Invasive Buttonweed Cotula coronopifolia (Asteraceae) Is Halotolerant and Has High Potential for Dispersal by Endozoochory
Abstract
1. Introduction
2. Results
2.1. Effects of Salinity, Population, and Gut Passage on Germinability
2.2. Effects of Gut Passage and Salinity on Time to Germination
3. Discussion
3.1. Effect of Gut Passage on Germination Patterns
3.2. Implications for Long-Distance Dispersal
3.3. The Importance of Halotolerance
4. Materials and Methods
4.1. Study Species
4.2. Study Sites and Plant Material
4.3. Gut Passage Simulation
4.4. Germination Experiment
4.5. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van der Velde, G.; Rajagopal, S.; Kuyper-Kollenaar, M.; Bij de Vaate, A.; Thieltges, D.W.; MacIsaac, H.J. Biological Invasions: Concepts to understand and predict a global threat. In Ecological Studies, Wetlands: Functioning, Biodiversity Conservation, and Restoration; Bobbink, R., Beltman, B., Verhoeven, J.T.A., Whigham, D.F., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; Volume 191, pp. 61–85. [Google Scholar] [CrossRef]
- Downey, P.O.; Richardson, D.M. Alien plant invasions and native plant extinctions: A six-threshold framework. AoB Plants 2016, 8, plw047. [Google Scholar] [CrossRef] [PubMed]
- Foxcroft, L.C.; Pyšek, P.; Richardson, D.M.; Genovesi, P.; MacFadyen, S. Plant invasion science in protected areas: Progress and priorities. Biol. Invasions 2017, 19, 1353–1378. [Google Scholar] [CrossRef]
- van Kleunen, M.; Pyšek, P.; Dawson, W.; Essl, F.; Kreft, H.; Pergl, J.; Weigelt, P.; Stein, A.; Dullinger, S.; König, C.; et al. The Global Naturalized Alien Flora (GloNAF) database. Ecology 2019, 100, e02542. [Google Scholar] [CrossRef]
- Hovick, S.M.; Peterson, C.J.; Carson, W.P. Predicting invasiveness and range size in wetland plants using biological traits: A multivariate experimental approach. J. Ecol. 2012, 100, 1373–1382. [Google Scholar] [CrossRef]
- Palma, E.; Vesk, P.A.; White, M.; Baumgartner, J.B.; Catford, J.A. Plant functional traits reflect different dimensions of species invasiveness. Ecology 2021, 102, e03317. [Google Scholar] [CrossRef]
- Moyano, J.; Essl, F.; Heleno, R.; Vargas, P.; Nuñez, M.A.; Rodriguez-Cabal, M.A. Diaspore traits specialized to animal adhesion and sea current dispersal are positively associated with the naturalization of European plants across the world. Ecography 2022, 2022, e06423. [Google Scholar] [CrossRef]
- Lososová, Z.; Axmanová, I.; Chytrý, M.; Midolo, G.; Abdulhak, S.; Karger, D.N.; Renaud, J.; Van Es, J.; Vittoz, P.; Thuiller, W. Seed dispersal distance classes and dispersal modes for the European flora. Glob. Ecol. Biogeogr. 2023, 32, 1485–1494. [Google Scholar] [CrossRef]
- González-Varo, J.P.; Rumeu, B.; Bracho-Estévanez, C.A.; Acevedo-Limón, L.; Baltzinger, C. Lovas-Kiss Á, Green A.J. Overlooked seed-dispersal modes and underestimated distances. Glob. Ecol. Biogeogr. 2024, 33, e13835. [Google Scholar] [CrossRef]
- Green, A.J.; Baltzinger, C.; Lovas-Kiss, Á. Plant dispersal syndromes are unreliable, especially for predicting zoochory and long-distance dispersal. Oikos 2021, 2022, e08327. [Google Scholar] [CrossRef]
