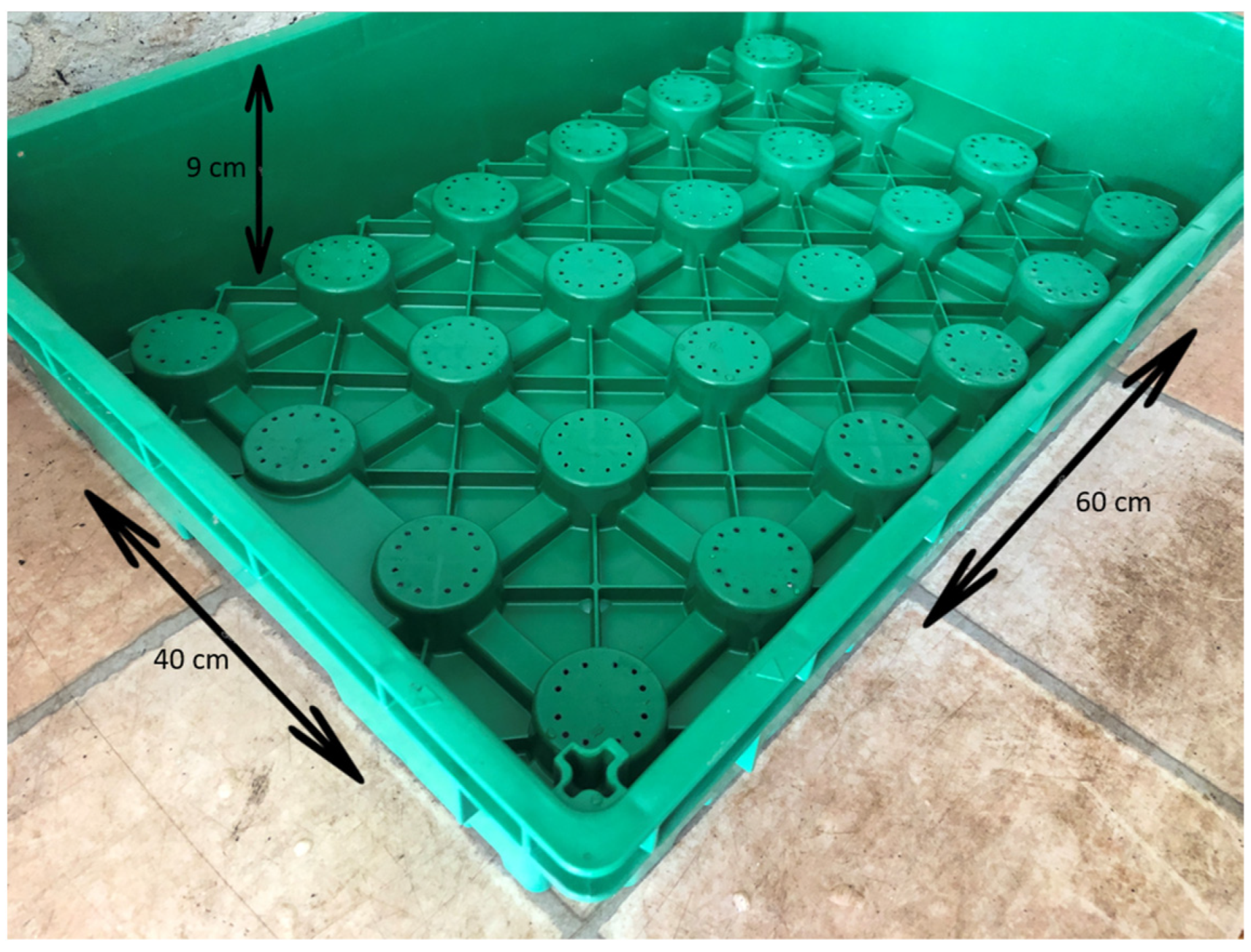
4. Materials and Methods
- 1st: condition assessment after planting, 10 November 2021;
- 2nd: sprouting after winter dormancy, 10 April 2022;
- 3rd: state before summer dormancy, 15 May 2022;
- 4th: culture finishing, elimination of the stock, 15 October 2022.
