Tissue Culture Innovations for Propagation and Conservation of Myrteae—A Globally Important Myrtaceae Tribe
Abstract
:1. Introduction
2. Micropropagation of Myrteae
2.1. Introduction to Micropropagation
2.2. Tissue Culture for Micropropagation of Myrteae
2.3. Tissue Culture of Eugenia Species
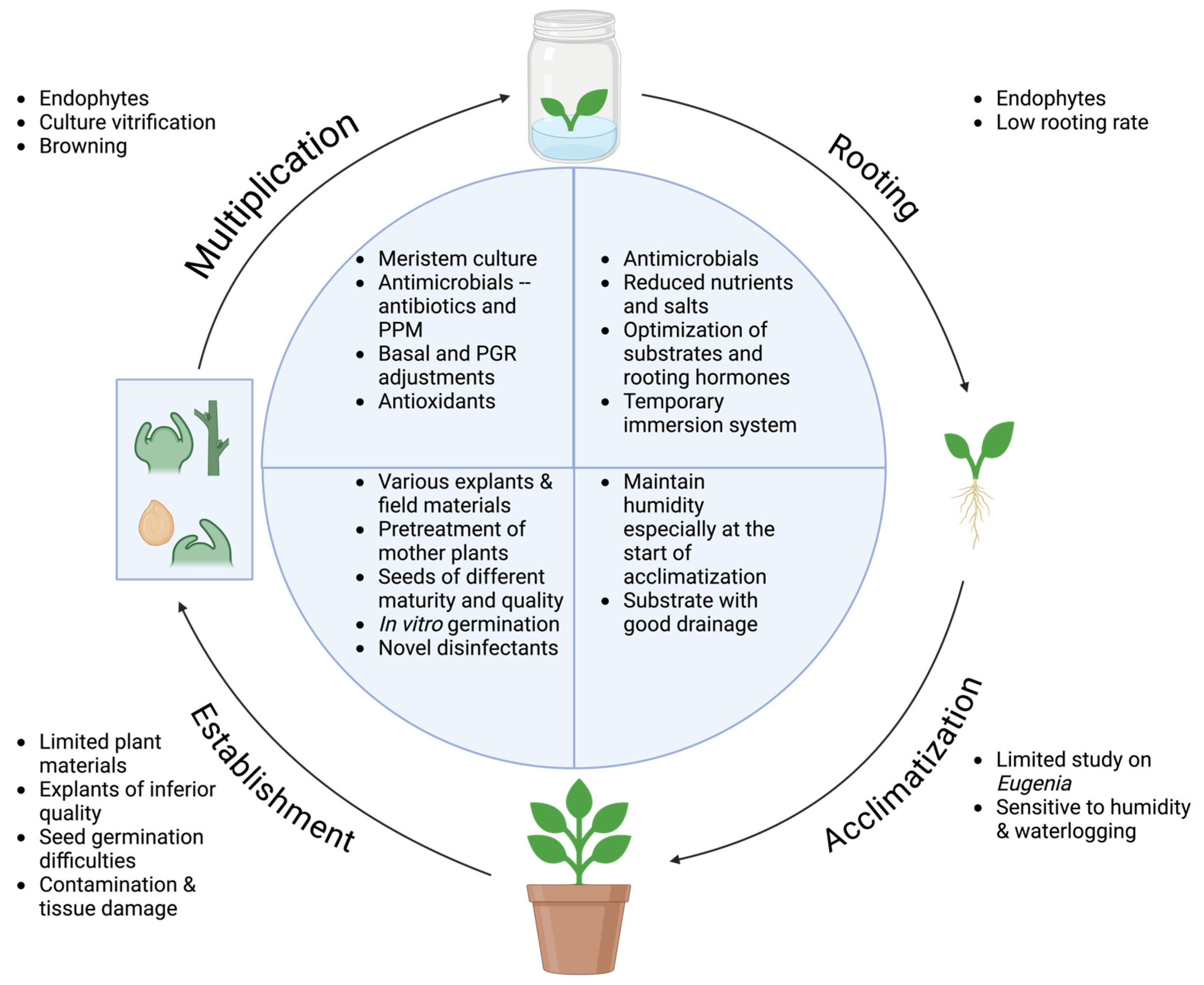
2.3.1. Establishment
2.3.2. Multiplication
2.3.3. Rooting and Acclimatization
2.3.4. Summary of Eugenia Tissue Culture and Potential Challenges
| Species | Micro- Propagation Technique | Explant Type and Sterilization Method | Initiation Medium | Multiplication Medium | Rooting Medium | Acclimatization Substrate | References |
|---|---|---|---|---|---|---|---|
| E. involucrata | In vitro germination; Node culture | Seeds; apical buds germinated from seeds (1 cm) 70% ethanol for 1 min, 3% Ca(OCl)2 for 15 min, 3% NaOCl for 15 min | Water agar (in vitro germination) | Half-strength MS with 87.6 mM sucrose, 277.5 μM myo-inositol, 0, 0.1 or 0.2 μM BAP; 0, 10, 20 or 30 µM IBA, and 7 g L−1 agar, pH 5.8 | - | - | [146] |
| E. involucrata | Node culture | Non-woody axillary buds between the third and fourth nodal segments (1 g L−1 benomyl and 0.1 g L−1 streptomycin sulfate for 30 min) 70% ethanol for 30 s, agitation in 1.5% (v/v) NaOCl for 15 min, 0.05% (w/v) HgCl2 for 10 min, 1.5% (v/v) NaOCl solution with three drops of commercial detergent for 10 min; sterile water wash between treatments; explants maintained in 100 mg L−1 ascorbic acid | - | Half-strength MS with 87.6 mM sucrose, 277.5 μM myo-inositol, 0.5 µM NAA, 32 µM TDZ, and 7 g L−1 agar, pH 5.8 | - | - | [82] |
| E. involucrata | Shoot culture | Apical and nodal shoots (1.5 cm) germinated from seeds 70% ethanol, 1.25% NaOCl 15 min, rinse with autoclaved distilled water 3 times, 10 min silver nanoparticles (prepared by reducing 95 mL solution containing 5 mg AgNO3 with 5 mL 1% sodium citrate solution, heating in a water bath at 90 °C) | Vermiculite and sand (1:1) substrate, watered daily and fortnightly with a one fourth MS solution (seed germination) | Half-strength MS with half-strength vitamins, 0.5 μM IBA, and 0.9 μM BAP, and 7.0 g L−1 agar, pH 5.8 | Seedlings developed a root system on multiplication medium | Vermiculite and sand (1:1) substrate in plastic containers, packed into transparent bags to keep moist for indoor acclimatization, then unpacked and placed under shaded environment for outdoor acclimatization | [100] |
