Improving Rice Quality by Regulating the Heading Dates of Rice Varieties without Yield Penalties
Abstract
:1. Introduction
2. Results
2.1. Identification of Significant Loci for Heading Dates Using GWAS
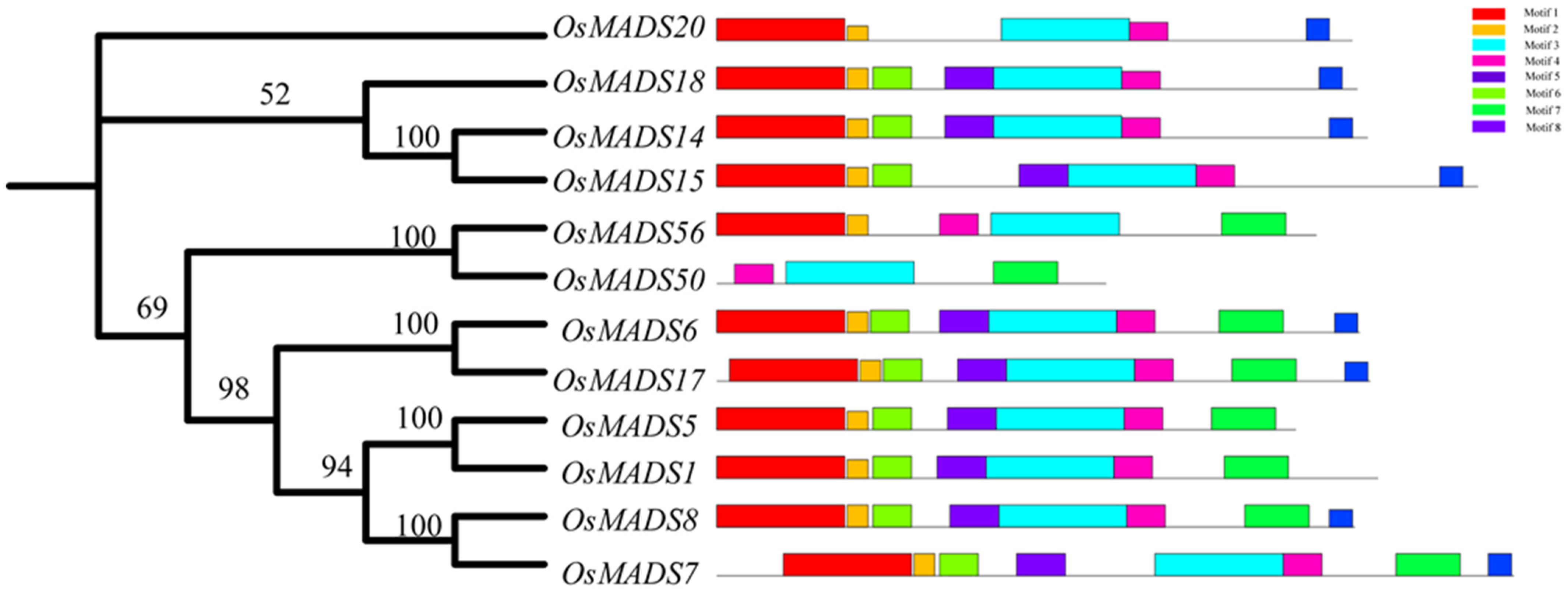
2.2. Phylogeny Analysis of OsMADS50
2.3. Protein Structural Analysis of OsMADS50
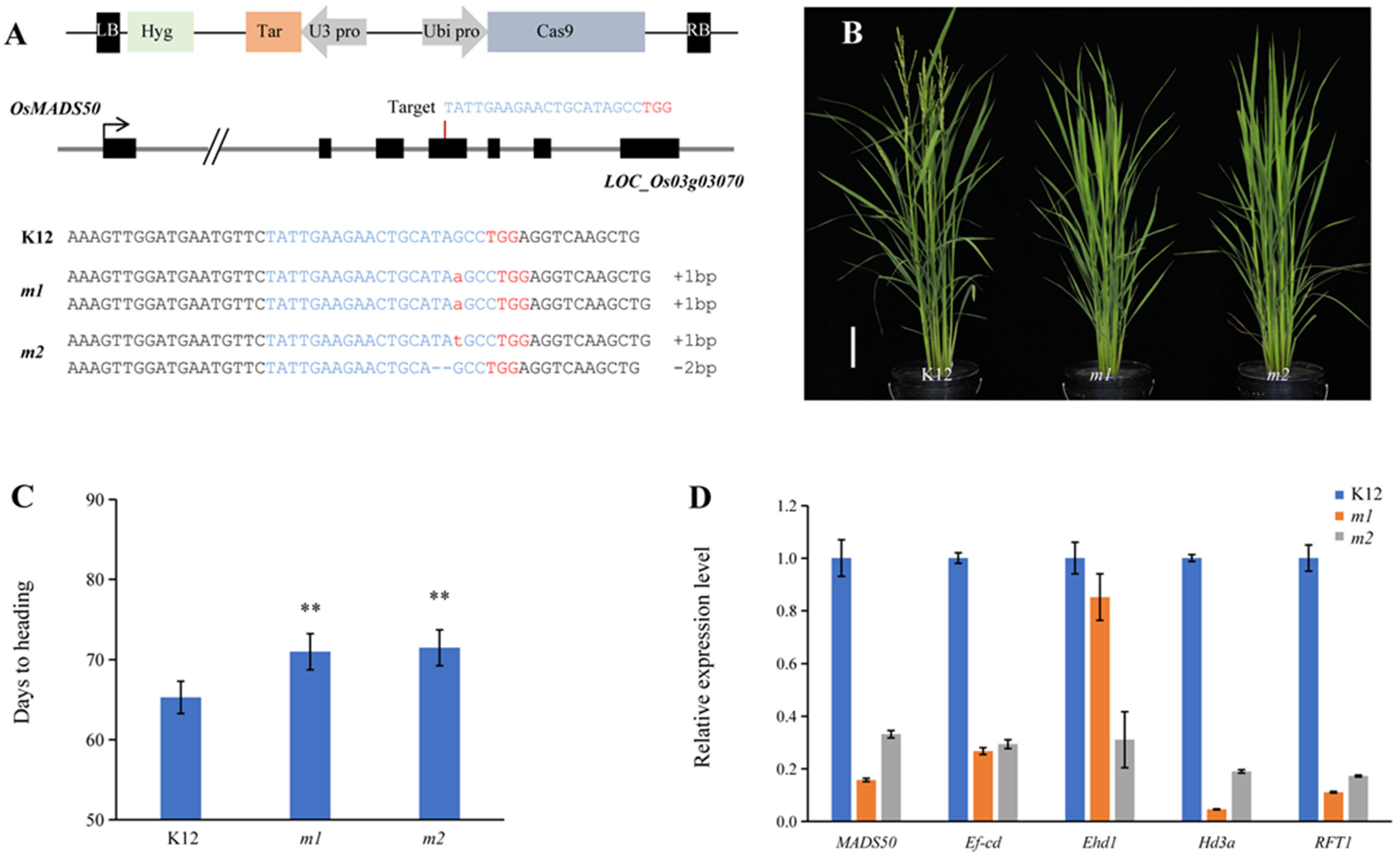
2.4. osmads50 Mutants’ Delayed Growth Period
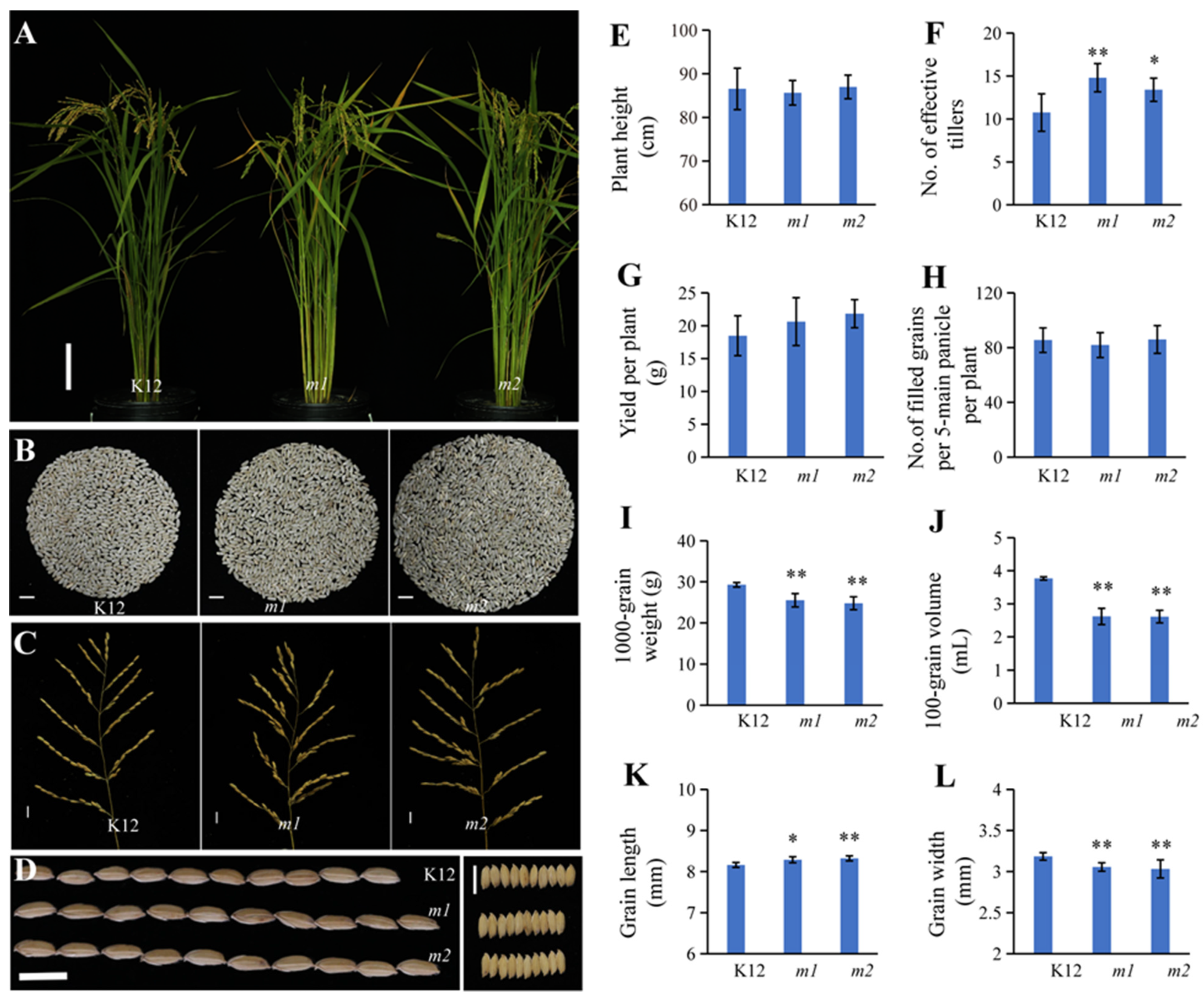
2.5. osmads50 Mutants’ Significantly Increased Tiller Numbers
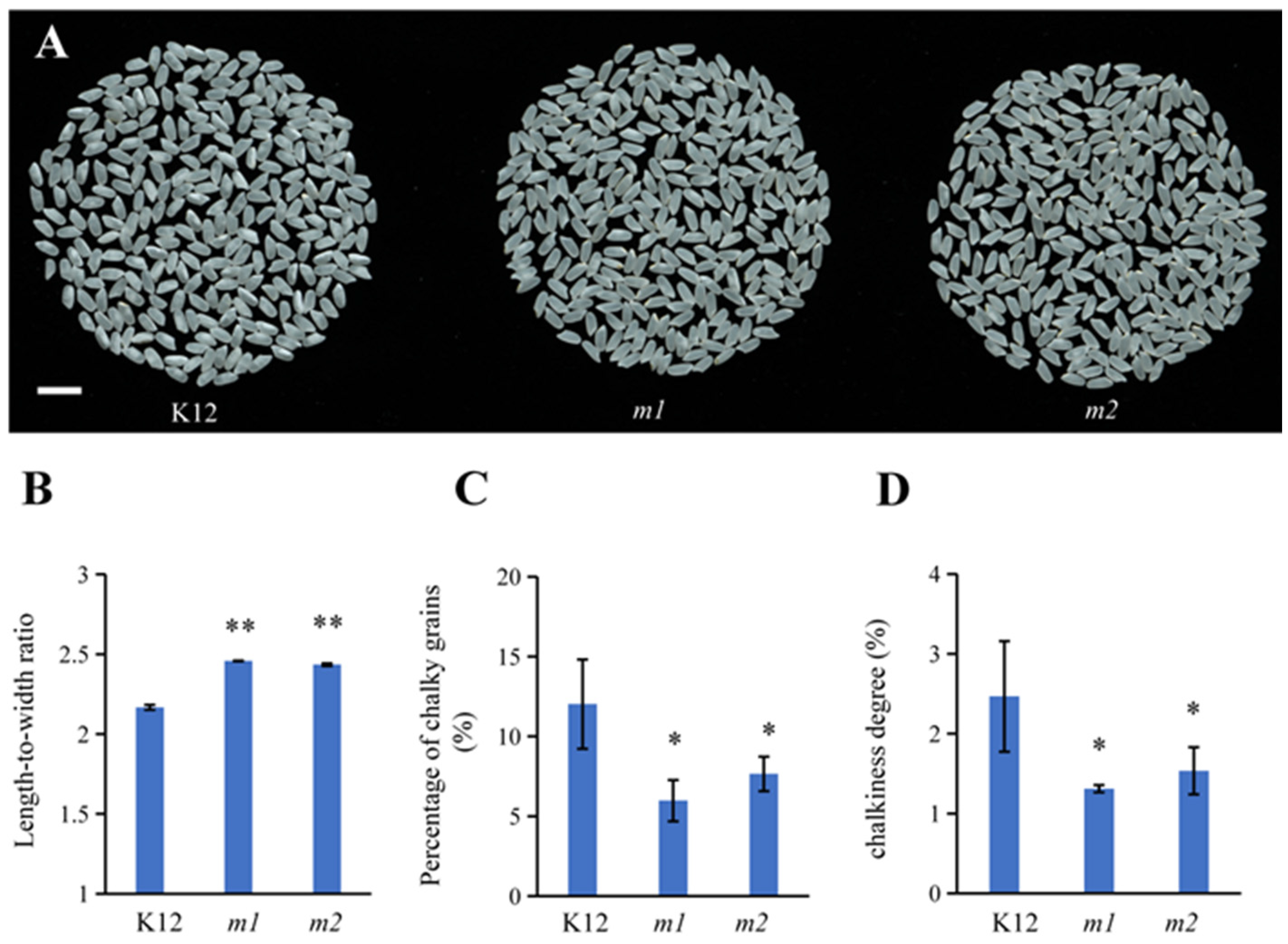
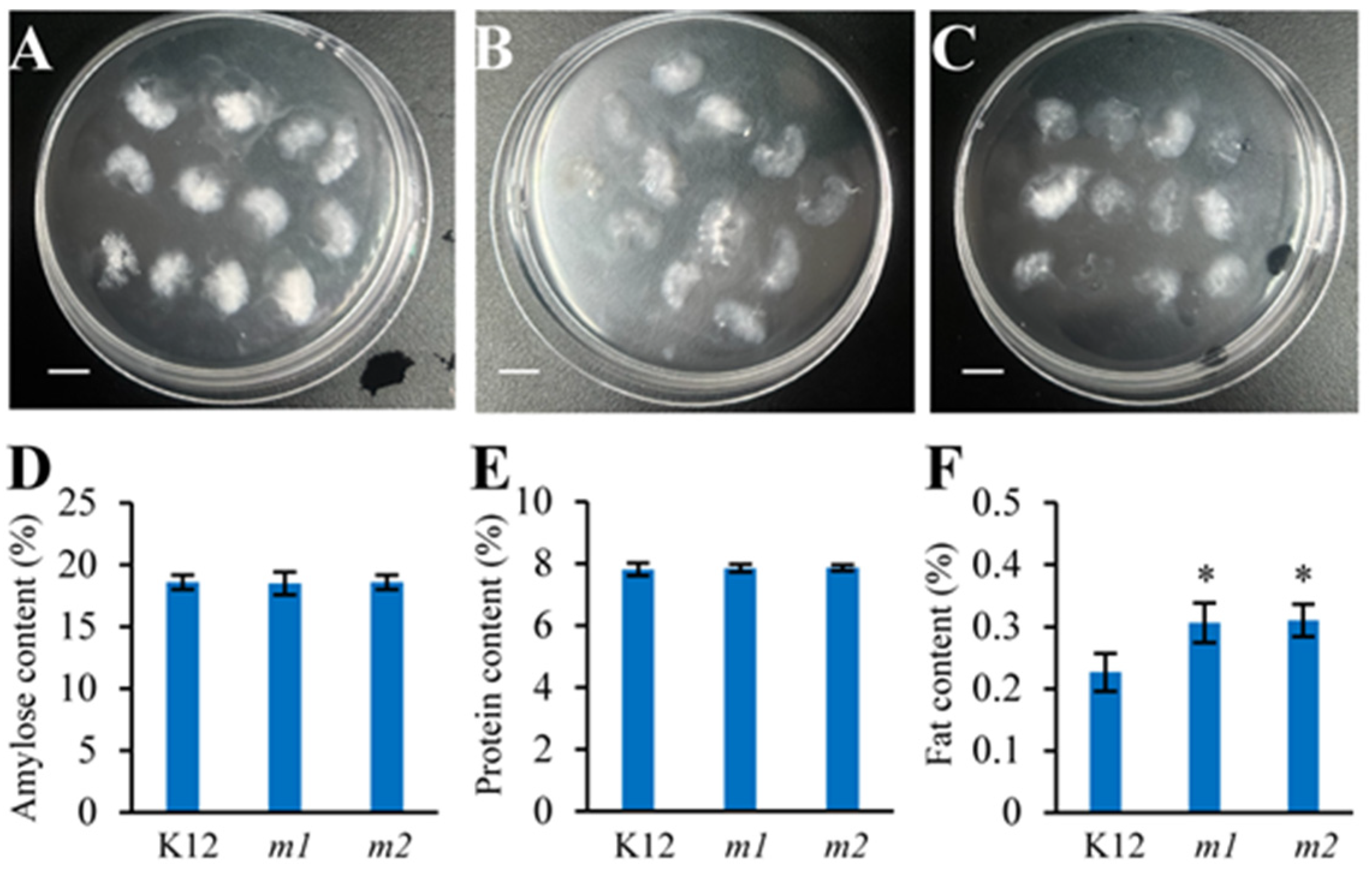
2.6. Improved Appearance Quality of osmads50 Seeds
2.7. Slightly Modified Physicochemical Properties of the osmads50 Seeds
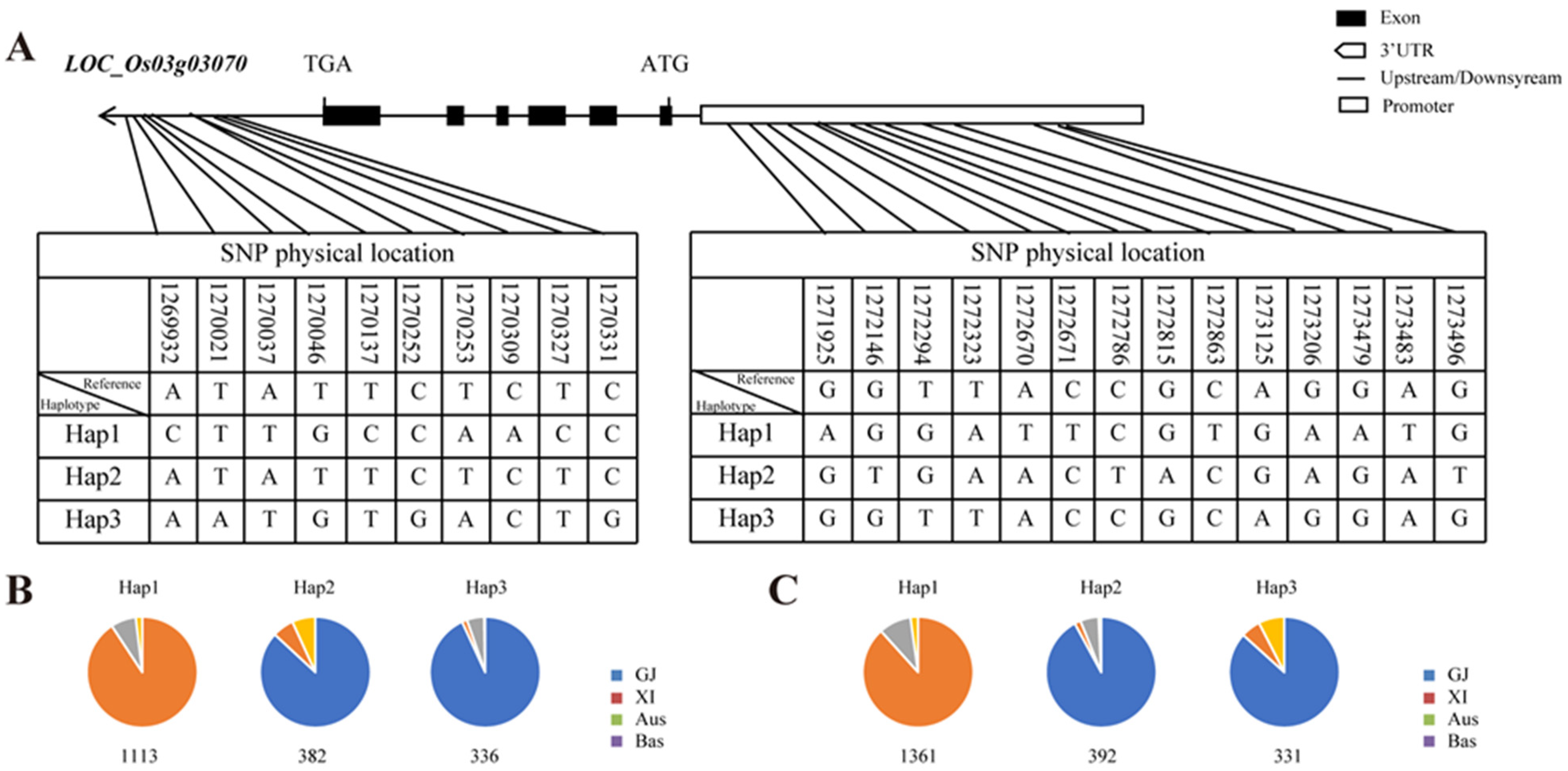
2.8. Haplotype Analysis of OsMADS50
3. Discussion
4. Materials and Methods
4.1. Genome-Wide Association Analysis (GWAS)
4.2. Plant Materials and Growth Conditions
4.3. Phylogenetic Analysis and Protein Structural Analysis
4.4. Plasmid Construction and Agrobacterium-Mediated Rice Transformation
4.5. Detection of Mutations and Transgenic-Free Plants
4.6. RNA Extraction and Quantitative Real-Time PCR (qRT-PCR)
4.7. Measurement of Rice-Yield-Related Traits
4.8. Evaluation of Rice Appearance Quality
4.9. Determination of Alkali Spreading Value in Rice
4.10. Physicochemical Analyses of Rice
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, Y.; Fu, D.B.; Zhu, C.M.; He, Y.Z.; Zhang, H.J.; Liu, T.; Li, X.H.; Wu, C.Y. The RING-Finger Ubiquitin Ligase HAF1 Mediates Heading date 1 Degradation during Photoperiodic Flowering in Rice. Plant Cell 2015, 27, 2455–2468. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Xing, Y.; Weng, X.; Zhao, Y.; Tang, W.; Wang, L.; Zhou, H.; Yu, S.; Xu, C.; Li, X.; et al. Natural variation in Ghd7 is an important regulator of heading date and yield potential in rice. Nat. Genet. 2008, 40, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Qian, Q. Rice Breeding: A Long Noncoding Locus with Great Potential. Mol. Plant 2019, 12, 1431–1433. [Google Scholar] [CrossRef] [PubMed]
- Cho, L.H.; Yoon, J.; An, G. The control of flowering time by environmental factors. Plant J. 2017, 90, 708–719. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, V.; Martignago, D.; Goretti, D.; Cerise, M.; Somssich, M.; de Rosa, M.; Galbiati, F.; Shrestha, R.; Lazzaro, F.; Simon, R.; et al. Antagonistic Transcription Factor Complexes Modulate the Floral Transition in Rice. Plant Cell 2017, 29, 2801–2816. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Teshima, K.M.; Yokoi, S.; Innan, H.; Shimamoto, K. Variations in Hd1 proteins, Hd3a promoters, and Ehd1 expression levels contribute to diversity of flowering time in cultivated rice. Proc. Natl. Acad. Sci. USA 2009, 106, 4555–4560. [Google Scholar] [CrossRef] [PubMed]