4.1. Measurement of Peroxidase Enzyme Activity
4.2. Proline Measurement
4.3. Data Evaluation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Francini, A.; Romano, D.; Toscano, S.; Ferrante, A. The contribution of Ornamental Plants to Urban Ecosystem Services. Earth 2022, 3, 1258–1274. [Google Scholar] [CrossRef]
- Gladkov, E.A.; Gladkova, O.V. Ornamental plants adapted to urban ecosystem pollution: Lawn grasses tolerating deicing reagents. Environ. Sci. Pollut. Res. 2022, 29, 22947–22951. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Hershkowitz, J.; Paudel, A.; Sun, Y.; Chen, J.J.; Dai, X.; Chappell, M. Morphological and physiological responses of ornamental grasses to saline water irrigation. HortScience 2021, 56, 678–686. [Google Scholar] [CrossRef]
- Hillock, D.; Schnelle, M.; Hentges, C. Ornamental Grasses and Grass-Like Plants for Oklahoma; Oklahoma Cooperative Extension Service: Stillwater, OK, USA, 2022. [Google Scholar]
- Pla, M. Evaluation of assortment of ornamental grasses and their environmental importance in the urban landscape. J. Environ. Prot. Ecol. 2020, 21, 1673–1682. [Google Scholar]
- Jiang, Y.; Kang, M.; Zhu, Y.; Xu, G. Plant biodiversity patterns on Helan mountain, China. Acta Oecologica 2007, 32, 125–133. [Google Scholar] [CrossRef]
- Fodorpataki, L.; Szigyártó, L.; Bartha, C. Növénytani Ismeretek; Scientia Kiadó: Kolozsvár, Romania, 2009; p. 200. [Google Scholar]
- Gladkov, E.A.; Tashlieva, I.I.; Gladkova, O.V. Ornamental plants adapted to urban ecosystem pollution: Lawn grasses and painted daisy tolerating copper. Environ. Sci. Pollut. Res. 2021, 28, 14115–14120. [Google Scholar] [CrossRef] [PubMed]
- Yan-sha, Z.; Jing, O.; Qian, L.; Zhi-ye, Z. Comprehensive Evaluation of the Ornamental Adaptability of 10 Species of Introduced Ornamental Grass in Guiyang. Subtrop. Plant Sci. 2021, 50, 26. [Google Scholar]
- RHS Gardening, Festuca. Available online: https://www.rhs.org.uk/plants/festuca (accessed on 1 August 2024).
- Meusel, H.; Jäger, E.; Weinert, E.; Fischer, G. Vergleichen de Chorologiebder Zentral-Europäischen Flora; VEB Gustav Fischer: Jena, Germany, 1965; pp. 1–2. [Google Scholar]
- Jardineiro.net, Festuca glauca. Available online: https://www.jardineiro.net/plantas/festuca-azul-festuca-glauca.html (accessed on 1 August 2024).
- Shahabzadeh, Z.; Mohammadi, R.; Darvishzadeh, R.; Jafari, M.; Alipour, H. Investigation of genetic diversity of forage yield and morphological traits in tall fescue (Festuca arundinacea Schreb.) populations. Iran. J. Rangel. For. Plant Breed. Genet. Res. 2020, 28, 17–36. [Google Scholar]
- Mohammadi, R.; Panahi, B.; Amiri, S. ISSR based study of fine fescue (Festuca ovina L.) highlighted the genetic diversity of Iranian accessions. Cytol. Genet. 2020, 54, 257–263. [Google Scholar] [CrossRef]
- Wang, J.P.; Bughrara, S.S. Detection of an efficient restriction enzyme combination for cDNA-AFLP analysis in Festuca mairei and evaluation of the identity of transcript-derived fragments. Mol. Biotechnol. 2005, 29, 211–220. [Google Scholar] [CrossRef]
- Chakrabarti, M.; Nagabhyru, P.; Schardl, C.L.; Dinkins, R.D. Differential gene expression in tall fescue tissues in response to water deficit. Plant Genome 2022, 15, e20199. [Google Scholar] [CrossRef] [PubMed]
- Kiedrzyński, M.; Zielińska, K.M.; Rewicz, A.; Kiedrzyńska, E. Habitat and spatial thinning improve the Maxent models performed with incomplete data. J. Geophys. Res. Biogeosci. 2017, 122, 1359–1370. [Google Scholar] [CrossRef]
- North Carolina State University, Plant Toolbox, Festuca amethystina. Available online: https://plants.ces.ncsu.edu/plants/festuca-amethystina/ (accessed on 1 August 2024).
