Effect of Hemp Extraction Procedures on Cannabinoid and Terpenoid Composition
Abstract
:1. Introduction
2. Results
2.1. Quantification of Terpenes, Cannabinoids, and Extraction Yield
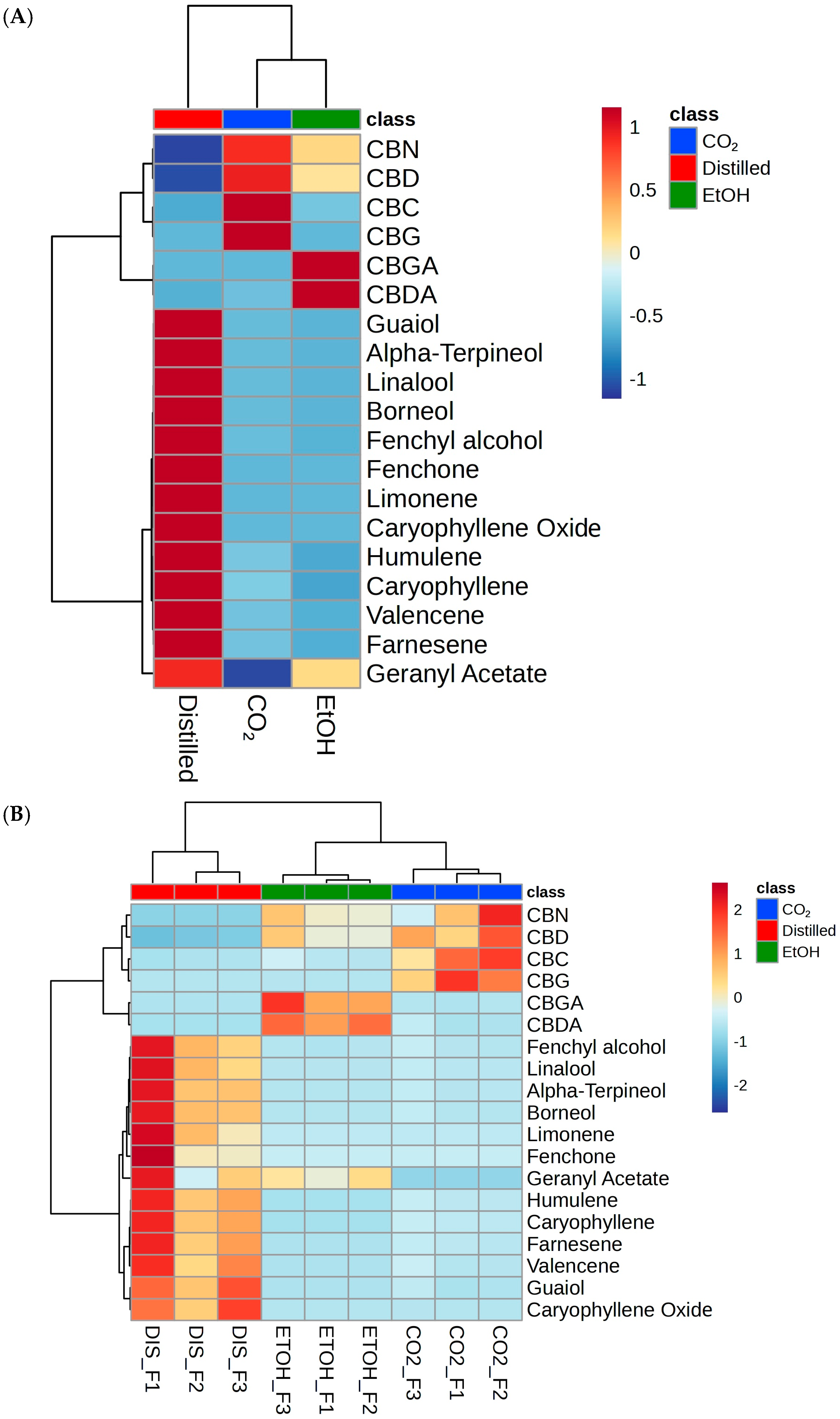
2.2. Statistical Analysis of Feature Relative Abundance
3. Discussion
4. Materials and Methods
4.1. Hemp Material and Solvents
4.2. Solvent Extraction
4.3. Hydrodistillation
4.4. Supercritical CO2 Extraction
4.5. LC-MS/MS
4.6. GC-MS
4.7. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sommano, S.R.; Chittasupho, C.; Ruksiriwanich, W.; Jantrawut, P. The cannabis terpenes. Molecules 2020, 25, 5792. [Google Scholar] [CrossRef] [PubMed]
- Breijyeh, Z.; Jubeh, B.; Bufo, S.A.; Karaman, R.; Scrano, L. Cannabis: A toxin-producing plant with potential therapeutic uses. Toxins 2021, 13, 117. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; Dai, K.; Xie, Z.; Chen, J. Secondary Metabolites Profiled in Cannabis Inflorescences, Leaves, Stem Barks, and Roots for Medicinal Purposes. Sci. Rep. 2020, 10, 3309. [Google Scholar] [CrossRef] [PubMed]
- Radwan, M.M.; Chandra, S.; Gul, S.; ElSohly, M.A. Cannabinoids, phenolics, terpenes and alkaloids of cannabis. Molecules 2021, 26, 2774. [Google Scholar] [CrossRef] [PubMed]
- Taura, F.; Sirikantaramas, S.; Shoyama, Y.; Yoshikai, K.; Shoyama, Y.; Morimoto, S. Cannabidiolic-acid synthase, the chemotype-determining enzyme in the fiber-type Cannabis sativa. FEBS Lett. 2007, 581, 2929–2934. [Google Scholar] [CrossRef] [PubMed]
- Kearsey, L.J.; Prandi, N.; Karuppiah, V. Structure of the Cannabis sativa olivetol-producing enzyme reveals cyclization plasticity in type III polyketide synthases. FEBS J. 2020, 287, 1511–1524. [Google Scholar] [CrossRef]
