Antioxidant Activity and UHPLC-MS/MS Characterization of Polyphenol and Nicotine Content in Nicotiana Glauca Leaf Extracts: A Comparative Study of Conventional and Deep Eutectic Solvent Extraction Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Methanol Conventional Extraction (MC)
2.3. Deep Eutectic Solvents (DES) Extraction
2.4. Determination of Total Phenolic
2.5. Determination of Total Flavonoids
2.6. In-Vitro Antioxidant Activity
2.7. UHPLC-MS/MS Methodology
Instrumentation and MS Parameters
2.8. Nicotine Content Determination
3. Results
3.1. Total Phenol Content
3.2. Total Flavonoid Content
3.3. Antioxidant Activity (DPPH Assay) for N. glauca Leaf Extracts
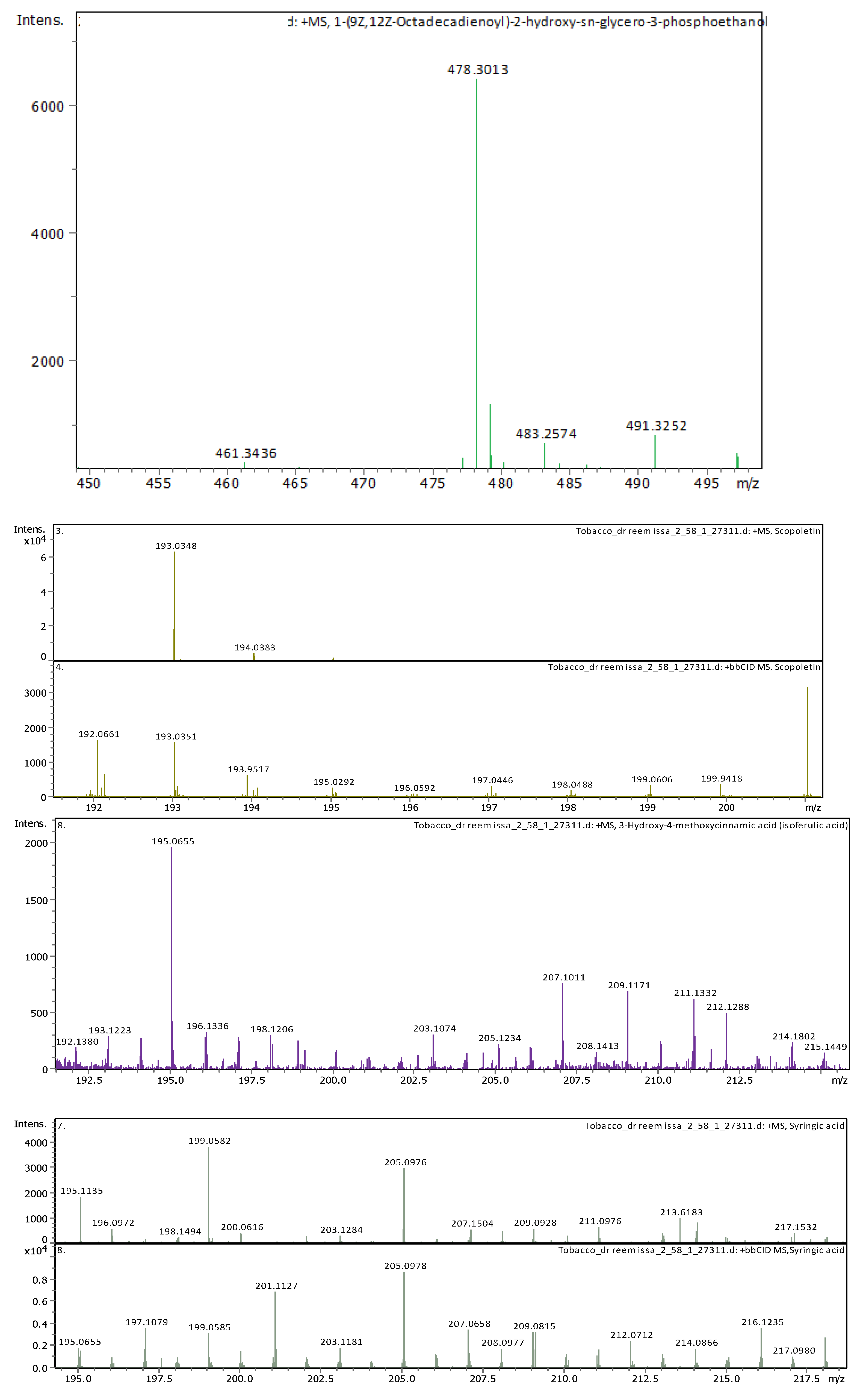
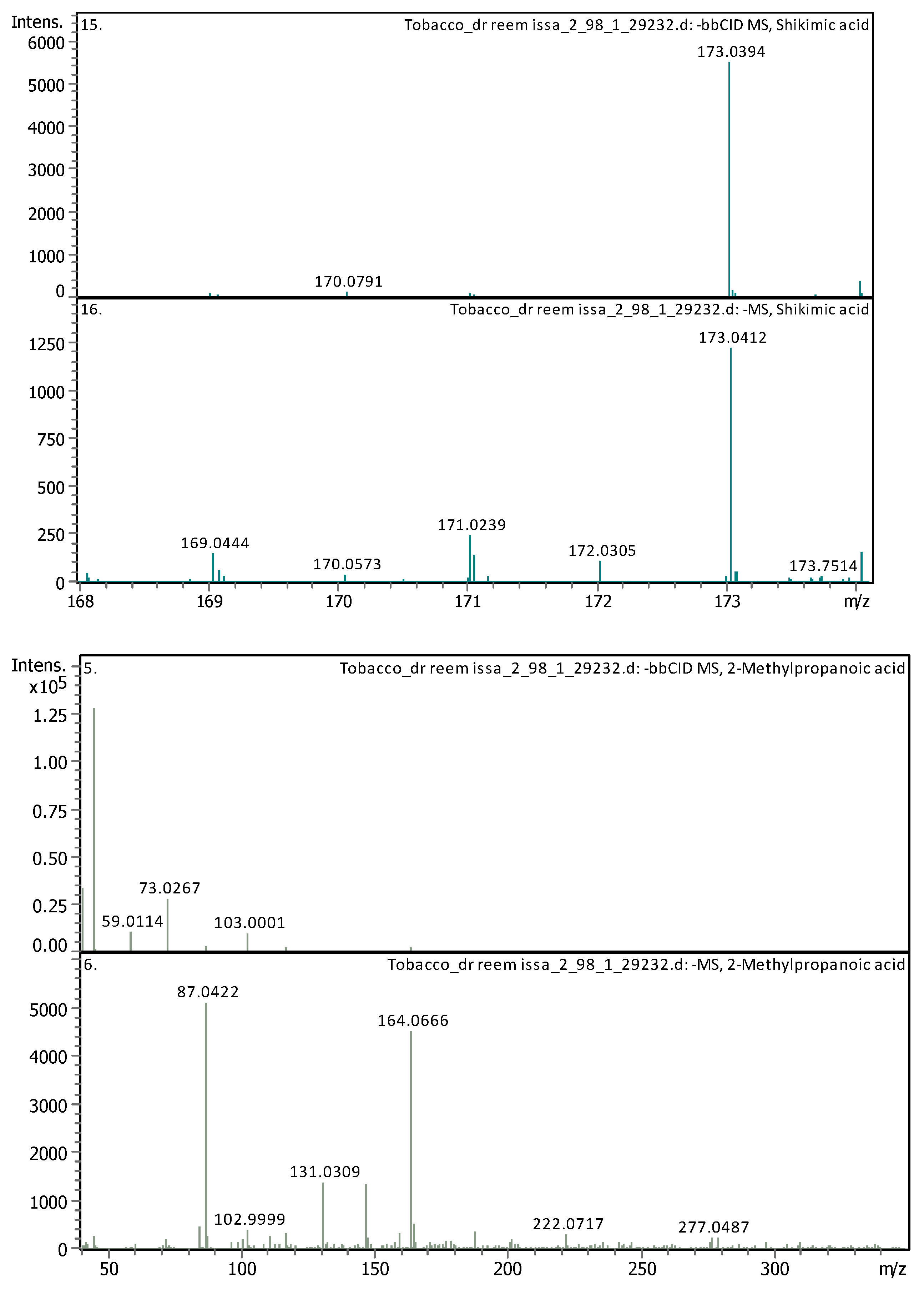
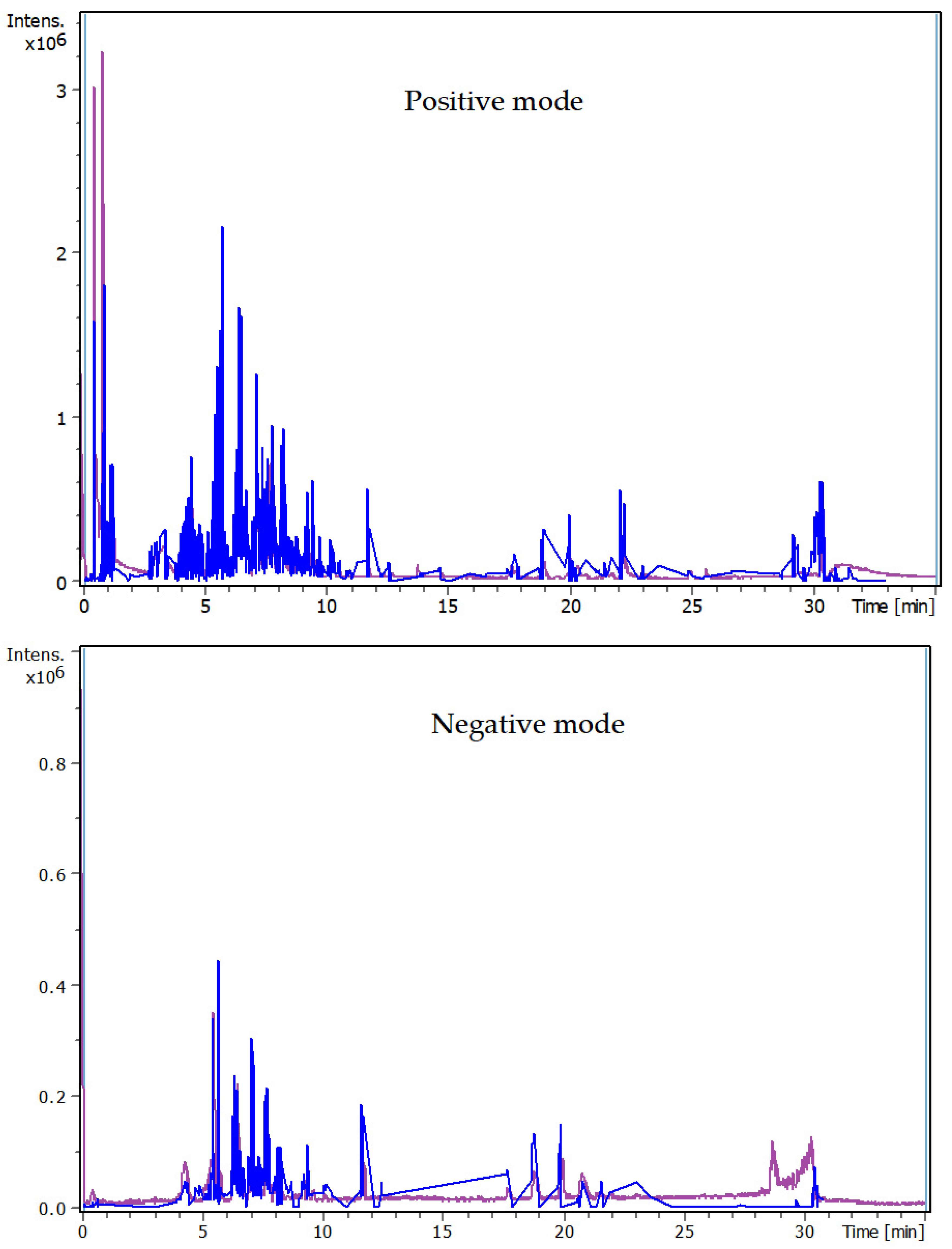
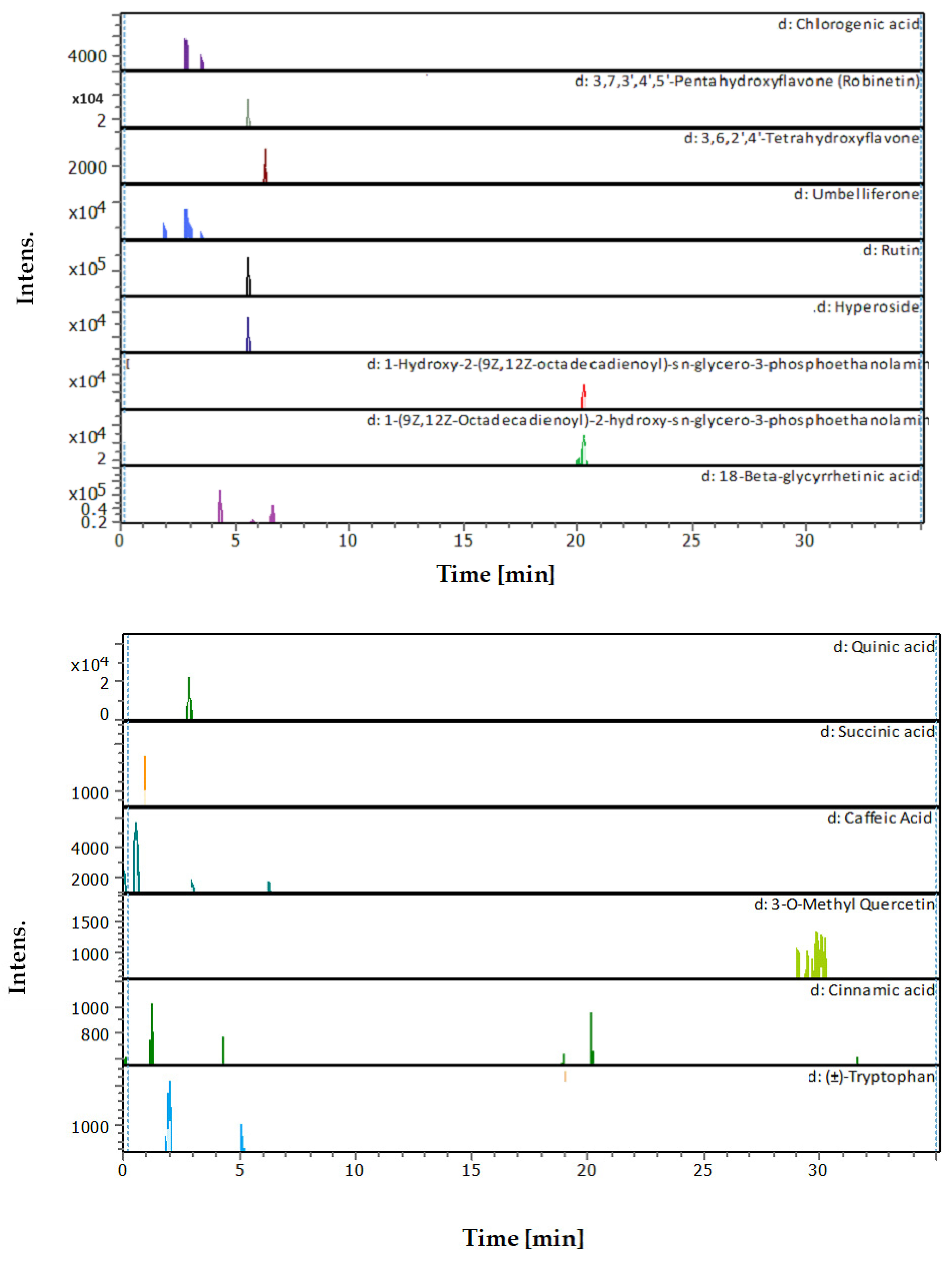
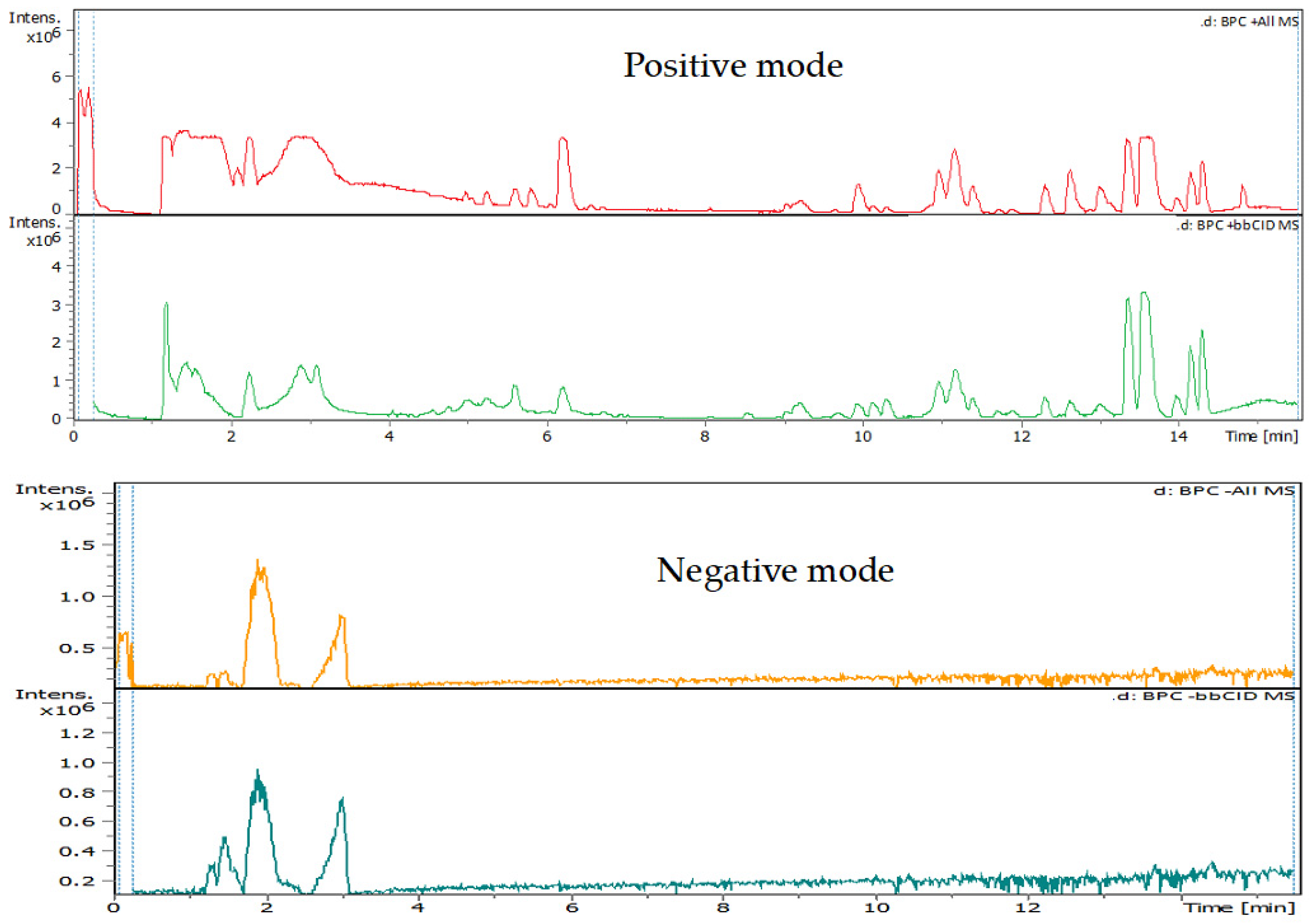
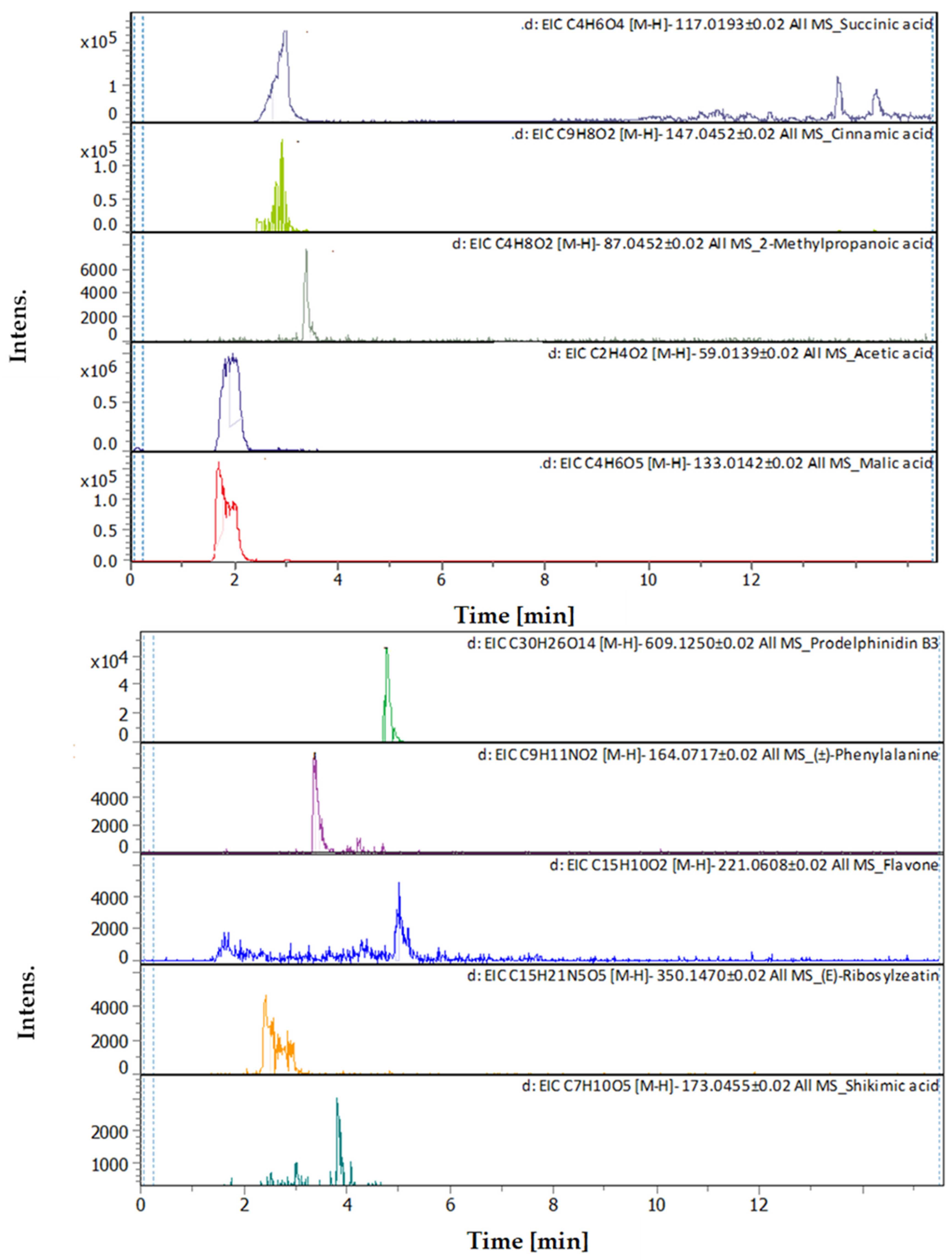
3.4. Identification of Phenols Using UHPLC-MS/MS Analysis
3.4.1. Methanol Conventional Extraction (MC)
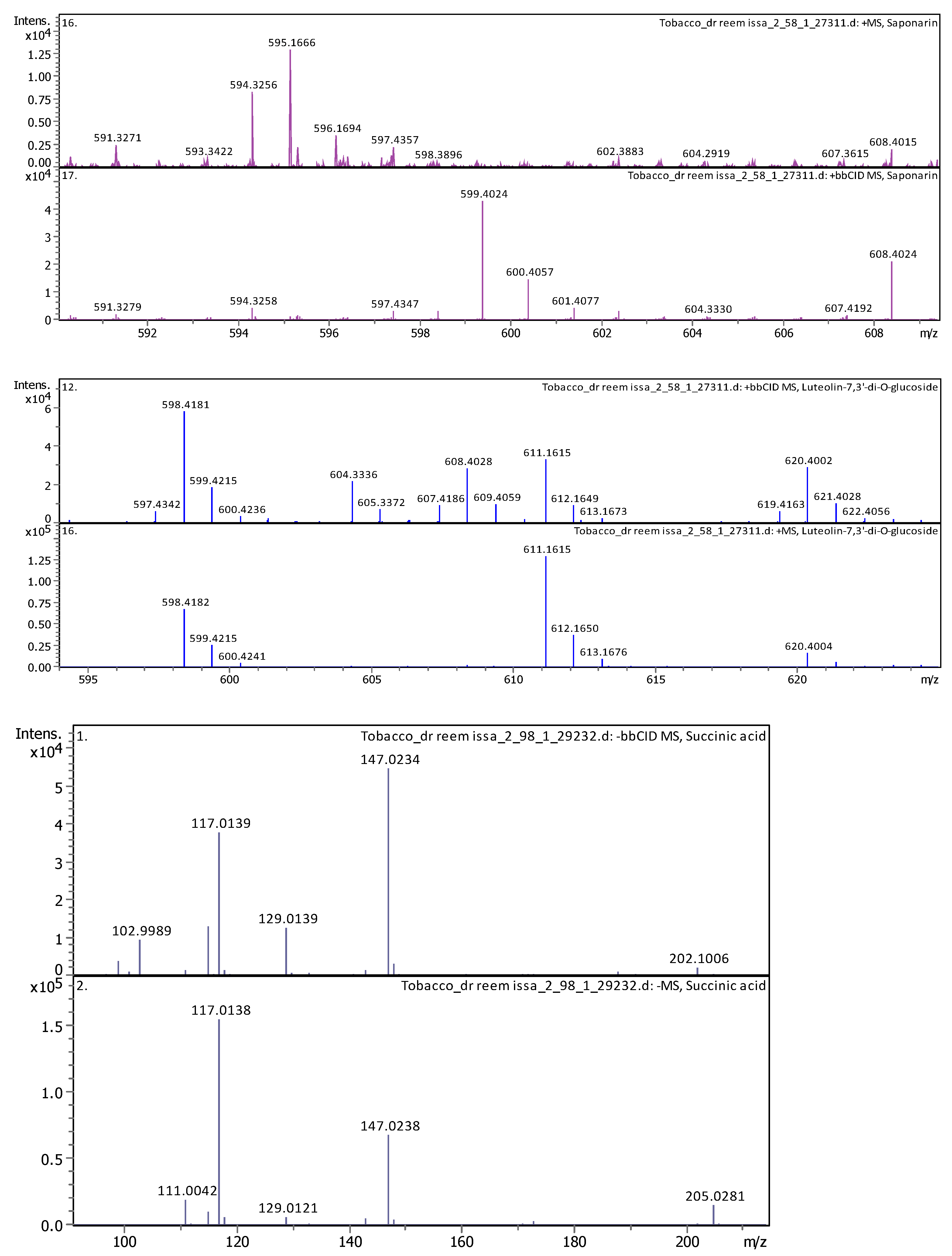
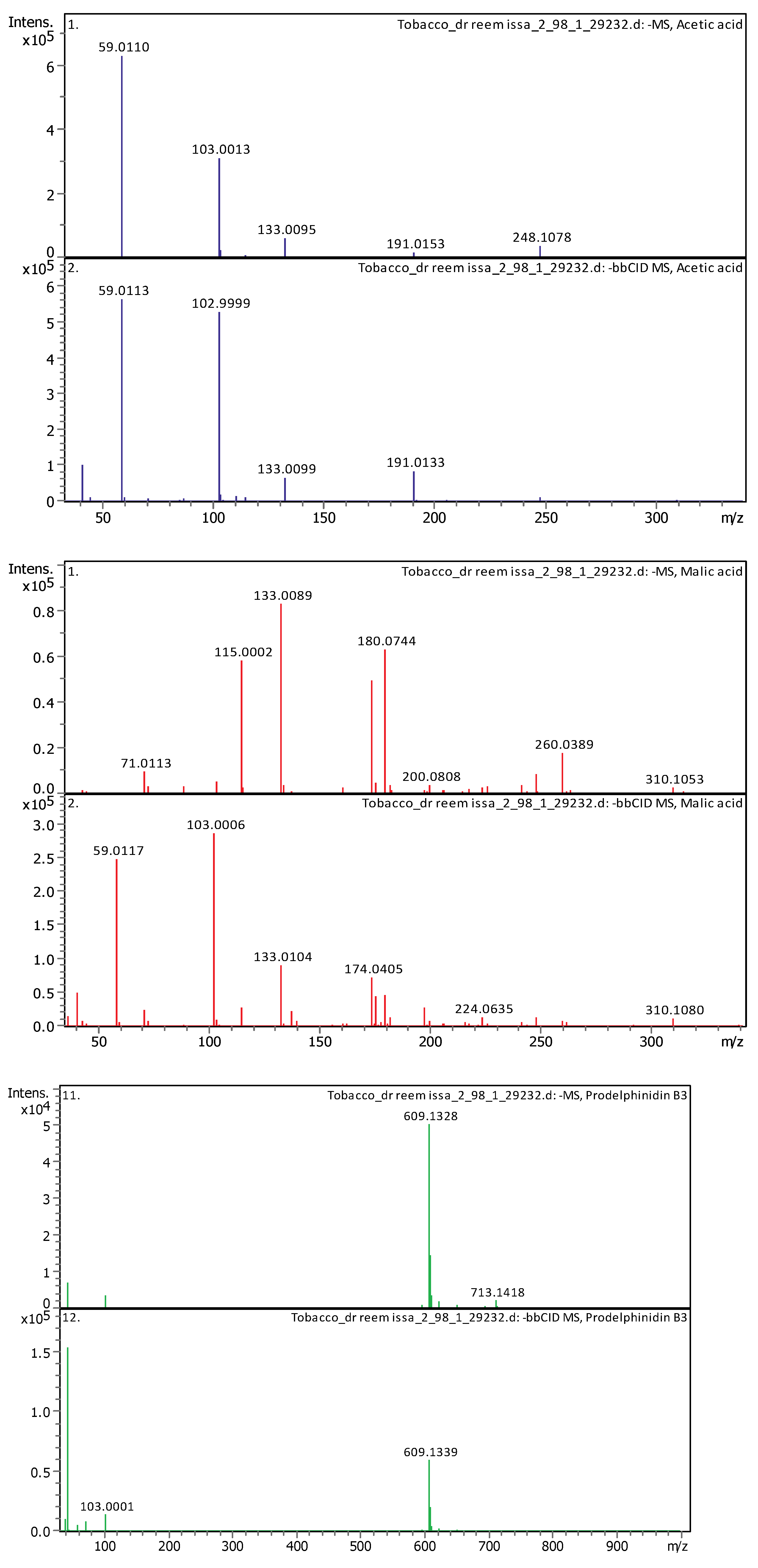
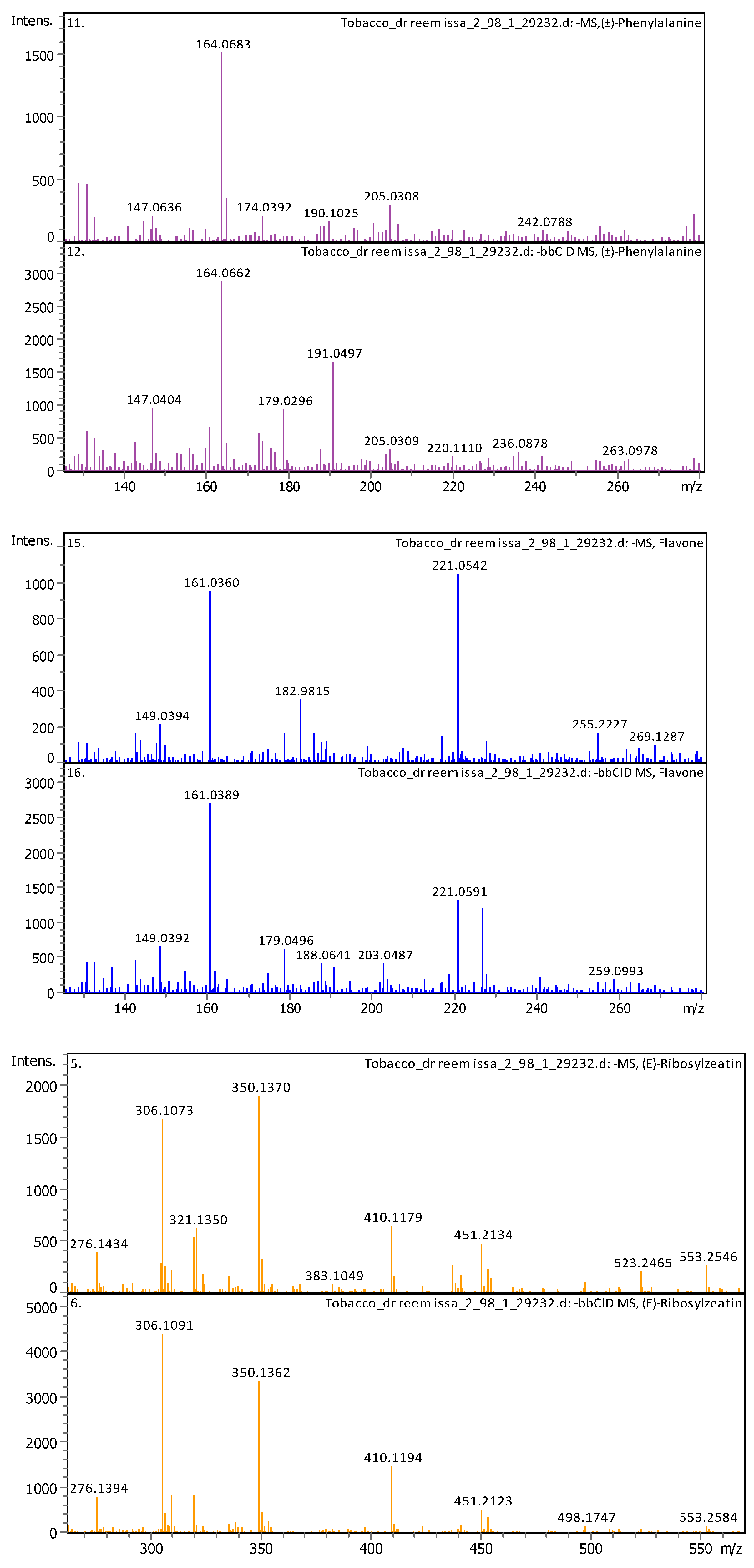
3.4.2. Deep Eutectic Extraction (DES)
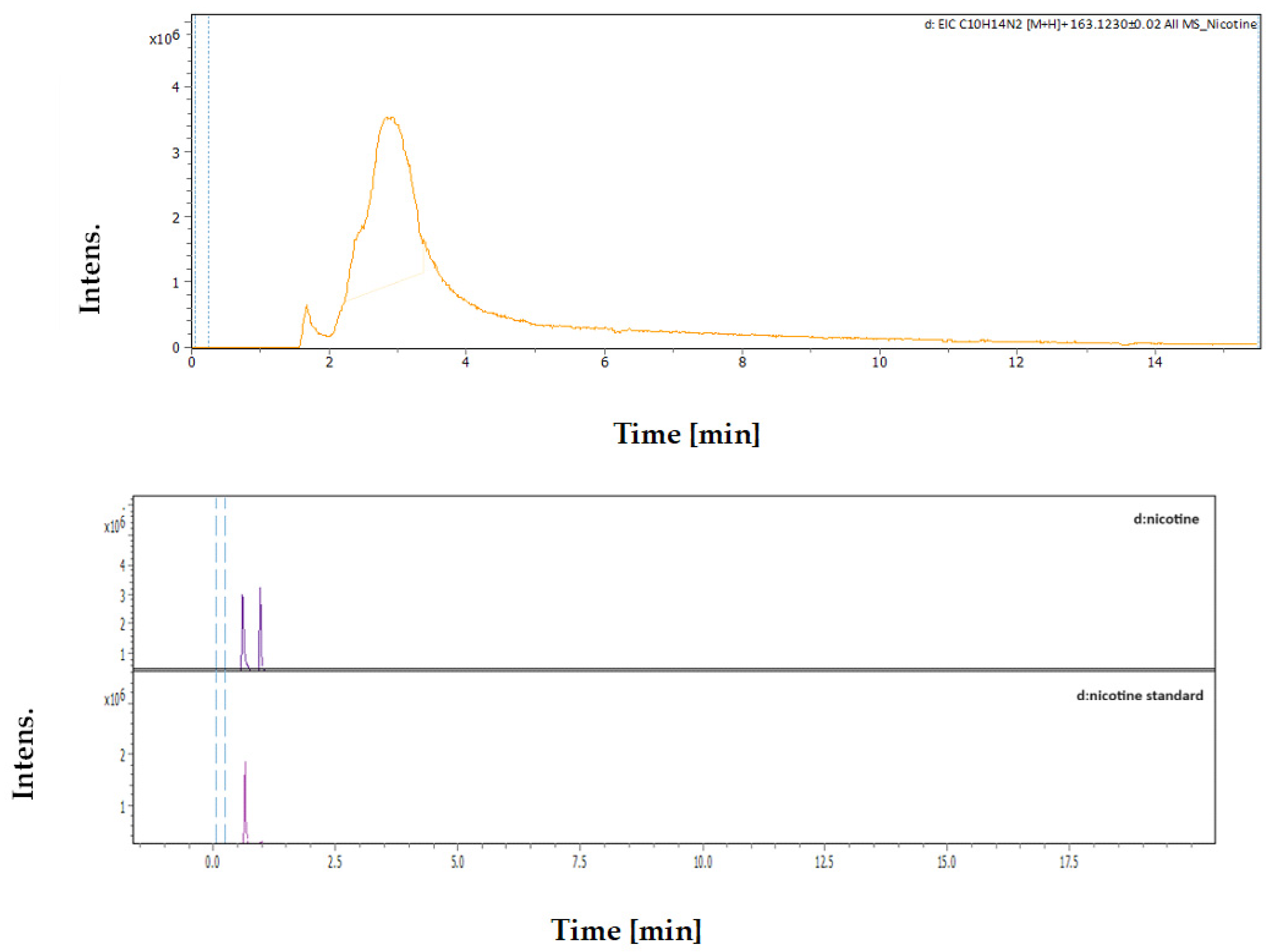
3.4.3. Identification and Quantification of Nicotine
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
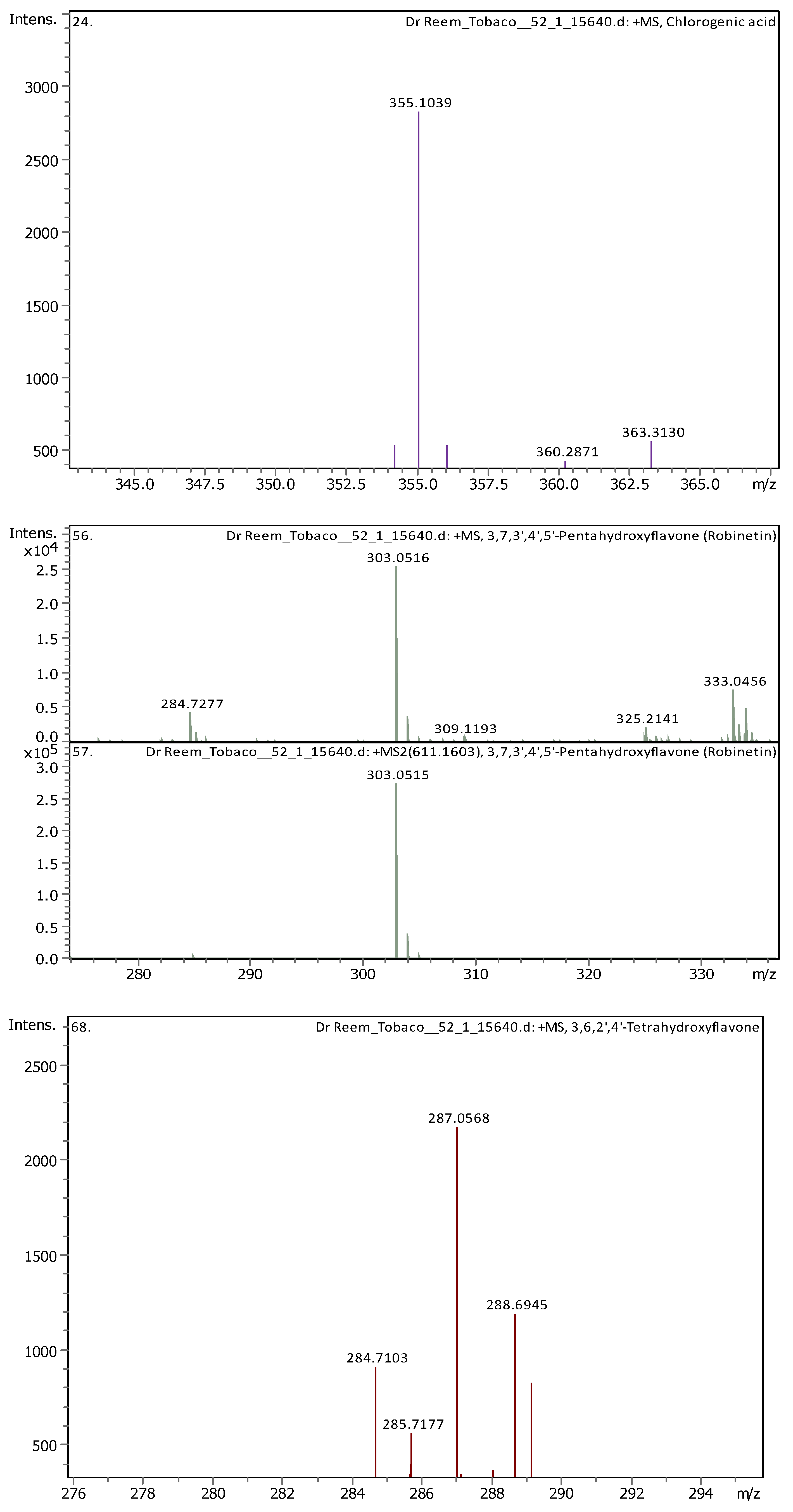
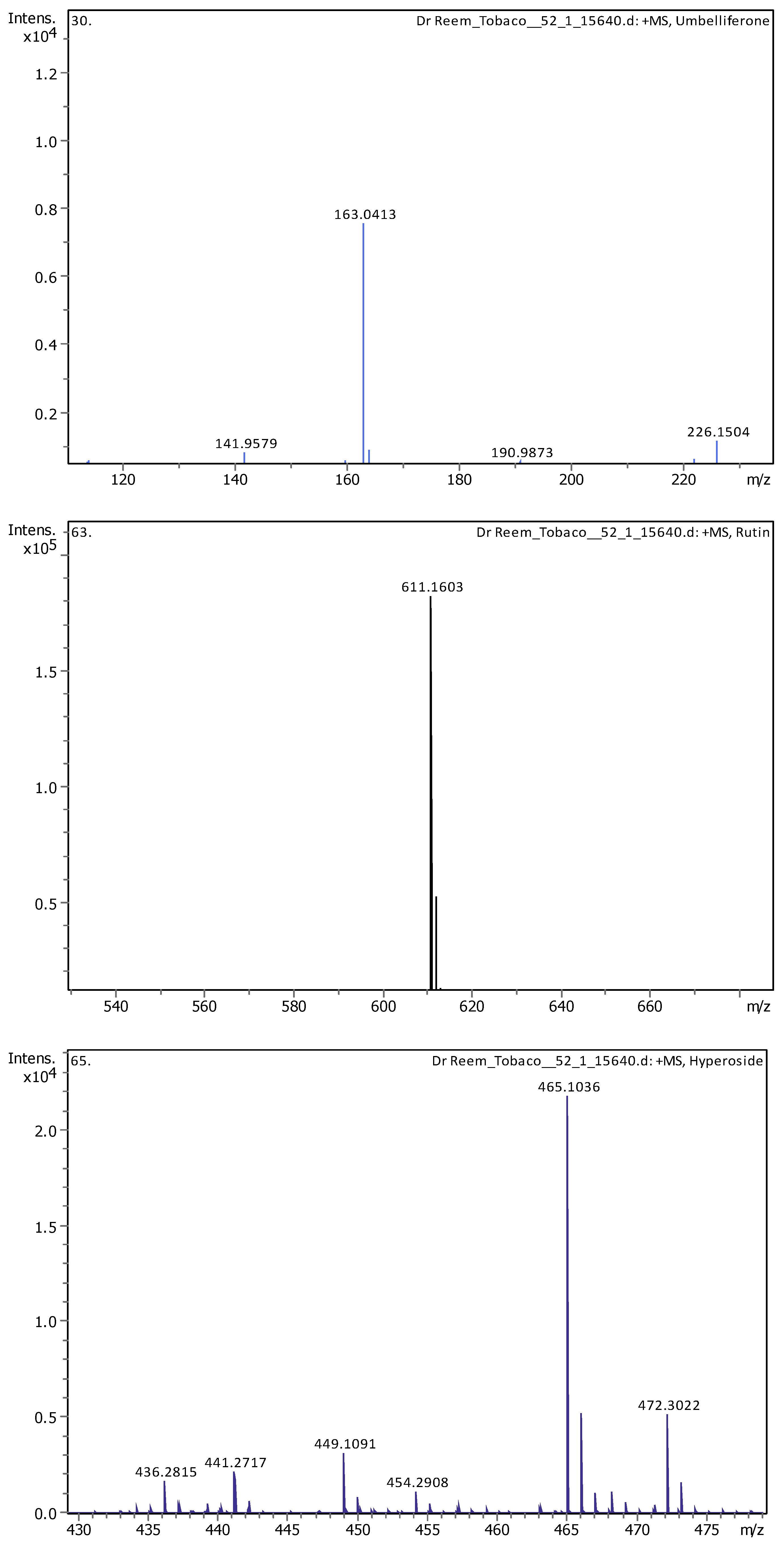
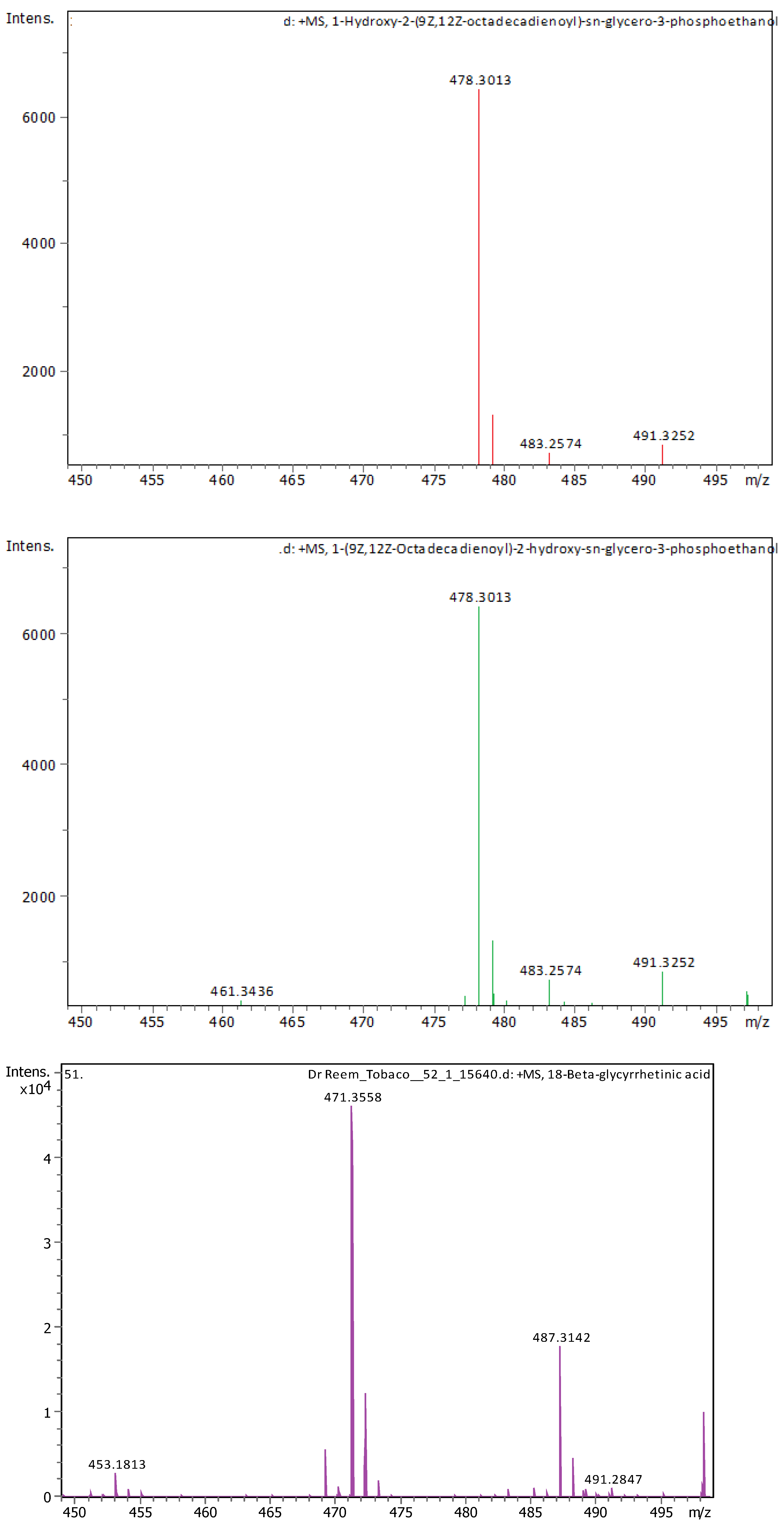
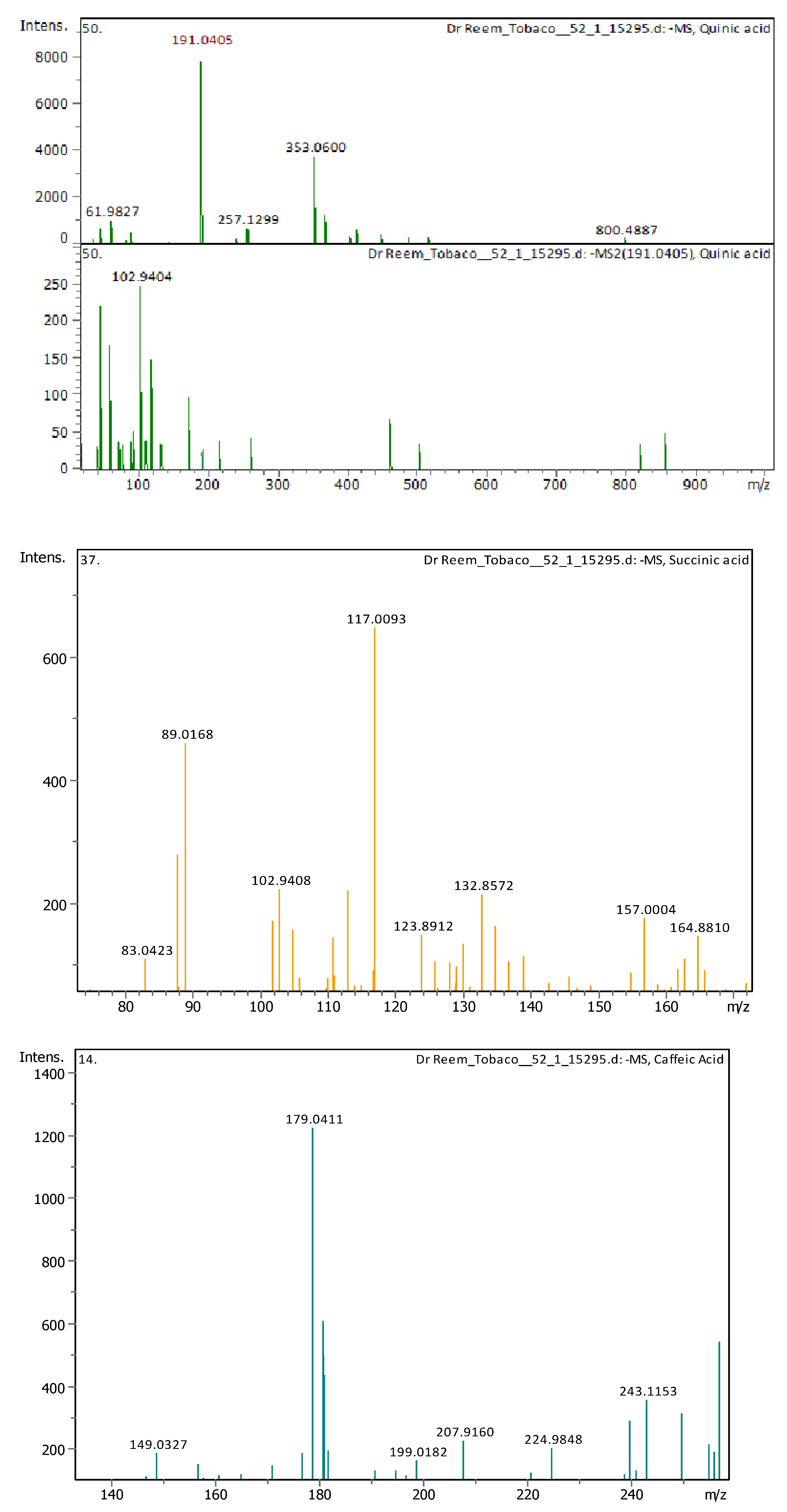
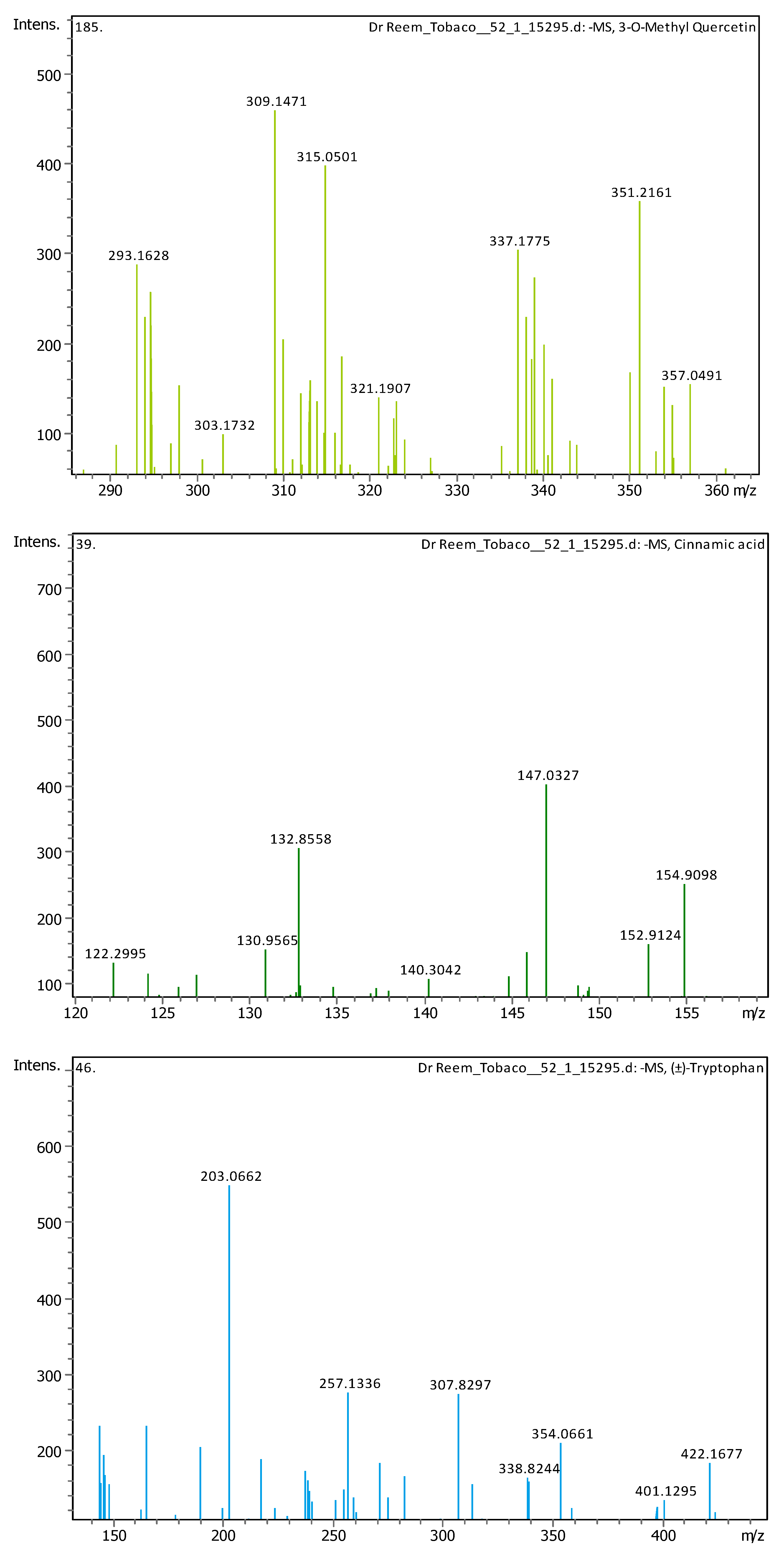
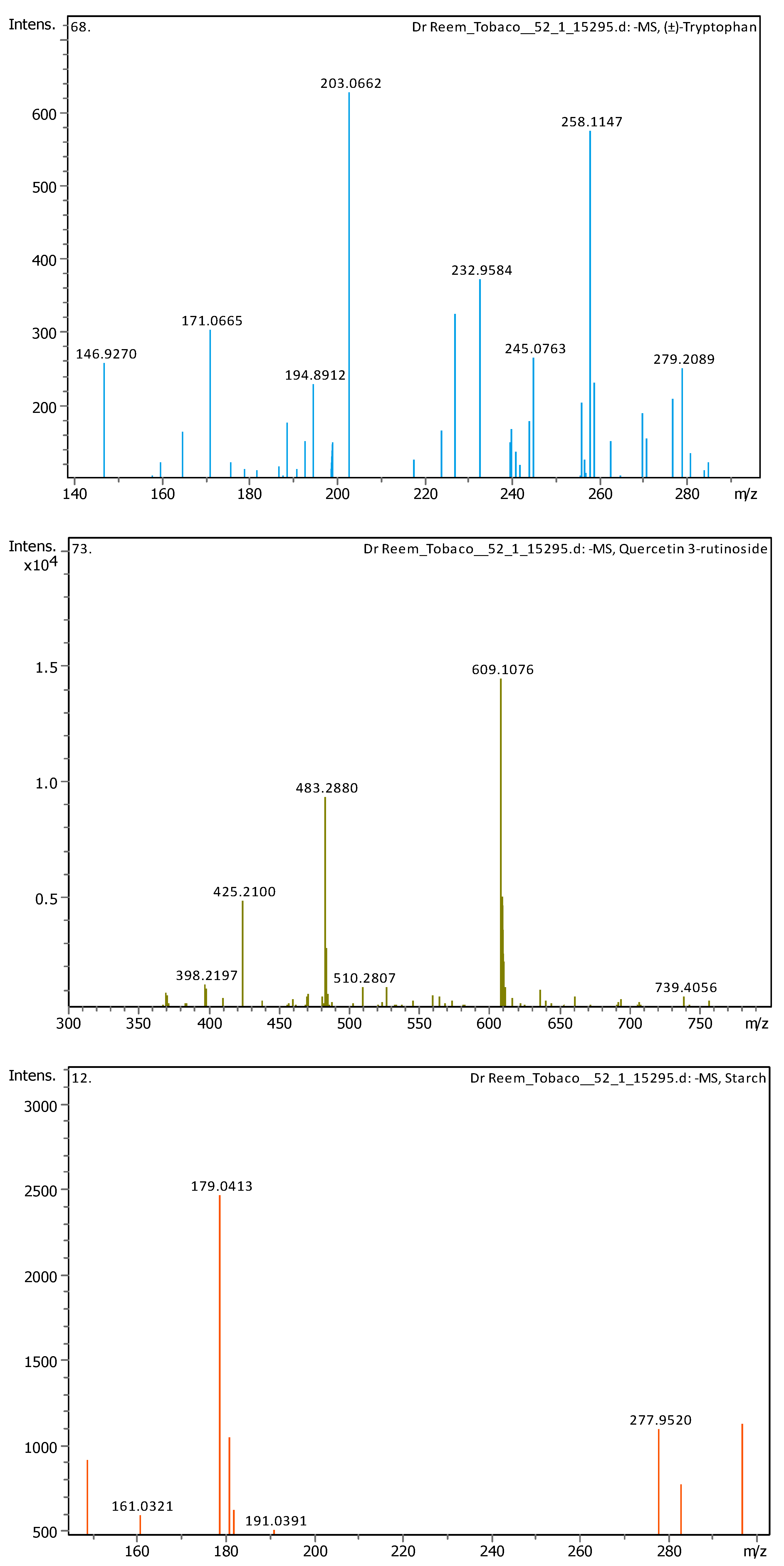

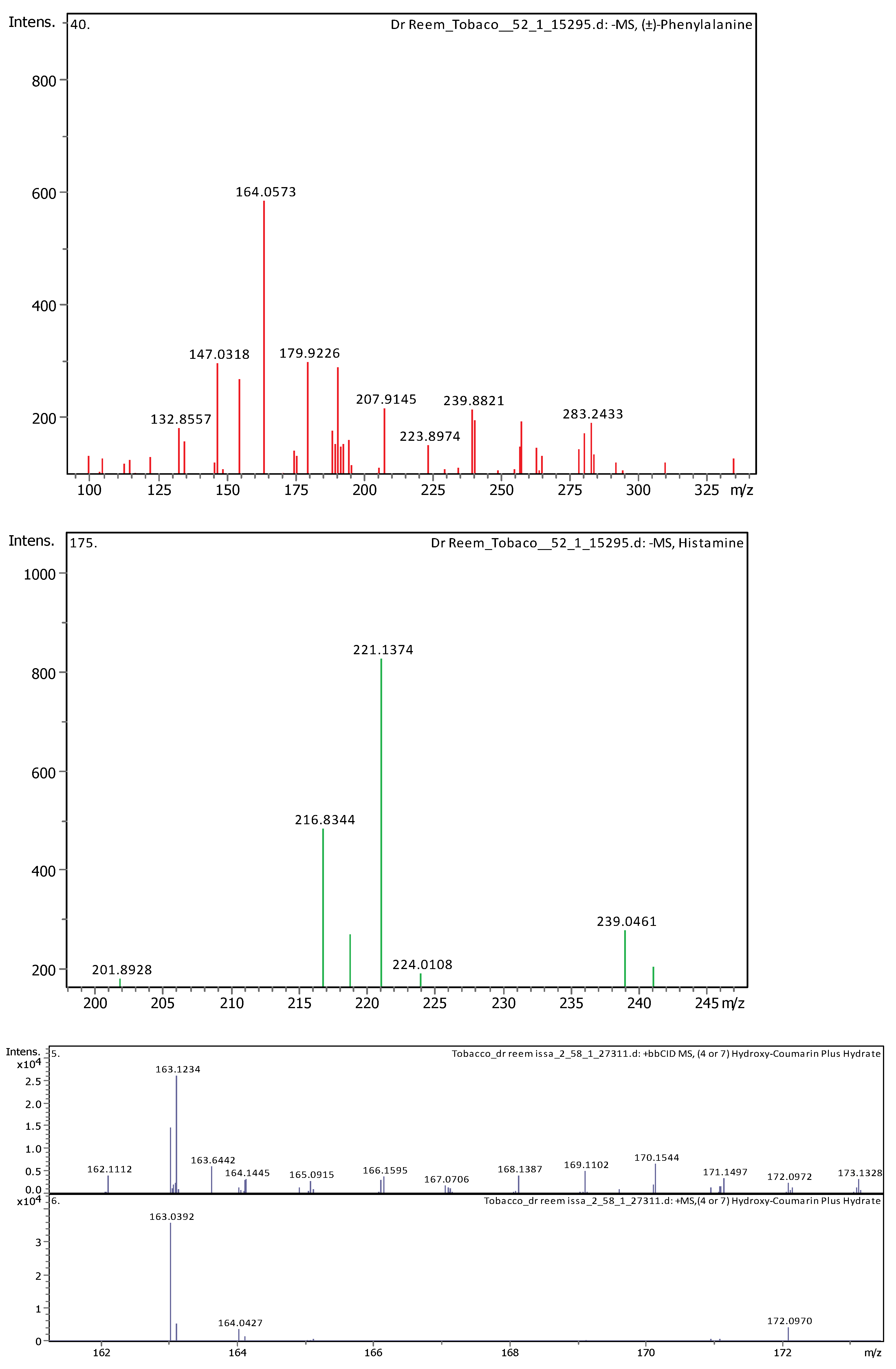
Appendix B
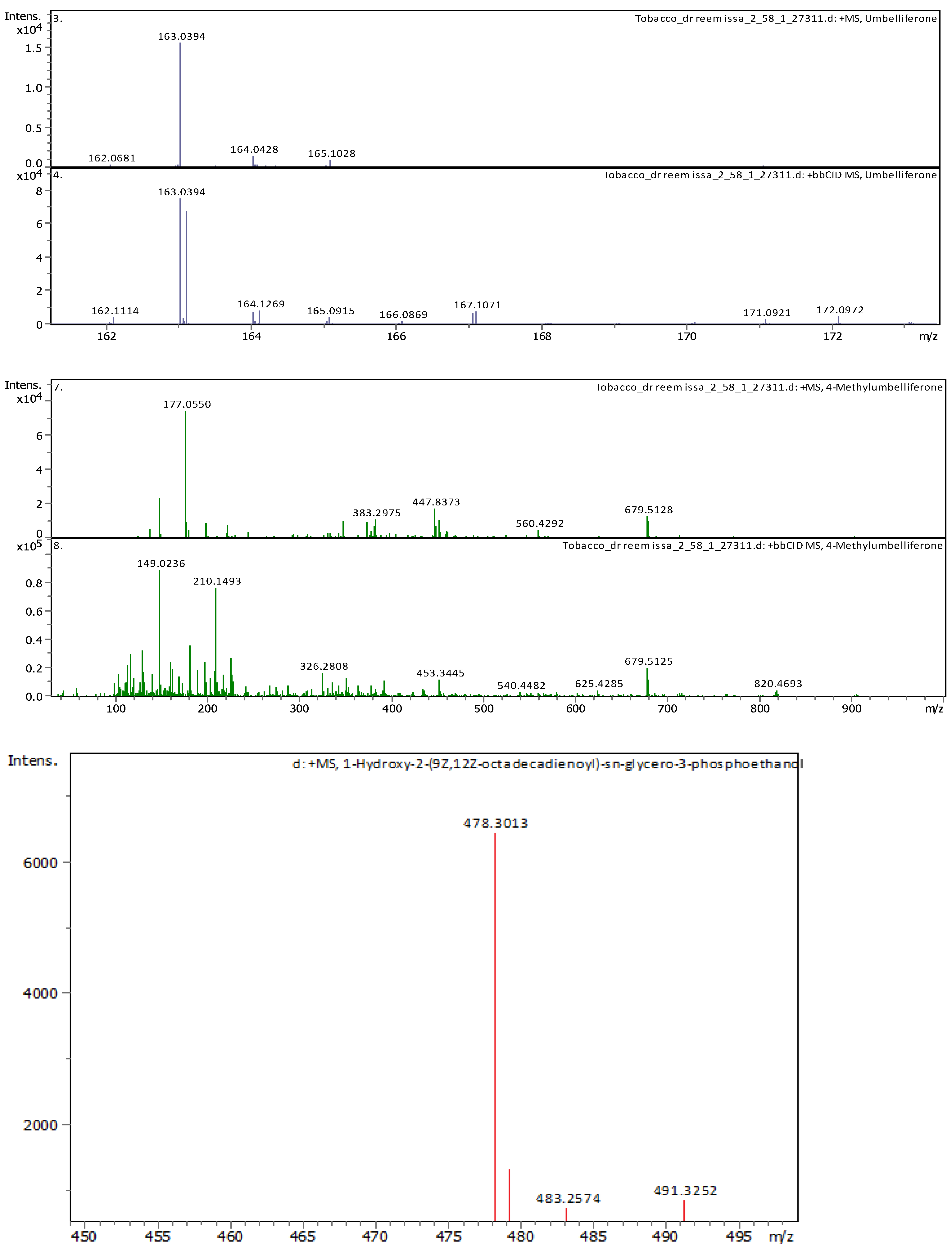

References
- Berlowitz, I.; Torres, E.G.; Walt, H.; Wolf, U.; Maake, C.; Martin-Soelch, C. “Tobacco Is the Chief Medicinal Plant in My Work”: Therapeutic Uses of Tobacco in Peruvian Amazonian Medicine Exemplified by the Work of a Maestro Tabaquero. Front. Pharmacol. 2020, 11, 594591. [Google Scholar] [CrossRef] [PubMed]
- Charlton, A. Medicinal Uses of Tobacco in History. J. R. Soc. Med. 2004, 97, 292–296. [Google Scholar] [CrossRef]
- Zou, X.; Bk, A.; Abu-Izneid, T.; Aziz, A.; Devnath, P.; Rauf, A.; Mitra, S.; Emran, T.B.; Mujawah, A.A.H.; Lorenzo, J.M.; et al. Current Advances of Functional Phytochemicals in Nicotiana Plant and Related Potential Value of Tobacco Processing Waste: A Review. Biomed. Pharmacother. 2021, 143, 112191. [Google Scholar] [CrossRef]
- Massadeh, R.; El-Elimat, T.; Al-Gharaibeh, M.; Tawaha, K.; Alali, F. UPLC-HRESI-MS and GC-MS Analysis of the Leaves of Nicotiana glauca. Acta Pharm. 2022, 72, 97–108. [Google Scholar] [CrossRef]