- RHS Gardening. Festuca amethystina. Available online: https://www.rhs.org.uk/plants/32846/festuca-amethystina/details (accessed on 1 August 2024).
- Roleček, J.; Dřevojan, P.; Šmarda, P. First record of Festuca amethystina L. from the Transylvanian Basin (Romania). Contrib. Bot. 2019, 54, 91–97. [Google Scholar] [CrossRef]
- Hameter GMBH. Available online: https://www.hameter.at/produkt/festuca-amethystina-walberla/ (accessed on 18 July 2023).
- Oprea, M.; Giosanu, D.; Vulpe, M. Decorative herbs, a green solution for urban arrangements. J. Nat. Sci. Res. 2022, 11, 356–364. [Google Scholar] [CrossRef]
- RHS Gardening. Festuca glauca. Available online: https://www.rhs.org.uk/plants/7181/festuca-glauca-vill/details (accessed on 1 August 2024).
- Cojocariu, M.; Chelariu, E.L.; Chiruţă, C. Study on behavior of some perennial flowering species used in vertical systems for green facades in Eastern European climate. Appl. Sci. 2022, 12, 474. [Google Scholar] [CrossRef]
- Ostapets, T. Comparative characteristics of main morphological indication and type of inheritance of leaf plate color in species Festuca glauca, Festuca rubra, Festuca ovina. Nor. J. Dev. Int. Sci. 2020, 51-2, 15–17. [Google Scholar]
- Yoon, H.S.; Cho, C.H.; Yun, M.S.; Jang, S.J.; You, H.J.; Kim, J.H.; Han, D.; Cha, K.H.; Moon, S.H.; Lee, K.; et al. Akkermansia muciniphila secretes a glucagon-like peptide-1-inducing protein that improves glucose homeostasis and ameliorates metabolic disease in mice. Nat. Microbiol. 2021, 6, 563–573. [Google Scholar] [CrossRef]
- North Carolina State University, Plant Toolbox, Festuca glauca. Available online: https://plants.ces.ncsu.edu/plants/festuca-glauca/ (accessed on 1 August 2024).
- Il Lavandeto Di Assisi di Fastellini Lorena. Available online: https://illavandetodiassisi.it/products/festuca-glauca-ucthe (accessed on 18 July 2023).
- Ellenberg, H. Zeigerwerte von pflanzen in Mitteleuropa. Scr. Geobot. 1991, 18, 1–248. [Google Scholar]
- Sonkoly, J.; Tóth, E.; Balogh, N.; Balogh, L.; Bartha, D.; Bata, K.; Bátori, Z.; Békefi, N.; Botta-Dukát, Z.; Bölöni, J.; et al. PADAPT 1.0—The Pannonian Database of Plant Traits. bioRxiv 2022. [Google Scholar] [CrossRef]
- Pladias—Database of the Czech Flora and Vegetation. FloraVeg.eu 11.7. Festuca glauca. 2024. Available online: www.pladias.cz (accessed on 1 August 2024).