- Tahir, M.N.; Shahbazi, F.; Rondeau-Gagné, S.; Trant, J.F. The biosynthesis of the cannabinoids. J. Cannabis Res. 2021, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Gagne, S.J.; Stout, J.M.; Liu, E.; Boubakir, Z.; Clark, S.M.; Page, J.E. Identification of olivetolic acid cyclase from Cannabis sativa reveals a unique catalytic route to plant polyketides. Proc. Natl. Acad. Sci. USA 2012, 109, 12811–12816. [Google Scholar] [CrossRef]
- Fellermeier, M.; Zenkm, M.H. Prenylation of olivetolate by a hemp transferase yields cannabigerolic acid, the precursor of tetrahydrocannabinol. FEBS Lett. 1998, 427, 283–285. [Google Scholar] [CrossRef]
- Flores-Sanchez, I.J.; Verpoorte, R. Plant Polyketide Synthases: A fascinating group of enzymes. Plant Physiol. Biochem. 2009, 47, 167–174. [Google Scholar] [CrossRef]
- Booth, J.K.; Page, J.E.; Bohlmann, J. Terpene synthases from Cannabis sativa. PLoS ONE 2017, 12, e0173911. [Google Scholar] [CrossRef]
- Günnewich, N.; Page, J.E.; Köllner, T.G.; Degenhardt, J.; Kutchan, T.M. Functional expression and characterization of trichome-specific (-)-limonene synthase and (+)-α-pinene synthase from Cannabis sativa. Nat. Prod. Commun. 2007, 2, 1934578X0700200. [Google Scholar] [CrossRef]
- Booth, J.K.; Yuen, M.M.S.; Jancsik, S.; Madilao, L.L.; Page, J.E.; Bohlmann, J. Terpene synthases and terpene variation in Cannabis sativa. Plant Physiol. 2020, 184, 130–147. [Google Scholar] [CrossRef]
- Miziorko, H.M. Enzymes of the mevalonate pathway of isoprenoid biosynthesis. Arch. Biochem. Biophys. 2011, 505, 131–143. [Google Scholar] [CrossRef]
- McGarvey, D.J.; Croteau, R. Terpenoid metabolism. Plant Cell 1995, 7, 1015–1026. [Google Scholar] [CrossRef]
