Genetic Evaluation of Water Use Efficiency and Nutrient Use Efficiency in Populus deltoides Bartr. ex Marsh. Seedlings in China
Abstract
1. Introduction
2. Results
2.1. Genetic Variation in Growth and Biomass
2.2. Genetic Variation in Leaf δ13C and δ15N
2.3. Heritability of Traits
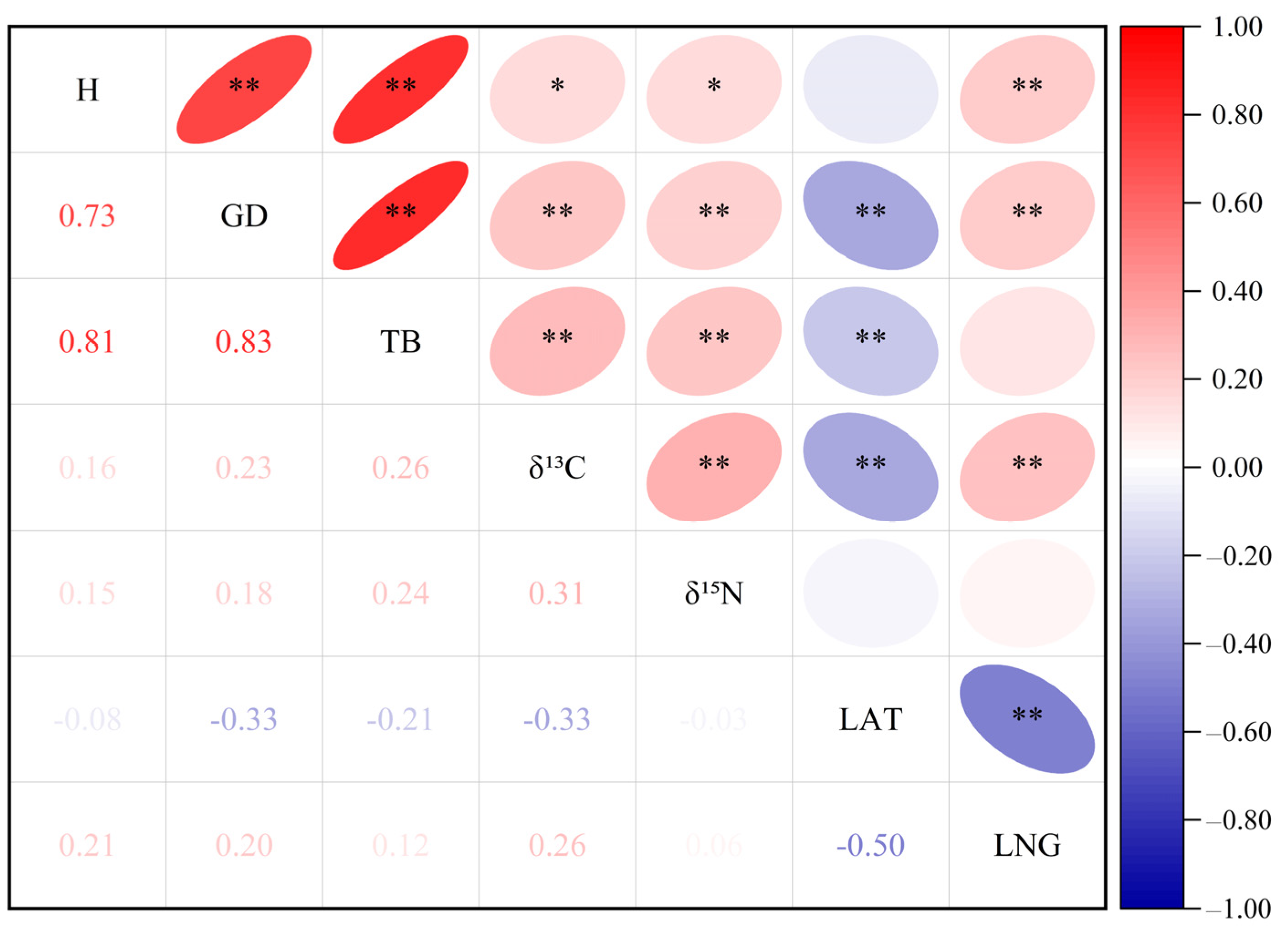
2.4. Correlation Analysis of Parameters
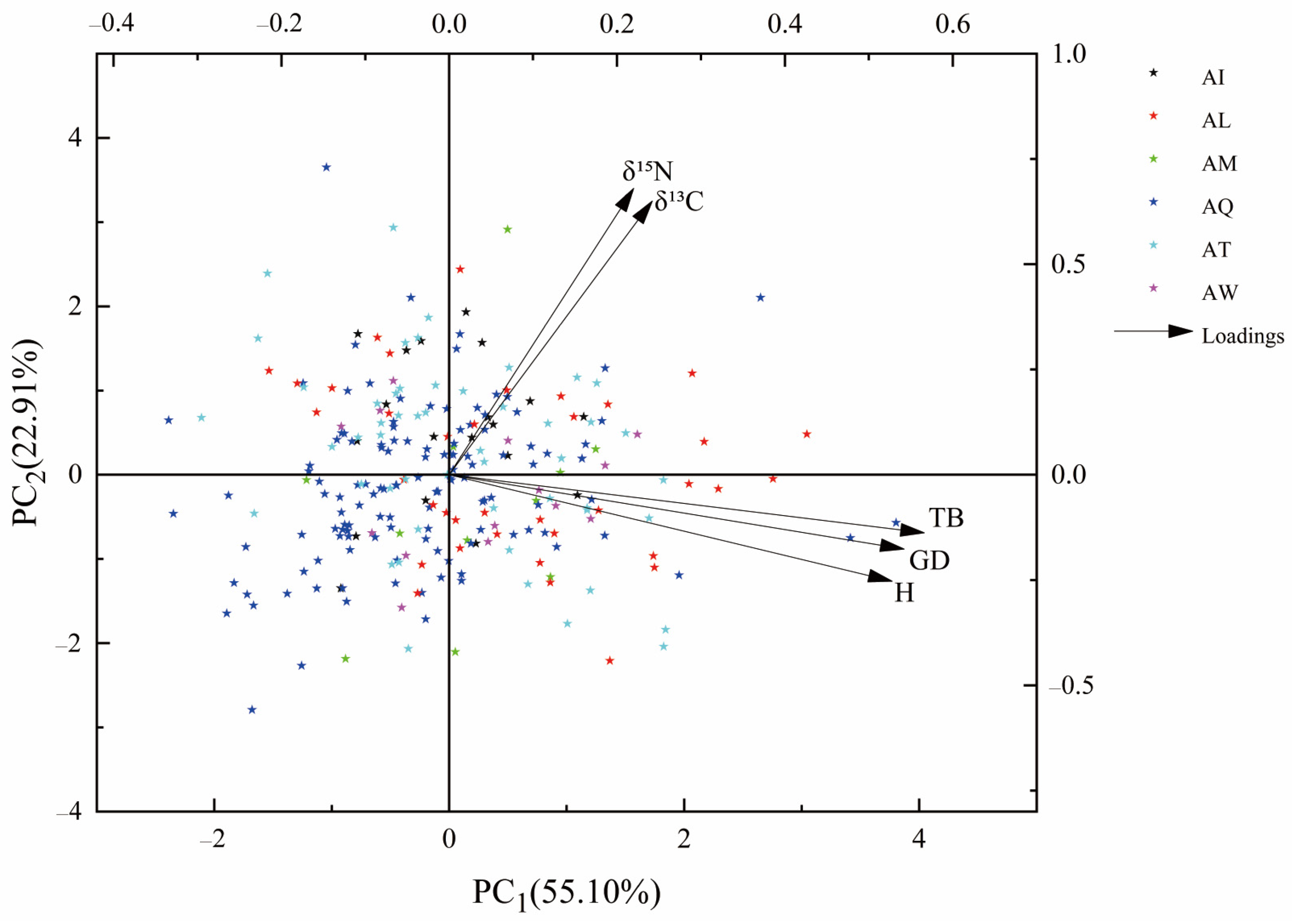
2.5. Comprehensive Evaluation
3. Discussion
3.1. Genetic Variation of P. deltoides
3.2. Heritability of Traits in P. deltoides
3.3. Correlation of Traits and Geographic Location in P. deltoides
3.4. A Comprehensive Evaluation of P. deltoides
4. Materials and Methods
4.1. Test Materials
4.2. Trait Measurement
4.2.1. Subsubsection Carbon and Nitrogen Isotope Ratios in Leaves
4.2.2. Growth Trait
4.3. Data Processing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Navarro, A.; Portillo-Estrada, M.; Ceulemans, R. Identifying the best plant water status indicator for bio-energy poplar genotypes. GCB Bioenergy 2020, 12, 426–444. [Google Scholar] [CrossRef]
- Du, K.; Jiang, S.X.; Chen, H.; Xia, Y.F.; Guo, R.H.; Ling, A.Y.; Liao, T.; Wu, W.Q.; Kang, X.Y. Spatiotemporal miRNA and transcriptomic network dynamically regulate the developmental and senescence processes of poplar leaves. Hortic. Res. 2023, 10, uhad186. [Google Scholar] [CrossRef]
- He, D.; Wan, X.; Wang, B.; Wan, X.; Lu, M. Poplars and Willows, Sustaining Livelihoods in Urban and Periurban Forests in China; FAO: Rome, Italy, 2018. [Google Scholar]
- Tun, T.N.; Guo, J.; Fang, S.Z.; Tian, Y. Planting spacing affects canopy structure, biomass production and stem roundness in poplar plantations. Scand. J. For. Res. 2018, 33, 464–474. [Google Scholar] [CrossRef]
- Fahrenkrog, A.M.; Neves, L.G.; Resende, M.F.R.; Dervinis, C.; Davenport, R.; Barbazuk, W.B.; Kirst, M. Population genomics of the eastern cottonwood (Populus deltoides). Ecol. Evol. 2017, 7, 9426–9440. [Google Scholar] [CrossRef] [PubMed]
- Su, X.H.; Ma, C.G.; Ding, C.J. Establish & improve scientific development system of poplar breeding in China. Prot. For. Sci. Technol. 2011, 1, 11–14. (In Chinese) [Google Scholar]
- Zhao, M.Q.; Lei, Y.J.; Wu, L.; Qi, H.R.; Song, Z.H.; Xu, M. The miR159a-PeMYB33 module regulates poplar adventitious rooting through the abscisic acid signal pathway. Plant J. 2024, 18, 879–891. [Google Scholar] [CrossRef] [PubMed]
- Li, J.P.; Zhang, Z.; Yao, C.S.; Yang, L.; Wang, Z.M.; Fang, B.T.; Zhang, Y.H. Improving winter wheat grain yield and water-/nitrogen-use efficiency by optimizing the micro-sprinkling irrigation amount and nitrogen application rate. J. Integr. Agric. 2021, 20, 606–621. [Google Scholar] [CrossRef]
- Chen, X.; Xing, H.L.; Liu, B.; Wang, Y.S.; Cui, N.B.; Wang, Z.H.; Zhang, Y.X. Changes induced by multi-stage water stress on maize growth, water and nitrogen utilization and hormone signaling under different nitrogen supplies. Agric. Water Manag. 2023, 290, 108570. [Google Scholar] [CrossRef]
- Querejeta, J.I.; Prieto, I.; Armas, C.; Casanoves, F.; Diémé, J.S.; Diouf, M.; Yossi, H.; Kaya, B.; Pugnaire, F.I.; Rusch, G.M. Higher leaf nitrogen content is linked to tighter stomatal regulation of transpiration and more efficient water use across dryland trees. New Phytol. 2022, 235, 1351–1364. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.W.; Jiang, L.; Ren, H.Y.; Han, X.G. Opposing responses of temporal stability of aboveground and belowground net primary productivity to water and nitrogen enrichment in a temperate grassland. Glob. Change Biol. 2024, 30, e17071. [Google Scholar] [CrossRef]
- Koutroulis, A.G. Dryland changes under different levels of global warming. Sci. Total Environ. 2019, 655, 482–511. [Google Scholar] [CrossRef] [PubMed]
- Lian, X.; Piao, S.L.; Chen, A.P.; Huntingford, C.; Fu, B.J.; Li, L.Z.; Huang, J.P.; Sheffield, J.; Berg, A.M.; Keenan, T.F. Multifaceted characteristics of dryland aridity changes in a warming world. Nat. Rev. Earth Environ. 2021, 2, 232–250. [Google Scholar] [CrossRef]
