UV-B Stress-Triggered Amino Acid Reprogramming and ABA-Mediated Hormonal Crosstalk in Rhododendron chrysanthum Pall.
Abstract
:1. Introduction
2. Result
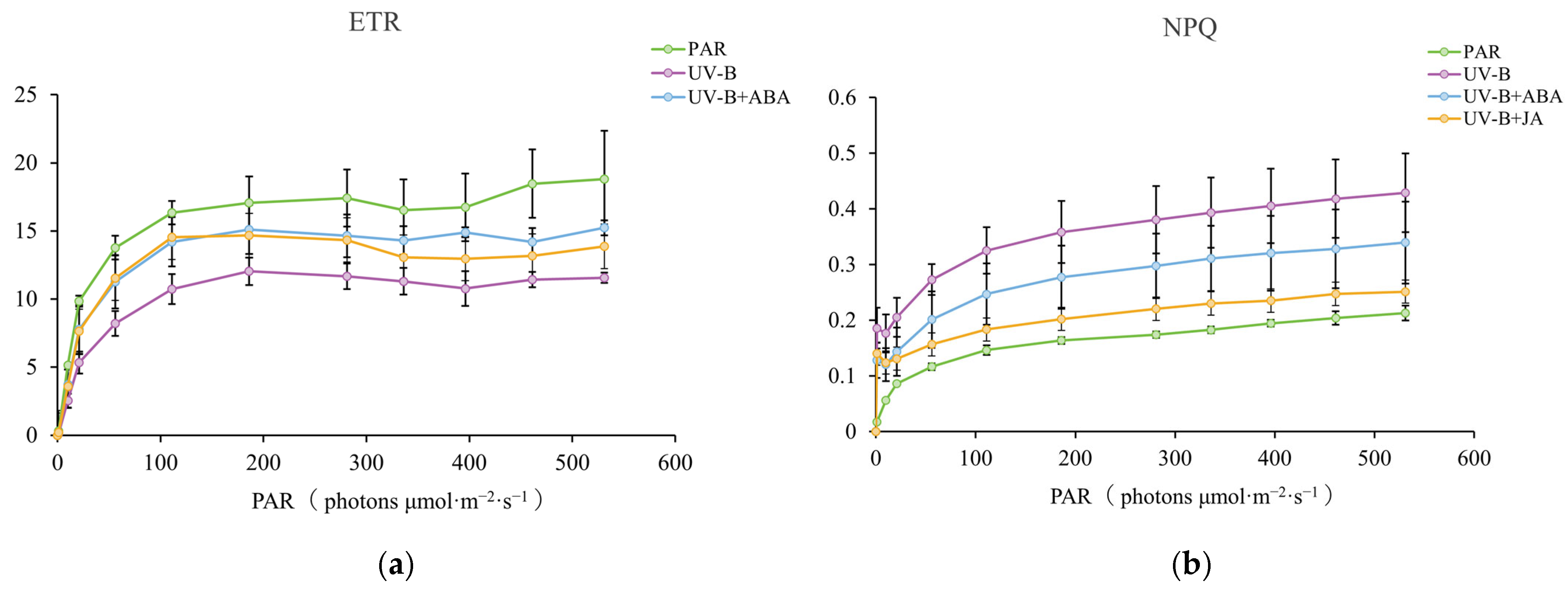
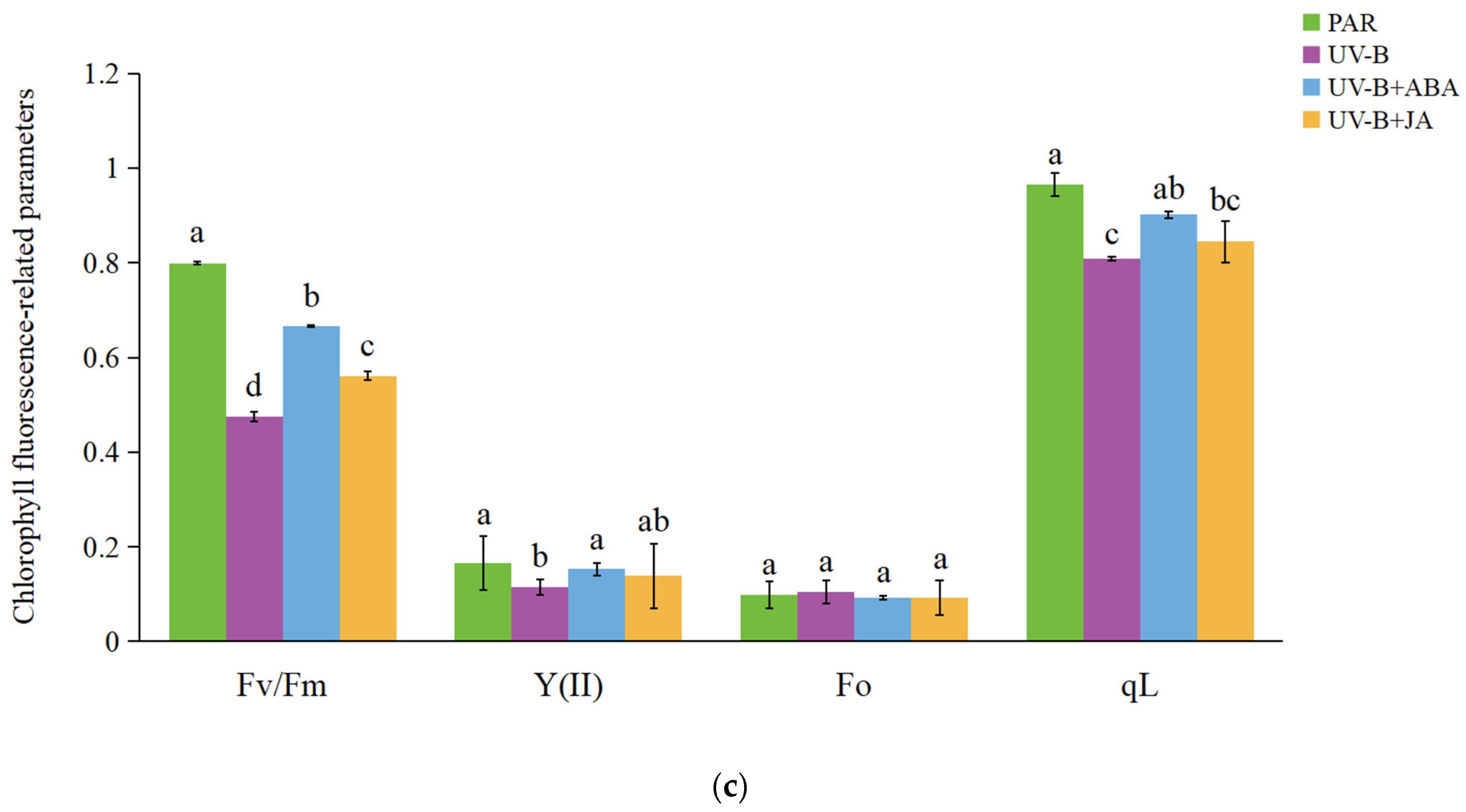
2.1. Exogenous Hormone Therapy Improves R. chrysanthum’s Ability to Withstand UV-B Damage
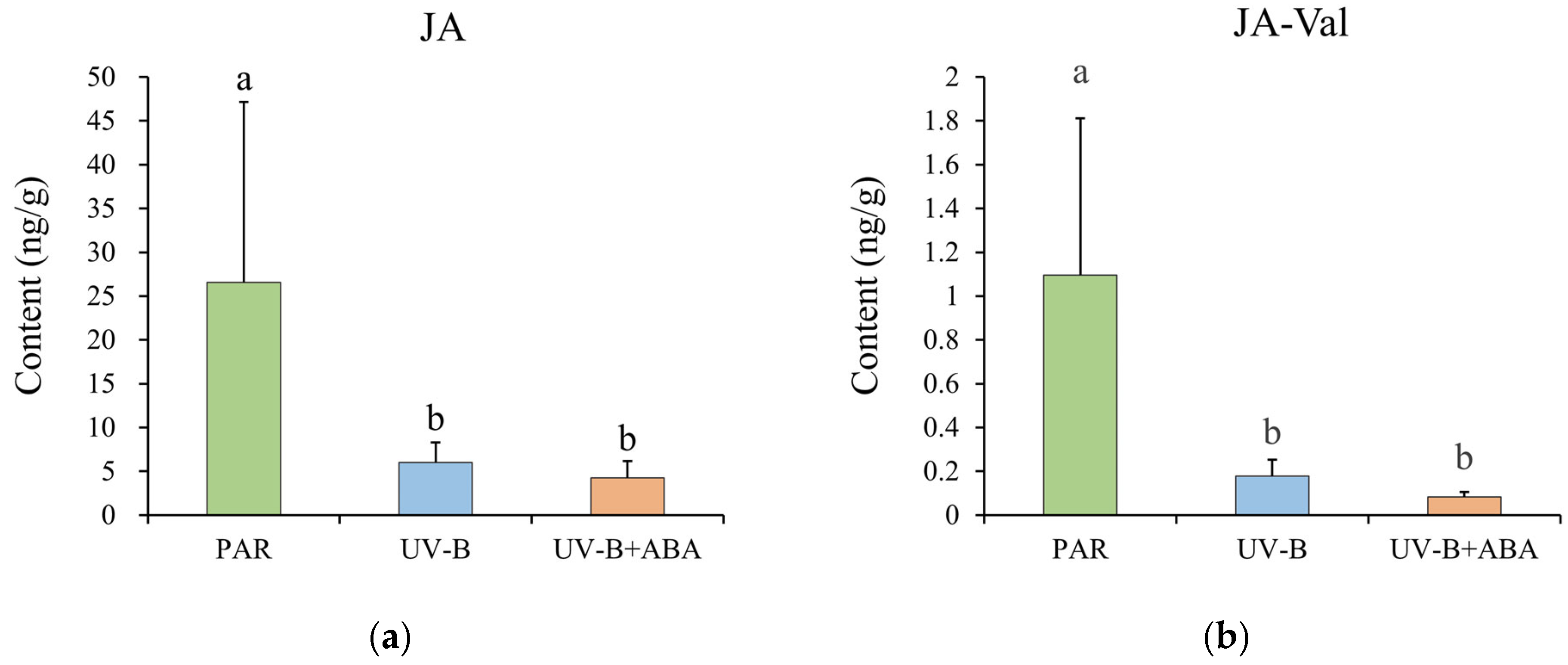
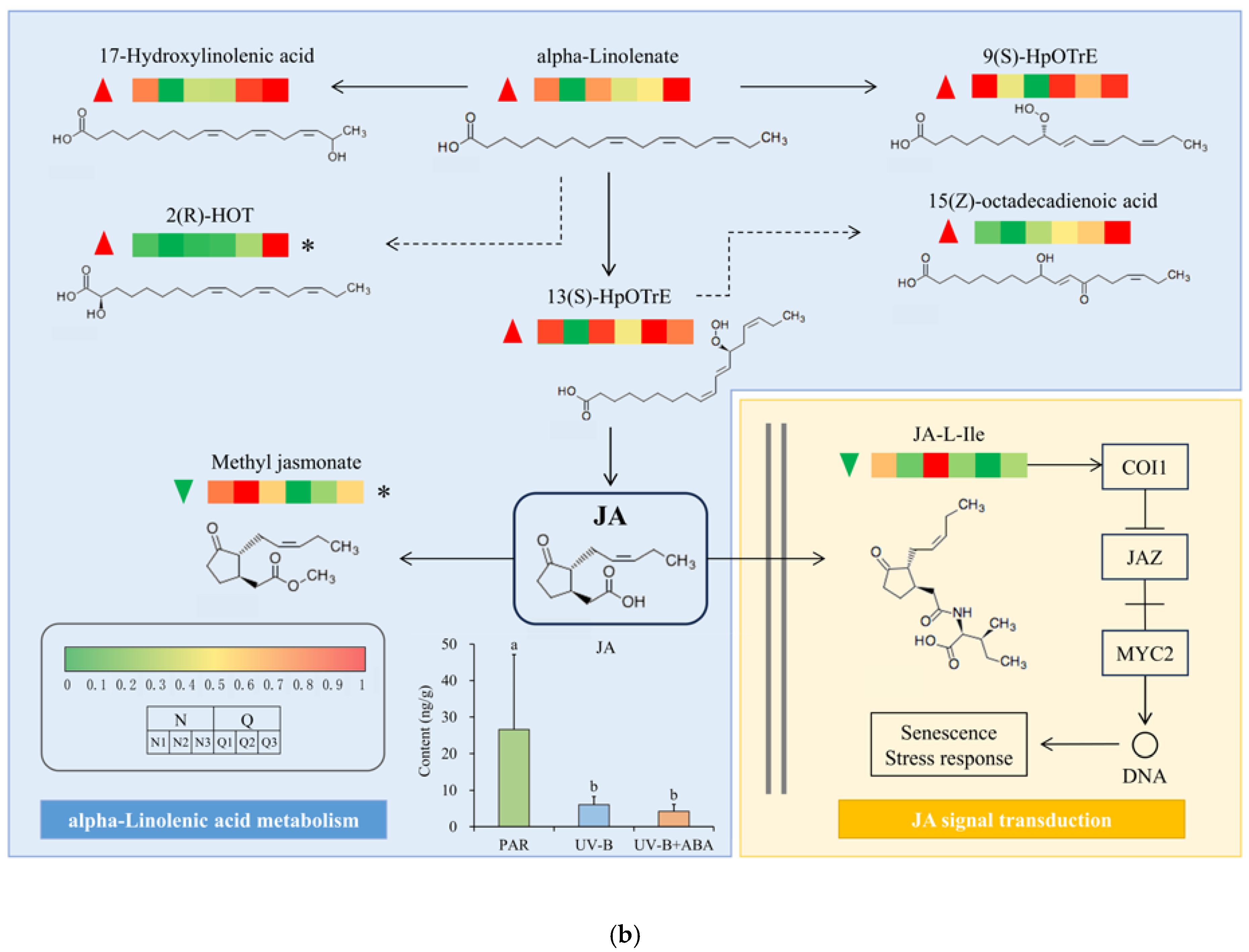
2.2. Negative Control of JA by ABA in R. chrysanthum during UV-B Stress
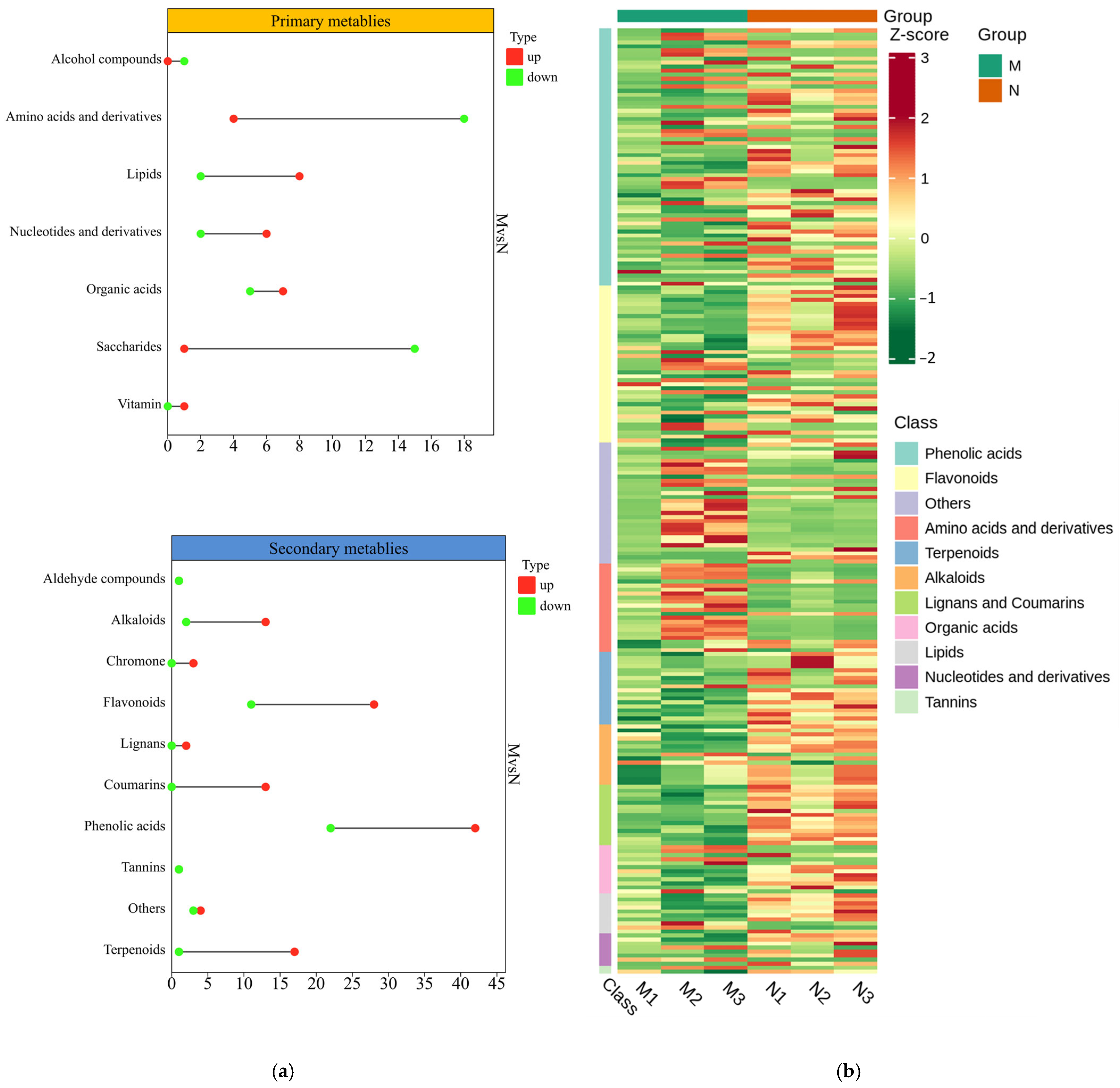
2.3. Metabolomic Analysis of R. chrysanthum
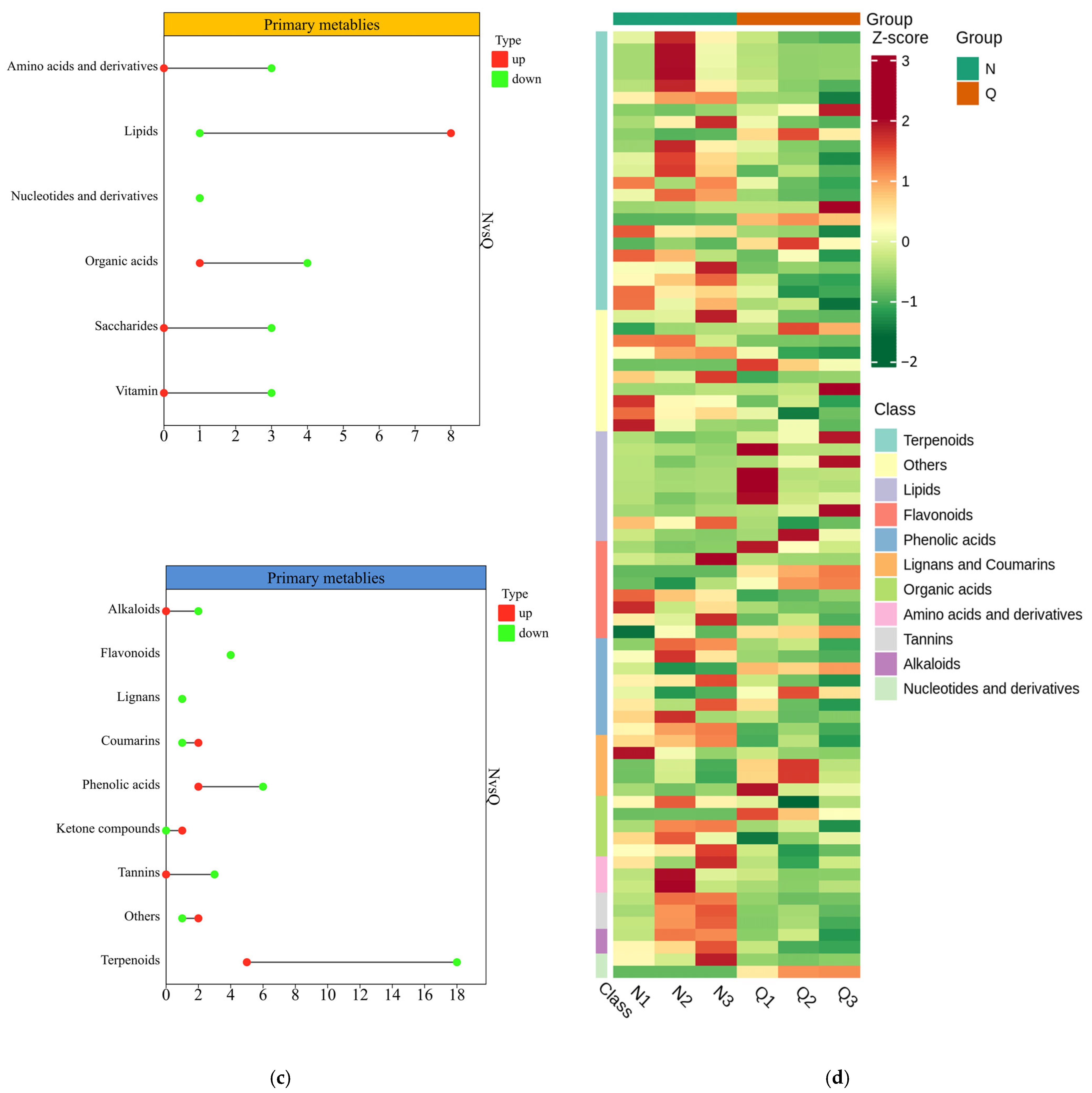
2.4. Response of Primary and Secondary Metabolites of R. chrysanthum to UV-B as Well as Exogenous Exogenous ABA
2.5. Primary and Secondary Main Contributing Metabolites
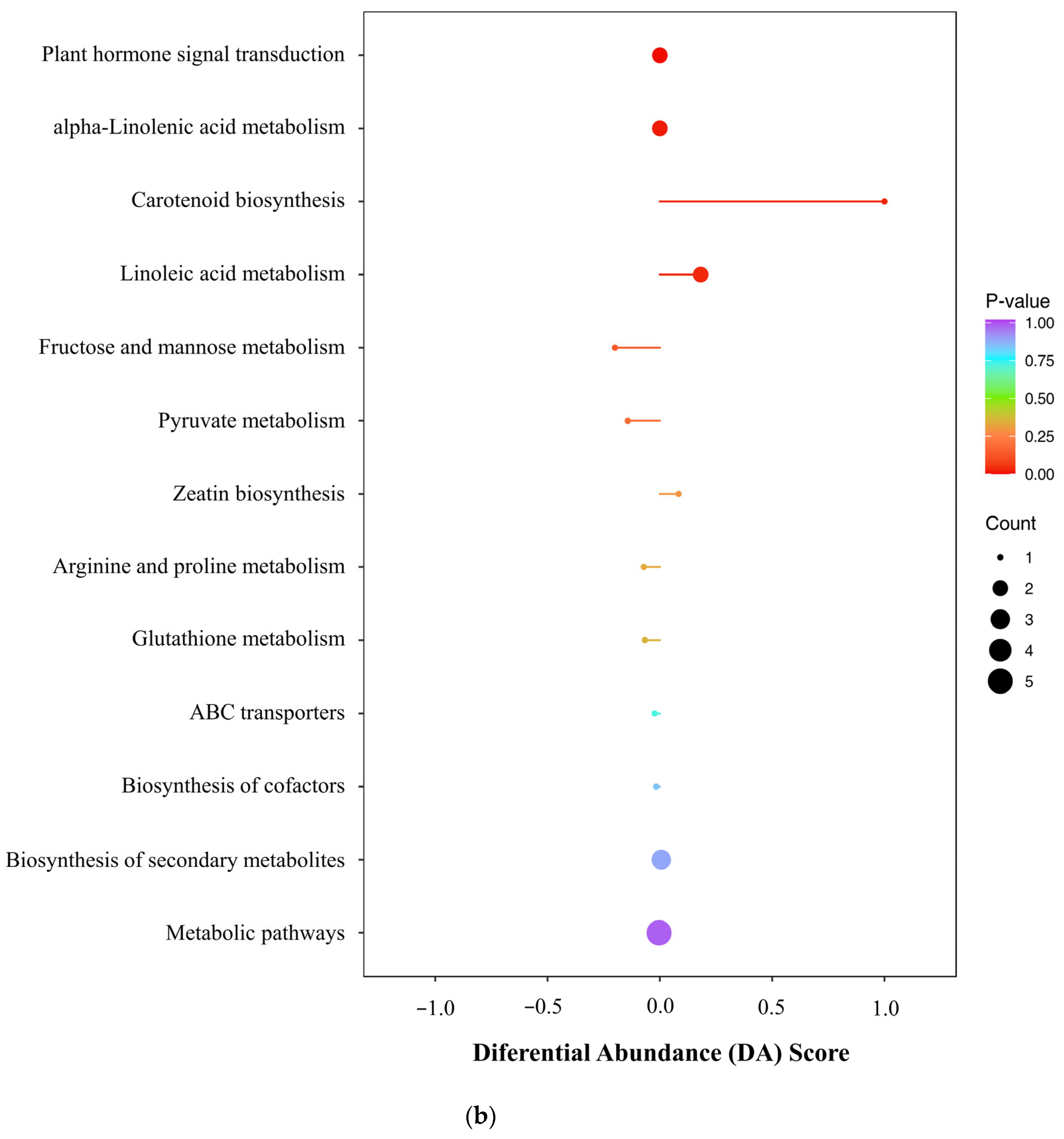
2.6. Effects of UV-B and Exogenous ABA Treatment on the Metabolic Pathway of R. chrysanthum
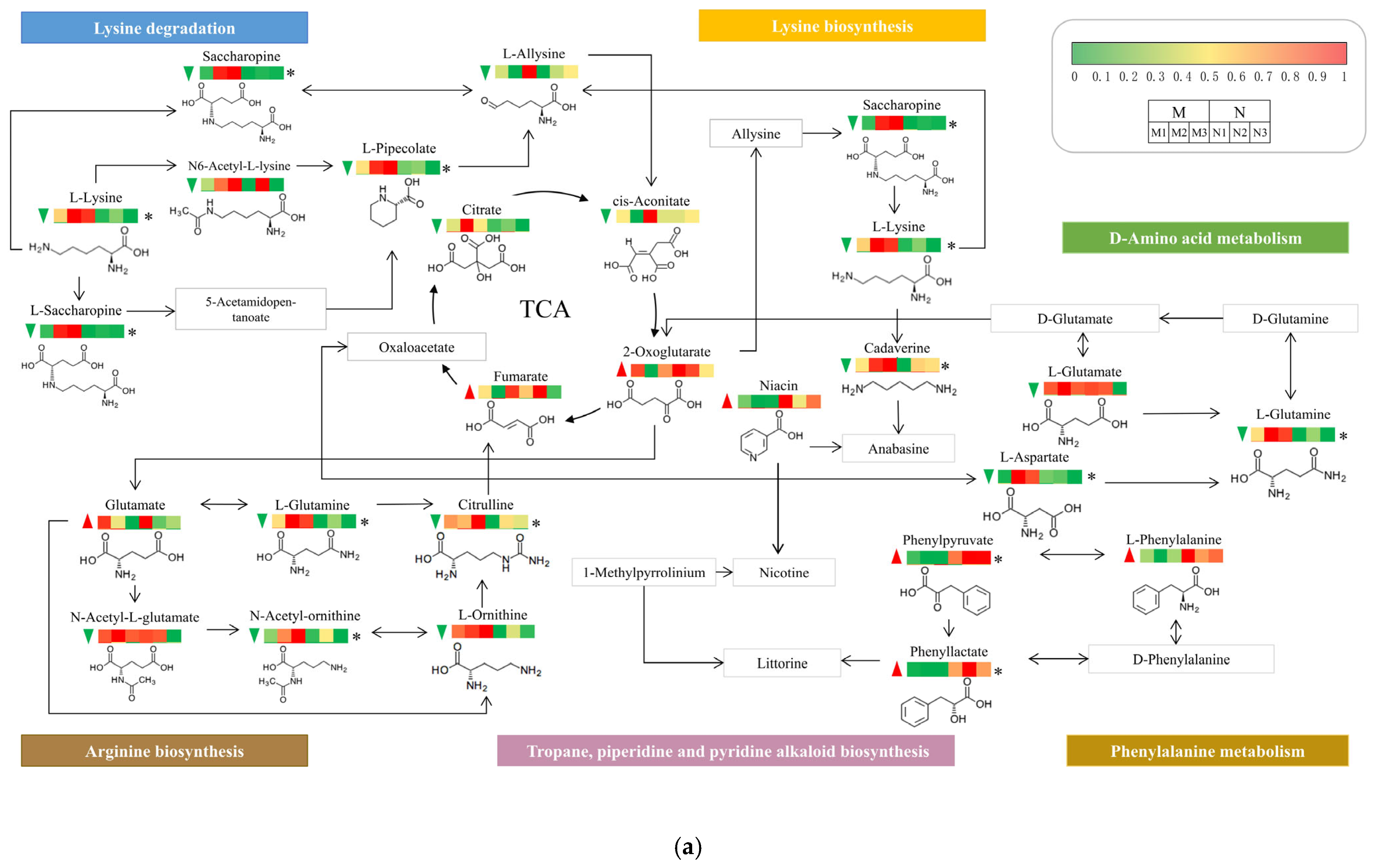
2.7. UV-B and Exogenous ABA Prompt Rearrangement of the Metabolic Pathway Network of R. chrysanthum
3. Discussion
4. Materials and Methods
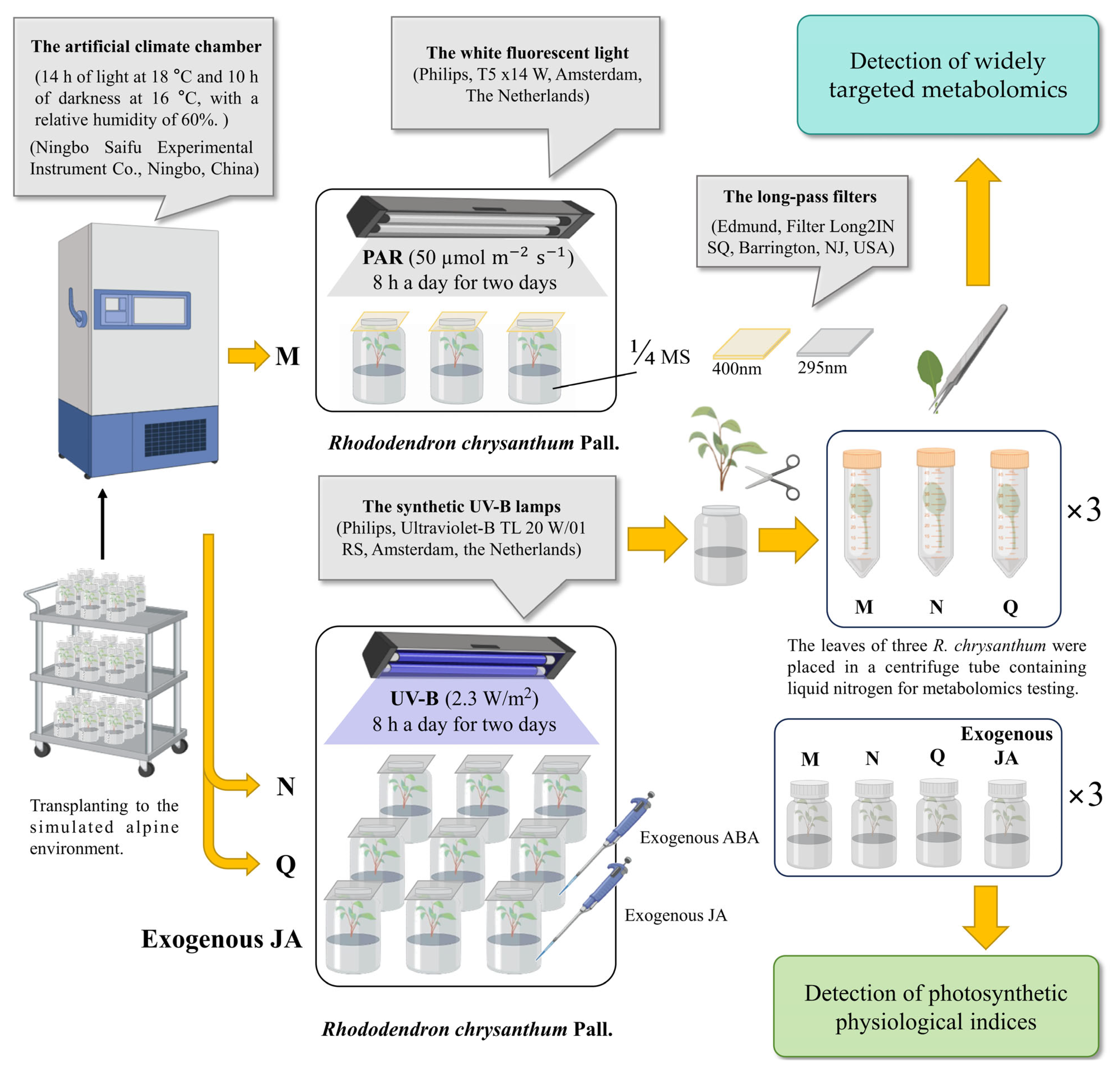
4.1. Material Preparation and Experimental Treatments
4.2. Widely Targeted Metabolomics Testing
4.2.1. Dry-Sample Extraction Conditions
4.2.2. UPLC Conditions
4.2.3. ESI-Q TRAP-MS/MS
4.2.4. Qualitative and Quantitative Analysis of Metabolites
4.2.5. Differential Metabolites (DMs) Selected
4.2.6. OPLS-DA and Pearson Correlation Coefficients
4.3. Determination of Chlorophyll Fluorescence Parameters
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABA | Abscisic acid |
| ANOVA | Analysis of variance |
| DMs | Differential metabolites |
| ETR | Actual electron transport rate |
| FC | Fold change |
| Fm | Maximum fluorescence |
| Fm’ | Maximum fluorescence in the light |
| Fo | Minimal fluorescence |
| Fv/Fm | Maximal photochemical efficiency of PSII |
| JA | Jasmonic acid |
| JA-L-Ile | Jasmonoyl-L-isoleucine |
| JA-Val | Jasmonoyl-L-valine |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| MWDB | Metware database |
| NPQ | Non-photochemical quenching |
| OPLS-DA | Orthogonal partial least-squares discriminant analysis |
| PAR | Photosynthetic active radiation |
| PCCs | Pearson’s correlation coefficients |
| PSII | Photosystem II |
| QCs | Quality control samples |
| R. chrysanthum | Rhododendron chrysanthum Pall. |
| SD | Standard deviation |
| UPLC-MS/MS | Ultra-performance liquid chromatography–tandem mass spectrometry |
| UV-B | Ultraviolet radiation b |
| VIP | Variable importance in projection |
| Y(II) | Photochemical yield of PSII |
References
- Bais, A.F.; Bernhard, G.; McKenzie, R.L.; Aucamp, P.J.; Young, P.J.; Ilyas, M.; Jockel, P.; Deushi, M. Ozone-climate interactions and effects on solar ultraviolet radiation. Photochem. Photobiol. Sci. 2019, 18, 602–640. [Google Scholar] [CrossRef] [PubMed]