| E. javanica (now accepted as Syzygium aqueum) | Meristem culture | Meristem (0.5 mm) from a 5-year-old tree 70% ethanol for 15 s, 1% NaOCl for 10 min | Liquid MS with 87.6 mM sucrose and 2.2 μM BAP (4 weeks) followed by MS with 87.6 mM sucrose and 2.2 μM BAP, solidified with agar | MS with 87.6 mM sucrose, 0.4 μM BAP, 0.5 μM NAA, and 8 g L−1 agar | 9.4 mM NAA dip for 5 s, then placed on half MS with 58.4 mM sucrose and 8 g L−1 agar; half- strength MS with 0.5 μM NAA, 58.4 mM sucrose, and 8 g L−1 agar | - | [128] |
| E. myrtifolia (now accepted as Syzygium austral) | In vitro germination; Shoot culture | Seeds at different maturity level (external integument removed); shoots germinated from seeds 80% ethanol 5 min, 30% (v/v) commercial bleach 20 min | MS (half- strength macronutrients and full- strength micronutrients) with 2.5 μM TDZ, pH 5.7 (optimal seeds regeneration; in dark) hormone- free half- strength MS, pH 5.7 (adventitious buds elongation) | MS with 4.4 μM BAP, 0.05 μM NAA, and 8 g L−1 agar, pH 5.7 | Hormone- free MS medium | Sterilized soil and peat (1:1) in clay pots, covered by a glass beaker for 2 weeks, then uncovered and moved to greenhouse for more than 2 weeks before transfer to open air | [114] |
| E. myrtifolia (now accepted as Syzygium austral) | Shoot culture | Buds collected in spring 70% ethanol for a few seconds, 50% ACE detergent for 30 min | Half- strength MS with half- strength vitamins, 87.6 mM sucrose, 1 g L−1 PVP, 2.2 μM BAP, 0.05 μM IBA, 0.1 μM GA3, 56.8 μM filter- sterilized ascorbic acid, and 7 g L−1 agar | MS with 87.6 mM sucrose, 1 g L−1 PVP, 2.2 μM BAP, 0.05 μM IBA, 0.1 μM GA3, 56.8 μM filter-sterilized ascorbic acid, and 7 g L−1 agar | - | - | [120] |
| E. pyriformis | Node culture | Nodal segments from adult plants 70% ethanol with Tween 20 (one drop per 100 mL) for 90 s, 1% NaOCl for 20 min | - | MS with 87.6 mM sucrose, 300 mg L−1 PVP or ascorbic acid, 2 g L−1 activated charcoal, and 5.5 g L−1 agar, pH 5.8 | - | - | [81] |
| E. singampattiana | Node culture | Nodal segments (0.5–1 cm) 70% ethanol for 1–5 min, 0.1% HgCl2 for 3 min | MS with 4.4 μM BAP, 4.5 μM TDZ, 87.6 mM sucrose, and 8 g L−1 agar, pH 5.7 | - | MS with 2.5 μM IBA, 87.6 mM sucrose, and 8 g L−1 agar, pH 5.7 | Sterilized garden soil, sand, and vermiculite (2:1:1) in plastic pots covered with polythene bags, watered with a one fourth MS solution every three days, transferred to field conditions after 15 days | [138] |
| E. smithii (now accepted as Syzygium smithii) | Shoot tip culture | Apical and nodal shoot tips from a 3-year-old plant; in vitro shoots (0.5 cm) 0.2% HgCl2 for 4 min | MS with 58.4 mM sucrose, 2.2 μM BAP, 0.5 μM IBA, and 7 g L−1 agar, pH 5.6–5.8 | Elongation on MS with 58.4 mM sucrose, 4.4 μM BAP, 2.5 μM IBA, and 7 g L−1 agar, pH 5.6–5.8 | One-fifth strength MS with 4.7 μM NAA | Garden mould and peat moss (1:1) in plastic pots maintained in a 22–25 °C greenhouse, sprayed with 5 g L−1 thiram (fungicide) two to three times | [116] |
