Cymbopogon citratus Allelochemical Volatiles as Potential Biopesticides against the Pinewood Nematode
Abstract
1. Introduction
2. Results
2.1. Lemongrass Essential Oil Composition
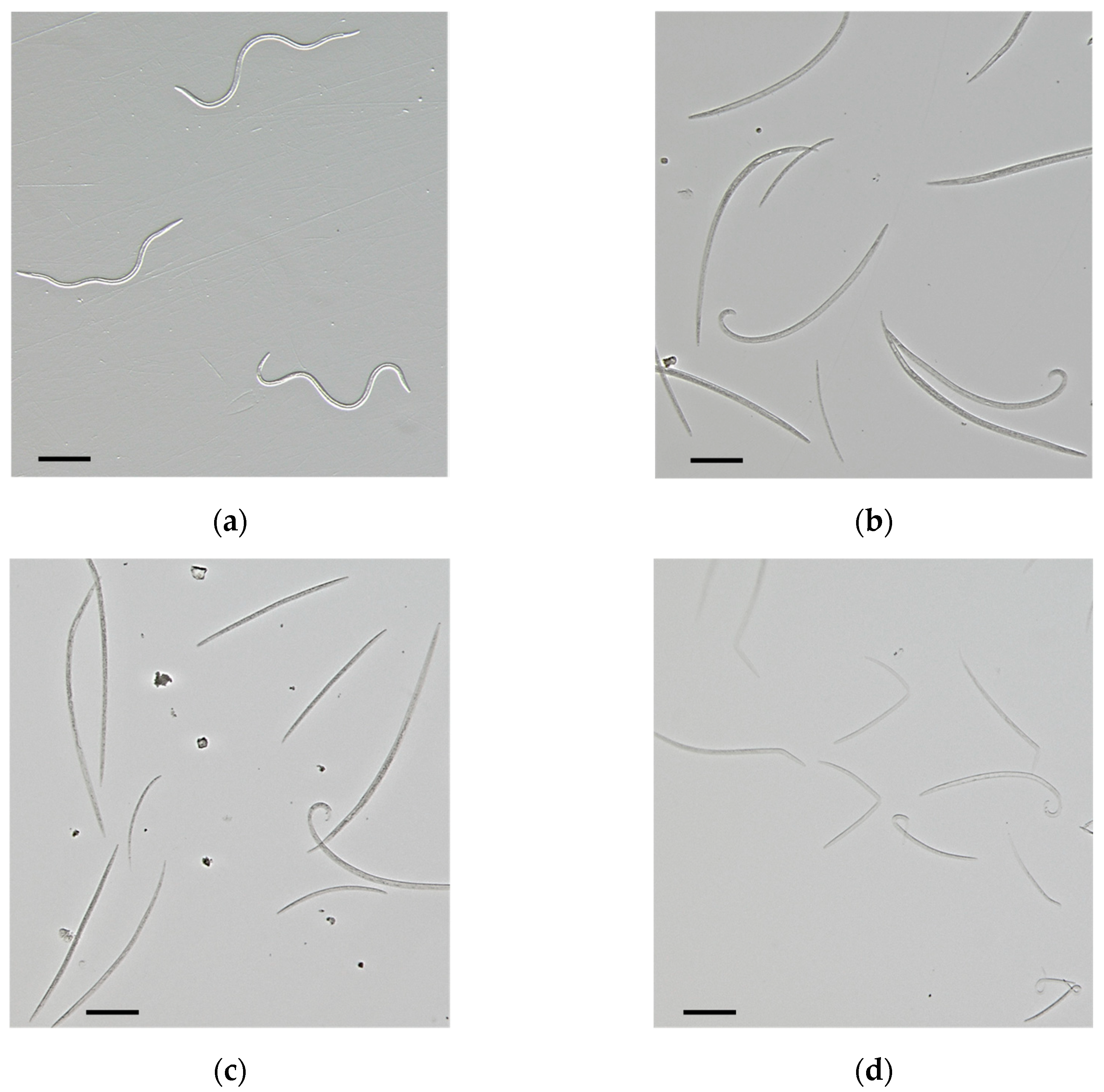
2.2. Nematicidal Activity of Lemongrass EOs
2.3. Potential Impacts on the Environment and Human Health
3. Discussion
4. Materials and Methods
4.1. Essential Oils, Fractions, and Volatiles
4.2. Rearing the Pinewood Nematode
4.3. In Vitro Direct-Contact Bioassays
4.4. Prediction of Environmental and Ecotoxicological Parameters
4.5. Reported Toxicological and Ecotoxicological Acute Toxicity Thresholds
4.6. Data Treatment and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kiruthika, S.; Vishali, S. Industrial Application of Essential Oils. In Essential Oils: Extraction Methods and Applications; Inamuddin, Ed.; Wiley: Hoboken, NJ, USA, 2024; pp. 49–67. ISBN 9781119829614. [Google Scholar]
- Nakatsu, T.; Lupo, A.T.; Chinn, J.W.; Kang, R.K.L. Biological Activity of Essential Oils and Their Constituents. Stud. Nat. Prod. Chem. 2000, 21, 571–631. [Google Scholar] [CrossRef]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Scheffer, J.J.C. Factors Affecting Secondary Metabolite Production in Plants: Volatile Components and Essential Oils. Flavour Fragr. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Faria, J.M.S.; Barbosa, P.; Vieira, P.; Vicente, C.S.L.; Figueiredo, A.C.; Mota, M. Phytochemicals as Biopesticides against the Pinewood Nematode Bursaphelenchus xylophilus: A Review on Essential Oils and Their Volatiles. Plants 2021, 10, 2614. [Google Scholar] [CrossRef]
- European Parliament and Council REGULATION (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 Concerning the Placing of Plant Protection Products on the Market and Repealing Council Directives 79/117/EEC and 91/414/EEC. Off. J. Eur. Union 2009, 309, 1–50.
- Eurostat Agri-Environmental Indicator—Consumption of Pesticides—Statistics Explained. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Agri-environmental_indicator_-_consumption_of_pesticides#cite_note-1 (accessed on 28 February 2024).