- Navarro-Ramos, M.J.; Van Leeuwen, C.H.A.; Olsson, C.; Elmberg, J.; Månsson, J.; Martín-Vélez, V.; Lovas-Kiss, Á.; Green, A.J. Seed dispersal between aquatic and agricultural habitats by greylag geese. Agric. Ecosyst. Environ. 2024, 359, 108741. [Google Scholar] [CrossRef]
- Almeida, B.A.; Lukács, B.A.; Lovas-Kiss, Á.; Reynolds, C.; Green, A.J. Functional traits drive dispersal interactions between European waterfowl and seeds. Front. Plant Sci. 2022, 12, 795288. [Google Scholar] [CrossRef]
- Martín-Vélez, V.; van Leeuwen, C.H.A.; Sánchez, M.I.; Hortas, F.; Shamoun-Baranes, J.; Thaxter, C.B.; Lens, L.; Camphuysen, C.J.; Green, A.J. Spatial patterns of weed dispersal by wintering gulls within and beyond an agricultural landscape. J. Ecol. 2021, 109, 1947–1958. [Google Scholar] [CrossRef]
- Green, A.J.; Lovas-Kiss, Á.; Reynolds, C.; Sebastián-González, E.; Silva, G.G.; Van Leeuwen, C.H.A.; Wilkinson, D.M. Dispersal of aquatic and terrestrial organisms by waterbirds: A review of current knowledge and future priorities. Freshw. Biol. 2023, 68, 173–190. [Google Scholar] [CrossRef]
- Vandertoorn, J. On the Ecology of Cotula coronopifolia L. and Ranunculus sceleratus L. 1 Geographic-Distribution, Habitat, and Field Observations. Acta Bot. Neerl. 1980, 29, 385–396. [Google Scholar] [CrossRef]
- Ridley, H.N. The Dispersal of Plants throughout the World; L. Reeve and Co., Ltd.: London, UK, 1930. [Google Scholar]
- Sanz Elorza, M.; Sánchez, E.D.; Vesperinas, E.S. Las especies invasoras, Cotula coronopifolia. In Atlas de las Plantas Alóctonas Invasoras en España; Dirección General para la Biodiversidad, Ministerio de Medio Ambiente: Madrid, Spain, 2004; pp. 130–132. Available online: https://www.gisandbeers.com/GeoBazar/Libros/Atlas%20biodiversidad/Atlas%20de%20las%20Plantas%20Aloctonas%20Invasoras%20de%20Espana.pdf (accessed on 10 April 2024).
- Costea, M.; El Miari, H.; Laczkó, L.; Fekete, R.; Molnár, A.V.; Lovas-Kiss, Á.; Green, A.J. The effect of gut passage by waterbirds on the seed coat and pericarp of diaspores lacking “external flesh”: Evidence for widespread adaptation to endozoochory in angiosperms. PLoS ONE 2019, 14, e0226551. [Google Scholar] [CrossRef] [PubMed]
- Killick, H.J. Cotula coronopifolia L. In BSBI Online Plant Atlas 2020; Stroh, P.A., Walker, K.J., Humphrey, T.A., Pescott, O.L., Burkmar, R.J., Eds.; Botanical Society of Britain and Ireland & Princeton University Press: Princeton, UK, 2023; Volume 2, Available online: https://plantatlas2020.org/atlas (accessed on 5 March 2024).
- Marfella, L.; Rufino, F.; Glanville, H.C.; Mastrocicco, M.; Strumia, S. Distribution of the invasive alien species Cotula coronopifolia L. (Asteraceae) relating to water halinity and sodicity in the Variconi wetland (Campania, southern Italy). Hydrobiologia 2023, 850, 1653–1668. [Google Scholar] [CrossRef]
- Tomasson, L. Four Years with Cotula coronopifolia: Monitoring and Climate Suitability Modelling. Bachelor Student Dissertation, Umeå University, Umeå, Sweden, 2022. Available online: https://urn.kb.se/resolve?urn=urn:nbn:se:umu:diva-192974 (accessed on 20 February 2024).