- Silva, F.D.S.; Albuquerque, U.P.; Costa Júnior, L.M.; Lima, A.D.S.; Nascimento, A.L.B.D.; Monteiro, J.M. An Ethnopharmacological Assessment of the Use of Plants against Parasitic Diseases in Humans and Animals. J. Ethnopharmacol. 2014, 155, 1332–1341. [Google Scholar] [CrossRef] [PubMed]
- Ameya, G.; Manilal, A.; Merdekios, B. In Vitro Antibacterial Activity and Phytochemical Analysis of Nicotiana tabacum, L. Extracted in Different Organic Solvents. Open Microbiol. J. 2017, 11, 352–359. [Google Scholar] [CrossRef]
- Anumudu, C.; Mi, N.; Cc, O.; Io, N.; Ihenetu, F. Antimicrobial Activities of Extracts of Tobacco Leaf (Nicotiana tabacum) and Its Grounded Snuff (Utaba) on Candida albicans and Streptococcus pyogenes. PLoS Negl. Trop. Dis. 2019, 7, 000300. [Google Scholar] [CrossRef]
- Fernanda, S.A.; Amru, B.A.; Rahmani, H.A.; Gozan, M.; Irsyad, N.S.; Bahar, M.; Puspita, O.S.; Zulfa, F.; Pramono, A. Antibacterial Potential of Nicotiana tabacum L. Var Virginia Pyrolysis Extract Against Staphylococcus aureus, Enterococcus faecalis, Escherichia coli, and Pseudomonas aeruginosa. IOP Conf. Ser. Earth Environ. Sci. 2021, 755, 012013. [Google Scholar] [CrossRef]
- Gutiérrez, D.; Bah, M.; Garduño, M.; Mendoza, S.; Serrano, V. Anti-Inflammatory and Antioxidant Activities of Methanol Extracts and Alkaloid Fractions of Four Mexican Medicinal Plants of Solanaceae. Afr. J. Tradit. Complement. Altern. Med. AJTCAM/Afr. Netw. Ethnomed. 2014, 11, 259–267. [Google Scholar] [CrossRef]
- Ali Alghamdi, A. Phytoconstituents Screening and Antimicrobial Activity of the Invasive Species Nicotiana glauca Collected from Al-Baha Region of Saudi Arabia. Saudi J. Biol. Sci. 2021, 28, 1544–1547. [Google Scholar] [CrossRef]
- Al-Akayleh, F.; Ali, H.H.M.; Ghareeb, M.M.; Al-Remawi, M. Therapeutic deep eutectic system of capric acid and menthol: Characterization and pharmaceutical application. J. Drug Deliv. Sci. Technol. 2019, 53, 101159. [Google Scholar] [CrossRef]
- Al-Mawla, L.; Al-Akayleh, F.; Daadoue, S.; Mahyoob, W.; Al-Tameemi, B.; Al-Remawi, M.; Adwan, S.; Agha, A.S.A.A. Development, characterization, and ex vivo permeation assessment of diclofenac diethylamine deep eutectic systems across human skin. J. Pharm. Innov. 2023, 18, 2196–2209. [Google Scholar] [CrossRef]
- Alkhawaja, B.; Al-Akayleh, F.; Nasereddin, J.; Kamran, M.; Woodman, T.; Al-Rubaye, Z.; Qinna, N.; Al-Remawi, M.; Olaimat, A.R. Structural insights into novel therapeutic deep eutectic systems with capric acid using 1D, 2D NMR and DSC techniques with superior gut permeability. RSC Adv. 2024, 14, 14793–14806. [Google Scholar] [CrossRef] [PubMed]