- Bais, A.F.; McKenzie, R.L.; Bernhard, G.; Aucamp, P.J.; Ilyas, M.; Madronich, S.; Tourpali, K. Ozone depletion and climate change: Impacts on UV radiation. Photochem. Photobiol. Sci. 2015, 14, 19–52. [Google Scholar] [CrossRef] [PubMed]
- Albert, K.R.; Mikkelsen, T.N.; Ro-Poulsen, H.; Michelsen, A.; Arndal, M.F.; Bredahl, L.; Håkansson, K.B.; Boesgaard, K.; Schmidt, N.M. Improved UV-B screening capacity does not prevent negative effects of ambient UV irradiance on PSII performance in high arctic plants. Results from a six year UV exclusion study. J. Plant Physiol. 2010, 167, 1542–1549. [Google Scholar] [CrossRef] [PubMed]
- Hideg, E.; Jansen, M.A.; Strid, A. UV-B exposure, ROS, and stress: Inseparable companions or loosely linked associates? Trends Plant Sci. 2013, 18, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Widel, M.; Krzywon, A.; Gajda, K.; Skonieczna, M.; Rzeszowska-Wolny, J. Induction of bystander effects by UVA, UVB, and UVC radiation in human fibroblasts and the implication of reactive oxygen species. Free Radic. Biol. Med. 2014, 68, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, R.P.; Richa, N.; Kumar, A.; Tyagi, M.B.; Sinha, R.P. Molecular mechanisms of ultraviolet radiation-induced DNA damage and repair. J. Nucleic Acids 2010, 2010, 592980. [Google Scholar] [CrossRef] [PubMed]
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Changing scenario in plant UV-B research:UV-B from a generic stressor to a specific regulator. J. Photochem. Photobiol. B 2015, 153, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Falara, V.; Amarasinghe, R.; Poldy, J.; Pichersky, E.; Barrow, R.A.; Peakall, R. The production of a key floral volatile is dependent on UV light in a sexually deceptive orchid. Ann. Bot. 2013, 111, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Turunen, M.; Latola, K. UV-B radiation and acclimation in timberline plants. Environ. Pollut. 2005, 137, 390–403. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Q.; Shang, S.; Chen, J.; Lu, P.; Zang, Y.; Tang, X. Physiological responses and metabonomics analysis of male and female Sargassum thunbergii macroalgae exposed to ultraviolet-B stress. Front. Plant Sci. 2022, 13, 778602. [Google Scholar] [CrossRef]
- Zhou, X.; Lyu, J.; Sun, L.; Dong, J.; Xu, H. Metabolic programming of Rhododendron chrysanthum leaves following exposure to UVB irradiation. Funct. Plant Biol. 2021, 48, 1175–1185. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Liu, X.; Zhu, W.; Cheng, X.; Wang, X.; Wan, X.; Liu, L. Ambient ultraviolet B signal modulates tea flavor characteristics via shifting a metabolic flux in flavonoid biosynthesis. J. Agric. Food Chem. 2021, 69, 3401–3414. [Google Scholar] [CrossRef] [PubMed]