- Pokhrel, Y.; Felfelani, F.; Satoh, Y.; Boulange, J.; Burek, P.; Gädeke, A.; Gerten, D.; Gosling, S.N.; Grillakis, M.; Gudmundsson, L. Global terrestrial water storage and drought severity under climate change. Nat. Clim. Change 2021, 11, 226–233. [Google Scholar] [CrossRef]
- Meusburger, K.; Trotsiuk, V.; Schmidt-Walter, P.; Baltensweiler, A.; Brun, P.; Bernhard, F.; Gharun, M.; Habel, R.; Hagedorn, F.; Köchli, R. Soil–plant interactions modulated water availability of Swiss forests during the 2015 and 2018 droughts. Glob. Change Biol. 2022, 28, 5928–5944. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Deng, M.Z.; Lin, Y.R.; Liu, P.Z.; Wang, X.L.; Yang, S.F.; Ren, X.L.; Chen, X.L.; Liu, T.N. Effects of the border on yield and water use in wheat/maize intercropping in rain-fed areas with different nitrogen levels. Field Crops Res. 2023, 302, 109105. [Google Scholar] [CrossRef]
- Ye, T.Y.; Ma, J.F.; Zhang, P.; Shan, S.; Liu, L.L.; Tang, L.; Cao, W.X.; Liu, B.; Zhu, Y. Interaction effects of irrigation and nitrogen on the coordination between crop water productivity and nitrogen use efficiency in wheat production on the North China Plain. Agric. Water Manag. 2022, 271, 107787. [Google Scholar] [CrossRef]
- Wang, H.D.; Wu, L.F.; Wang, X.K.; Zhang, S.H.; Cheng, M.H.; Feng, H.; Fan, J.L.; Zhang, F.C.; Xiang, Y.Z. Optimization of water and fertilizer management improves yield, water, nitrogen, phosphorus and potassium uptake and use efficiency of cotton under drip fertigation. Agric. Water Manag. 2021, 245, 106662. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Thapa, G.B. Is ‘Better cotton’ better than conventional cotton in terms of input use efficiency and financial performance? Land Use Policy 2016, 52, 136–143. [Google Scholar] [CrossRef]
- Shah, A.N.; Javed, T.; Singhal, R.K.; Shabbir, R.; Wang, D.; Hussain, S.; Anuragi, H.; Jinger, D.; Pandey, H.; Abdelsalam, N.R. Nitrogen use efficiency in cotton: Challenges and opportunities against environmental constraints. Front. Plant Sci. 2022, 13, 970339. [Google Scholar] [CrossRef]
- Cannell, M.G. Environmental impacts of forest monocultures: Water use, acidification, wildlife conservation, and carbon storage. Planted For. 1999, 17, 239–262. [Google Scholar]
- Monclus, R.; Dreyer, E.; Villar, M.; Delmotte, F.M.; Delay, D.; Petit, J.M.; Barbaroux, C.; Le Thiec, D.; Bréchet, C.; Brignolas, F. Impact of drought on productivity and water use efficiency in 29 genotypes of Populus deltoides × Populus nigra. New Phytol. 2006, 169, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Toillon, J.; Dallé, E.; Bodineau, G.; Berthelot, A.; Bastien, J.C.; Brignolas, F.; Marron, N. Plasticity of yield and nitrogen removal in 56 Populus deltoides× P. nigra genotypes over two rotations of short-rotation coppice. For. Ecol. Manag. 2016, 375, 55–65. [Google Scholar] [CrossRef]
- Galloway, J.N.; Dentener, F.J.; Capone, D.G.; Boyer, E.W.; Howarth, R.W.; Seitzinger, S.P.; Asner, G.P.; Cleveland, C.C.; Green, P.; Holland, E.A. Nitrogen cycles: Past, present, and future. Biogeochemistry 2004, 70, 153–226. [Google Scholar] [CrossRef]
- Khan, A.; Tan, D.K.Y.; Munsif, F.; Afridi, M.Z.; Shah, F.; Wei, F.; Fahad, S.; Zhou, R.Y. Nitrogen nutrition in cotton and control strategies for greenhouse gas emissions: A review. Environ. Sci. Pollut. Res. 2017, 24, 23471–23487. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.E.; Zeng, H.H.; Zhao, F.; Chen, C.F.; Liu, W.J.; Yang, B.; Zhang, W.J. Recognizing the role of plant species composition in the modification of soil nutrients and water in rubber agroforestry systems. Sci. Total Environ. 2020, 723, 138042. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.H.; Fan, X.R.; Miller, A.J. Plant nitrogen assimilation and use efficiency. Annu. Rev. Plant Biol. 2012, 63, 153–182. [Google Scholar] [CrossRef] [PubMed]
- Cooley, S.S.; Fisher, J.B.; Goldsmith, G.R. Convergence in water use efficiency within plant functional types across contrasting climates. Nat. Plants 2022, 8, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.W.; Yu, X.X.; Jia, G.D.; Li, H.Z.; Liu, Z.Q. Variation characteristics of long-term water use efficiency based on tree-ring carbon isotope discrimination. Acta Ecol. Sin. 2017, 37, 2093–2100. (In Chinese) [Google Scholar]