- Zong, W.; Ren, D.; Huang, M.; Sun, K.; Feng, J.; Zhao, J.; Xiao, D.; Xie, W.; Liu, S.; Zhang, H.; et al. Strong photoperiod sensitivity is controlled by cooperation and competition among Hd1, Ghd7 and DTH8 in rice heading. New Phytol. 2020, 229, 1635–1649. [Google Scholar] [CrossRef]
- Wang, G.K.; Wang, C.G.; Lu, G.H.; Wang, W.; Mao, G.F.; Habben, J.E.; Song, C.; Wang, J.T.; Chen, J.; Gao, Y.; et al. Knockouts of a late flowering gene via CRISPR-Cas9 confer early maturity in rice at multiple field locations. Plant Mol. Biol. 2020, 104, 137–150. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, H.Y.; Ren, D.; Tang, H.W.; Qiu, R.; Feng, J.L.; Long, Y.M.; Niu, B.X.; Chen, D.P.; Zhong, T.Y.; et al. Genetic interactions between diverged alleles of Early heading date 1 (Ehd1) and Heading date 3a (Hd3a)/ RICE FLOWERING LOCUS T1 (RFT1) control differential heading and contribute to regional adaptation in rice (Oryza sativa). New Phytol. 2015, 208, 936–948. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhu, M.M.; Xu, Z.J.; Xu, Q. Assessment of the effect of ten heading time genes on reproductive transition and yield components in rice using a CRISPR/Cas9 system. Theor. Appl. Genet. 2019, 132, 1887–1896. [Google Scholar] [CrossRef]
- Zhan, P.L.; Ma, S.P.; Xiao, Z.L.; Li, F.P.; Wei, X.; Lin, S.J.; Wang, X.L.; Ji, Z.; Fu, Y.; Pan, J.H.; et al. Natural variations in grain length 10 (GL10) regulate rice grain size. J. Genet. Genom. 2022, 49, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Endo-Higashi, N.; Izawa, T. Flowering time genes Heading date 1 and Early heading date 1 together control panicle development in rice Panicle Development in Rice. Plant Cell Physiol. 2011, 52, 1083–1094. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Zhang, F.T.; Wang, H.R.; Wang, W.; Zhao, F.; Li, Z.J.; Sun, C.H.; Chen, F.M.; Xu, F.; Chang, S.Q.; et al. Ef-cd locus shortens rice maturity duration without yield penalty. Proc. Natl. Acad. Sci. USA 2019, 116, 18717–18722. [Google Scholar] [CrossRef]