- Cao, K.; Zhang, Z.; Fan, H.; Tan, Y.; Xu, H.; Zhou, X. Comparative transcriptomic analysis reveals gene expression in response to cold stress in Rhododendron aureum Georgi. Theor. Exp. Plant Physiol. 2022, 34, 347–366. [Google Scholar] [CrossRef]
- Bornman, J.F.; Barnes, P.W.; Robinson, S.A.; Ballaré, C.L.; Flint, S.D.; Caldwell, M.M. Solar ultraviolet radiation and ozone depletion-driven climate change: Effects on terrestrial ecosystems. Photochem. Photobiol. Sci. 2015, 14, 88–107. [Google Scholar] [CrossRef] [PubMed]
- Kuttippurath, J.; Lefèvre, F.; Pommereau, J.P.; Roscoe, H.K.; Goutail, F.; Pazmiño, A.; Shanklin, J.D. Antarctic ozone loss in 1979–2010: First sign of ozone recovery. Atmos. Chem. Phys. 2013, 13, 1625–1635. [Google Scholar] [CrossRef]
- Wang, F.; Xu, Z.; Fan, X.; Zhou, Q.; Cao, J.; Ji, G.; Jing, S.; Feng, B.; Wang, T. Transcriptome analysis reveals complex molecular mechanisms underlying UV tolerance of Wheat (Triticum aestivum, L.). J. Agric. Food Chem. 2019, 67, 563–577. [Google Scholar] [CrossRef]
- Cutler, S.R.; Rodriguez, P.L.; Finkelstein, R.R.; Abrams, S.R. Abscisic acid: Emergence of a core signaling network. Annu. Rev. Plant Biol. 2010, 61, 651–679. [Google Scholar] [CrossRef] [PubMed]
- Vishwakarma, K.; Upadhyay, N.; Kumar, N.; Yadav, G.; Singh, J.; Mishra, R.K.; Kumar, V.; Verma, R.; Upadhyay, R.G.; Pandey, M.; et al. Abscisic acid signaling and abiotic stress tolerance in plants: A review on current knowledge and future prospects. Front. Plant Sci. 2017, 8, 161. [Google Scholar] [CrossRef] [PubMed]
- Swamy, P.M.; Smith, B.N. Role of abscisic acid in plant stress tolerance. Curr. Sci. 1999, 76, 1220–1227. [Google Scholar]
- Martínez-Mazón, P.; Bahamonde, C.; Herrera-Rodríguez, M.B.; Fernández-Ocaña, A.M.; Rexach, J.; González-Fontes, A.; Camacho-Cristóbal, J.J. Role of ABA in the adaptive response of arabidopsis plants to long-term boron toxicity treatment. Plant Physiol. Biochem. 2023, 202, 107965. [Google Scholar] [CrossRef]
- Segarra-Medina, C.; Alseekh, S.; Fernie, A.R.; Rambla, J.L.; Pérez-Clemente, R.M.; Gómez-Cádenas, A.; Zandalinas, S.I. Abscisic acid promotes plant acclimation to the combination of salinity and high light stress. Plant Physiol. Biochem. 2023, 203, 108008. [Google Scholar] [CrossRef] [PubMed]
- Habibpourmehraban, F.; Wu, Y.; Masoomi-Aladizgeh, F.; Amirkhani, A.; Atwell, B.J.; Haynes, P.A. Pre-Treatment of rice plants with aba makes them more tolerant to multiple abiotic stress. Int. J. Mol. Sci. 2023, 24, 9628. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Chen, M.-H.; Yang, L.-T.; Li, Y.-R.; Wu, J.-M. Effects of exogenous abscisic acid on cell membrane and endogenous hormone contents in leaves of Sugarcane seedlings under cold stress. Sugar Tech. 2015, 17, 59–64. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Choi, K.-I.; Khan, A.L.; Waqas, M.; Lee, I.-J. Exogenous application of abscisic acid regulates endogenous gibberellins homeostasis and enhances resistance of oriental melon (Cucumis melo var. L.) against low temperature. Sci. Hortic. 2016, 207, 41–47. [Google Scholar] [CrossRef]