- Robinson, D. δ15N as an integrator of the nitrogen cycle. Trends Ecol. Evol. 2001, 16, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Kahmen, A.; Wanek, W.; Buchmann, N. Foliar δ15N values characterize soil N cycling and reflect nitrate or ammonium preference of plants along a temperate grassland gradient. Oecologia 2008, 156, 861–870. [Google Scholar] [CrossRef]
- Fang, H.J.; Yu, G.R.; Cheng, S.L.; Zhu, T.H.; Zheng, J.J.; Mo, J.M.; Yan, J.H.; Luo, Y.Q. Nitrogen-15 signals of leaf-litter-soil continuum as a possible indicator of ecosystem nitrogen saturation by forest succession and N loads. Biogeochemistry 2011, 102, 251–263. [Google Scholar] [CrossRef]
- Liu, F.; Liu, P.; Cao, M.; Yang, C.; Chen, T.T.; Zhou, H.K.; Wang, W.Y. Review on application of stable isotope technique to the study of plant water relations. Ecol. Sci. 2020, 39, 224–232. (In Chinese) [Google Scholar]
- Farquhar, G.D.; Ehleringer, J.R.; Hubick, K.T. Carbon isotope discrimination and photosynthesis. Annu. Rev. Plant Biol. 1989, 40, 503–537. [Google Scholar] [CrossRef]
- Schulze, E.D.; Gebauer, G.; Ziegler, H.; Lange, O.L. Estimates of nitrogen fixation by trees on an aridity gradient in Namibia. Oecologia 1991, 88, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Peter, H.; Ian, J.A. Roles of root symbioses in African woodland and forest evidence from 15N abundance and foliar analysis. J. Ecol. 1995, 83, 217–224. [Google Scholar]
- Anyia, A.; Slaski, J.; Nyachiro, J.; Archambault, D.; Juskiw, P. Relationship of carbon isotope discrimination to water use efficiency and productivity of barley under field and greenhouse conditions. J. Agron. Crop Sci. 2007, 193, 313–323. [Google Scholar] [CrossRef]
- Akhter, J.; Monneveux, P.; Sabir, S.; Ashraf, M.; Lateef, Z.; Serraj, R. Selection of drought tolerant and high water use efficient rice cultivars through 13C isotope discrimination technique. Pak. J. Bot. 2010, 42, 3887–3897. [Google Scholar]
- Mininni, A.N.; Tuzio, A.C.; Brugnoli, E.; Dichio, B.; Sofo, A. Carbon isotope discrimination and water use efficiency in interspecific Prunus hybrids subjected to drought stress. Plant Physiol. Biochem. 2022, 175, 33–43. [Google Scholar] [CrossRef]
- Rabarijaona, A.; Ponton, S.; Bert, D.; Ducousso, A.; Richard, B.; Levillain, J.; Brendel, O. Provenance differences in water-use efficiency among sessile oak populations grown in a mesic common garden. Front. For. Glob. Change 2022, 5, 914199. [Google Scholar] [CrossRef]
- Arifuzzaman, M.; Mamidi, S.; Sanz-Saez, A.; Zakeri, H.; Scaboo, A.; Fritschi, F.B. Identification of loci associated with water use efficiency and symbiotic nitrogen fixation in soybean. Front. Plant Sci. 2023, 14, 1271849. [Google Scholar] [CrossRef]
- Jiang, X.Y.; Song, M.Y.; Qiao, Y.; Liu, M.Z.; Ma, L.; Fu, S.L. Long-term water use efficiency and non-structural carbohydrates of dominant tree species in response to nitrogen and water additions in a warm temperate forest. Front. Plant Sci. 2022, 13, 1025162. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wen, X.F. Limiting resource and leaf functional traits jointly determine distribution patterns of leaf intrinsic water use efficiency along aridity gradients. Front. Plant Sci. 2022, 13, 909603. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.B.; Lai, Y.; Tang, X.L.; Phillips, O.L.; Liu, J.F.; Chen, D.X.; Wen, D.Z.; Wang, S.L.; Chen, L.C.; Tian, X.J. Multiple environmental factors regulate the large-scale patterns of plant water use efficiency and nitrogen availability across China’s forests. Environ. Res. Lett. 2021, 16, 034026. [Google Scholar] [CrossRef]
- Cao, X.; Jia, J.B.; Zhang, C.; Li, H.; Liu, T.X.; Jiang, X.N.; Polle, A.; Peng, C.H.; Luo, Z.B. Anatomical, physiological and transcriptional responses of two contrasting poplar genotypes to drought and re-watering. Physiol. Plant. 2014, 151, 480–494. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Li, H.; Liu, T.X.; Polle, A.; Peng, C.H.; Luo, Z.B. Nitrogen metabolism of two contrasting poplar species during acclimation to limiting nitrogen availability. J. Exp. Bot. 2013, 64, 4207–4224. [Google Scholar] [CrossRef] [PubMed]