- Nakaweh, A.; Al-Akayleh, F.; Al-Remawi, M.; Abdallah, Q.; Agha, A.S. Deep Eutectic System-Based Liquisolid Nanoparticles as Drug Delivery System of Curcumin for In-Vitro Colon Cancer Cells. J. Pharm. Innov. 2024, 19, 18. [Google Scholar] [CrossRef]
- Al-Akayleh, F.; Al-Remawi, M.; Agha, A.; Abu-Nameh, E. Applications and Risk Assessments of Ionic Liquids in Chemical and Pharmaceutical Domains: An Updated Overview. Jordan J. Chem. (JJC) 2023, 18, 53–76. [Google Scholar]
- Alkhawaja, B.; Al-Akayleh, F.; Nasereddin, J.; Malek, S.A.; Alkhawaja, N.; Kamran, M.; Al-Rubaye, Z.; Smairat, M.; Al-Remawi, M.; Aburayyan, W.S. Levofloxacin–Fatty Acid Systems: Dual Enhancement Through Deep Eutectic Formation and Solubilization for Pharmaceutical Potential and Antibacterial Activity. AAPS PharmSciTech 2023, 24, 244. [Google Scholar] [CrossRef]
- Alkhawaja, B.; Al-Akayleh, F.; Al-Khateeb, A.; Nasereddin, J.; Ghanim, B.Y.; Bolhuis, A.; Jaber, N.; Al-Remawi, M.; Qinna, N.A. Deep eutectic liquids as a topical vehicle for tadalafil: Characterisation and potential wound healing and antimicrobial activity. Molecules 2023, 28, 2402. [Google Scholar] [CrossRef] [PubMed]
- Luhaibi, D.K.; Ali, H.H.M.; Al-Ani, I.; Shalan, N.; Al-Akayleh, F.; Al-Remawi, M.; Nasereddin, J.; Qinna, N.A.; Al-Adham, I.; Khanfar, M. The formulation and evaluation of deep eutectic vehicles for the topical delivery of azelaic acid for acne treatment. Molecules 2023, 28, 6927. [Google Scholar] [CrossRef]
- Daadoue, S.; Al-Remawi, M.; Al-Mawla, L.; Idkaidek, N.; Khalid, R.M.; Al-Akayleh, F. Deep eutectic liquid as transdermal delivery vehicle of Risperidone. J. Mol. Liq. 2022, 345, 117347. [Google Scholar] [CrossRef]
- Al-Akayleh, F.; Adwan, S.; Khanfar, M.; Idkaidek, N.; Al-Remawi, M. A novel eutectic-based transdermal delivery system for risperidone. AAPS PharmSciTech 2021, 22, 4. [Google Scholar] [CrossRef]
- Alkhawaja, B.; Al-Akayleh, F.; Al-Rubaye, Z.; Bustami, M.; Smairat, M.A.; Agha, A.S.; Nasereddin, J.; Qinna, N.; Michael, A.; Watts, A.G. Dissecting the stability of Atezolizumab with renewable amino acid-based ionic liquids: Colloidal stability and anticancer activity under thermal stress. Int. J. Biol. Macromol. 2024, 270, 132208. [Google Scholar] [CrossRef] [PubMed]
- Airouyuwa, J.O.; Sivapragasam, N.; Redha, A.A.; Maqsood, S. Sustainable green extraction of anthocyanins and carotenoids using natural deep eutectic solvents (NaDES): A review of recent developments. Food Chem. 2024, 448, 139061. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, D.; Fang, J.; Song, Z.; Geng, J.; Zhao, J.; Fang, Y.; Wang, C.; Li, M. Green and efficient extraction of flavonoids from Perilla frutescens (L.) Britt. leaves based on natural deep eutectic solvents: Process optimization, component identification, and biological activity. Food Chem. 2024, 452, 139508. [Google Scholar] [CrossRef] [PubMed]