- Rao, N.N.; Prasad, K.; Kumar, P.R.; Vijayraghavan, U. Distinct regulatory role for RFL, the rice LFY homolog, in determining flowering time and plant architecture. Proc. Natl. Acad. Sci. USA 2008, 105, 3646–3651. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.F.; Liu, X.; Zhao, Z.G.; Jiang, L.; Gao, H.; Zhang, Y.H.; Zheng, M.; Chen, L.M.; Liu, S.J.; Zhai, H.Q.; et al. Heading date gene, dth3 controlled late flowering in O. Glaberrima Steud. by down-regulating Ehd1. Plant Cell Rep. 2011, 30, 2243–2254. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.R.; Zhu, S.S.; Cui, S.; Hou, H.G.; Wu, H.Q.; Hao, B.Y.; Cai, L.; Xu, Z.; Liu, L.L.; Jiang, L.; et al. Transcriptional and post-transcriptional regulation of heading date in rice. New Phytol. 2021, 230, 943–956. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.R.; Cai, L.; Wu, H.Q.; Wang, B.X.; Gu, B.; Cui, S.; Huang, X.L.; Xu, Z.; Hao, B.Y.; Hou, H.G.; et al. Fine-tuning rice heading date through multiplex editing of the regulatory regions of key genes by CRISPR-Cas9. Plant Biotechnol. J. 2024, 22, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.Y.; Wang, T.; Wang, L.L.; Li, X.M.; Jia, Y.C.; Liu, C.; Huang, X.H.; Xie, W.B.; Wang, X.L. Natural selection of a GSK3 determines rice mesocotyl domestication by coordinating strigolactone and brassinosteroid signaling. Nat. Commun. 2018, 9, 2523. [Google Scholar] [CrossRef]
- Yano, K.; Yamamoto, E.; Aya, K.; Takeuchi, H.; Lo, P.C.; Hu, L.; Yamasaki, M.; Yoshida, S.; Kitano, H.; Hirano, K.; et al. Genome-wide association study using whole-genome sequencing rapidly identifies new genes influencing agronomic traits in rice. Nat. Genet. 2016, 48, 927–934. [Google Scholar] [CrossRef]
- Chen, J.X.; Zhou, H.; Xie, W.B.; Xia, D.; Gao, G.J.; Zhang, Q.L.; Wang, G.W.; Lian, X.M.; Xiao, J.H.; He, Y.Q. Genome-wide association analyses reveal the genetic basis of combining ability in rice. Plant Biotechnol. J. 2019, 17, 2211–2222. [Google Scholar] [CrossRef]
- Huang, X.H.; Zhao, Y.; Wei, X.H.; Li, C.Y.; Wang, A.; Zhao, Q.; Li, W.J.; Guo, Y.L.; Deng, L.W.; Zhu, C.R.; et al. Genome-wide association study of flowering time and grain yield traits in a worldwide collection of rice germplasm. Nat. Genet. 2012, 44, 32-U53. [Google Scholar] [CrossRef] [PubMed]
- Meyer, R.S.; Choi, J.Y.; Sanches, M.; Plessis, A.; Flowers, J.M.; Amas, J.; Dorph, K.; Barretto, A.; Gross, B.; Fuller, D.Q.; et al. Domestication history and geographical adaptation inferred from a SNP map of African rice. Nat. Genet. 2016, 48, 1083–1088. [Google Scholar] [CrossRef]