- Tomasson, L. Cotula coronopifolia–Invasive or Just Another Alien Species? Master’s Thesis, Department of Ecology, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2020. [Google Scholar]
- Raulings, E.; Morris, K.; Thompson, R.; Nally, R.M. Do birds of a feather disperse plants together?: Plant dispersal by ducks. Freshw. Biol. 2011, 56, 1390–1402. [Google Scholar] [CrossRef]
- Lovas-Kiss, Á.; Sánchez, M.I.; Wilkinson, D.M.; Coughlan, N.E.; Alves, J.A.; Green, A.J. Shorebirds as important vectors for plant dispersal in Europe. Ecography 2019, 42, 956–967. [Google Scholar] [CrossRef]
- Navarro-Ramos, M.J.; Green, A.J.; Lovas-Kiss, A.; Roman, J.; Brides, K.; van Leeuwen, C.H.A. A predatory waterbird as a vector of plant seeds and aquatic invertebrates. Freshw. Biol. 2022, 67, 657–671. [Google Scholar] [CrossRef]
- van Leeuwen, C.H.A.; Soons, M.B.; Vandionant, L.G.V.T.I.; Green, A.J.; Bakker, E.S. Seed dispersal by waterbirds: A mechanistic understanding by simulating avian digestion. Ecography 2023, 2023, e06470. [Google Scholar] [CrossRef]
- Lovas-Kiss, Á.; Martín-Vélez, V.; Brides, K.; Wilkinson, D.M.; Griffin, L.R.; Green, A.J. Migratory geese allow plants to disperse to cooler latitudes across the ocean. J. Biogeogr. 2023, 50, 1602–1614. [Google Scholar] [CrossRef]
- Espinar, J.L.; García, L.V.; Figuerola, J.; Green, A.J.; Clemente, L. Helophyte germination in a Mediterranean salt marsh: Gut-passage by ducks changes seed response to salinity. J. Veg. Sci. 2004, 15, 315–322. [Google Scholar] [CrossRef]
- Espinar, J.L.; Figuerola, J.; Green, A.J. Long term impacts of endozoochory and salinity on germination of wetland plants after entering simulated seed banks. Front. Plant Sci. 2023, 14, 1275622. [Google Scholar] [CrossRef] [PubMed]
- Carbonell, J.A.; Céspedes, V.; Green, A.J. Is the spread of the alien water boatman Trichocorixa verticalis verticalis (Hemiptera, Corixidae) aided by zoochory and drought resistant eggs? Freshw. Biol. 2021, 66, 409–420. [Google Scholar] [CrossRef]
- Muñoz-Rodríguez, A.F.; Sanjosé, I.; Márquez-García, B.; Infante-Izquierdo, M.D.; Polo-Ávila, A.; Nieva, F.J.J.; Castillo, J.M. Germination syndromes in response to salinity of Chenopodiaceae halophytes along the intertidal gradient. Aquat. Bot. 2017, 139, 48–56. [Google Scholar] [CrossRef]
- Castillo, J.M.; Curado, G.; Muñoz-Rodríguez, A.F.; Grewell, B.J. Germination syndrome divergence among pairs of sympatric sister species along an estuarine salinity gradient. Environ. Exp. Bot. 2021, 181, 104274. [Google Scholar] [CrossRef]
- Traveset, A.; Robertson, A.W.; Rodríguez-Pérez, J. A review on the role of endozoochory on seed germination. In Seed Dispersal: Theory and Its Application in a Changing World; CABI Publising, Wallingford, UK, 2007; pp. 78–103.