- Siegwolf, R.T.W.; Matyssek, R.; Saurer, M.; Maurer, S.; Günthardt-Goerg, M.S.; Schmutz, P.; Bucher, J.B. Stable isotope analysis reveals differential effects of soil nitrogen and nitrogen dioxide on the water use efficiency in hybrid poplar leaves. New Phytol. 2008, 149, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.S.; Zhou, P.Y.; Tong, C.F.; Bi, C.W.; Xu, L.A. Assembly and analysis of the Populus deltoides mitochondrial genome: The first report of a multicircular mitochondrial conformation for the genus Populus. J. For. Res. 2023, 34, 717–733. [Google Scholar] [CrossRef]
- Yang, S.L.; Shi, J.; Chen, L.H.; Zhang, J.; Zhang, D.J.; Xu, Z.F.; Xiao, J.J.; Zhu, P.; Liu, Y.; Lin, T.T. Physiological and biomass partitioning shifts to water stress under distinct soil types in Populus deltoides saplings. J. Plant Ecol. 2020, 13, 545–553. [Google Scholar] [CrossRef]
- Wei, S.Y.; Wu, H.T.; Li, X.P.; Chen, Y.N.; Yang, Y.H.; Dai, M.L.; Yin, T.M. Identification of genes underlying the resistance to Melampsora larici-populina in an R gene supercluster of the Populus deltoides genome. Plant Dis. 2020, 104, 1133–1143. [Google Scholar] [CrossRef]
- Yan, Y.B.; Pan, H.X. Genetic variation and selection of seedling traits in hybrid progeny of Populus deltoides. J. Zhejiang A&F Univ. 2021, 38, 1144–1152. (In Chinese) [Google Scholar]
- Chen, C.; Ding, C.J.; Zhang, J.; Li, B.; Chu, Y.G.; Su, X.H.; Huang, Q.J. Population structure analysis and core collection construction of Populus deltoides. Sci. Silvae Sin. 2020, 56, 67–76. (In Chinese) [Google Scholar]
- Mwase, W.F.; Savill, P.S.; Hemery, G. Genetic parameter estimates for growth and form traits in common ash (Fraxinus excelsior, L.) in a breeding seedling orchard at Little Wittenham in England. New For. 2008, 36, 225–238. [Google Scholar] [CrossRef]
- Metougui, M.L.; Mokhtari, M.; Maughan, P.J.; Jellen, E.N.; Benlhabib, O. Morphological variability, heritability and correlation studies within an argan tree population (Argania spinosa (L.) Skeels) preserved in situ. Int. J. Agric. For. 2017, 7, 42–51. [Google Scholar]
- Ewool, M.B.; Akromah, R. Genetic variability, coefficient of variance, heritability and genetic advance of pro-vitamin a maize hybrids. Int. J. Agric. Inn. Res. 2017, 6, 84–90. [Google Scholar]
- Scribner, K.T.; Uhrig, G.; Kanefsky, J.; Sard, N.M.; Holtgren, M.; Jerome, C.; Ogren, S. Pedigree-based decadal estimates of lake sturgeon adult spawning numbers and genetic diversity of stream-side hatchery produced offspring. J. Great Lakes Res. 2022, 48, 551–564. [Google Scholar] [CrossRef]
- Li, A.R.; Ma, M.; Li, H.T.; He, S.F.; Wang, S.G. Genetic diversity and population differentiation of a Chinese endangered plant Ammopiptanthus nanus (M. Pop.) Cheng f. Genes 2023, 14, 1020. [Google Scholar] [CrossRef] [PubMed]
- Fred, F.; Mwabumba, L.; Mhango, J.; Missanjo, E.; Kadzuwa, H.; Likoswe, M. Genetic and phenotypic parameters for growth traits of Widdringtonia whytei-Rendle translocation provenance Trials in Malawi. J. Glob. Ecol. Environ. 2023, 17, 32–48. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, X.; Gu, W.; Wang, L.; Li, W.; Gao, Y.; Wu, L.; Guo, X.; Tigabu, M.; Xia, D.; et al. Genetic stability of Larix olgensis provenances planted in different sites in northeast China. For. Ecol. Manag. 2021, 485, 118988. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Wang, C.; Li, S.C.; Hou, W.; Zhang, S.Q.; Han, G.J.; Pan, D.; Wang, P.; Cheng, Y.F.; Liu, G.F. Genetic variation and selection of introduced provenances of Siberian Pine (Pinus sibirica) in frigid regions of the Greater Xing’an Range, Northeast China. J. For. Res. 2014, 25, 549–556. [Google Scholar] [CrossRef]
- Villani, F.; Sansotta, A.; Cherubini, M.; Cesaroni, D.; Sbordoni, V. Genetic structure of natural populations of Castanea sativa in Turkey: Evidence of a hybrid zone. J. Evol. Biol. 1999, 12, 233–244. [Google Scholar] [CrossRef]