- Hanuš, L.O.; Hod, Y. Terpenes/Terpenoids in Cannabis: Are they important? Med. Cannabis Cannabinoids 2020, 3, 25–60. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, E.; Lin, F. Terpene Biosynthesis: Modularity rules. Angew. Chem. Int. Ed. 2012, 51, 1124–1137. [Google Scholar] [CrossRef]
- Jiang, S.Y.; Jin, J.; Sarojam, R.; Ramachandran, S. A comprehensive survey on the terpene synthase gene family provides new insight into its evolutionary patterns. Genome Biol. Evol. 2019, 11, 2078–2098. [Google Scholar] [CrossRef]
- Chacon, F.T.; Raup-Konsavage, W.M.; Vrana, K.E.; Kellogg, J.J. Secondary terpenes in Cannabis sativa L.: Synthesis and synergy. Biomedicines 2022, 10, 3142. [Google Scholar] [CrossRef]
- Baratta, F.; Pignata, I.; Ravetto, E.L.; Brusa, P. Cannabis for medical use: Analysis of recent clinical trials in view of current legislation. Front. Pharmacol. 2022, 13, 888903. [Google Scholar] [CrossRef] [PubMed]
- Adesso, M.; Laser, P.; Mills, A. An overview of industrial hemp law in the United States. UDC Law Rev. 2019, 22, 85–102. [Google Scholar]
- Ramirez, C.L.; Fanovich, M.A.; Churio, M.S. Chapter 4—Cannabinoids: Extraction Methods, Analysis, and Physicochemical Characterization. In Studies in Natural Products Chemistry; Atta-ur-Rahman, F., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 61, pp. 143–173. [Google Scholar] [CrossRef]
- Karğılı, U.; Ezgi, A. Evaluation of cannabinoid (CBD and THC) content of four different strains of cannabis grown in four different regions. Eur. Food Res. Technol. 2022, 248, 1351–1364. [Google Scholar] [CrossRef]