| E. anthacanthoides (now accepted as E. squarrosa) | Seed culture | Seeds 2.0% NaOCl and Tween 20 for 20 min | Half- strength MS with 3.8 μM thiamine, 555.1 μM myo-inositol, 29.2 μM sucrose, and 2.5 g L−1 Gelrite®, pH 5.8 | - | Seedlings developed roots on initiation medium | Rich substrate in organic matter and abundant watering | [83] |
| E. subdisticha | Seed culture | Seeds 2.0% NaOCl for 20 min | Half- strength MS with 3.8 μM thiamine, 555.1 μM myo-inositol, 29.2 μM sucrose, and 2.5 g L−1 Gelrite®, pH 5.8 | - | Seedlings developed roots on initiation medium | Rich substrate in organic matter and abundant watering | [83] |
| E. sulcata | Seed culture | Seeds 70% ethanol for 1 min, 2.5% NaOCl for 20 min | WPM with activated charcoal, 7 g L−1 agar, pH 5.8 ± 0.1 | - | Seedlings developed roots on initiation medium | Maintained in 50% shade greenhouse with a micro- sprinkler watering system | [86] |
| E. uniflora | In vitro germination; Node culture | Seeds from wild genotypes; apical and nodal segments (1.5 cm) 70% ethanol for 1 min, 1.25% NaOCl for 25 min | Water agar with 6.0 g L−1 agar (in vitro germination); water agar with 87.6 mM sucrose and 7.5 g L−1 agar (to verify the existence of endogenous bacteria and fungi) | Half-strength MS with 87.6 mM sucrose, 0.5 μM IBA, 0.9 μM BAP, and 6.0 g L−1 agar, pH 5.8 | Autoclaved sand in pots and maintained in a nursery environment with daily watering | Sand: organic soil (1:1) and cultivated in a greenhouse with daily watering | [129] |
| E. uniflora | In vitro germination; Shoot tip culture | Seeds from the ripe fruits; apical and axillary shoot tips (0.5 mm) 70% ethanol for 1 min, 0.5% NaOCl for 20 min | WPM with 87.6 mM sucrose and 25 g L−1 Gelsan® (in vitro germination) | WPM with 87.6 mM sucrose, 4.44 μM BAP, and 25 g L−1 Gelsan® (in conventional system) WPM with 87.6 mM sucrose, 11.1 μM or 17.76 μM BAP, and 25 g L−1 Gelsan® (in the natural ventilation system) | - | - | [130] |
| E. uniflora | Shoot tip culture | Shoot segments (2–3 mm) from plants maintained in a greenhouse 70% ethanol for 5 min and 0.3% NaOCl for 10 min | MS with 0.9 μM BAP and 2 g L−1 Gelrite® (kept in dark before shoot regeneration) | Half-strength MS (shoot elongation) | Half- strength MS | Sterilized vermiculite in pots covered with a glass beaker, kept in an incubator maintained at 25 °C, 14 h photoperiod, then transferred to a greenhouse after 2 months | [117] |