- Park, I.K.; Park, J.Y.; Kim, K.H.; Choi, K.S.; Choi, I.H.; Kim, C.S.; Shin, S.C. Nematicidal Activity of Plant Essential Oils and Components from Garlic (Allium sativum) and Cinnamon (Cinnamomum verum) Oils against the Pine Wood Nematode (Bursaphelenchus xylophilus). Nematology 2005, 7, 767–774. [Google Scholar] [CrossRef]
- Kong, J.O.; Lee, S.M.; Moon, Y.S.; Lee, S.G.; Ahn, Y.J. Nematicidal Activity of Plant Essential Oils against Bursaphelenchus xylophilus (Nematoda: Aphelenchoididae). J. Asia Pac. Entomol. 2006, 9, 173–178. [Google Scholar] [CrossRef]
- Plata-Rueda, A.; Martínez, L.C.; da Rolim, G.S.; Coelho, R.P.; Santos, M.H.; de Tavares, W.S.; Zanuncio, J.C.; Serrão, J.E. Insecticidal and Repellent Activities of Cymbopogon citratus (Poaceae) Essential Oil and Its Terpenoids (Citral and Geranyl Acetate) against Ulomoides dermestoides. Crop Prot. 2020, 137, 105299. [Google Scholar] [CrossRef]
- Assadpour, E.; Can Karaça, A.; Fasamanesh, M.; Mahdavi, S.A.; Shariat-Alavi, M.; Feng, J.; Kharazmi, M.S.; Rehman, A.; Jafari, S.M. Application of Essential Oils as Natural Biopesticides; Recent Advances. Crit. Rev. Food Sci. Nutr. 2023, 64, 6477–6497. [Google Scholar] [CrossRef] [PubMed]
- Avoseh, O.; Oyedeji, O.; Rungqu, P.; Nkeh-Chungag, B.; Oyedeji, A. Cymbopogon Species; Ethnopharmacology, Phytochemistry and the Pharmacological Importance. Molecules 2015, 20, 7438–7453. [Google Scholar] [CrossRef]
- Lawal, O.A.; Ogundajo, A.L.; Avoseh, N.O.; Ogunwande, I.A. Cymbopogon citratus. In Medicinal Spices and Vegetables from Africa: Therapeutic Potential against Metabolic, Inflammatory, Infectious and Systemic Diseases; Elsevier: Amsterdam, The Netherlands, 2017; pp. 397–423. ISBN 9780128094419. [Google Scholar]
- Leite, M.C.A.; De Brito Bezerra, A.P.; De Sousa, J.P.; Guerra, F.Q.S.; De Oliveira Lima, E. Evaluation of Antifungal Activity and Mechanism of Action of Citral against Candida albicans. Evid. Based Complement. Altern. Med. 2014, 2014, 378280. [Google Scholar] [CrossRef]