- Montero, L.; Meckelmann, S.W.; Kim, H.; Ayala-Cabrera, J.F.; Schmitz, O.J. Differentiation of industrial hemp strains by their cannabinoid and phenolic compounds using LC × LC-HRMS. Anal. Bioanal. Chem. 2022, 414, 5445–5459. [Google Scholar] [CrossRef]
- Peterswald, T.J.; Mieog, J.C.; Azman, H.R.; Magner, N.J.; Trebilco, A.; Kretzschmar, T.; Purdy, S.J. Moving Away from 12:12; the Effect of Different Photoperiods on Biomass Yield and Cannabinoids in Medicinal Cannabis. Plants 2023, 12, 1061. [Google Scholar] [CrossRef] [PubMed]
- Addo, P.W.; Poudineh, Z.; Shearer, M.; Taylor, N.; MacPherson, S.; Raghavan, V.; Orsat, V.; Lefsrud, M. Relationship between Total Antioxidant Capacity, Cannabinoids and Terpenoids in Hops and Cannabis. Plants 2023, 12, 1225. [Google Scholar] [CrossRef] [PubMed]
- Birenboim, M.; Brikenstein, N.; Duanis-Assaf, D.; Maurer, D.; Chalupowicz, D.; Kenigsbuch, D.; Shimshoni, J.A. In Pursuit of Optimal Quality: Cultivar-Specific Drying Approaches for Medicinal Cannabis. Plants 2024, 13, 1049. [Google Scholar] [CrossRef] [PubMed]
- Rožanc, J.; Kotnik, P.; Milojević, M. Different Cannabis sativa extraction methods result in different biological activities against a colon cancer cell line and healthy colon cells. Plants 2021, 10, 566. [Google Scholar] [CrossRef] [PubMed]
- Lazarjani, M.P.; Young, O.; Kebede, L.; Seyfoddin, A. Processing and extraction methods of medicinal cannabis: A narrative review. J. Cannabis Res. 2021, 3, 32. [Google Scholar] [CrossRef]
- Pattnaik, F.; Nanda, S.; Mohanty, S. Cannabis: Chemistry, extraction and therapeutic applications. Chemosphere 2022, 289, 133012. [Google Scholar] [CrossRef]
- Al Ubeed, H.M.S.; Bhuyan, D.J.; Alsherbiny, M.A.; Basu, A.; Vuong, Q.V. A comprehensive review on the techniques for extraction of bioactive compounds from medicinal cannabis. Molecules 2022, 27, 604. [Google Scholar] [CrossRef]