- Sun, Q.; Zhou, X.; Yang, L.; Xu, H.; Zhou, X. Integration of phosphoproteomics and transcriptome studies reveals ABA signaling pathways regulate UV-B tolerance in Rhododendron chrysanthum leaves. Genes 2023, 14, 1153. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Wu, Y.; Wu, D.; Rao, W.; Guo, J.; Ma, Y.; Wang, Z.; Shangguan, X.; Wang, H.; Xu, C.; et al. The coiled-coil and nucleotide binding domains of brown planthopper resistance14 function in signaling and resistance against planthopper in rice. Plant Cell 2017, 29, 3157–3185. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Q.; Du, L.; Hallerman, E.M.; Li, Y. Transcriptomic and metabolomic responses of rice plants to cnaphalocrocis medinalis caterpillar infestation. Insects 2020, 11, 705. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lei, Y.; Lu, C.; Wang, L.; Wu, J. MYC2, MYC3, and MYC4 function additively in wounding-induced jasmonic acid biosynthesis and catabolism. J. Integr. Plant Biol. 2020, 62, 1159–1175. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Su, L.; Sun, X.; Li, X.; Sun, M.; Karungo, S.K.; Fang, S.; Chu, J.; Li, S.; Xin, H. Expression of vitis amurensis NAC26 in arabidopsis enhances drought tolerance by modulating jasmonic acid synthesis. J. Exp. Bot. 2016, 67, 2829–2845. [Google Scholar] [CrossRef]
- Wu, S.; Wang, Y.; Zhang, J.; Wang, Y.; Yang, Y.; Chen, X.; Wang, Y. How does Malus crabapple resist ozone? Transcriptomics and metabolomics analyses. Ecotoxicol. Environ. Saf. 2020, 201, 110832. [Google Scholar] [CrossRef]
- Chapman, K.M.; Marchi-Werle, L.; Hunt, T.E.; Heng-Moss, T.M.; Louis, J. Abscisic and jasmonic acids contribute to soybean tolerance to the Soybean Aphid (Aphis glycines Matsumura). Sci. Rep. 2018, 8, 15148. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Yang, C.; Cao, J.; Chen, H.; Pang, J.; Zhao, Q.; Wang, Z.; Qing Fu, Z.; Liu, J. A bHLH transcription activator regulates defense signaling by nucleo-cytosolic trafficking in rice. J. Integr. Plant Biol. 2020, 62, 1552–1573. [Google Scholar] [CrossRef] [PubMed]
- Shu, J.; Ma, X.; Ma, H.; Huang, Q.; Zhang, Y.; Guan, M.; Guan, C. Transcriptomic, proteomic, metabolomic, and functional genomic approaches of Brassica napus L. during salt stress. PLoS ONE 2022, 17, e0262587. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Lam, S.M.; Shui, G. Metabolomics, a powerful tool for agricultural research. Int. J. Mol. Sci. 2016, 17, 1871. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Li, X.; Sun, L.; Sun, M.; Xu, H.; Zhou, X. Plant hormones and phenolic acids response to UV-B stress in Rhododendron chrysanthum pall. Biol. Direct 2024, 19, 40. [Google Scholar] [CrossRef] [PubMed]