- Li, H.Y.; Du, H.P.; Huang, Z.R.; He, M.L.; Kong, L.P.; Fang, C.; Chen, L.Y.; Yang, H.; Zhang, Y.H.; Liu, B.H.; et al. The AP2/ERF transcription factor TOE4b regulates photoperiodic flowering and grain yield per plant in soybean. Plant Biotechnol. J. 2023, 21, 1682–1694. [Google Scholar] [CrossRef] [PubMed]
- Misra, G.; Badoni, S.; Parween, S.; Singh, R.K.; Leung, H.; Ladejobi, O.; Mott, R.; Sreenivasulu, N. Genome-wide association coupled gene to gene interaction studies unveil novel epistatic targets among major effect loci impacting rice grain chalkiness. Plant Biotechnol. J. 2021, 19, 910–925. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.F.; Zhao, X.R.; Wang, X.M.; Hathorn, A.; Hunt, C.; Cruickshank, A.W.; van Oosterom, E.J.; Godwin, I.D.; Mace, E.S.; Jordan, D.R. Large-scale GWAS in sorghum reveals common genetic control of grain size among cereals. Plant Biotechnol. J. 2020, 18, 1093–1105. [Google Scholar] [CrossRef]
- Zhao, L.; Zheng, Y.T.; Wang, Y.; Wang, S.S.; Wang, T.Z.; Wang, C.G.; Chen, Y.; Zhang, K.P.; Zhang, N.; Dong, Z.D.; et al. A HST1-like gene controls tiller angle through regulating endogenous auxin in common wheat. Plant Biotechnol. J. 2023, 21, 122–135. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.T.; Zou, J.P.; Yang, X.; Wang, K.J.; Rao, Y.C.; Wang, C. Development and Application of Prime Editing in Plants. Rice Sci. 2023, 30, 509–522. [Google Scholar]
- Cui, Y.; Xu, Z.J.; Xu, Q. Elucidation of the relationship between yield and heading date using CRISPR/Cas9 system-induced mutation in the flowering pathway across a large latitudinal gradient. Mol. Breed. 2021, 41, 23. [Google Scholar] [CrossRef]
- Li, S.T.; Luo, Y.Q.; Wei, G.L.; Zong, W.B.; Zeng, W.Y.; Xiao, D.D.; Zhang, H.; Song, Y.A.; Hao, Y.; Sun, K.L.; et al. Improving yield-related traits by editing the promoter of the heading date gene Ehd1 in rice. Theor. Appl. Genet. 2023, 136, 239. [Google Scholar] [CrossRef]
- Liu, X.; Liu, H.; Zhang, Y.; He, M.; Li, R.; Meng, W.; Wang, Z.; Li, X.; Bu, Q. Fine-tuning Flowering Time via Genome Editing of Upstream Open Reading Frames of Heading Date 2 in Rice. Rice 2021, 14, 59. [Google Scholar] [CrossRef]