- Sainz Martinez, A.; Lanaridi, O.; Stagel, K.; Halbwirth, H.; Schnürch, M.; Bica-Schröder, K. Extraction techniques for bioactive compounds of cannabis. Nat. Prod. Rep. 2023, 40, 676–717. [Google Scholar] [CrossRef]
- Qamar, S.; Torres, Y.J.M.; Parekh, H.S.; Falconer, R.J. Extraction of medicinal cannabinoids through supercritical carbon dioxide technologies: A review. J. Chromatogr. B 2021, 1167, 122581. [Google Scholar] [CrossRef]
- Uwineza, P.A.; Waśkiewicz, A. Recent advances in supercritical fluid extraction of natural bioactive compounds from natural plant materials. Molecules 2020, 25, 3847. [Google Scholar] [CrossRef]
- Dhawan, D.; Gupta, J. Comparison of different solvents for phytochemical extraction potential from Datura metel plant leaves. Int. J. Biol. Chem. 2016, 11, 17–22. [Google Scholar] [CrossRef]
- Bowen, J.K.; Chaparro, J.M.; McCorkle, A.M. The impact of extraction protocol on the chemical profile of cannabis extracts from a single cultivar. Sci. Rep. 2021, 11, 2180. [Google Scholar] [CrossRef]
- Isidore, E.; Karim, H.; Ioannou, I. Extraction of phenolic compounds and terpenes from Cannabis sativa L. by-products: From conventional to intensified processes. Antioxidants 2021, 10, 942. [Google Scholar] [CrossRef]
- Tiago, F.J.; Paiva, A.; Matias, A.A.; Duarte, A.R.C. Extraction of Bioactive Compounds from Cannabis sativa L. Flowers and/or Leaves Using Deep Eutectic Solvents. Front. Nutr. 2022, 9, 892314. [Google Scholar] [CrossRef]
- Haji-Moradkhani, A.; Rezaei, R.; Moghimi, M. Optimization of pulsed electric field-assisted oil extraction from cannabis seeds. Food Process 2019, 42, e13028. [Google Scholar] [CrossRef]