- Kusano, M.; Tohge, T.; Fukushima, A.; Kobayashi, M.; Hayashi, N.; Otsuki, H.; Kondou, Y.; Goto, H.; Kawashima, M.; Matsuda, F.; et al. Metabolomics reveals comprehensive reprogramming involving two independent metabolic responses of arabidopsis to UV-B light. Plant J. 2011, 67, 354–369. [Google Scholar] [CrossRef] [PubMed]
- Maher, M.; Ahmad, H.; Nishawy, E.; Li, Y.; Luo, J. Novel transcriptome study and detection of metabolic variations in UV-B-treated Date Palm (Phoenix dactylifera cv. Khalas). Int. J. Mol. Sci. 2021, 22, 2564. [Google Scholar] [CrossRef] [PubMed]
- Appenroth, K.J.; Stöckel, J.; Srivastava, A.; Strasser, R.J. Multiple effects of chromate on the photosynthetic apparatus of Spirodela polyrhiza as probed by OJIP chlorophyll a fluorescence measurements. Environ. Pollut. 2001, 115, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Yang, G.; Tan, C.; Zhao, C. Effects of nitrogen stress on the photosynthetic CO2 assimilation, chlorophyll fluorescence, and sugar-nitrogen ratio in corn. Sci. Rep. 2015, 5, 9311. [Google Scholar] [CrossRef]
- Laible, P.D.; Zipfel, W.; Owens, T.G. Excited state dynamics in chlorophyll-based antennae: The role of transfer equilibrium. Biophys. J. 1994, 66, 844–860. [Google Scholar] [CrossRef]
- Hu, T.; Hu, L.; Zhang, X.; Zhang, P.; Zhao, Z.; Fu, J. Differential responses of CO2 assimilation, carbohydrate allocation and gene expression to NaCl stress in perennial ryegrass with different salt tolerance. PLoS ONE 2013, 8, e66090. [Google Scholar] [CrossRef]
- Yoon, J.; Cho, L.H.; Tun, W.; Jeon, J.S.; An, G. Sucrose signaling in higher plants. Plant Sci. 2021, 302, 110703. [Google Scholar] [CrossRef] [PubMed]
- Berli, F.J.; Alonso, R.; Bressan-Smith, R.; Bottini, R. UV-B impairs growth and gas exchange in grapevines grown in high altitude. Physiol. Plant 2013, 149, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Lüscher, J.; Morales, F.; Delrot, S.; Sánchez-Díaz, M.; Gomés, E.; Aguirreolea, J.; Pascual, I. Short- and long-term physiological responses of grapevine leaves to UV-B radiation. Plant Sci. 2013, 213, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Beckles, D.M. Dynamic changes in the starch-sugar interconversion within plant source and sink tissues promote a better abiotic stress response. J. Plant Physiol. 2019, 234–235, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.A.; Bush, D.R. Carbohydrate export from the leaf: A highly regulated process and target to enhance photosynthesis and productivity. Plant Physiol. 2011, 155, 64–69. [Google Scholar] [CrossRef]