- Soltani, E.; Baskin, C.C.; Baskin, J.M.; Heshmati, S.; Mirfazeli, M.S. A meta-analysis of the effects of frugivory (endozoochory) on seed germination: Role of seed size and kind of dormancy. Plant Ecol. 2018, 219, 1283–1294. [Google Scholar] [CrossRef]
- Costa, J.C.; Neto, C.; Arsenio, P.; Capelo, J. Geographic variation among Iberian communities of the exotic halophyte Cotula coronopifolia. Alp. Bot. 2009, 119, 53–61. [Google Scholar] [CrossRef]
- Roger Clive, O.; Muhali Olaide, J.; Charles Petrus, L. Germination ecology of three Asteraceae annuals Arctotis hirsuta, Oncosiphon suffruticosum, and Cotula duckittiae in the winter-rainfall region of South Africa: A review. Open Agric. 2022, 7, 656–667. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Seed dormancy in Asteraceae: A global vegetation zone and taxonomic/phylogenetic assessment. Seed Sci. Res. 2023, 33, 135–169. [Google Scholar] [CrossRef]
- Peralta-Sánchez, J.M.; Ansotegui, A.; Hortas, F.; Redón, S.; Martín-Vélez, V.; Green, A.J.; Navarro-Ramos, M.J.; Lovas-Kiss, A.; Sánchez, M.I. Seed Size, Not Dispersal Syndrome, Determines Potential for Spread of Ricefield Weeds by Gulls. Plants 2023, 12, 1470. [Google Scholar] [CrossRef]
- Lovas-Kiss, Á.; Navarro-Ramos, M.J.; Vincze, O.; Löki, V.; Urgyán, R.; Pallér-Kapusi, F.; van Leeuwen, C.H.A.; Green, A.J.; Lukács, B.A. Traits for transport: Alien wetland plants gain an advantage during endozoochorous seed dispersal by waterfowl. Freshw. Biol. 2023, 68, 1703–1715. [Google Scholar] [CrossRef]
- Urgyán, R.; Lukács, B.A.; Fekete, R.; Molnár, V.A.; Nagy, A.; Vincze, O.; Green, A.J.; Lovas-Kiss, Á. Plants dispersed by a non-frugivorous migrant change throughout the annual cycle. Glob. Ecol. Biogeogr. 2023, 32, 70–82. [Google Scholar] [CrossRef]
- Martín-Vélez, V.; Lovas-Kiss, Á.; Sánchez, M.I.; Green, A.J. Endozoochory of the same community of plants lacking fleshy fruits by storks and gulls. J. Veg. Sci. 2021, 32, e12967. [Google Scholar] [CrossRef]
- Reynolds, C.; Cumming, G.S.; Vilà, M.; Green, A.J. Birds as key vectors for the dispersal of some alien species: Further thoughts. Divers. Distrib. 2017, 23, 577–580. [Google Scholar] [CrossRef]
- Wilkinson, D.M. Plants on the wing: Waterbirds and plant dispersal in Britain. Br. Wildl. 2023, 35, 167–171. [Google Scholar]
- Noe, G.B.; Zedler, J.B. Differential effects of four abiotic factors on the germination of salt marsh annuals. Am. J. Bot. 2000, 87, 1679–1692. [Google Scholar] [CrossRef]
- Goodman, A.M.; Ganf, G.G.; Dandy, G.C.; Maier, H.R.; Gibbs, M.S. The response of freshwater plants to salinity pulses. Aquat. Bot. 2010, 93, 59–67. [Google Scholar] [CrossRef]
- Lopez-Archilla, A.I.; Coleto, M.; Montes, C.; Penin, I.; Guerrero, M.C. Temporal variation of phytoplankton in two neighbouring Mediterranean shallow lakes in Doñana National Park (Spain). Limnetica 2012, 31, 289–304. [Google Scholar] [CrossRef]
- Daliakopoulos, I.N.; Tsanis, I.K.; Koutroulis, A.; Kourgialas, N.N.; Varouchakis, A.E.; Karatzas, G.P.; Ritsema, C.J. The threat of soil salinity: A European scale review. Sci. Total Environ. 2016, 573, 727–739. [Google Scholar] [CrossRef] [PubMed]
- Gassó, N.; Thuiller, W.; Pino, J.; Vilà, M. Potential distribution range of invasive plant species in Spain. NeoBiota 2012, 12, 25–40. [Google Scholar] [CrossRef]
- Julve, P. Index Botanique, écologique et Chorologique de la Flore de France. Baseflor. 1998. Available online: http://philippe.julve.pagesperso-orange.fr/catminat.htm (accessed on 12 May 2024).