- Liu, H.; Kong, F.; Yin, H.; Middel, A.; Zheng, X.; Huang, J.; Xu, H.; Wang, D.; Wen, Z. Impacts of green roofs on water, temperature, and air quality: A bibliometric review. Build. Environ. 2021, 196, 107794. [Google Scholar] [CrossRef]
- Manso, M.; Teotónio, I.; Silva, C.M.; Cruz, C.O. Green roof and green wall benefits and costs: A review of the quantitative evidence. Renew. Sustain. Energy Rev. 2021, 135, 110111. [Google Scholar] [CrossRef]
- Tang, M.; Zheng, X. Experimental study of the thermal performance of an extensive green roof on sunny summer days. Appl. Energy 2019, 242, 1010–1021. [Google Scholar] [CrossRef]
- Filazzola, A.; Shrestha, N.; MacIvor, J.S. The contribution of constructed green infrastructure to urban biodiversity: A synthesis and meta-analysis. J. Appl. Ecol. 2019, 56, 2131–2143. [Google Scholar] [CrossRef]
- Van Der Kolk, H.J.; van den Berg, P.; Korthals, G.; Bezemer, T.M. Shading enhances plant species richness and diversity on an extensive green roof. Urban. Ecosyst. 2020, 23, 935–943. [Google Scholar] [CrossRef]
- Aguiar, A.C.; Robinson, S.A.; French, K. Friends with benefits: The effects of vegetative shading on plant survival in a green roof environment. PLoS ONE 2019, 14, e0225078. [Google Scholar] [CrossRef] [PubMed]
- Shetty, N.H.; Elliott, R.M.; Wang, M.; Palmer, M.I.; Culligan, P.J. Comparing the hydrological performance of an irrigated native vegetation green roof with a conventional Sedum spp. green roof in New York City. PLoS ONE 2022, 17, e0266593. [Google Scholar] [CrossRef] [PubMed]
- Erwin, J.; Hensley, J. Plants with horticultural and ecological attributes for green roofs in a cool, dry climate. HortScience 2019, 54, 1703–1711. [Google Scholar] [CrossRef]
- Koehler, M. Extensive Green Roof Study on a Retrofit Building in Berlin, Germany: Report after 34 Years of Monitoring. In Proceedings of the Green Roof and Wall Conference, Philadelphia, PA, USA, 16–19 October 2022. [Google Scholar]
- Zanin, G.; Bortolini, L. Performance of Three Different Native Plant Mixtures for Extensive Green Roofs in a Humid Subtropical Climate Context. Water 2020, 12, 3484. [Google Scholar] [CrossRef]
- de Carvalho, R.C.; Paço, T.; Branquinho, C.; Marques Da Silva, J. Using Chlorophyll a Fluorescence Imaging to Select Desiccation-Tolerant Native Moss Species for Water-Sustainable Green Roofs. Water 2020, 12, 1748. [Google Scholar] [CrossRef]
- Koppány, A. Zöldtetők. Magyar Építőipar. 1994, 11–12, 366–368. [Google Scholar]
- Tuttolomondo, T.; Fascella, G.; Licata, M.; Schicchi, R.; Gennaro, M.C.; Bella, S.L.; Leto, C.; Aprile, S. Studies on Sedum taxa found in Sicily (Italy) for mediterranean extensive green roofs. Ital. J. Agron. 2018, 13, 148–154. [Google Scholar] [CrossRef]