- Zemke, N.R.; Armand, E.J.; Wang, W.; Lee, S.; Zhou, J.; Li, Y.E.; Liu, H.; Tian, W.; Nery, J.R.; Castanon, R.G.; et al. Conserved and divergent gene regulatory programs of the mammalian neocortex. Nature 2023, 624, 390–402. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Kong, D.; Mo, X.; Wang, Y.; Yang, Q.; Kardol, P.; Barrantes, O.J.V.; Simpson, M.J.; Zeng, H.; Reich, P.B.; et al. Molecular-level carbon traits underlie the multidimensional fine root economics space. Nat. Plants 2024, 10, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Sterner, R.W.; Elser, J.J.; Vitousek, P. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere; Princeton University Press: Princeton, NJ, USA, 2017; p. 464. [Google Scholar]
- Müller, A.; Horna, V.; Kleemann, F.; Vornam, B.; Leuschner, C. Physiological vs. morphological traits controlling the productivity of six aspen full-sib families. Biomass Bioenergy 2013, 56, 274–283. [Google Scholar] [CrossRef]
- Safavi, S.A.; Pourdad, S.S.; Taeb, M.; Khosroshahli, M. Assessment of genetic variation among safflower (Carthamus tinctorius L.) accessions using agro-morphological traits and molecular markers. J. Food Agric. Envir. Sci. Pollut. Res. 2010, 8, 616–625. [Google Scholar]
- Xu, R.; Cheng, S.; Zhou, J.; Tigabu, M.; Ma, X.; Li, M. Intraspecific variations in leaf functional traits of Cunninghamia lanceolata provenances. BMC Plant Biol. 2023, 23, 92. [Google Scholar] [CrossRef] [PubMed]
- White, T.L.; Adams, W.T.; Neale, D.B. Forest Genetics; CABI: London, UK, 2007. [Google Scholar]
- Gomaa, N.H.; Picó, F.X. Depicting the phenotypic space of the annual plant Diplotaxis acris in hyperarid deserts. Ecol. Evol. 2021, 11, 15708–15719. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chu, Y.G.; Ding, C.G.; Su, X.H.; Huang, Q.J. Genetic diversity and population structure of black cottonwood (Populus deltoides) revealed using simple sequence repeat markers. BMC Genet. 2020, 21, 2. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Han, Q.; Xia, Y.; Geng, X.; Du, K.; Yang, J.; Kang, X. Construction of a breeding parent population of Populus tomentosa based on SSR genetic distance analysis. Sci. Rep. 2020, 10, 18573. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.Z.; Zhao, X.; Pan, W.; Zhang, J.F.; Li, B.L.; Zhang, D.Q. Phenotypic variation among five provenances of Populus simonii in northern China. For. Stud. China 2011, 13, 97–103. [Google Scholar] [CrossRef]
- McKown, A.D.; Guy, R.D.; Klápště, J.; Geraldes, A.; Friedmann, M.; Cronk, Q.C.; El-Kassaby, Y.A.; Mansfield, S.D.; Douglas, C. Geographical and environmental gradients shape phenotypic trait variation and genetic structure in Populus trichocarpa. New Phytol. 2014, 201, 1263–1276. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Chen, X.; Gai, Z.; Zhai, J.; Guo, X.; Han, X.; Zhang, S.; Wu, Z.; Li, Z. Phenotypic diversity and variation in natural Populus euphratica populations shaped by environmental factors. Contemp. Probl. Ecol. 2023, 16, 230–252. [Google Scholar] [CrossRef]
- Difazio, S.P.; Slavov, G.T.; Joshi, C.P. Genetics Genomics and Breeding of Poplar; Science Publishers: New York, NY, USA; CRC Press: New York, NY, USA, 2011. [Google Scholar]
- Baltunis, B.S.; Gapare, W.J.; Wu, H.X. Genetic parameters and genotype by environment interaction in radiata pine for growth and wood quality traits in Australia. Silvae Genet. 2009, 59, 113–124. [Google Scholar] [CrossRef]
- Galeano, E.; Thomas, B.R. Unraveling genetic variation among white spruce families generated through different breeding strategies: Heritability, growth, physiology, hormones and gene expression. Front. Plant Sci. 2023, 14, 1052425. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.D. Plant Breeding: Principles and Methods, 6th ed.; Kalyani Publishers: New Delhi, India, 2001. [Google Scholar]
- Kanaga, M.K.; Ryel, R.J.; Mock, K.E.; Pfrender, M.E. Quantitative-genetic variation in morphological and physiological traits within a quaking aspen (Populus tremuloides) population. Can. J. For. Res. 2008, 38, 1690–1694. [Google Scholar] [CrossRef]