- Al-Akayleh, F.; Khalid, R.M.; Hawash, D.; Al-Kaissi, E.; Al-Adham, I.S.I.; Al-Muhtaseb, N.; Jaber, N.; Al-Remawi, M.; Collier, P.J. Antimicrobial potential of natural deep eutectic solvents. Lett. Appl. Microbiol. 2022, 75, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Xu, S.; Goksen, G.; Yi, C.; Shao, P. Chitosan films plasticized with choline-based deep eutectic solvents: UV shielding, antioxidant, and antibacterial properties. Food Hydrocoll. 2023, 135, 108196. [Google Scholar] [CrossRef]
- Nystedt, H.L.; Grønlien, K.G.; Rolfsnes, R.R.; Winther-Larsen, H.C.; Økstad, O.A.L.; Tønnesen, H.H. Neutral natural deep eutectic solvents as anti-biofilm agents. Biofilm 2023, 5, 100114. [Google Scholar] [CrossRef] [PubMed]
- Tsvetov, N.; Pasichnik, E.; Korovkina, A.; Gosteva, A. Extraction of bioactive components from Chamaenerion angustifolium (L.) Scop. with choline chloride and organic acids natural deep eutectic solvents. Molecules 2022, 27, 4216. [Google Scholar] [CrossRef] [PubMed]
- Edrisi, S.; Bakhshi, H. Separation of Polyphenolic Compounds from Citrus aurantium L. peel by deep eutectic solvents and their recovery using a new DES-based aqueous two-phase system. J. Mol. Liq. 2024, 402, 124790. [Google Scholar] [CrossRef]
- Hong, J.; Deng, M.; Zhao, L. Natural Deep Eutectic Solvent Combined with Ultrasonic Enhancement: A Green Extraction Strategy for Solanesol in Tobacco Leaves. Ind. Crops Prod. 2022, 187, 115355. [Google Scholar] [CrossRef]
- Yu, T.; Yang, L.; Shang, X.; Bian, S. Recovery of Cembratrien-Diols from Waste Tobacco (Nicotiana tabacum L.) Flowers by Microwave-Assisted Deep Eutectic Solvent Extraction: Optimization, Separation, and In Vitro Bioactivity. Molecules 2024, 29, 1563. [Google Scholar] [CrossRef]
- Nasr, H. Ecological and phytochemical studies on Nicotiana glauca from Egypt. Egypt. J. Exp. Biol. 2014, 10, 87–95. [Google Scholar]
- Alnsour, L.; Issa, R.; Awwad, S.; Albals, D.; Al-Momani, I. Quantification of Total Phenols and Antioxidants in Coffee Samples of Different Origins and Evaluation of the Effect of Degree of Roasting on Their Levels. Molecules 2022, 27, 1591. [Google Scholar] [CrossRef]
- Ubaydee, A.H.N.; Issa, R.; Hajleh, M.N.A.; Ghanim, B.Y.; Al-Akayleh, F.; Qinna, N.A. The Effect of Medicago sativa Extract and Light on Skin Hypopigmentation Disorders in C57/BL6 Mice. J. Cosmet. Dermatol. 2022, 21, 6270–6280. [Google Scholar] [CrossRef]