| E. uniflora | In vitro germination; indirect somatic embryogenesis | Seeds; nodal segments (1.5 cm) 70% ethanol for 10 min, 1.5% NaOCl for 20 min | Water agar with 6.0 g L−1 agar (in vitro germination) | Callogenesis on MS with Morel vitamins [147], 555.1 μM myo-inositol, 87.6 mM sucrose, 56.8 μM ascorbic acid, 5.2 μM citric acid, 10.0 μM NAA, 5.0 μM TDZ, and 6 g L−1 agar, pH 5.8; somatic embryogenesis on callus induction medium | - | - | [148] |
| E. uniflora | Seed culture | Seeds - | Water agar with 8.0 g L−1 agar (seeds germination) | Half-strength liquid WPM added to water agar every 4 weeks | Seedlings developed roots on initiation medium | - | [115] |
| E. uniflora | In vitro germination; seed culture | Ripe seeds (dehydrated for 7 d) 70% ethanol for 30 s, 5.0% NaOCl for 20 min | MS with 66.7 μM GA3, 3% sucrose, 8.0 g L−1 agar, pH 5.8 ± 0.1 | - | - | After 120 d, plants transferred to Basaplant® commercial substrate with Osmocote® 15-09-12 and covered with a polyethylene plastic bag, which was gradually opened after 7 d. The plants were then kept in a nursery with 50% sunlight and watered twice a day. | [149] |
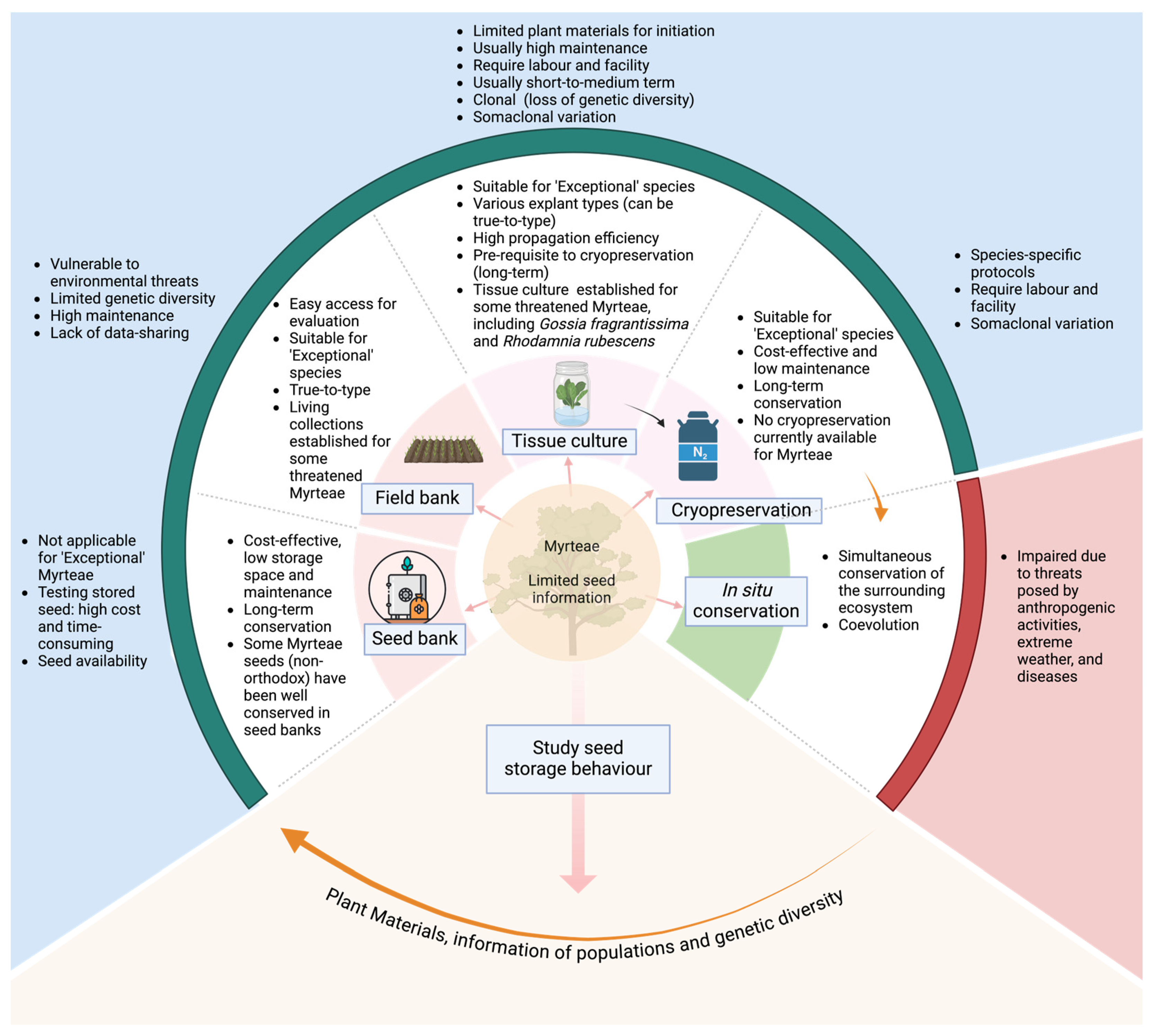
3. Importance of Tissue Culture in Conservation of Other Myrteae Genera
3.1. Seed Banking
3.2. Field Banking
3.3. Tissue-Culture-Based Methods for Plant Conservation
3.3.1. Slow-Growth Tissue Culture
3.3.2. Cryopreservation
3.4. Conservation of Myrteae—Overview
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Lists of Field Repositories of Common Guava Cultivars
| Country | Guava Cultivars | Field Repositories | Reference |
| Brazil | 59 guava accessions | Psidium genebank of Embrapa Semiarid | [186] |
| China | - | the National Field Genebank for Tropical Fruits | [187] |
| Cuba | At least 40 cultivars, including ‘Enana Cubana roja’, ‘N6’, and ‘Suprema Roja’ | Germplasm Bank of Tropical and Subtropical Fruit Trees (the principal Cuban guava collection) | [188] |
| Mexico | 113 guava accessions, including native, cultivated, and backyard guavas | Germplasm bank of INIFAP (National Institute for Agriculture, Forestry and Livestock Research) | [189] |
| USA | 40 cultivars including ‘Rica’, ‘Gema de Doro’, and ‘RED INDIAN’ | Hilo, Hawaii-National Germplasm Repository (NGR) | [162] |
| USA | ‘Malherbe’ | Miami, Florida-NGR | [162] |
| Venezuela | 50 accessions of Guava | The Myrtaceae germplasm bank | [190] |
References
- Govaerts, R.; Sobral, M.; Ashton, P.; Barrie, F.; Holst, B.K.; Landrum, L.L.; Matsumoto, K.; Mazine, F.F.; Lughadha, E.N.; Proneça, C. World Checklist of Myrtaceae; Royal Botanic Gardens: Kew, UK, 2008. [Google Scholar]
- Plants of the World Online. Myrtaceae Juss. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:30000056-2 (accessed on 19 June 2024).