- Lojewski, N.R.; Fischer, D.G.; Bailey, J.K.; Schweitzer, J.A.; Whitham, T.G.; Hart, S.C. Genetic basis of aboveground productivity in two native Populus species and their hybrids. Tree Physiol. 2009, 29, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Guet, J.; Fabbrini, F.; Fichot, R.; Sabatti, M.; Bastien, C.; Brignolas, F. Genetic variation for leaf morphology, leaf structure and leaf carbon isotope discrimination in European populations of black poplar (Populus nigra L.). Tree Physiol. 2015, 35, 850–863. [Google Scholar] [CrossRef] [PubMed]
- Su, B.Q.; Shangguan, Z.P. Response of water use efficiency and plant-soil C:N:P stoichiometry to stand quality in Robinia pseudoacacia on the Loess Plateau of China. Catena 2021, 206, 105571. [Google Scholar] [CrossRef]
- Chen, J.S.; Chen, Y.P.; Wang, K.B.; Wang, G.L.; Wu, J.H.; Zhang, Y.Y. Differences in soil water storage, consumption, and use efficiency of typical vegetation types and their responses to precipitation in the Loess Plateau, China. Sci. Total Environ. 2023, 869, 161710. [Google Scholar] [CrossRef]
- Houlton, B.Z.; Sigman, D.M.; Schuur, E.A.; Hedin, L.O. A climate-driven switch in plant nitrogen acquisition within tropical forest communities. Proc. Natl. Acad. Sci. USA 2007, 104, 8902–8906. [Google Scholar] [CrossRef]
- Flexas, J.; Carriquí, M.; Coopman, R.E.; Gago, J.; Galmés, J.; Martorell, S.; Morales, F.; Diaz-Espejo, A. Stomatal and mesophyll conductances to CO2 in different plant groups: Underrated factors for predicting leaf photosynthesis responses to climate change? Plant Sci. 2014, 226, 41–48. [Google Scholar] [CrossRef]
- Soolanayakanahally, R.Y.; Guy, R.D.; Silim, S.N.; Drewes, E.C.; Schroeder, W.R. Enhanced assimilation rate and water use efficiency with latitude through increased photosynthetic capacity and internal conductance in balsam poplar (Populus balsamifera L.). Plant Cell Environ. 2009, 32, 1821–1832. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Liu, S.; Xu, G.X.; Chen, J.; Xing, H.S.; Li, F.F.; Zhang, M.M.; Cao, X.W.; Shi, Z.M. Differences and drivers of leaf stable carbon and nitrogen isotope in herbs under different vegetation types on the eastern Qinghai-Tibetan Plateau. Chin. J. Appl. Ecol. 2024, 35, 877. (In Chinese) [Google Scholar]
- Peri, P.L.; Ladd, B.; Pepper, D.A.; Bonser, S.P.; Laffan, S.W.; Amelung, W. Carbon (δ13C) and nitrogen (δ15N) stable isotope composition in plant and soil in Southern Patagonia’s native forests. Global Change Biol. 2012, 18, 311–321. [Google Scholar] [CrossRef]
- Li, J.M.; Du, L.S.; Guan, W.B.; Yu, F.H.; Mark, V.K. Latitudinal and longitudinal clines of phenotypic plasticity in the invasive herb Solidago canadensis in China. Oecologia 2016, 182, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Dawson, H.R.; Silva, L.C.R.; Peñuelas, J.; Sardans, J.; Lambers, H.; Zeng, F.; Lai, Y.; Jia, Y.; Zhou, G.; et al. Atmospheric factors outweigh species traits and soil properties in explaining spatiotemporal variation in water-use efficiency of tropical and subtropical forest species. Agric. For. Meteorol. 2022, 323, 109056. [Google Scholar] [CrossRef]
- Chen, M.; Shi, Z.M.; Liu, S.; Xu, G.X.; Cao, X.W.; Chen, J.; Zhang, M.M.; Feng, Q.H.; Centritto, M.; Cao, J. Leaf functional traits have more contributions than climate to the variations of leaf stable carbon isotope of different plant functional types on the eastern Qinghai–Tibetan Plateau. Sci. Total Environ. 2023, 871, 162036. [Google Scholar] [CrossRef]
- Evans, J.R.; Von Caemmerer, S. Temperature response of carbon isotope discrimination and mesophyll conductance in tobacco. Plant Cell Environ. Sci. Pollut. Res. 2013, 36, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.D.; Li, Y.; Yao, N.; Biswas, A.; Zou, Y.F.; Meng, Q.T.; Liu, F.G. The lagged effect and impact of soil moisture drought on terrestrial ecosystem water use efficiency. Ecol. Indicators 2021, 133, 108349. [Google Scholar] [CrossRef]
- Field, C.; Merino, J.; Mooney, H.A. Compromises between water-use efficiency and nitrogen-use efficiency in five species of California evergreens. Oecologia 1983, 60, 384–389. [Google Scholar] [CrossRef]
- Kloeppel, B.; Gower, S.; Vogel, J.; Reich, P. Leaf-level resource use for evergreen and deciduous conifers along a resource availability gradient. Funct. Ecol. 2000, 14, 281–292. [Google Scholar] [CrossRef]