- Al-Bayati, M.; Issa, R.; Abu-Samak, M.; Alnsour, L.; Awwad, S. Phytochemical Analysis and Evaluation of Anti-Hyperlipidaemic Effect for Ethanolic Leaf Extract of Equisetum ramosissimum L.: In Vivo Study Rats’ Models. Pharmacia 2023, 70, 557–568. [Google Scholar] [CrossRef]
- Kheawfu, K.; Kaewpinta, A.; Chanmahasathien, W.; Rachtanapun, P.; Jantrawut, P. Extraction of Nicotine from Tobacco Leaves and Development of Fast Dissolving Nicotine Extract Film. Membranes 2021, 11, 403. [Google Scholar] [CrossRef]
- Dai, Y.; Van Spronsen, J.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Ionic liquids and deep eutectic solvents in natural products research: Mixtures of solids as extraction solvents. J. Nat. Prod. 2013, 76, 2162–2173. [Google Scholar] [CrossRef]
- Chanioti, S.; Tzia, C. Extraction of phenolic compounds from olive pomace by using natural deep eutectic solvents and innovative extraction techniques. Innov. Food Sci. Emerg. Technol. 2018, 48, 228–239. [Google Scholar] [CrossRef]
- Zannou, O.; Koca, I.; Aldawoud, T.M.; Galanakis, C.M. Recovery and stabilization of anthocyanins and phenolic antioxidants of roselle (Hibiscus sabdariffa L.) with hydrophilic deep eutectic solvents. Molecules 2020, 25, 3715. [Google Scholar] [CrossRef]
- Alsaud, N.; Shahbaz, K.; Farid, M. Application of deep eutectic solvents in the extraction of polyphenolic antioxidants from New Zealand Manuka leaves (Leptospermum scoparium): Optimization and antioxidant activity. J. Mol. Liq. 2021, 337, 116385. [Google Scholar] [CrossRef]
- Pavić, V.; Flačer, D.; Jakovljević, M.; Molnar, M.; Jokić, S. Assessment of total phenolic content, in vitro antioxidant and antibacterial activity of Ruta graveolens L. extracts obtained by choline chloride based natural deep eutectic solvents. Plants 2019, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Bakirtzi, C.; Triantafyllidou, K.; Makris, D.P. Novel lactic acid-based natural deep eutectic solvents: Efficiency in the ultrasound-assisted extraction of antioxidant polyphenols from common native Greek medicinal plants. J. Appl. Res. Med. Aromat. Plants 2016, 3, 120–127. [Google Scholar] [CrossRef]
- Alsenidi, M.; Moustafa, M. Variation in Chemicals and Antimicrobial Activities of Nicotiana glauca Graham. Bangladesh J. Bot. 2023, 52, 403–410. [Google Scholar] [CrossRef]
- Tayoub, G.; Sulaiman, H.; Alorfi, M. Determination of nicotine levels in the leaves of some Nicotiana tabacum varieties cultivated in Syria. Herba Pol. 2015, 61, 23–30. [Google Scholar] [CrossRef]