- Nahar, L.; Uddin, S.J.; Alam, M.d.A.; Sarker, S.D. Extraction of naturally occurring cannabinoids: An update. Phytochem. Anal. 2021, 32, 228–241. [Google Scholar] [CrossRef]
- Mazzara, E.; Torresi, J.; Fico, G.; Papini, A.; Kulbaka, N.; Dall’Acqua, S.; Sut, S.; Garzoli, S.; Mustafa, A.M.; Cappellacci, L.; et al. A Comprehensive Phytochemical Analysis of Terpenes, Polyphenols and Cannabinoids, and Micromorphological Characterization of 9 Commercial Varieties of Cannabis sativa L. Plants 2022, 11, 891. [Google Scholar] [CrossRef]
- Addo, P.W.; Sagili, S.U.K.R.; Bilodeau, S.E. Cold ethanol extraction of cannabinoids and terpenes from cannabis using response surface methodology: Optimization and comparative study. Molecules 2022, 27, 8780. [Google Scholar] [CrossRef] [PubMed]
- Rovetto, L.J.; Aieta, N.V. Supercritical carbon dioxide extraction of cannabinoids from Cannabis sativa L. J. Supercrit. Fluids 2017, 129, 16–27. [Google Scholar] [CrossRef]
- Karğılı, U.; Aytaç, E. Supercritical fluid extraction of cannabinoids (THC and CBD) from four different strains of cannabis grown in different regions. J. Supercrit. Fluids 2022, 179, 105410. [Google Scholar] [CrossRef]
- Gallo-Molina, A.C.; Castro-Vargas, H.I.; Garzón-Méndez, W.F. Extraction, isolation and purification of tetrahydrocannabinol from the Cannabis sativa L. plant using supercritical fluid extraction and solid phase extraction. J. Supercrit. Fluids 2019, 146, 208–216. [Google Scholar] [CrossRef]
- López-Olmos, C.; García-Valverde, M.T.; Hidalgo, J.; Ferrerio-Vera, C.; Sánchez De Medina, V. Comprehensive comparison of industrial cannabinoid extraction techniques: Evaluation of the most relevant patents and studies at pilot scale. Front. Nat. Prod. 2022, 1, 1043147. [Google Scholar] [CrossRef]