- Du, B.; Zheng, J.; Ji, H.; Zhu, Y.; Yuan, J.; Wen, J.; Kang, H.; Liu, C. Stable carbon isotope used to estimate water use efficiency can effectively indicate seasonal variation in leaf stoichiometry. Ecol. Indicators 2021, 121, 107250. [Google Scholar] [CrossRef]
- Shao, X.; Chen, S.; Chen, Y.; Yang, Z.; Liu, Q.; Zhang, G.; Chen, D.; Sui, M.; Zang, L. A Review of the relationship between plant water use efficiency and plant functional community structure. World For. Res. 2024, 37, 37–44. (In Chinese) [Google Scholar]
- Cao, Q.J.; Lu, B.R.; Xia, H.; Rong, J.; Sala, F.; Spada, A.; Grassi, F. Genetic diversity and origin of weedy rice (Oryza sativa f. spontanea) populations found in North-eastern China revealed by simple sequence repeat (SSR) markers. Ann. Bot. 2006, 98, 1241–1252. [Google Scholar] [PubMed]
- Singh, A.; Kumar, A.; Kumar, R.; Prakash, J.; Kumar, N.; Verma Arvind, K. Evaluation of salt tolerance in jamun (Syzygium cumini L. Skeels) using morpho-physiological traits and membership function analysis. Sci. Hortic. 2024, 326, 112742. [Google Scholar] [CrossRef]
- Ramesh, K.J. A study of variability, associations, and path analysis in poplar (Populus deltoides Bartr. ex Marsh). J. Sustain. For. 2012, 31, 185–204. [Google Scholar]
- Xu, Y.; Li, P.; Zou, C.; Lu, Y.; Xie, C.; Zhang, X.; Prasanna, B.M.; Olsen, M.S. Enhancing genetic gain in the era of molecular breeding. J. Exp. Bot. 2017, 68, 2641–2666. [Google Scholar] [CrossRef] [PubMed]
- Gylander, T.; Hamann, A.; Brouard, J.S.; Thomas, B.R. The potential of aspen clonal forestry in Alberta: Breeding regions and estimates of genetic gain from selection. PLoS ONE 2012, 7, e44303. [Google Scholar] [CrossRef] [PubMed]
- Niemczyk, M.; Thomas, B.R. Growth parameters and resistance to Sphaerulina musiva-induced canker are more important than wood density for increasing genetic gain from selection of Populus spp. hybrids for northern climates. Ann. For. Sci. 2020, 77, 26. [Google Scholar] [CrossRef]
- Jiang, L.P.; Pei, X.N.; Hu, Y.B.; Chiang, V.L.; Zhao, X.Y. Effects of environment and genotype on growth traits in poplar clones in Northeast China. Euphytica 2021, 217, 169. [Google Scholar] [CrossRef]
- Liu, N.; Ding, C.J.; Li, B.; Ding, M.; Su, X.H.; Huang, Q.J. Analysis of the genotype interaction of four-year-old Populus euramericana using the BLUP-GGE technique. Forests 2021, 12, 1759. [Google Scholar] [CrossRef]
- Nagamitsu, T.; Nagasaka, K.; Yoshimaru, H.; Tsumura, Y. Provenance tests for survival and growth of 50-year-old Japanese larch (Larix kaempferi) trees related to climatic conditions in central Japan. Tree Genet. Genom. 2013, 10, 87–99. [Google Scholar] [CrossRef]
- Porth, I.; Klápště, J.; McKown, A.D.; La Mantia, J.; Guy, R.D.; Ingvarsson, P.K.; Hamelin, R.; Mansfield, S.D.; Ehlting, J.; Douglas, C.J.; et al. Evolutionary quantitative genomics of Populus trichocarpa. PLoS ONE 2015, 10, e0142864. [Google Scholar] [CrossRef] [PubMed]

| Trait | AW | AT | AQ | AM | AL | AI | Mean |
|---|---|---|---|---|---|---|---|
| Height (cm) | 77.67 a (21.27%) | 61.56 d (29.91%) | 62.49 cd (22.7%) | 70.67 b (17.23%) | 70.16 b (25.1%) | 66.17 c (18.66%) | 68.12 (22.48%) |
| Ground Diameter (mm) | 7.38 bc (9.54%) | 7.64 b (14.1%) | 7.02 d (16.98%) | 7.54 b (12.74%) | 8.20 a (17.89%) | 7.12 cd (10.19%) | 7.48 (13.57%) |
| Total Biomass (g) | 20.12 bc (26.49%) | 20.67 bc (31.92%) | 19.18 c (30.62%) | 21.53 b (23.63%) | 23.28 a (31.1%) | 19.10 c (18.91%) | 20.65 (27.11%) |
| Trait | Quadratic Sum | Mean Square | F | Percentage of Variation (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Intra-Provenances | Inter-Provenances | Intra-Provenances | Inter-Provenances | Intra-Provenances | Inter-Provenances | Intra-Provenances | Inter-Provenances | |
| Height (cm) | 196,889.5 | 14,969.44 | 772.122 | 2993.89 | 6.500 ** | 25.222 ** | 90.53 | 9.47 |
| Ground Diameter (mm) | 1142.81 | 136.64 | 4.48 | 27.33 | 6.29 ** | 38.34 ** | 83.48 | 16.52 |
| Total Biomass (g) | 29,099.52 | 1597.38 | 114.12 | 319.48 | 5.28 ** | 14.79 ** | 93.75 | 6.25 |
| Trait | AW | AT | AQ | AM | AL | AI | Mean |
|---|---|---|---|---|---|---|---|