- Wang, T.Y.; He, W.C.; Li, X.X.; Zhang, C.; He, H.Y.; Yuan, Q.L.; Zhang, B.; Zhang, H.; Leng, Y.; Wei, H.; et al. A rice variation map derived from 10 548 rice accessions reveals the importance of rare variants. Nucleic Acids Res. 2023, 51, 10924–10933. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.G.; Li, X.X.; He, H.Y.; Yuan, Q.L.; Song, Y.N.; Wei, Z.R.; Lin, H.; Hu, M.; Zhao, F.L.; Zhang, C.; et al. A super pan-genomic landscape of rice. Cell Res. 2022, 32, 878–896. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, J.; Han, J.J.; Han, M.J.; An, G. Functional analyses of the flowering time gene OsMADS50, the putative SUPPRESSOR OF OVEREXPRESSION OF CO 1/AGAMOUS-LIKE 20 (SOC1/AGL20) ortholog in rice. Plant J. 2004, 38, 754–764. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.L.; Zhou, H.Z.; Wu, Y.R.; Zhang, H.; Lin, J.; Jiang, X.Y.; He, Q.J.; Zhu, J.S.; Li, Y.; Yu, H.; et al. OsSPL3, an SBP-Domain Protein, Regulates Crown Root Development in Rice. Plant Cell 2019, 31, 1257–1275. [Google Scholar] [CrossRef] [PubMed]
- Ryu, C.H.; Lee, S.; Cho, L.H.; Kim, S.L.; Lee, Y.S.; Choi, S.C.; Jeong, H.J.; Yi, J.; Park, S.J.; Han, C.D.; et al. OsMADS50 and OsMADS56 function antagonistically in regulating long day (LD)-dependent flowering in rice. Plant Cell Environ. 2009, 32, 1412–1427. [Google Scholar] [CrossRef]
- Latif, A.; Azam, S.; Shahid, N.; Javed, M.R.; Haider, Z.; Yasmeen, A.; Sadaqat, S.; Shad, M.; Husnain, T.; Rao, A.Q. Overexpression of the AGL42 gene in cotton delayed leaf senescence through downregulation of NAC transcription factors. Sci. Rep. 2022, 12, 21093. [Google Scholar] [CrossRef]
- Chen, W.H.; Lin, P.T.; Hsu, W.H.; Hsu, H.F.; Li, Y.C.; Tsao, C.W.; Hsu, M.C.; Mao, W.T.; Yang, C.H. Regulatory network for FOREVER YOUNG FLOWER-like genes in regulating Arabidopsis flower senescence and abscission. Commun. Biol. 2022, 5, 662. [Google Scholar] [CrossRef]
- Mateo-Bonmati, E.; Esteve-Bruna, D.; Juan-Vicente, L.; Nadi, R.; Candela, H.; Lozano, F.M.; Ponce, M.R.; Pérez-Pérez, J.M.; Micol, J.L. INCURVATA11 and CUPULIFORMIS2 Are Redundant Genes That Encode Epigenetic Machinery Components in Arabidopsis. Plant Cell 2018, 30, 1596–1616. [Google Scholar] [CrossRef]
- Ma, J.; Yang, Y.J.; Luo, W.; Yang, C.C.; Ding, P.Y.; Liu, Y.X.; Qiao, L.Y.; Chang, Z.J.; Geng, H.W.; Wang, P.H.; et al. Genome-wide identification and analysis of the MADS-box gene family in bread wheat (Triticum aestivum L.). PLoS ONE 2017, 12, e0181443. [Google Scholar] [CrossRef]
- Fujino, K. Days to heading, controlled by the heading date genes, Hd1 and DTH8, limits rice yield-related traits in Hokkaido, Japan. Breed. Sci. 2020, 70, 277–282. [Google Scholar] [CrossRef]