- Wilson, P.G. Myrtaceae. In Flowering Plants. Eudicots: Sapindales, Cucurbitales, Myrtaceae; Kubitzki, K., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 212–271. [Google Scholar]
- Johnson, L.A.S.; Briggs, B.G. Three old southern families—Myrtaceae, Proteaceae and Restionaceae. In Ecological Biogeography of Australia; Keast, A., Ed.; Dr. W. Junk b.v. Publishers: The Hague, The Netherlands, 1981; pp. 429–469. [Google Scholar]
- Vasconcelos, T.N.C.; Proença, C.E.B.; Ahmad, B.; Aguilar, D.S.; Aguilar, R.; Amorim, B.S.; Campbell, K.; Costa, I.R.; De-Carvalho, P.S.; Faria, J.E.Q. Myrteae phylogeny, calibration, biogeography and diversification patterns: Increased understanding in the most species rich tribe of Myrtaceae. Mol. Phylogenetics Evol. 2017, 109, 113–137. [Google Scholar] [CrossRef] [PubMed]
- Plants of the World Online. Eugenia P.Micheli ex L. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:326088-2#children (accessed on 16 January 2023).
- Plants of the World Online. Myrcia DC. ex Guill. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:30001220-2 (accessed on 16 January 2023).
- Castuera-Oliveira, L.; de Oliveira-Filho, A.T.; Eisenlohr, P.V. Emerging hotspots of tree richness in Brazil. Acta Bot. Bras. 2020, 34, 117–134. [Google Scholar] [CrossRef]
- Staggemeier, V.G.; Diniz-Filho, J.A.F.; Morellato, L.P.C. The shared influence of phylogeny and ecology on the reproductive patterns of Myrteae (Myrtaceae). J. Ecol. 2010, 98, 1409–1421. [Google Scholar] [CrossRef]
- Pizo, M.A. The seed dispersers and fruit syndromes of Myrtaceae in the Brazilian Atlantic forest. In Seed Dispersal and Frugivory: Ecology, Evolution and Conservation; Levey, D., Silva, W., Galetti, M., Eds.; CABI Publishing: Wallingford, UK, 2002; pp. 129–143. [Google Scholar]
- Margis, R.; Felix, D.; Caldas, J.F.; Salgueiro, F.; De Araujo, D.S.D.; Breyne, P.; Van Montagu, M.; De Oliveira, D.; Margis-Pinheiro, M. Genetic differentiation among three neighboring Brazil-cherry (Eugenia uniflora L.) populations within the Brazilian Atlantic rain forest. Biodivers. Conserv. 2002, 11, 149–163. [Google Scholar] [CrossRef]
- Amorim, A.C.L.; Lima, C.K.F.; Hovell, A.M.C.; Miranda, A.L.P.; Rezende, C.M. Antinociceptive and hypothermic evaluation of the leaf essential oil and isolated terpenoids from Eugenia uniflora L. (Brazilian Pitanga). Phytomedicine 2009, 16, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Sardi, J.d.C.O.; Freires, I.A.; Lazarini, J.G.; Infante, J.; de Alencar, S.M.; Rosalen, P.L. Unexplored endemic fruit species from Brazil: Antibiofilm properties, insights into mode of action, and systemic toxicity of four Eugenia spp. Microb. Pathog. 2017, 105, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.K. Important Myrtaceae fruit crops. In Proceedings of the II International Symposium on Guava and other Myrtaceae, Mérida, Mexico, 10–13 November 2008; Aguascalientes, Mexico, 17–18 November 2008. pp. 33–38. [Google Scholar]
- Wu, S.-B.; Long, C.; Kennelly, E.J. Phytochemistry and health benefits of jaboticaba, an emerging fruit crop from Brazil. Food Res. Int. 2013, 54, 148–159. [Google Scholar] [CrossRef]
- Guo, R.; Canter, P.H.; Ernst, E. Herbal medicines for the treatment of rhinosinusitis: A systematic review. Otolaryngol.-Head Neck Surg. 2006, 135, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Dafni, A. Myrtle (Myrtus communis) as a ritual plant in the Holy Land—A comparative study in relation to ancient traditions. Econ. Bot. 2016, 70, 222–234. [Google Scholar] [CrossRef]
- Logan City Council. Gossia gonoclada Recovery Plan 2019–2029; Logan City Council: Brisbane, Australia, 2019; pp. 1–18.