- Shi, C.; Song, K.; Zhang, X.; Sun, Y.; Sui, Y.; Chen, Y.; Jia, Z.; Sun, H.; Sun, Z.; Xia, X.X. Antimicrobial Activity and Possible Mechanism of Action of Citral against Cronobacter sakazakii. PLoS ONE 2016, 11, e0159006. [Google Scholar] [CrossRef] [PubMed]
- Mota, M.; Braasch, H.; Bravo, M.A.; Penas, A.C.; Burgermeister, W.; Metge, K.; Sousa, E. First Report of Bursaphelenchus xylophilus in Portugal and in Europe. Nematology 1999, 1, 727–734. [Google Scholar] [CrossRef]
- Back, M.A.; Bonifácio, L.; Inácio, M.L.; Mota, M.; Boa, E. Pine Wilt Disease: A Global Threat to Forestry. Plant Pathol. 2024, 73, 1026–1041. [Google Scholar] [CrossRef]
- EPPO. Bursaphelenchus xylophilus. EPPO Datasheets on Pests Recommended for Regulation. 2024. Available online: https://gd.eppo.int (accessed on 11 August 2024).
- Andrés, M.F.; González-Coloma, A.; Sanz, J.; Burillo, J.; Sainz, P. Nematicidal Activity of Essential Oils: A Review. Phytochem. Rev. 2012, 11, 371–390. [Google Scholar] [CrossRef]
- Sarri, K.; Mourouzidou, S.; Ntalli, N.; Monokrousos, N. Recent Advances and Developments in the Nematicidal Activity of Essential Oils and Their Components against Root-Knot Nematodes. Agronomy 2024, 14, 213. [Google Scholar] [CrossRef]
- Faria, J.M.S.; Barbosa, P.; Bennett, R.N.; Mota, M.; Figueiredo, A.C. Bioactivity against Bursaphelenchus xylophilus: Nematotoxics from Essential Oils, Essential Oils Fractions and Decoction Waters. Phytochemistry 2013, 94, 220–228. [Google Scholar] [CrossRef]
- Trent University Level I Model. Available online: https://www.trentu.ca/cemc/resources-and-models/level-i-model (accessed on 29 March 2024).
- Mackay, D.; Di Guardo, A.; Paterson, S.; Cowan, C.E. Evaluating the Environmental Fate of a Variety of Types of Chemicals Using the EQC Model. Environ. Toxicol. Chem. 1996, 15, 1627–1637. [Google Scholar] [CrossRef]
- US EPA. Estimation Programs Interface Suite™ for Microsoft® Windows; v 4.11; United States Environmental Protection Agency: Washington, DC, USA, 2023.