- Benedí, C.; Cotula, L. Flora Iberica; Benedí, C., Buira, A., Rico, E., Crespo, M.B., Quintanar, A., Aedo, C., Eds.; Real Jardín Botánico CSIC: Madrid, Spain, 2019; Volume XVI(III), pp. 275–279. [Google Scholar]
- Müller, J.D. Ocean Acidification in the Baltic Sea: Involved Processes, Metrology of pH in Brackish Waters, and Calcification under Fluctuating Conditions; Universität Rostock, Rostock, Germany, 2018.
- Dawson, W.R.; Whittow, G.C. Regulation of body temperature. In Sturkie’s Avian Physiology, 5th ed.; Whittow, G.C., Ed.; Academic Press: Honolulu, HI, USA, 2000; pp. 343–390. [Google Scholar] [CrossRef]
- Navarro-Ramos, M.J.; Green, A.J.; de Vries, R.; van Leeuwen, C.H.A. Float, fly, then sink: Wetland plant seed buoyancy is lost after internal dispersal by waterbirds. Hydrobiologia, 2024; in press. [Google Scholar]

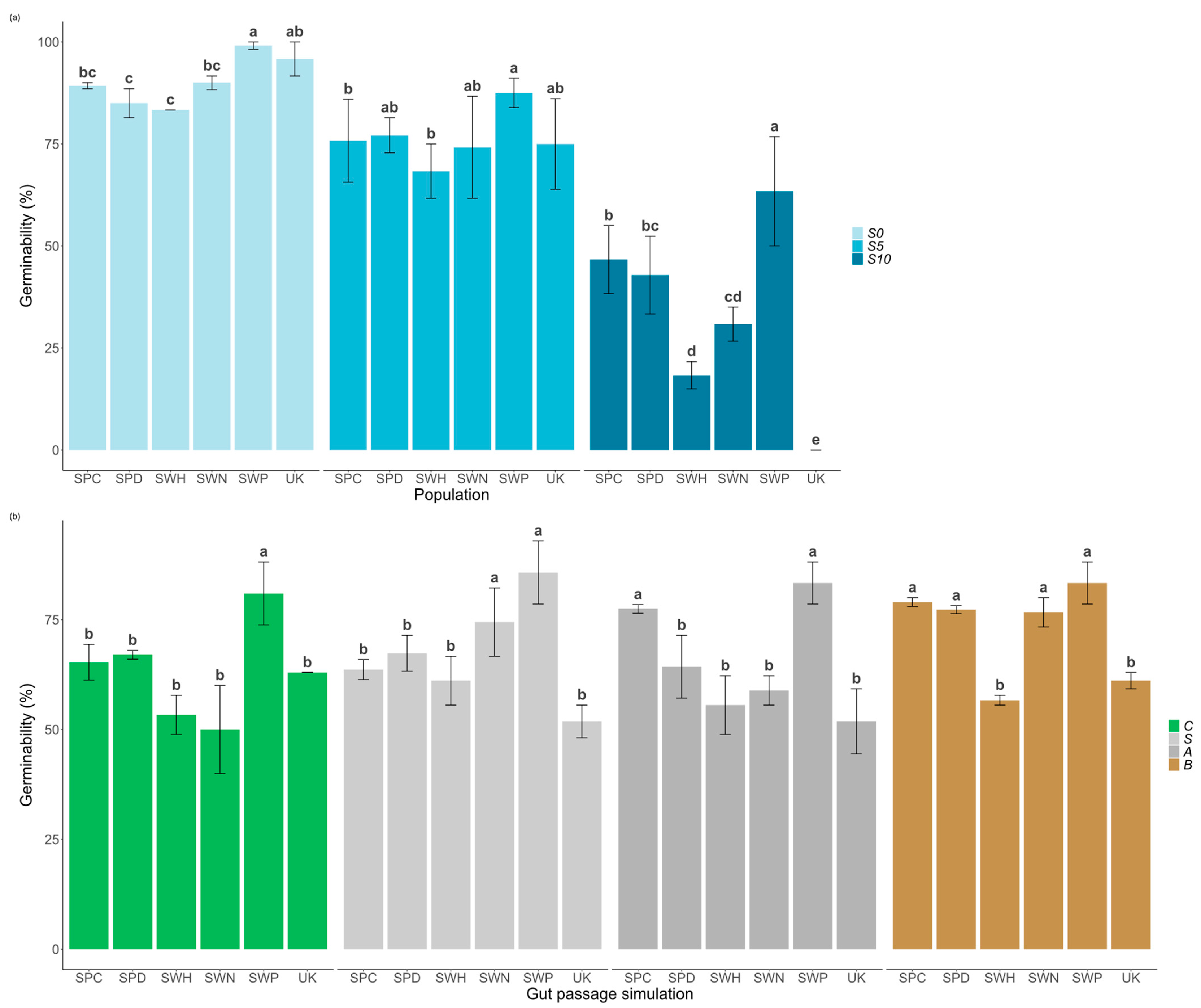
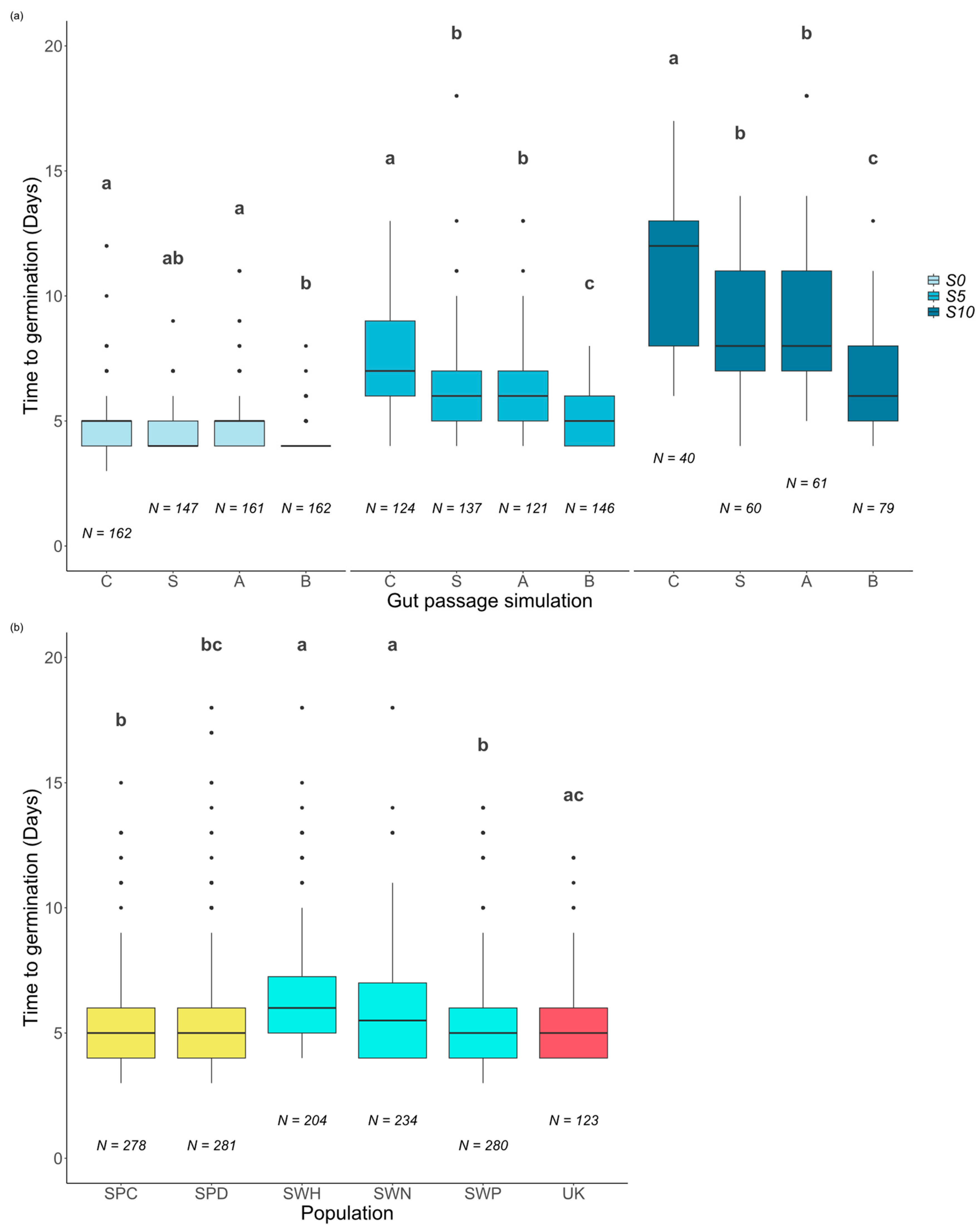
| Germinability | Df | Sum Sq | Mean Sq | F Value | Pr (>|F|) |
|---|---|---|---|---|---|
| Salinity | 2 | 107.51 | 53.75 | 355.326 | <0.001 |
| Population | 5 | 15.59 | 3.12 | 20.615 | <0.001 |