- MacNeill, G.J.; Mehrpouyan, S.; Minow, M.A.A.; Patterson, J.A.; Tetlow, I.J.; Emes, M.J. Starch as a source, starch as a sink: The bifunctional role of starch in carbon allocation. J. Exp. Bot. 2017, 68, 4433–4453. [Google Scholar] [CrossRef] [PubMed]
- Galili, G. The aspartate-family pathway of plants. Plant Signal. Behav. 2011, 6, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, D.; Li, M.; Shi, L. Metabolic profiles reveal changes in wild and cultivated Soybean seedling leaves under salt stress. PLoS ONE 2016, 11, e0159622. [Google Scholar] [CrossRef]
- Baena-González, E.; Sheen, J. Convergent energy and stress signaling. Trends Plant Sci. 2008, 13, 474–482. [Google Scholar] [CrossRef]
- Forde, B.G.; Lea, P.J. Glutamate in plants: Metabolism, regulation, and signalling. J. Exp. Bot. 2007, 58, 2339–2358. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, A.; Paździora, W.; Ekiert, H. The influence of exogenous phenylalanine on the accumulation of secondary metabolites in agitated shoot cultures of Ruta graveolens L. Molecules 2023, 28, 727. [Google Scholar] [CrossRef] [PubMed]
- Snaidr, V.A.; Damian, D.L.; Halliday, G.M. Nicotinamide for photoprotection and skin cancer chemoprevention: A review of efficacy and safety. Exp. Dermatol. 2019, 28 (Suppl. S1), 15–22. [Google Scholar] [CrossRef] [PubMed]
- Gibon, Y.; Pyl, E.T.; Sulpice, R.; Lunn, J.E.; Höhne, M.; Günther, M.; Stitt, M. Adjustment of growth, starch turnover, protein content and central metabolism to a decrease of the carbon supply when arabidopsis is grown in very short photoperiods. Plant Cell Environ. 2009, 32, 859–874. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.K.; Dubeaux, G.; Takahashi, Y.; Schroeder, J.I. Signaling mechanisms in abscisic acid-mediated stomatal closure. Plant J. 2021, 105, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jia, W.; Yang, J.; Ismail, A.M. Role of ABA in integrating plant responses to drought and salt stresses. Field Crops Res. 2006, 97, 111–119. [Google Scholar] [CrossRef]
- Weber, H. Fatty acid-derived signals in plants. Trends Plant Sci. 2002, 7, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Upchurch, R.G. Fatty acid unsaturation, mobilization, and regulation in the response of plants to stress. Biotechnol. Lett. 2008, 30, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Savchenko, T.; Walley, J.W.; Chehab, E.W.; Xiao, Y.; Kaspi, R.; Pye, M.F.; Mohamed, M.E.; Lazarus, C.M.; Bostock, R.M.; Dehesh, K. Arachidonic acid: An evolutionarily conserved signaling molecule modulates plant stress signaling networks. Plant Cell 2010, 22, 3193–3205. [Google Scholar] [CrossRef]