- National Library of Medicine PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 29 March 2024).
- University of Hertfordshire PPDB: Pesticide Properties DataBase. Available online: http://sitem.herts.ac.uk/aeru/ppdb/en/ (accessed on 29 March 2024).
- ECHA. ECHA European Chemicals Agency. Available online: https://echa.europa.eu/pt/home (accessed on 29 March 2023).
- Kundu, A.; Dutta, A.; Mandal, A.; Negi, L.; Malik, M.; Puramchatwad, R.; Antil, J.; Singh, A.; Rao, U.; Saha, S.; et al. A Comprehensive in Vitro and in Silico Analysis of Nematicidal Action of Essential Oils. Front. Plant Sci. 2021, 11, 614143. [Google Scholar] [CrossRef]
- Ibrahim, S.K.; Traboulsi, A.F.; El-Haj, S. Effect of Essential Oils and Plant Extracts on Hatching, Migration and Mortality of Meloidogyne incognita. Phytopathol. Mediterr. 2006, 45, 238–246. [Google Scholar] [CrossRef]
- Barbosa, P.; Lima, A.S.; Vieira, P.; Dias, L.S.; Tinoco, M.T.; Barroso, J.G.; Pedro, L.G.; Figueiredo, A.C.; Mota, M. Nematicidal Activity of Essential Oils and Volatiles Derived from Portuguese Aromatic Flora against the Pinewood Nematode, Bursaphelenchus xylophilus. J. Nematol. 2010, 42, 8–16. [Google Scholar] [CrossRef]
- Kong, J.-O.; Park, I.; Choi, K.-S.; Shin, S.-C.; Ahn, Y.-J. Nematicidal and Propagation Activities of Thyme Red and White Oil Compounds toward Bursaphelenchus xylophilus (Nematoda: Parasitaphelenchidae). J. Nematol. 2007, 39, 237–242. [Google Scholar] [PubMed]
- Choi, I.H.; Kim, J.; Shin, S.C.; Park, I.K. Nematicidal Activity of Monoterpenoids against the Pine Wood Nematode (Bursaphelenchus xylophilus). Russ. J. Nematol. 2007, 15, 35–40. [Google Scholar]
- Park, I.-K.K.; Kim, J.; Lee, S.-G.G.; Shin, S.-C.C. Nematicidal Activity of Plant Essential Oils and Components from Ajowan (Trachyspermum ammi), Allspice (Pimenta dioica) and Litsea (Litsea cubeba) Essential Oils against Pine Wood Nematode (Bursaphelenchus Xylophilus). J. Nematol. 2007, 39, 275–279. [Google Scholar] [PubMed]
- GHS (United Nations) Globally Harmonized System of Classification and Labelling of Chemicals (GHS) (4th Edition). Available online: http://digitallibrary.un.org/record/660120 (accessed on 29 March 2024).