| Gut passage | 3 | 3.07 | 1.02 | 6.770 | <0.001 |
| Salinity × Population | 10 | 12.43 | 1.24 | 8.215 | <0.001 |
| Gut passage × Population | 15 | 5.61 | 0.37 | 2.470 | 0.00133 |
| Time to Germination | |||||
| Salinity | 2 | 2686 | 1343.1 | 454.56 | <0.001 |
| Population | 5 | 298 | 59.7 | 20.20 | <0.001 |
| Gut passage | 3 | 604 | 201.4 | 68.17 | <0.001 |
| Salinity × Gut passage | 6 | 330 | 54.9 | 18.60 | <0.001 |
| Sampling Site | Locality | Latitude | Longitude | Sampling Date | Conductivity/ Salinity |
|---|---|---|---|---|---|
| SPC | Salinas de Cetina, Cádiz, Spain | 36°34′31″ N | 6°08′32″ W | 8 April 2022 | 4.66 mS/cm 1 |
| SPD | Laguna Dulce, Doñana, Spain | 36°58′51.95″ N | 6°29′5.53″ W | 8 April 2022 | 0.79 mS/cm 1 |
| SWH | Halland. Sweden | 57°01′20.3″ N | 12°19′53.4″ E | 21 August 2022 | 18–26 ppt 2 |
| SWN | Nyköping. Sweden | 58°42′53.7″ N | 17°05′15.1″ E | 12 August 2022 | 6–7 ppt 2 |
| SWP | Pulken. Sweden | 55°52′59.8″ N | 14°12′21.6″ E | 5 August 2022 | <1 ppt 2 |