- Tantullavetch, Y.; Chutrtong, W.; Nimkulrat, S. The Development and Production of Nicotine Gum for Use in Smoking Cessation Therapy (the Second Period); Srinakharinwirot University: Bangkok, Thailand, 2007. [Google Scholar]
- Banožić, M.; Banjari, I.; Flanjak, I.; Paštar, M.; Vladić, J.; Jokić, S. Optimization of MAE for the Separation of Nicotine and Phenolics from Tobacco Waste by Using the Response Surface Methodology Approach. Molecules 2021, 26, 4363. [Google Scholar] [CrossRef]
- Furer, V.; Hersch, M.; Silvetzki, N.; Breuer, G.S.; Zevin, S. Nicotiana glauca (Tree Tobacco) Intoxication—Two Cases in One Family. J. Med. Toxicol. 2011, 7, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Schep, L.J.; Slaughter, R.J.; Beasley, D.M.G. Nicotinic Plant Poisoning. Clin. Toxicol. 2009, 47, 771–781. [Google Scholar] [CrossRef]
- Janakat, S.; Al-Merie, H. Evaluation of Hepatoprotective Effect of Pistacia lentiscus, Phillyrea latifolia and Nicotiana glauca. J. Ethnopharmacol. 2002, 83, 135–138. [Google Scholar] [CrossRef]
- Rodgman, A.; Perfetti, T. The Chemical Components of Tobacco and Tobacco Smoke, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2013; ISBN 978-1-4665-1548-2. [Google Scholar]
- Wang, H.; Zhao, M.; Yang, B.; Jiang, Y.; Rao, G. Identification of Polyphenols in Tobacco Leaf and Their Antioxidant and Antimicrobial Activities. Food Chem. 2008, 107, 1399–1406. [Google Scholar] [CrossRef]
- Trifa, W.; Akkal, S.; Lefahal, M.; Benmekhebi, L.; Khennouf, S. Preliminary Screening of Extracts for Determination of Antioxidant Activity by Different Methods. Curr. Issues Pharm. Med. Sci. 2020, 33, 32–37. [Google Scholar] [CrossRef]
- Özdenefe, M.S.; Takcı, A.M.; Kayış, F.B. Antibacterial, Antioxidant, Antidiabetic Potentials and Chemical Composition of Nicotiana glauca Graham Leaf Extract. J. Anatol. Environ. Anim. Sci. 2023, 8, 700–706. [Google Scholar] [CrossRef]
- Puripattanavong, J.; Songkram, C.; Lomlim, L.; Amnuaikit, T. Development of Concentrated Emulsion Containing Nicotiana tabacum Extract for Use as Pesticide. J. Appl. Pharm. Sci. 2013, 3, 16–21. [Google Scholar] [CrossRef]


| Extraction Media | mg/mL ± SD (Equivalent to Gallic Acid) |
|---|---|
| DES 30% | 0.326 ± 0.11 |
| DES 70% | 0.300 ± 0.03 |
| DES 90% | 0.311 ± 0.02 |
| MC | 0.119 ± 0.01 |
| Extraction Media | mg/mL ± SD (Equivalent to Rutin) |
|---|---|
| DES 30% | 0.128 ± 0.03 |
| DES 70% | 0.115 ± 0.14 |
| DES 90% | 0.928 ± 0.09 |
| MC | 0.011 ± 0.01 |
| # | Rt [min] | m/z Meas. | M Meas. | Ions | Name | Molecular Formula |
|---|---|---|---|---|---|---|