- Narayanan, S.; Tamura, P.J.; Roth, M.R.; Prasad, P.V.; Welti, R. Wheat leaf lipids during heat stress: I. High day and night temperatures result in major lipid alterations. Plant Cell Environ. 2016, 39, 787–803. [Google Scholar] [CrossRef]
- Kachroo, A.; Kachroo, P. Fatty acid-derived signals in plant defense. Annu. Rev. Phytopathol. 2009, 47, 153–176. [Google Scholar] [CrossRef]
- Kumar, P.P. Regulation of biotic and abiotic stress responses by plant hormones. Plant Cell Rep. 2013, 32, 943. [Google Scholar] [CrossRef]
- Bari, R.; Jones, J.D. Role of plant hormones in plant defence responses. Plant Mol. Biol. 2009, 69, 473–488. [Google Scholar] [CrossRef]
- Navarro, L.; Bari, R.; Achard, P.; Lisón, P.; Nemri, A.; Harberd, N.P.; Jones, J.D. Dellas control plant immune responses by modulating the balance of jasmonic acid and salicylic acid signaling. Curr. Biol. 2008, 18, 650–655. [Google Scholar] [CrossRef]
- Nishiyama, R.; Watanabe, Y.; Leyva-Gonzalez, M.A.; Ha, C.V.; Fujita, Y.; Tanaka, M.; Seki, M.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Herrera-Estrella, L.; et al. Arabidopsis AHP2, AHP3, and AHP5 histidine phosphotransfer proteins function as redundant negative regulators of drought stress response. Proc. Natl. Acad. Sci. USA 2013, 110, 4840–4845. [Google Scholar] [CrossRef]
- Katsir, L.; Chung, H.S.; Koo, A.J.; Howe, G.A. Jasmonate signaling: A conserved mechanism of hormone sensing. Curr. Opin. Plant Biol. 2008, 11, 428–435. [Google Scholar] [CrossRef]
- Pauwels, L.; Barbero, G.F.; Geerinck, J.; Tilleman, S.; Grunewald, W.; Pérez, A.C.; Chico, J.M.; Bossche, R.V.; Sewell, J.; Gil, E.; et al. NINJA connects the co-repressor topless to jasmonate signalling. Nature 2010, 464, 788–791. [Google Scholar] [CrossRef]
- Sabzehzari, M.; Naghavi, M.R. Phyto-miRNAs-based regulation of metabolites biosynthesis in medicinal plants. Gene 2019, 682, 13–24. [Google Scholar] [CrossRef]
- Jwa, N.S.; Agrawal, G.K.; Tamogami, S.; Yonekura, M.; Han, O.; Iwahashi, H.; Rakwal, R. Role of defense/stress-related marker genes, proteins and secondary metabolites in defining rice self-defense mechanisms. Plant Physiol. Biochem. 2006, 44, 261–273. [Google Scholar] [CrossRef]
- Cheng, H.P.; Yang, X.H.; Lan, L.; Xie, L.J.; Chen, C.; Liu, C.; Chu, J.; Li, Z.Y.; Liu, L.; Zhang, T.Q.; et al. Chemical deprenylation of N(6) -Isopentenyladenosine (i(6) A) RNA. Angew. Chem. Int. Ed. Engl. 2020, 59, 10645–10650. [Google Scholar] [CrossRef]
- Colombo, F.; Falvella, F.S.; De Cecco, L.; Tortoreto, M.; Pratesi, G.; Ciuffreda, P.; Ottria, R.; Santaniello, E.; Cicatiello, L.; Weisz, A.; et al. Pharmacogenomics and analogues of the antitumour agent N6-isopentenyladenosine. Int. J. Cancer 2009, 124, 2179–2185. [Google Scholar] [CrossRef]