| UK | Hoylake, Wirral. United Kingdom | 53°23′36.1″ N | 3°11′09.7″ W | 29 July 2022 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-García, R.; Green, A.J.; Tomasson, L.; Hortas, F.; Ortiz, M.A. Invasive Buttonweed Cotula coronopifolia (Asteraceae) Is Halotolerant and Has High Potential for Dispersal by Endozoochory. Plants 2024, 13, 2219. https://doi.org/10.3390/plants13162219
Sánchez-García R, Green AJ, Tomasson L, Hortas F, Ortiz MA. Invasive Buttonweed Cotula coronopifolia (Asteraceae) Is Halotolerant and Has High Potential for Dispersal by Endozoochory. Plants. 2024; 13(16):2219. https://doi.org/10.3390/plants13162219
Chicago/Turabian StyleSánchez-García, Raúl, Andy J. Green, Lina Tomasson, Francisco Hortas, and Maria A. Ortiz. 2024. "Invasive Buttonweed Cotula coronopifolia (Asteraceae) Is Halotolerant and Has High Potential for Dispersal by Endozoochory" Plants 13, no. 16: 2219. https://doi.org/10.3390/plants13162219
APA StyleSánchez-García, R., Green, A. J., Tomasson, L., Hortas, F., & Ortiz, M. A. (2024). Invasive Buttonweed Cotula coronopifolia (Asteraceae) Is Halotolerant and Has High Potential for Dispersal by Endozoochory. Plants, 13(16), 2219. https://doi.org/10.3390/plants13162219