- Choi, S.C.; Lee, S.; Kim, S.R.; Lee, Y.S.; Liu, C.Y.; Cao, X.F.; An, G. Trithorax group protein Oryza sativa Trithorax1 controls flowering time in rice via interaction with early heading date3. Plant Physiol. 2014, 164, 1326–1337. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.S.; Liu, Q.Q.; Zhang, C.Q.; Li, Q.F. Genetic control of grain appearance quality in rice. Biotechnol. Adv. 2022, 60, 108014. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lu, J.; Shi, W.J.; Chen, Y.H.; Yu, J.W.; Chen, S.H.; Zhao, D.S.; Huang, L.C.; Fan, X.L.; Zhang, C.Q.; et al. RGA1 regulates grain size, rice quality and seed germination in the small and round grain mutant srg5. BMC Plant Biol. 2024, 24, 167. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.; Jighly, A.; Joukhadar, R.; Niazi, N.K.; Al-Misned, F. Current Status and Future Prospects of Head Rice Yield. Agriculture 2023, 13, 705. [Google Scholar] [CrossRef]
- Zhou, Z.; Robards, K.; Helliwell, S.; Blanchard, C. Composition and functional properties of rice. Int. J. Food Sci. Technol. 2002, 37, 849–868. [Google Scholar] [CrossRef]
- Wang, C.C.; Yu, H.; Huang, J.; Wang, W.S.; Faruquee, M.; Zhang, F.; Zhao, X.Q.; Fu, B.Y.; Chen, K.; Zhang, H.L.; et al. Towards a deeper haplotype mining of complex traits in rice with RFGB v2.0. Plant Biotechnol. J. 2020, 18, 14–16. [Google Scholar] [CrossRef]
- Wang, L.W.; Wang, C.M.; Wang, Y.H.; Niu, M.; Ren, Y.L.; Zhou, K.N.; Zhang, H.; Lin, Q.B.; Wu, F.Q.; Cheng, Z.J.; et al. WSL3, a component of the plastid-encoded plastid RNA polymerase, is essential for early chloroplast development in rice. Plant Mol. Biol. 2016, 92, 581–595. [Google Scholar] [CrossRef]
- Fu, F.F.; Xue, H.W. Coexpression analysis identifies Rice Starch Regulator1, a rice AP2/EREBP family transcription factor, as a novel rice starch biosynthesis regulator. Plant Physiol. 2010, 154, 927–938. [Google Scholar] [CrossRef]
- Qiu, L.L.; Zhou, P.; Wang, H.; Zhang, C.; Du, C.X.; Tian, S.J.; Wu, Q.Q.; Wei, L.T.; Wang, X.Y.; Zhou, Y.M.; et al. Photoperiod Genes Contribute to Daylength-Sensing and Breeding in Rice. Plants 2023, 12, 899. [Google Scholar] [CrossRef]
- Cho, L.H.; Yoon, J.; Tun, W.; Baek, G.; Peng, X.; Hong, W.J.; Mori, I.C.; Hojo, Y.; Matsuura, T.; Kim, S.R.; et al. Cytokinin increases vegetative growth period by suppressing florigen expression in rice and maize. Plant J. 2022, 110, 1619–1635. [Google Scholar] [CrossRef]
- Hori, K.; Matsubara, K.; Yano, M. Genetic control of flowering time in rice: Integration of Mendelian genetics and genomics. Theor. Appl. Genet. 2016, 129, 2241–2252. [Google Scholar] [CrossRef] [PubMed]