- Martin, M.P.; Peters, C.M.; Ashton, M.S. Revisiting camu-camu (Myrciaria dubia): Twenty-seven years of fruit collection and flooding at an Oxbow Lake in Peruvian Amazonia. Econ. Bot. 2014, 68, 169–176. [Google Scholar] [CrossRef]
- Ribeiro, M.C.; Metzger, J.P.; Martensen, A.C.; Ponzoni, F.J.; Hirota, M.M. The Brazilian Atlantic Forest: How much is left, and how is the remaining forest distributed? Implications for conservation. Biol. Conserv. 2009, 142, 1141–1153. [Google Scholar] [CrossRef]
- The Nature Conservancy. Places We Protect—The Atlantic Forest. Available online: https://www.nature.org/en-us/get-involved/how-to-help/places-we-protect/atlantic-forest/?tab_q=tab_container-tab_element (accessed on 2 April 2022).
- Lucas, E.J.; Bünger, M.O. Myrtaceae in the Atlantic Forest: Their role as a ‘model’ group. Biodivers. Conserv. 2015, 24, 2165–2180. [Google Scholar] [CrossRef]
- Tabarelli, M.; Cardoso da Silva, J.M.; Gascon, C. Forest fragmentation, synergisms and the impoverishment of neotropical forests. Biodivers. Conserv. 2004, 13, 1419–1425. [Google Scholar] [CrossRef]
- Rigueira, D.M.G.; da Rocha, P.L.B.; Mariano-Neto, E. Forest cover, extinction thresholds and time lags in woody plants (Myrtaceae) in the Brazilian Atlantic Forest: Resources for conservation. Biodivers. Conserv. 2013, 22, 3141–3163. [Google Scholar] [CrossRef]
- Enquist, B.J.; Enquist, C.A. Long-term change within a Neotropical forest: Assessing differential functional and floristic responses to disturbance and drought. Glob. Change Biol. 2011, 17, 1408–1424. [Google Scholar] [CrossRef]
- Phillips, O.L.; Aragão, L.E.; Lewis, S.L.; Fisher, J.B.; Lloyd, J.; López-González, G.; Malhi, Y.; Monteagudo, A.; Peacock, J.; Quesada, C.A. Drought sensitivity of the Amazon rainforest. Science 2009, 323, 1344–1347. [Google Scholar] [CrossRef] [PubMed]
- Harms, K.E.; Condit, R.; Hubbell, S.P.; Foster, R.B. Habitat associations of trees and shrubs in a 50-ha neotropical forest plot. J. Ecol. 2001, 89, 947–959. [Google Scholar] [CrossRef]
- Condit, R.; Hubbell, S.P.; Foster, R.B. Changes in tree species abundance in a Neotropical forest: Impact of climate change. J. Trop. Ecol. 1996, 12, 231–256. [Google Scholar] [CrossRef]
- Fensham, R.J.; Radford-Smith, J. Unprecedented extinction of tree species by fungal disease. Biol. Conserv. 2021, 261, 109276–109284. [Google Scholar] [CrossRef]
- Carnegie, A.J.; Lidbetter, J.R.; Walker, J.; Horwood, M.A.; Tesoriero, L.; Glen, M.; Priest, M.J. Uredo rangelii, a taxon in the guava rust complex, newly recorded on Myrtaceae in Australia. Australas. Plant Pathol. 2010, 39, 463–466. [Google Scholar] [CrossRef]
- Galbraith, M.; Large, M. Implications for selected indigenous fauna of Tiritiri Matangi of the establishment of Austropuccinia psidii (G. Winter) Beenken (myrtle rust) in northern New Zealand. Perspect. Biosecurity 2017, 2, 6–26. [Google Scholar]
- Coutinho, T.; Wingfield, M.; Alfenas, A.; Crous, P. Eucalyptus rust: A disease with the potential for serious international implications. Plant Dis. 1998, 82, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Glen, M.; Alfenas, A.C.; Zauza, E.A.V.; Wingfield, M.J.; Mohammed, C. Puccinia psidii: A threat to the Australian environment and economy—A review. Australas. Plant Pathol. 2007, 36, 1–16. [Google Scholar] [CrossRef]
- Carnegie, A.J.; Kathuria, A.; Pegg, G.S.; Entwistle, P.; Nagel, M.; Giblin, F.R. Impact of the invasive rust Puccinia psidii (myrtle rust) on native Myrtaceae in natural ecosystems in Australia. Biol. Invasions 2016, 18, 127–144. [Google Scholar] [CrossRef]
- Pegg, G.S.; Giblin, F.R.; McTaggart, A.R.; Guymer, G.P.; Taylor, H.; Ireland, K.B.; Shivas, R.G.; Perry, S. Puccinia psidii in Queensland, Australia: Disease symptoms, distribution and impact. Plant Pathol. 2014, 63, 1005–1021. [Google Scholar] [CrossRef]
- Yepes, M.S.; de Carvalho Junior, A.A. Uredinales (rust fungi) biota of the Parque Nacional do Itatiaia, Brazil: An analysis of composition, species diversity and altitudinal distribution. Caldasia 2013, 35, 165–176. [Google Scholar]
- Ruiz, R.A.R.; Alfenas, A.C.; Maffia, L.A.; Barbosa, M.B. Progress of the eucalypt rust, caused by Puccinia psidii in the field. Fitopatol. Bras. 1989, 14, 73–81. [Google Scholar]
- CABI. Austropuccinia psidii (myrtle rust). Available online: https://www.cabi.org/isc/datasheet/45846#3DA0F26F-4272-4014-86D6-1D93377DDA32 (accessed on 3 April 2022).