- Rocha, B.; Paco, T.A.; Luz, A.C.; Palha, P.; Milliken, S.; Kotzen, B.; Branquinho, C.; Pinho, P.; de Carvalho, R.C. Are biocrusts and xerophytic vegetation a viable green roof typology in a mediterranean climate? A comparison between differently vegetated green roofs in water runoff and water quality. Water 2021, 13, 94. [Google Scholar] [CrossRef]
- Carlos, V.R.; Franco, N.; Peyre, G.; Juan, P.R. Green roof design with engineered extensive substrates and native species to evaluate stormwater runoff and plant establishment in a neotropical mountain climate. Sustainability 2020, 12, 6534. [Google Scholar] [CrossRef]
- Fahrig, L. Effects of habitat fragmentation on biodiversity. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 487–515. [Google Scholar] [CrossRef]
- Hao, J.; He, D.; Wu, Y.; Fu, L.; He, K. A study of the emission and concentration distribution of vehicular pollutants in the urban area of Beijing. Atmos. Environ. 2000, 34, 453–465. [Google Scholar] [CrossRef]
- Kátai, J. Talajtan, Talajökológia; Észak-Alföldi Régiókért Kht.: Debrecen, Hungary, 2008; 190p, ISBN 9789639874053. [Google Scholar]
- Fan, P.; Wang, K. Evaluation of cold resistance of ornamental species for planting as urban rooftop greening. For. Stud. China 2011, 13, 239–244. [Google Scholar] [CrossRef]
- Martín, G.; Márquez, Y.; Mantica, F.; Duque, P.; Irimia, M. Alternative splicing landscapes in Arabidopsis thaliana across tissues and stress conditions highlight major functional differences with animals. Genome Biol. 2021, 22, 35. [Google Scholar] [CrossRef]
- Dossa, K.; Diouf, D.; Cissé, N. Genome-wide investigation of Hsf genes in sesame reveals their segmental duplication expansion and their active role in drought stress response. Front. Plant Sci. 2016, 7, 1522. [Google Scholar] [CrossRef]
- Bachle, S.; Zaricor, M.; Griffith, D.; Qui, F.; Still, C.J.; Ungerer, M.C.; Nippert, J.B. Physiological responses to drought stress and recovery reflect differences in leaf function and anatomy among grass lineages. BioRxiv 2022. [Google Scholar] [CrossRef]
- Rai, G.K.; Bhat, B.A.; Mushtaq, M.; Tariq, L.; Rai, P.K.; Basu, U.; Dar, A.A.; Islam, S.T.; Hassan, T.I.; Bhat, J.A. Insights into decontamination of soils by phytoremediation: A detailed account on heavy metal toxicity and mitigation strategies. Physiol. Plant. 2021, 173, 287–304. [Google Scholar] [CrossRef]
- Mur, L.A.; Mandon, J.; Persijn, S.; Cristescu, S.M.; Moshkov, I.E.; Novikova, G.V.; Hall, M.A.; Harren, F.J.M.; Hebelsturp, K.H.; Gupta, K.J. Nitric oxide in plants: An assessment of the current state of knowledge. AoB Plants 2013, 5, pls052. [Google Scholar] [CrossRef]