- Nakamichi, N.; Kudo, T.; Makita, N.; Kiba, T.; Kinoshita, T.; Sakakibara, H. Flowering time control in rice by introducing Arabidopsis clock-associated PSEUDO-RESPONSE REGULATOR 5. Biosci. Biotechnol. Biochem. 2020, 84, 970–979. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.N.; Feng, Q.; Miao, J.S.; Zhu, J.J.; Zhou, C.C.; Fan, D.L.; Lu, Y.Q.; Tian, Q.L.; Wang, Y.C.; Zhan, Q.L.; et al. The WD40 domain-containing protein Ehd5 positively regulates flowering in rice (Oryza sativa). Plant Cell 2023, 35, 4002–4019. [Google Scholar] [CrossRef] [PubMed]
- Komiya, R.; Ikegami, A.; Tamaki, S.; Yokoi, S.; Shimamoto, K. Hd3a and RFT1 are essential for flowering in rice. Development 2008, 135, 767–774. [Google Scholar] [CrossRef]
- Komiya, R.; Yokoi, S.; Shimamoto, K. A gene network for long-day flowering activates RFT1 encoding a mobile flowering signal in rice. Development 2009, 136, 3443–3450. [Google Scholar] [CrossRef] [PubMed]
- Doi, K.; Izawa, T.; Fuse, T.; Yamanouchi, U.; Kubo, T.; Shimatani, Z.; Yano, M.; Yoshimura, A. Ehd1, a B-type response regulator in rice, confers short-day promotion of flowering and controls FT-like gene expression independently of Hd1. Genes Dev. 2004, 18, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.H.; Fang, J.; Zhao, T.L.; Xu, B.; Zhang, F.T.; Liu, L.C.; Tang, J.Y.; Zhang, G.F.; Deng, X.J.; Chen, F.; et al. The histone methyltransferase SDG724 mediates H3K36me2/3 deposition at MADS50 and RFT1 and promotes flowering in rice. Plant Cell 2012, 24, 3235–3247. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.B.; Yan, Z.Q.; Guan, J.N.; Huo, Y.Q.; Wang, T.Q.; Li, T.; Cui, Z.B.; Ma, W.H.; Wang, X.X.; Chen, W.F. Two interacting basic helix-loop-helix transcription factors control flowering time in rice. Plant Physiol. 2023, 192, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, H.; Tachibana, C.; Tamaki, S.; Taoka, K.; Kyozuka, J.; Shimamoto, K. Hd3a promotes lateral branching in rice. Plant J. 2015, 82, 256–266. [Google Scholar] [CrossRef]
- Kobayashi, K.; Maekawa, M.; Miyao, A.; Hirochika, H.; Kyozuka, J. PANICLE PHYTOMER2 (PAP2), encoding a SEPALLATA subfamily MADS-box protein, positively controls spikelet meristem identity in rice. Plant Cell Physiol. 2010, 51, 47–57. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, H.P.; Liu, J.; Wang, W.; Sun, J.; Gao, Q.; Zhang, Y.H.; Ma, D.R.; Wang, J.Y.; Xu, Z.J.; et al. Loss of function of OsMADS34 leads to large sterile lemma and low grain yield in rice (Oryza sativa L.). Mol. Breed. 