| 1 | 0.61 | 131.04612 | 132.05339 | [M–H]− | L-Asparagine | C4H8N2O3 |
| 2 | 0.62 | 114.05616 | 115.06344 | [M–H]− | Proline | C5H9NO2 |
| 3 | 0.97 | 180.06594 | 181.07322 | [M–H]− | L-Tyrosine | C9H11NO3 |
| 4 | 1 | 117.01933 | 118.02661 | [M–H]− | Succinic acid | C4H6O4 |
| 5 | 1.28 | 147.04508 | 148.05236 | [M–H]− | Cinnamic acid | C9H8O2 |
| 6 | 1.28 | 164.0718 | 165.07908 | [M–H]− | (±)-Phenylalanine | C9H11NO2 |
| 7 | 2.03 | 203.08226 | 204.08954 | [M–H]− | (±)-Tryptophan | C11H12N2O2 |
| 8 | 2.9 | 191.05607 | 192.06335 | [M–H]− | Quinic acid | C7H12O6 |
| 9 | 2.96 | 355.10248 | 354.09521 | [M+H]+ | Chlorogenic acid | C16H18O9 |
| 10 | 3.05 | 179.03487 | 180.04214 | [M–H]− | Caffeic Acid | C9H8O4 |
| 11 | 5.07 | 163.03979 | 162.03251 | [M+H]+ | Umbelliferone | C9H6O3 |
| 12 | 5.12 | 203.08228 | 204.08955 | [M–H]− | (±)-Tryptophan | C11H12N2O2 |
| 13 | 5.57 | 609.1455 | 610.15278 | [M–H]− | Quercetin 3-rutinoside | C27H30O16 |
| 14 | 5.61 | 303.05014 | 302.04287 | [M+H]+ | Robinetin | C15H10O7 |
| 15 | 5.61 | 465.10293 | 464.09566 | [M+H]+ | Hyperoside | C21H20O12 |
| 16 | 5.62 | 611.16099 | 610.15353 | [M+H]+, [M+Na]+ | Rutin | C27H30O16 |
| 17 | 6.31 | 179.05592 | 180.0632 | [M–H]− | Starch | C6H12O6 |
| 18 | 6.37 | 287.0557 | 286.04842 | [M+H]+ | 3,6,2′,4′-Tetrahydroxyflavone | C15H10O6 |
| 19 | 9.1 | 315.05061 | 316.05788 | [M–H]− | 3-O-Methyl Quercetin | C16H12O7 |
| 20 | 21.09 | 478.28898 | 477.2817 | [M+H]+ | 1-Hydroxy-2-(9Z,12Z-octadecadienoyl)-sn-glycero-3-phosphoethanolamine (NMR) | C23H44NO7P |
| 21 | 22.31 | 478.28916 | 477.28189 | [M+H]+ | 1-(9Z,12Z-Octadecadienoyl)-2-hydroxy-sn-glycero-3-phosphoethanolamine (NMR) | C23H44NO7P |
| 22 | 22.49 | 471.35137 | 470.34409 | [M+H]+ | 18-Beta-glycyrrhetinic acid | C30H46O4 |
| 23 | 28.62 | 221.15517 | 222.16244 | [M–H]− | Histamine | C10H18N6 |
| # | Rt [min] | m/z Meas. | M Meas. | Ions | Name | Molecular Formula |
|---|---|---|---|---|---|---|
| 1 | 1.65 | 133.00998 | 134.0174 | [M–H]−, [M–H H2O]− | Malic acid | C4H6O5 |
| 2 | 1.91 | 59.01128 | 60.01856 | [M–H]− | Acetic acid | C2H4O2 |
| 3 | 2.39 | 350.14397 | 351.15125 | [M–H]− | (E)-Ribosylzeatin | C15H21N5O5 |
| 4 | 2.96 | 117.01473 | 118.022 | [M–H]− | Succinic acid | C4H6O4 |
| 5 | 3.32 | 147.04052 | 148.0478 | [M–H]− | Cinnamic acid | C9H8O2 |
| 6 | 3.35 | 87.04212 | 88.04939 | [M–H]− | 2-Methylpropanoic acid | C4H8O2 |
| 7 | 3.36 | 164.06681 | 165.07409 | [M–H]− | (±)-Phenylalanine | C9H11NO2 |
| 8 | 3.69 | 163.038920 | 162.031640 | [M+H]+ | Umbelliferone | C9H6O3 |
| 9 | 3.7 | 355.102500 | 354.095190 | [M+H]+, [M+K]+, [M+Na]+ | Chlorogenic acid | C16H18O9 |