- Gao, X.; Zhao, X.; Li, H.; Guo, L.; Lv, T.; Wu, P. Exotic shrub species (Caragana korshinskii) is more resistant to extreme natural drought than native species (Artemisia gmelinii) in a semiarid revegetated ecosystem. Agric. For. Meteorol. 2018, 263, 207–216. [Google Scholar] [CrossRef]
- Abdelaal, K.A.; Attia, K.A.; Alamery, S.F.; El-Afry, M.M.; Ghazy, A.I.; Tantawy, D.S.; Al-Doss, A.A.; El-Shawy, E.-S.E.; Abu-Elsaoud, A.M.; Hafez, Y.M. Exogenous application of proline and salicylic acid can mitigate the injurious impacts of drought stress on barley plants associated with physiological and histological characters. Sustainability 2020, 12, 1736. [Google Scholar] [CrossRef]
- Chen, L.; Liu, J.R.; Hu, W.F.; Gao, J.; Yang, J.Y. Vanadium in soil-plant system: Source, fate, toxicity, and bioremediation. J. Hazard. Mater. 2021, 405, 124200. [Google Scholar] [CrossRef]
- Krishnamurthy, S.R.; Khobragade, A.M.; Kale, A.M.; Asewar, B.V.; Khandare, V.S. Studies on the effect of osmoprotectants on microclimate of soybean (Glycine max L. Merrill). IJCS 2019, 7, 2686–2690. [Google Scholar]
- Ali, M.; Bakht, J.; Khan, D.G. Effect of water deficiency and potassium application on plant growth, osmolytes and grain yield of Brassica napus cultivars. Acta Bot. Croat. 2014, 73, 299–300. [Google Scholar] [CrossRef]
- Bandurska, H.; Jozwiak, W. A comparison of the effects of drought on proline accumulation and peroxidases activity in leaves of Festuca rubra L. and Lolium perenne L. Acta Soc. Bot. Pol. 2010, 79, 111–116. [Google Scholar] [CrossRef]
- Selahvarzi, Y.; Sarfaraz, S.; Kamali, M.; Zabihi, M.; Alizadeh, B. Effect of zeolite on some physiological and morphological traits of Festuca arundinacea grass under drought stress. IJID 2020, 14, 103–114. [Google Scholar]
- Farizani, M.S.; Khazaee, H.R.; Gazanchian, G.A. Improving Drought Stress Tolerance using Municipal Solid Waste (MSW) Compost in Native Grass–(Festuca arundinacea). J. Hortic. Sci. 2021, 2, 253–266. [Google Scholar]
- Chow, M.F.; Azlan, A.A.B.A. Assessing the performance of bio compost as soil media in extensive green roof. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2020; Volume 713, p. 012012. [Google Scholar]
- Xue, M.; Farrell, C. Use of organic wastes to create lightweight green roof substrates with increased plant-available water. Urban. For. Urban. Green. 2020, 48, 126569. [Google Scholar] [CrossRef]
- Xiao, C.; Guo, X.; Liu, L.; Luo, C.; Li, R.; Liu, G. Greening waste compost as a new substrate for green roofs. J. Zhejiang A&F Univ. 2019, 36, 598–604. [Google Scholar]