- Thornhill, A.H.; Ho, S.Y.W.; Külheim, C.; Crisp, M.D. Interpreting the modern distribution of Myrtaceae using a dated molecular phylogeny. Mol. Phylogenetics Evol. 2015, 93, 29–43. [Google Scholar] [CrossRef]
- IUCN. The IUCN Red List of Threatened Species. Version 2024-1. Available online: https://www.iucnredlist.org (accessed on 25 July 2024).
- Department of Environment and Science Queensland. Rare or Threatened Plants of Southeast Queensland (SEQ) Bioregion. Available online: https://wetlandinfo.des.qld.gov.au/wetlands/facts-maps/wildlife/?AreaID=bioregion-southeast-queensland-seq&Kingdom=plants&SpeciesFilter=RareOrThreatened (accessed on 27 August 2023).
- Hardstaff, L.K.; Sommerville, K.D.; Funnekotter, B.; Bunn, E.; Offord, C.A.; Mancera, R.L. Myrtaceae in Australia: Use of cryobiotechnologies for the conservation of a significant plant family under threat. Plants 2022, 11, 1017. [Google Scholar] [CrossRef]
- Sommerville, K.D.; Cuneo, P.; Errington, G.; Makinson, R.O.; Pederson, S.; Phillips, G.; Rollason, A.; Viler, V.; Offord, C.A. Conservation in the wake of myrtle rust—A case study on two critically endangered Australian rainforest plants. Pac. Conserv. Biol. 2019, 26, 218–229. [Google Scholar] [CrossRef]
- Martyn Yenson, A.J.; Sommerville, K.D.; Guja, L.K.; Merritt, D.J.; Dalziell, E.L.; Auld, T.D.; Broadhurst, L.; Coates, D.J.; Commander, L.; Crawford, A.D. Ex situ germplasm collections of exceptional species are a vital part of the conservation of Australia’s national plant treasures. Plants People Planet 2024, 6, 44–66. [Google Scholar] [CrossRef]
- Guerra, M.P.; Cangahuala-Inocente, G.C.; Vesco, L.L.D.; Pescador, R.; Caprestano, C.A. Micropropagation systems of feijoa (Acca sellowiana (O. Berg) Burret). In Protocols for Micropropagation of Selected Economically-Important Horticultural Plants; Lambardi, M., Ozudogru, E.A., Jain, S.M., Eds.; Humana Press: Totowa, NJ, USA, 2013; pp. 45–62. [Google Scholar]
- Singh, K.K.; Singh, S.P. A review: Micropropagation of guava (Psidium spp.). Hortic. Int. J. 2018, 2, 462–467. [Google Scholar] [CrossRef]
- Smith, R.H. In vitro propagation for commercial production of ornamentals. In Plant Tissue Culture, 3rd ed.; Smith, R.H., Ed.; Academic Press: San Diego, CA, USA, 2013; pp. 127–145. [Google Scholar]
- Burley, J. Applications of biotechnology in forestry and rural development. In Applications of Biotechnology in Forestry and Horticulture; Dhawan, V., Ed.; Springer: Boston, MA, USA, 1989; pp. 9–20. [Google Scholar]
- Bhatia, S.; Sharma, K. Plant tissue culture based industries. In Modern Applications of Plant Biotechnology in Pharmaceutical Sciences; Bhatia, S., Sharma, K., Dahiya, R., Bera, T., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 405–417. [Google Scholar]
- Rout, G.R.; Mohapatra, A.; Jain, S.M. Tissue culture of ornamental pot plant: A critical review on present scenario and future prospects. Biotechnol. Adv. 2006, 24, 531–560. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, J.C.; Sheng Gerald, L.T.; Teixeira da Silva, J.A. Micropropagation in the twenty-first century. In Plant Cell Culture Protocols; Loyola-Vargas, V.M., Ochoa-Alejo, N., Eds.; Springer: New York, NY, USA, 2018; pp. 17–46. [Google Scholar]
- Rout, G.R.; Jain, S.M. Advances in tissue culture techniques for ornamental plant propagation. In Achieving Sustainable Cultivation of Ornamental Plants; Reid, M., Ed.; Burleigh Dodds Science Publishing: London, UK, 2020; pp. 149–188. [Google Scholar]
- Chandler, S.F.; Sanchez, C. Genetic modification; the development of transgenic ornamental plant varieties. Plant Biotechnol. J. 2012, 10, 891–903. [Google Scholar] [CrossRef] [PubMed]