- Ranieri, R.; Ciaglia, E.; Amodio, G.; Picardi, P.; Proto, M.C.; Gazzerro, P.; Laezza, C.; Remondelli, P.; Bifulco, M.; Pisanti, S. N6-isopentenyladenosine dual targeting of AMPK and Rab7 prenylation inhibits melanoma growth through the impairment of autophagic flux. Cell Death Differ. 2018, 25, 353–367. [Google Scholar] [CrossRef]
- Zhou, X.; Gong, F.; Dong, J.; Lin, X.; Cao, K.; Xu, H.; Zhou, X. Abscisic acid affects phenolic acid content to increase tolerance to UV-B stress in Rhododendron chrysanthum Pall. Int. J. Mol. Sci. 2024, 25, 1234. [Google Scholar] [CrossRef]
- Lyu, J.; Wang, C.; Liang, D.; Liu, L.; Pandey, L.; Xu, H.; Zhou, X. Sensitivity of wild and domesticated Rhododendron chrysanthum to different light regime (UVA, UVB, and PAR). Photosynthetica 2019, 57, 841–849. [Google Scholar] [CrossRef]
- Gong, F.; Yu, W.; Zeng, Q.; Dong, J.; Cao, K.; Xu, H.; Zhou, X. Rhododendron chrysanthum’s primary metabolites are converted to phenolics more quickly when exposed to UV-B radiation. Biomolecules 2023, 13, 1700. [Google Scholar] [CrossRef]
- Chen, W.; Gong, L.; Guo, Z.; Wang, W.; Zhang, H.; Liu, X.; Yu, S.; Xiong, L.; Luo, J. A novel integrated method for large-scale detection, identification, and quantification of widely targeted metabolites: Application in the study of rice metabolomics. Mol. Plant 2013, 6, 1769–1780. [Google Scholar] [CrossRef]
- Giovannoni, J. Tomato multiomics reveals consequences of crop domestication and improvement. Cell 2018, 172, 6–8. [Google Scholar] [CrossRef]
- Fraga, C.G.; Clowers, B.H.; Moore, R.J.; Zink, E.M. Signature-discovery approach for sample matching of a nerve-agent precursor using liquid chromatography−mass spectrometry, XCMS, and chemometrics. Anal. Chem. 2010, 82, 4165–4173. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Dong, J.; Lin, X.; Zhou, X.; Xu, H. Isolation and identification of Acer truncatum Endophytic Fungus Talaromyces verruculosus and evaluation of its effects on insoluble phosphorus absorption capacity and growth of Cucumber seedlings. J. Fungi 2024, 10, 136. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, W.; Zhou, X.; Xu, H.; Zhou, X. UV-B Stress-Triggered Amino Acid Reprogramming and ABA-Mediated Hormonal Crosstalk in Rhododendron chrysanthum Pall. Plants 2024, 13, 2232. https://doi.org/10.3390/plants13162232
Yu W, Zhou X, Xu H, Zhou X. UV-B Stress-Triggered Amino Acid Reprogramming and ABA-Mediated Hormonal Crosstalk in Rhododendron chrysanthum Pall. Plants. 2024; 13(16):2232. https://doi.org/10.3390/plants13162232
Chicago/Turabian StyleYu, Wang, Xiangru Zhou, Hongwei Xu, and Xiaofu Zhou. 2024. "UV-B Stress-Triggered Amino Acid Reprogramming and ABA-Mediated Hormonal Crosstalk in Rhododendron chrysanthum Pall." Plants 13, no. 16: 2232. https://doi.org/10.3390/plants13162232