| δ13C (‰) | −30.277 b | −29.937 a | −30.514 c | −30.555 c | −29.894 a | −29.804 a | −30.164 |
| (1.84%) | (2.48%) | (2.35%) | (1.90%) | (2.26%) | (1.81%) | (2.11%) | |
| δ15N (‰) | −0.798 | −0.916 | −0.967 | −0.814 | −0.834 | −0.656 | −0.831 |
| (97.90%) | (88.36%) | (114.89%) | (85.54%) | (91.49%) | (91.24%) | (94.90%) |
| Trait | Quadratic Sum | Mean Square | F | Percentage of Variation (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Intra- Provenances | Inter- Provenances | Intra- Provenances | Inter- Provenances | Intra- Provenances | Inter- Provenances | Intra- Provenances | Inter- Provenances | |
| δ13C | 428.19 | 69.29 | 1.68 | 13.86 | 9.85 ** | 81.31 ** | 78.36 | 21.64 |
| δ15N | 1055.17 | 6.27 | 4.14 | 1.25 | 5.51 ** | 1.67 | 99.49 | 0.51 |
| Mean | 88.93 | 11.08 | ||||||
| Heritability (h2) | Height | Ground Diameter | Total Biomass | WUE | NUE |
|---|---|---|---|---|---|
| AM | 0.36 | 0.49 | 0.48 | 0.62 | 0.60 |
| AI | 0.71 | 0.60 | 0.55 | 0.73 | 0.42 |
| AW | 0.61 | 0.47 | 0.42 | 0.73 | 0.39 |
| AQ | 0.69 | 0.67 | 0.66 | 0.77 | 0.71 |
| AL | 0.51 | 0.58 | 0.48 | 0.69 | 0.66 |
| AT | 0.68 | 0.51 | 0.50 | 0.61 | 0.32 |
| Trait | Total Biomass | Ground Diameter | Height | δ15N | δ13C | Eigenvalue | Contribution Rate/% | Cumulative Contribution Rate/% |
|---|---|---|---|---|---|---|---|---|
| PC1 | 0.94 | 0.90 | 0.88 | 0.37 | 0.40 | 2.76 | 55.10 | 55.10 |
| PC2 | −0.15 | −0.19 | −0.27 | 0.73 | 0.70 | 1.14 | 22.91 | 78.01 |
| Comprehensive evaluation model: PCE−i = 0.707 × y(PCi1) + 0.293 × y(PCi2) | ||||||||
| Genotype Number | Population | Comprehensive Score | Ranking | Genetic Gain (ΔG) | ||||
|---|---|---|---|---|---|---|---|---|
| Height (cm) | Ground Diameter (mm) | Total Biomass (g) | WUE (‰) | NUE (‰) | ||||
| 178-2-141 | AQ | 0.81 | 1 | 22.17 | 3.85 | 16.40 | 0.221 | 1.265 |
| 174-1-2 | AQ | 0.80 | 2 | 23.09 | 1.41 | 8.86 | 0.721 | 3.145 |
| LA05-N15 | AL | 0.77 | 3 | 23.53 | 1.82 | 7.49 | 0.852 | 1.104 |
| 178-2-106 | AQ | 0.76 | 4 | 27.46 | 2.80 | 15.78 | 0.008 | 1.167 |
| LA05-N25 | AL | 0.71 | 5 | 22.34 | 1.48 | 7.96 | 0.532 | 0.807 |
| LA05-N27 | AL | 0.70 | 6 | 9.93 | 1.44 | 4.89 | 0.774 | 1.374 |
| LA09-N23 | AL | 0.67 | 7 | 11.46 | 1.60 | 6.08 | 0.110 | 1.499 |
| LA01-N3 | AL | 0.65 | 8 | 13.50 | 1.66 | 6.94 | 0.447 | 0.615 |
| Mean | 19.19 | 2.01 | 9.30 | 0.458 | 1.372 | |||
| Provenance | Longitude (W) | Latitude (N) | Type of Climate | Genotype Number |
|---|---|---|---|---|
| Iowa, America (AI) | 93°05′60″ | 41°52′48″ | Temperate continental climate | 19 |
| Louisiana, America (AL) | 91°52′48″ | 31°18′36″ | Subtropical humid climate | 37 |
| Missouri, America (AM) | 89°50′24″ | 38°03′36″ | Subtropical humid climate | 11 |
| Tennessee, America (AT) | 89°24′00″ | 36°09′36″ | Subtropical humid climate | 51 |
| Quebec, Canada (AQ) | 72°29′24″ | 46°20′24″ | Temperate continental climate | 124 |
| Washington, America (AW) | 119°04′48″ | 46°13′12″ | Temperate continental climate | 14 |
| Total | 256 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, C.; Liu, C.; Chen, C.; Liu, N.; Liu, F.; Su, X.; Huang, Q. Genetic Evaluation of Water Use Efficiency and Nutrient Use Efficiency in Populus deltoides Bartr. ex Marsh. Seedlings in China. Plants 2024, 13, 2228. https://doi.org/10.3390/plants13162228
Gao C, Liu C, Chen C, Liu N, Liu F, Su X, Huang Q. Genetic Evaluation of Water Use Efficiency and Nutrient Use Efficiency in Populus deltoides Bartr. ex Marsh. Seedlings in China. Plants. 2024; 13(16):2228. https://doi.org/10.3390/plants13162228
Chicago/Turabian StyleGao, Chengcheng, Chenggong Liu, Cun Chen, Ning Liu, Fenfen Liu, Xiaohua Su, and Qinjun Huang. 2024. "Genetic Evaluation of Water Use Efficiency and Nutrient Use Efficiency in Populus deltoides Bartr. ex Marsh. Seedlings in China" Plants 13, no. 16: 2228. https://doi.org/10.3390/plants13162228
APA StyleGao, C., Liu, C., Chen, C., Liu, N., Liu, F., Su, X., & Huang, Q. (2024). Genetic Evaluation of Water Use Efficiency and Nutrient Use Efficiency in Populus deltoides Bartr. ex Marsh. Seedlings in China. Plants, 13(16), 2228. https://doi.org/10.3390/plants13162228