2016, 36, 147. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Wang, C.; Fu, Y.P.; Wang, J.J.; Liu, Q.; Zhang, X.M.; Yan, C.J.; Qian, Q.; Wang, K.J. QTL editing confers opposing yield performance in different rice varieties. J. Integr. Plant Biol. 2018, 60, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.L.; Chen, L.T.; Zhu, Q.L.; Chen, Y.L.; Liu, Y.G. Rapid Decoding of Sequence-Specific Nuclease-Induced Heterozygous and Biallelic Mutations by Direct Sequencing of PCR Products. Mol. Plant 2015, 8, 1285–1287. [Google Scholar] [CrossRef]
- Febina, M.; John, D.; Raman, M. Physicochemical properties, eating and cooking quality and genetic variability: A comparative analysis in selected rice varieties of South India. Food Prod. Process. Nutr. 2023, 5, 49. [Google Scholar]
- Lu, H.; Peng, B.; Feng, X.; Shen, X. Model Optimization for Determination of Amylose, Protein, Fat and Moisture Content in Rice by Near-infrared Spectroscopy. China Rice 2020, 26, 55–59. [Google Scholar]


| QTL | Chr. | Physical Region (nt) | Significant | Lead SNP | Group | Co-Location Loci | |
|---|---|---|---|---|---|---|---|
| SNPs | Position (nt) | LMM-log10(P) | |||||
| q1 | 6 | 9,138,220–9,538,220 | 1 | 9,338,220 | 7.29 | All; XI | Hd1 |
| q2 | 5 | 960,013–1,360,013 | 1 | 1,160,013 | 7.99 | XI | RSR1 |
| q3 | 3 | 1,070,331–1,470,331 | 6 | 1,270,331 | 10.63 | All; GJ; XI | OsMADS50 |
| q4 | 10 | 16,609,046–17,021,303 | 26 | 16,809,046 | 8.82 | All | / |
| q5 | 10 | 17,029,581–17,429,581 | 25 | 17,228,554 | 7.71 | All; GJ | WSL3; FLO7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Yi, Q.; Dong, G.; Chen, Y.; Guo, L.; Gao, Z.; Zhu, L.; Ren, D.; Zhang, Q.; Li, Q.; et al. Improving Rice Quality by Regulating the Heading Dates of Rice Varieties without Yield Penalties. Plants 2024, 13, 2221. https://doi.org/10.3390/plants13162221
Liu J, Yi Q, Dong G, Chen Y, Guo L, Gao Z, Zhu L, Ren D, Zhang Q, Li Q, et al. Improving Rice Quality by Regulating the Heading Dates of Rice Varieties without Yield Penalties. Plants. 2024; 13(16):2221. https://doi.org/10.3390/plants13162221
Chicago/Turabian StyleLiu, Jianguo, Qinqin Yi, Guojun Dong, Yuyu Chen, Longbiao Guo, Zhenyu Gao, Li Zhu, Deyong Ren, Qiang Zhang, Qing Li, and et al. 2024. "Improving Rice Quality by Regulating the Heading Dates of Rice Varieties without Yield Penalties" Plants 13, no. 16: 2221. https://doi.org/10.3390/plants13162221