- Jiang, Y.; Huang, B. Osmotic adjustment and root growth associated with drought preconditioning-enhanced heat tolerance in Kentucky bluegrass. Crop Sci. 2001, 41, 1168–1173. [Google Scholar] [CrossRef]
- Suissa, J.S.; Friedman, W.E. From cells to stems: The effects of primary vascular construction on drought-induced embolism in fern rhizomes. New Phytol. 2021, 232, 2238–2253. [Google Scholar] [CrossRef] [PubMed]
- Fito System Kft. Instant Modular Greenroof. Available online: https://zoldtetoinstant.hu/ (accessed on 1 August 2024).
- Shannon, L.M.; Kay, E.; Lew, J.Y. Peroxidase Isozymes from Horseradish Roots. J. Biol. Chem. 1966, 241, 2166–2172. [Google Scholar] [CrossRef]
- Ábrahám, E.; Hourton-Cabassa, C.; Erdei, L.; Szabados, L. Methods for determination of proline in plants. In Plant Stress Tolerance (Methods in Molecular Biology); Humana Press: Clifton, NJ, USA, 2010; pp. 317–331. [Google Scholar] [CrossRef]
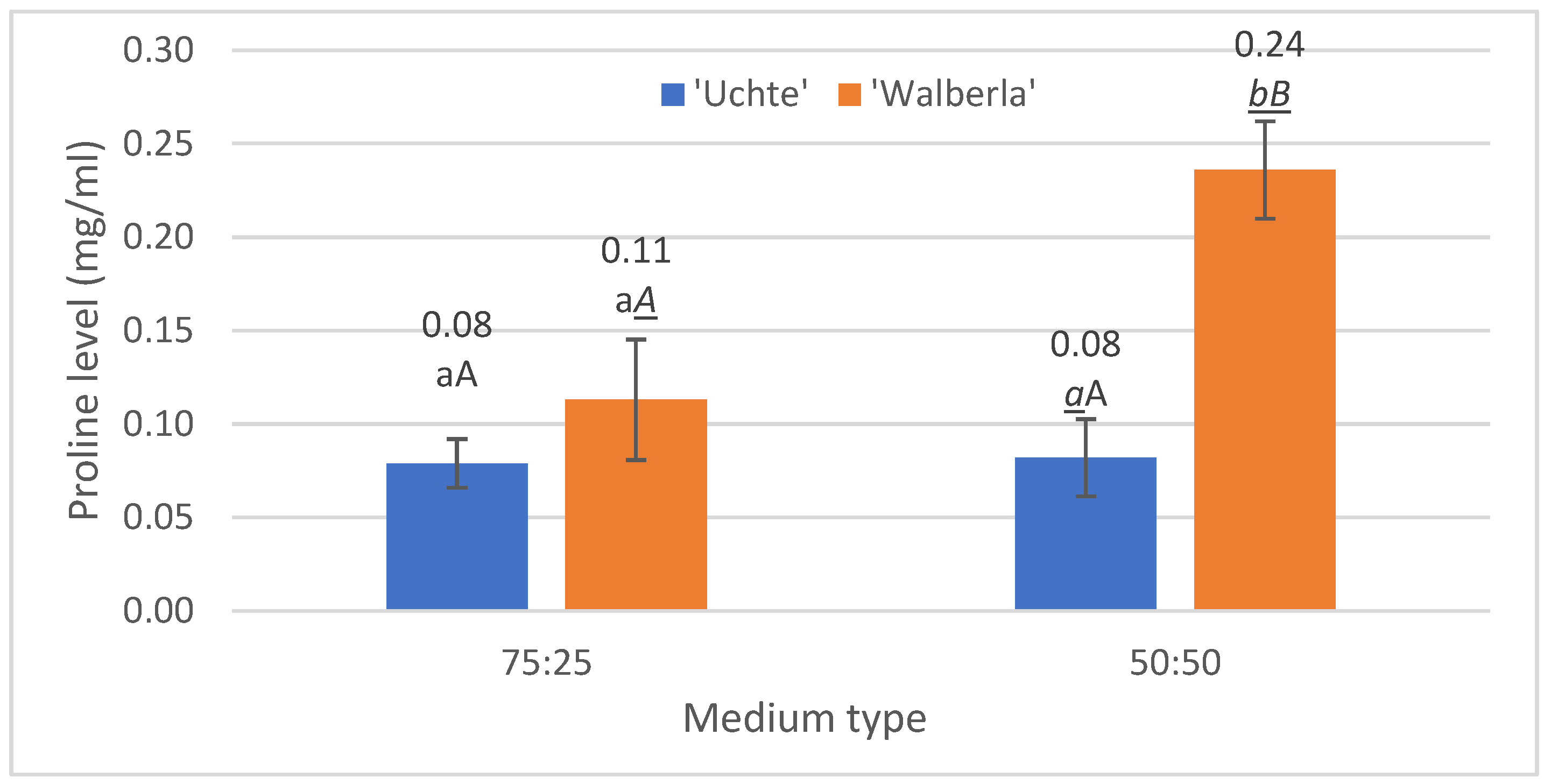

| Indicator Value | Light | Temperature | Moisture | Nutrients |
|---|---|---|---|---|
| F. amethystina | 6 | 5 | 3 | 2 |
| F. glauca | 8 | 9 | 5 | 5 |
| 75:25 ‘Uchte’ | 75:25 ‘Walberla’ | 50:50 ‘Uchte’ | 50:50 ‘Walberla’ | |
|---|---|---|---|---|
| Root length (cm) | 23.33 ± 1.26 aA | 23.40 ± 1.27 aA | 32.87 ± 1.96 aB | 26.70 ± 1.13 bA |
| Root circumference (cm) | 31.44 ± 1.00 bA | 21.91 ± 0.95 aA | 30.32 ± 0.71 bA | 22.44 ± 0.70 aA |
| 75:25 ‘Uchte’ | 75:25 ‘Walberla’ | 50:50 ‘Uchte’ | 50:50 ‘Walberla’ | |
|---|---|---|---|---|
| Fresh plant weight (g) | 115.36 ± 10.27 bA | 66.74 ± 7.54 aA | 161.47 ± 7.69 bB | 105.28 ± 11.16 aB |
| Dry plant weight (g) | 58.78 ± 5.23 bA | 32.35 ± 3.13 aA | 80.85 ± 4.00 bB | 46.32 ± 4.33 aB |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamar-Farkas, D.; Kisvarga, S.; Ördögh, M.; Orlóci, L.; Honfi, P.; Kohut, I. Comparison of Festuca glauca ‘Uchte’ and Festuca amethystina ‘Walberla’ Varieties in a Simulated Extensive Roof Garden Environment. Plants 2024, 13, 2216. https://doi.org/10.3390/plants13162216
Hamar-Farkas D, Kisvarga S, Ördögh M, Orlóci L, Honfi P, Kohut I. Comparison of Festuca glauca ‘Uchte’ and Festuca amethystina ‘Walberla’ Varieties in a Simulated Extensive Roof Garden Environment. Plants. 2024; 13(16):2216. https://doi.org/10.3390/plants13162216
Chicago/Turabian StyleHamar-Farkas, Dóra, Szilvia Kisvarga, Máté Ördögh, László Orlóci, Péter Honfi, and Ildikó Kohut. 2024. "Comparison of Festuca glauca ‘Uchte’ and Festuca amethystina ‘Walberla’ Varieties in a Simulated Extensive Roof Garden Environment" Plants 13, no. 16: 2216. https://doi.org/10.3390/plants13162216