- Fernández, S.; Carreras, T.; Castro, R. A comparative study of supercritical fluid and ethanol extracts of cannabis inflorescences: Chemical profile and biological activity. J. Supercrit. Fluids 2022, 179, 105385. [Google Scholar] [CrossRef]
- Pieracci, Y.; Ascrizzi, R.; Terreni, V. Essential Oil of Cannabis sativa L: Comparison of Yield and Chemical Composition of 11 Hemp Genotypes. Molecules 2021, 26, 4080. [Google Scholar] [CrossRef]
- Abdollahi, M.; Sefidkon, F.; Calagari, M.; Mousavi, A.; Mahomoodally, M.F. Impact of four hemp (Cannabis sativa L.) varieties and stage of plant growth on yield and composition of essential oils. Ind. Crops Prod. 2020, 155, 112793. [Google Scholar] [CrossRef]
- Abraham, E.J.; Wallace, E.D.; Kellogg, J.J. A comparison of high- and low-resolution gas chromatography-mass spectrometry for herbal product classification: A case study with Ocimum essential oils. Phytochem. Anal. 2023, 34, 680–691. [Google Scholar] [CrossRef]
- Kellogg, J.J.; Alonso, M.N.; Jordan, R.T.; Xiao, J.; Cafiero, J.H.; Bush, T.; Chen, X.; Towler, M.; Weathers, P.; Shell, S.S. A methoxylated flavone from Artemisia afra kills Mycobacterium tuberculosis. J. Ethnopharmacol. 2024, 333, 118500. [Google Scholar] [CrossRef]
- Szalata, M.; Dreger, M.; Zielińska, A.; Banach, J.; Szalata, M.; Wielgus, K. Simple Extraction of Cannabinoids from Female Inflorescences of Hemp (Cannabis sativa L.). Molecules 2022, 27, 5868. [Google Scholar] [CrossRef]
- Sepulveda, D.E.; Vrana, K.E.; Graziane, N.M.; Raup-Konsavage, W.M. Combinations of cannabidiol and Δ9-tetrahydrocannabinol in reducing chemotherapeutic induced neuropathic pain. Biomedicines 2022, 10, 2548. [Google Scholar] [CrossRef]