- Pavela, R.; Benelli, G. Essential Oils as Ecofriendly Biopesticides? Challenges and Constraints. Trends Plant Sci. 2016, 21, 1000–1007. [Google Scholar] [CrossRef]
- Council of Europe. European Directorate for the Quality of Medicines. In European Pharmacopoeia; Council of Europe: Strasbourg, France, 2010; p. 241. [Google Scholar]
- Whitehead, A.G.; Hemming, J.R. A Comparison of Some Quantitative Methods of Extracting Small Vermiform Nematodes from Soil. Ann. Appl. Biol. 1965, 55, 25–38. [Google Scholar] [CrossRef]
- Faria, J.M.S.; Cavaco, T.; Gonçalves, D.; Barbosa, P.; Teixeira, D.M.; Moiteiro, C.; Inácio, M.L. First Report on the Synergistic Interaction between Essential Oils against the Pinewood Nematode Bursaphelenchus xylophilus. Plants 2023, 12, 2438. [Google Scholar] [CrossRef]

| Compounds (≥1%) | FS | DS1 | DS2 | DS2 H 1 | DS2 O 1 |
|---|---|---|---|---|---|
| Hydrocarbons | |||||
| Monoterpenes | 25.1 | 38.2 | 19.5 | 74.0 | 1.4 |
| β-Myrcene | 24.7 ± 2.6 | 38.2 ± 4.4 | 19.5 ± 1.9 | 72.9 ± 0.3 | 1.4 ± 0.1 |
| cis-β-Ocimene | 0.4 ± 0.0 | t | t | 1.1 ± 0.0 | |
| Sesquiterpenes | 0.1 | 0.5 | 2.2 | 1.7 | |
| trans-α-Bergamotene | 0.0 ± 0.0 | 0.4 ± 0.1 | 1.0 ± 0.0 | 0.9 ± 0.1 | |
| β-Caryophyllene | 0.1 ± 0.0 | 0.1 ± 0.0 | 1.2 ± 0.0 | 0.8 ± 0.1 | |
| Oxygen-containing compounds | |||||
| Monoterpenes | 73.3 | 59.5 | 73.8 | 10.9 | 88.9 |
| Geranial | 42.5 ± 1.3 | 22.6 ± 1.2 | 33.7 ± 0.8 | 1.6 ± 0.1 | 45.4 ± 0.5 |
| Geraniol | 0.5 ± 0.1 | 13.8 ± 1.5 | 17.7 ± 0.3 | 5.2 ± 0.1 | |
| Geranyl acetate | 0.1 ± 0.0 | 0.5 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.0 | 1.3 ± 0.0 |
| Linalool | 0.7 ± 0.1 | 2.6 ± 0.5 | 0.2 ± 0.0 | 1.3 ± 0.1 | |
| Neral | 28.6 ± 0.7 | 19.6 ± 0.8 | 21.5 ± 1.1 | 9.0 ± 0.1 | 35.7 ± 0.1 |
| trans-Verbenol | 1.0 ± 0.1 | 0.4 ± 0.0 | 0.4 ± 0.1 | ||
| Sesquiterpenes | 0.1 | 0.3 | 1.6 | ||
| 10-epi-γ-Eudesmol | 0.1 ± 0.1 | 0.3 ± 0.0 | 1.6 ± 0.1 | ||
| FS | DS1 | DS2 | DS2 O 1 | Citral 2 | Geraniol | Emamectin Benzoate | |
|---|---|---|---|---|---|---|---|
| EC50 (µL/mL) | 0.429 ± 0.005 | 0.777 ± 0.003 | 0.275 ± 0.002 | 0.279 ± 0.002 | 0.266 ± 0.002 | 0.341 ± 0.001 | 0.364 ± 0.003 |
| p | 4.328 ± 0.272 | 5.144 ± 0.069 | 4.787 ± 0.113 | 3.707 ± 0.044 | 4.822 ± 0.097 | 4.626 ± 0.022 | 3.081 ± 0.524 |
| R2 | 0.99 | 0.99 | 0.97 | 0.99 | 0.97 | 0.99 | 0.96 |
| EC20 (µL/mL) | 0.290 ± 0.003 | 0.660 ± 0.006 | 0.222 ± 0.003 | 0.205 ± 0.001 | 0.141 ± 0.001 | 0.210 ± 0.000 | 0.168 ± 0.001 |
| EC100 (µL/mL) | 1.307–1.916 | 1.912–1.968 | 1.524–1.646 | 1.419–1.467 | 1.443–1.550 | 1.626–1.648 | 1.612–1.702 |
| Concentration (µL/mL) | 2 | 1 | 0.5 | 0.25 | 0.125 |
|---|---|---|---|---|---|
| Experimental mortality (%) | |||||