| 10 | 3.79 | 173.04114 | 174.04841 | [M–H]− | Shikimic acid | C7H10O5 |
| 11 | 3.8 | 199.057710 | 198.050440 | [M+H]+ | Syringic acid | C9H10O5 |
| 12 | 4.6 | 193.049450 | 192.042170 | [M+H]+ | Scopoletin | C10H8O4 |
| 13 | 4.76 | 465.102820 | 464.095540 | [M+H]+ | Hyperoside | C21H20O12 |
| 14 | 4.77 | 303.050010 | 302.042740 | [M+H]+ | Robietin | C15H10O7 |
| 15 | 4.77 | 611.160240 | 610.152970 | [M+H]+ | Luteolin-7,3′-di-O-glucoside | C27H30O16 |
| 16 | 4.91 | 609.1281 | 610.13537 | [M–H]− | Prodelphinidin B3 | C30H26O14 |
| 17 | 5 | 221.05958 | 222.06686 | [M–H]− | Flavone | C15H10O2 |
| 18 | 5.15 | 595.165470 | 594.158150 | [M+H]+, [M+Na]+ | Saponarin | C27H30O15 |
| 19 | 5.74 | 195.065060 | 194.057780 | [M+H]+ | 3-Hydroxy-4-methoxycinnamic acid (isoferulic acid) | C10H10O4 |
| 20 | 7.39 | 163.039120 | 162.031850 | [M+H]+ | (4 or 7) Hydroxy-Coumarin Plus Hydrate | C9H6O3 |
| 21 | 7.41 | 177.054460 | 176.047190 | [M+H]+ | 4-Methylumbelliferone | C10H8O3 |
| 22 | 8.54 | 495.125630 | 494.118360 | [M+H]+ | Salvianolic acid A | C26H22O10 |
| 23 | 11.6 | 249.148000 | 248.140730 | [M+H]+ | 3-Oxocostusic acid | C15H20O3 |
| Rt [min] | m/z Meas. | M Meas. | Ions | Name | Molecular Formula |
|---|---|---|---|---|---|
| 2.89 | 163.12293 | 324.2313 | [M+H+H]2+ | Nicotine | C10H14N2 |
| Sample | MC Extract | DES Extract |
|---|---|---|
| Area of Nicotine in Sample | 2,526,713 | 4,192,477 |
| Concentration of Nicotine | 635.07 ppm | 1194.91 ppm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Issa, R.; Al-Akayleh, F.; Alnsour, L.; Al-Sammarraie, T.R.; Omari, K.W.; Awwad, S.H. Antioxidant Activity and UHPLC-MS/MS Characterization of Polyphenol and Nicotine Content in Nicotiana Glauca Leaf Extracts: A Comparative Study of Conventional and Deep Eutectic Solvent Extraction Methods. Plants 2024, 13, 2240. https://doi.org/10.3390/plants13162240
Issa R, Al-Akayleh F, Alnsour L, Al-Sammarraie TR, Omari KW, Awwad SH. Antioxidant Activity and UHPLC-MS/MS Characterization of Polyphenol and Nicotine Content in Nicotiana Glauca Leaf Extracts: A Comparative Study of Conventional and Deep Eutectic Solvent Extraction Methods. Plants. 2024; 13(16):2240. https://doi.org/10.3390/plants13162240
Chicago/Turabian StyleIssa, Reem, Faisal Al-Akayleh, Lilian Alnsour, Tabarak R. Al-Sammarraie, Khaled W. Omari, and Shady H. Awwad. 2024. "Antioxidant Activity and UHPLC-MS/MS Characterization of Polyphenol and Nicotine Content in Nicotiana Glauca Leaf Extracts: A Comparative Study of Conventional and Deep Eutectic Solvent Extraction Methods" Plants 13, no. 16: 2240. https://doi.org/10.3390/plants13162240