- Anderson, B.D.; Sepulveda, D.E.; Nachnani, R.; Cortez-Resendiz, A.; Coates, M.D.; Beckett, A.; Bisanz, J.E.; Kellogg, J.J.; Raup-Konsavage, W.M. High cannabigerol hemp extract moderates colitis and modulates the microbiome in an inflammatory bowel disease model. J. Pharmacol. Exp. Ther. 2024, 390. [Google Scholar] [CrossRef] [PubMed]
- Schmid, R.; Heuckeroth, S.; Korf, A. Integrative analysis of multimodal mass spectrometry data in MZmine 3. Nat. Biotechnol. 2023, 41, 447–449. [Google Scholar] [CrossRef] [PubMed]

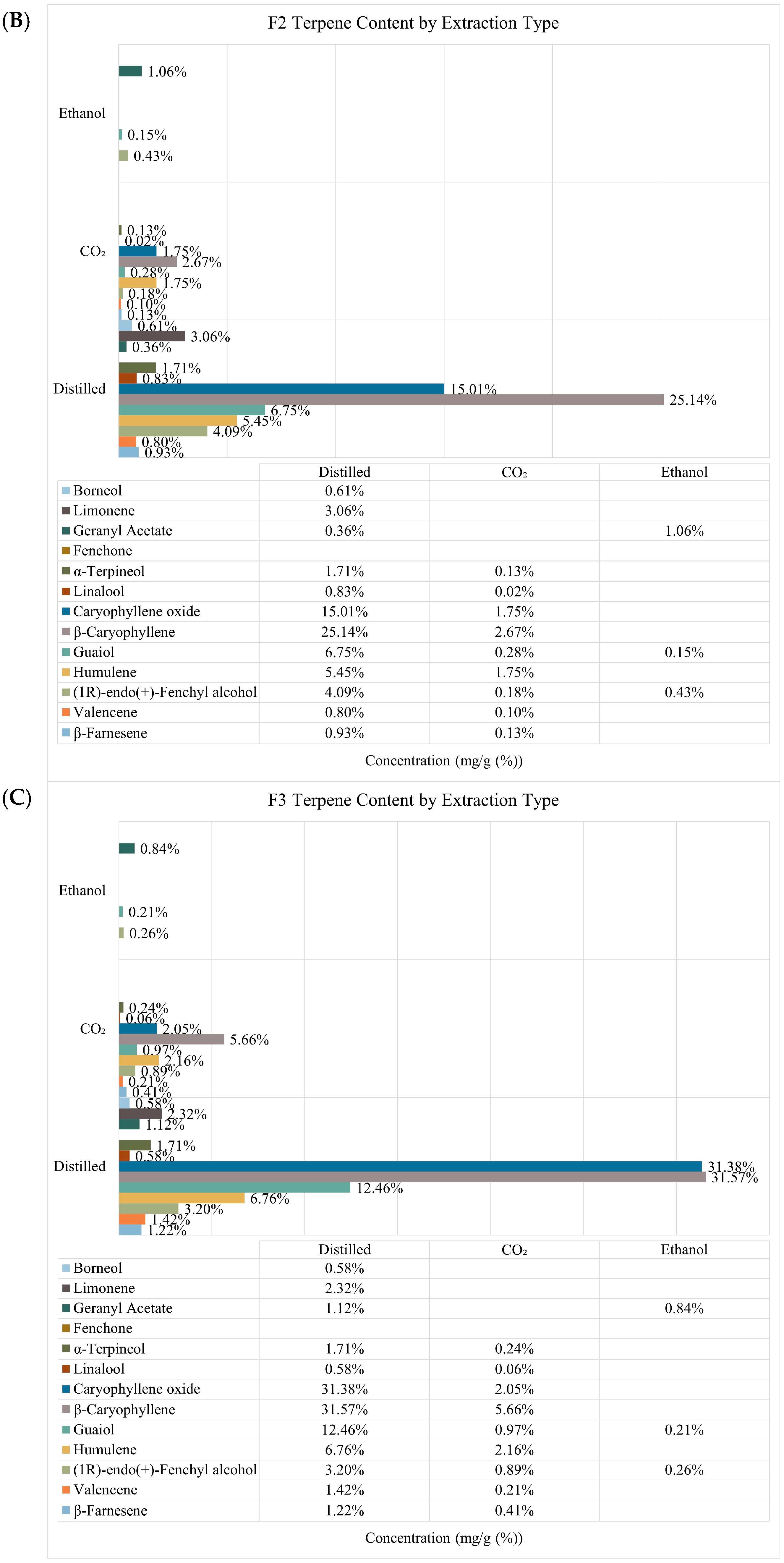
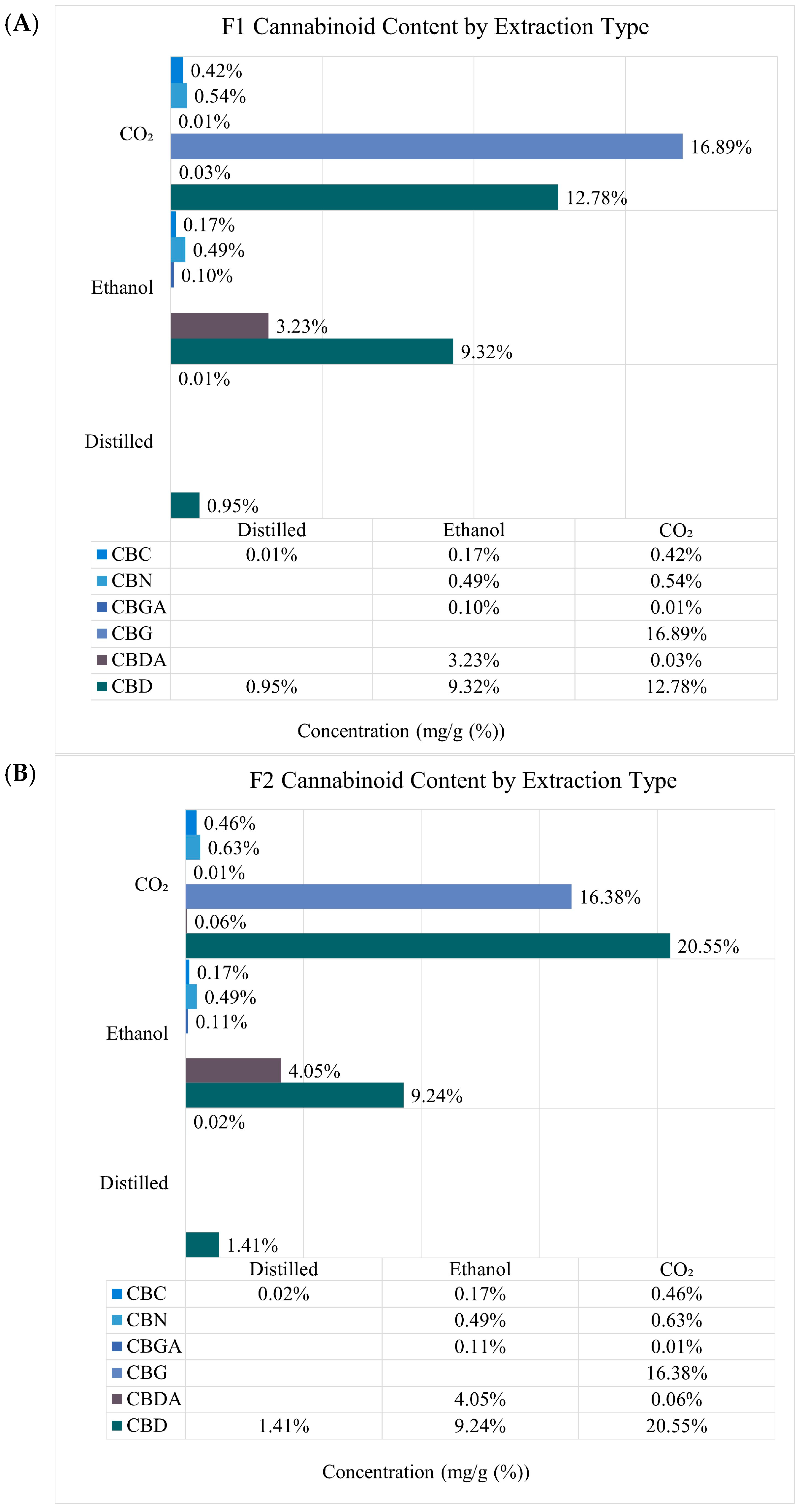
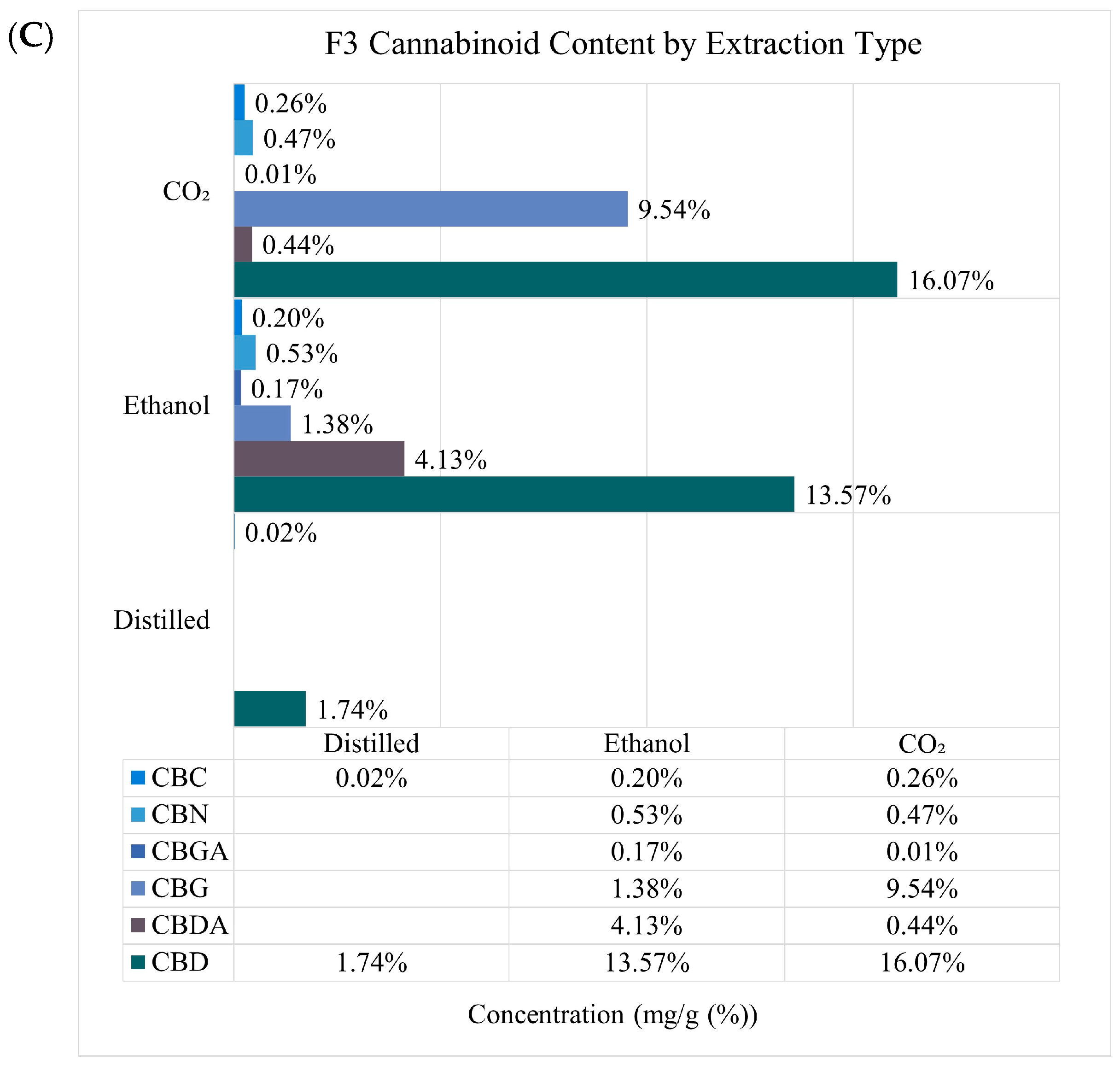

| F1 | F2 | F3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Distilled | Ethanol | CO2 | Distilled | Ethanol | CO2 | Distilled | Ethanol | CO2 | |
| Extraction yield w/w (%) | 1.11 | 24.29 ± 0.02 | 1.84 ± 0.01 | 0.18 | 22.03± 0.01 | 1.56 ± 0.01 | 0.08 | 24.27 ± 0.01 | 1.30 ± 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chacon, F.T.; Raup-Konsavage, W.M.; Vrana, K.E.; Kellogg, J.J. Effect of Hemp Extraction Procedures on Cannabinoid and Terpenoid Composition. Plants 2024, 13, 2222. https://doi.org/10.3390/plants13162222
Chacon FT, Raup-Konsavage WM, Vrana KE, Kellogg JJ. Effect of Hemp Extraction Procedures on Cannabinoid and Terpenoid Composition. Plants. 2024; 13(16):2222. https://doi.org/10.3390/plants13162222
Chicago/Turabian StyleChacon, Francisco T., Wesley M. Raup-Konsavage, Kent E. Vrana, and Joshua J. Kellogg. 2024. "Effect of Hemp Extraction Procedures on Cannabinoid and Terpenoid Composition" Plants 13, no. 16: 2222. https://doi.org/10.3390/plants13162222