| C. citratus (DS2) O 1 | 100.0 ± 0.0 | 99.5 ± 0.1 | 75.7 ± 0.7 | 26.9 ± 0.9 | 11.2 ± 0.6 |
| Mortality from fitted curves (%) | |||||
| Citral (geranial + neral), at 81% | 100.0–100.0 | 99.7–99.8 | 81.3–83.8 | 32.3–34.1 | 13.1–14.7 |
| Geraniol, at 5% | 7.3–7.6 | 4.3–4.5 | 3.3–3.5 | 2.9–3.1 | 2.7–2.9 |
| Sum of compounds activity 2 | >100 | >100 | 84.5–87.3 | 35.2–37.1 | 15.8–17.5 |
| Compartments | Air | Water | Soil | Sediment | |||||
|---|---|---|---|---|---|---|---|---|---|
| Compounds | Amount (%) | Half-Life (h) | Amount (%) | Half-Life (h) | Amount (%) | Half-Life (h) | Amount (%) | Half-Life (h) | Persistance (h) |
| Citral | 42.9 | 0.47 | 48.2 | 360 | 8.7 | 720 | 0.2 | 3240 | 374 |
| Geraniol | 16.4 | 0.26 | 69.7 | 360 | 3.6 | 720 | 0.3 | 3240 | 424 |
| Emamectin benzoate | 0.0 | 0.14 | 0.1 | 4320 | 97.6 | 8640 | 2.2 | 3890 | 1390 |
| Acute Toxicity Thresholds | Fish (LC50/96h, mg/L) | Invertebrates 1 (EC50/48h, mg/L) | Algae (EC50/72h, mg/L) |
|---|---|---|---|
| Citral | 6.8 2 | 6.8 | 103.8 3 |
| Geraniol | 22.0 4 | 16.1 | 48.0 5 |
| Emamectin benzoate | 0.2 4 | 0.001 | 0.007 5 |
| Compounds | Toxicity Thresholds (LD50 in mg/kg) | |
|---|---|---|
| Oral | Dermal | |
| Citral | 4960 | 2250 1 |
| Geraniol | 3600 | >5000 |
| Emamectin benzoate | 82 | 439 |
| Physicochemical Parameters | Citral 1 (Geranial + Neral) | Geraniol | Emamectin Benzoate |
|---|---|---|---|
| CAS number | 5392-40-5 | 106-24-1 | 155569-91-8 |
| Molecular mass (g/mol) | 152.23 | 154.25 | 1008.2 |
| Melting point (°C) | −10.0 | −15.0 | 146.0 |
| Vapor pressure (Pa) | 11.999 | 3.999 | 5 × 10−6 |
| Solubility in water (mg/L) | 1340.0 | 100.0 | 24.0 |
| Henry’s law Constant (Pa/m3/mol) | 4.408 | 1.165 | 0.0002 |
| LogKOW (unitless) | 3.45 | 3.56 | 5.00 |
| KOC (unitless) | 83 | 90 | 3.78 × 105 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faria, J.M.S.; Barbosa, P. Cymbopogon citratus Allelochemical Volatiles as Potential Biopesticides against the Pinewood Nematode. Plants 2024, 13, 2233. https://doi.org/10.3390/plants13162233
Faria JMS, Barbosa P. Cymbopogon citratus Allelochemical Volatiles as Potential Biopesticides against the Pinewood Nematode. Plants. 2024; 13(16):2233. https://doi.org/10.3390/plants13162233
Chicago/Turabian StyleFaria, Jorge M. S., and Pedro Barbosa. 2024. "Cymbopogon citratus Allelochemical Volatiles as Potential Biopesticides against the Pinewood Nematode" Plants 13, no. 16: 2233. https://doi.org/10.3390/plants13162233
APA StyleFaria, J. M. S., & Barbosa, P. (2024). Cymbopogon citratus Allelochemical Volatiles as Potential Biopesticides against the Pinewood Nematode. Plants, 13(16), 2233. https://doi.org/10.3390/plants13162233