- Rout, G.R.; Jain, S.M. Use of molecular markers in ornamental plants: A critical reappraisal. Eur. J. Hortic. Sci. 2006, 71, 53–68. [Google Scholar]
- da Silva, J.T.; Bolibok, H.; Rakoczy-Trojanowska, M. Molecular markers in micropropagation, tissue culture and in vitro plant research. Genes Genomes Genom. 2007, 1, 66–72. [Google Scholar]
- Gatehouse, J.A. Biotechnological prospects for engineering insect-resistant plants. Plant Physiol. 2008, 146, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Green, J.M.; Owen, M.D. Herbicide-resistant crops: Utilities and limitations for herbicide-resistant weed management. J. Agric. Food Chem. 2011, 59, 5819–5829. [Google Scholar] [CrossRef]
- Loyola-Vargas, V.M.; Avilez-Montalvo, R.N. Plant tissue culture: A battle horse in the genome editing using CRISPR/Cas9. In Plant Cell Culture Protocols; Loyola-Vargas, V.M., Ochoa-Alejo, N., Eds.; Springer: New York, NY, USA, 2018; pp. 131–148. [Google Scholar]
- Wochok, Z.S. The role of tissue culture in preserving threatened and endangered plant species. Biol. Conserv. 1981, 20, 83–89. [Google Scholar] [CrossRef]
- Opabode, J.T. Sustainable mass production, improvement, and conservation of African indigenous vegetables: The role of plant tissue culture, a review. Int. J. Veg. Sci. 2017, 23, 438–455. [Google Scholar] [CrossRef]
- Varshney, A.; Anis, M. Review of literature. In Trees: Propagation and Conservation; Varshney, A., Anis, M., Eds.; Springer: New Delhi, India, 2014; pp. 11–47. [Google Scholar]
- Thorpe, T.A.; Harry, I.S. Special problems and prospects in the propagation of woody species. In Plant Aging: Basic and Applied Approaches; Rodríguez, R., Tamés, R.S., Durzan, D.J., Eds.; Springer: Boston, MA, USA, 1990; pp. 67–74. [Google Scholar]
- Lynch, P.T. Tissue culture techniques in in vitro plant conservation. In Plant Conservation Biotechnology; Benson, E.E., Ed.; CRC Press: London, UK, 1999; pp. 67–88. [Google Scholar]
- Bunn, E.; Turner, S. Biotechnology and plant conservation in Australia: Tissue culture and cryogenic research for ex situ conservation and restoration of endangered plants. Australas. Plant Conserv. J. Aust. Netw. Plant Conserv. 2009, 17, 12–14. [Google Scholar] [CrossRef]
- George, E.F.; Hall, M.A.; Klerk, G.-J.D. Micropropagation: Uses and methods. In Plant Propagation by Tissue Culture; George, E.F., Hall, M.A., Klerk, G.-J.D., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 29–64. [Google Scholar]
- Ruffoni, B.; Mascarello, C.; Savona, M. In vitro propagation of ornamental Myrtus (Myrtus communis). In Protocols for In Vitro Propagation of Ornamental Plants; Humana Press: Totowa, NJ, USA, 2009; pp. 257–269. [Google Scholar]
- Ali, N.; Mulwa, R.M.S.; Norton, M.A.; Skirvin, R.M. Micropropagation of guava (Psidium guajava L.). J. Hortic. Sci. Biotechnol. 2003, 78, 739–741. [Google Scholar] [CrossRef]
- Shah, S.T.; Zamir, R.; Ahmad, J.; Ali, H.; Lutfullah, G. In vitro regeneration of plantlets from seedlings explants of guava (Psidium guajava L.) cv. Safeda. Pak. J. Bot. 2008, 40, 1195–1200. [Google Scholar]
- Lucas, E.J.; Harris, S.A.; Mazine, F.F.; Belsham, S.R.; Nic Lughadha, E.M.; Telford, A.; Gasson, P.E.; Chase, M.W. Suprageneric phylogenetics of Myrteae, the generically richest tribe in Myrtaceae (Myrtales). Taxon 2007, 56, 1105–1128. [Google Scholar] [CrossRef]
- Fensham, R.; Radford-Smith, J. Imminent Extinction of Australian Myrtaceae Trees and Shrubs by Myrtle Rust; Threatened Species Recovery Hub: Brisbane, Australia, 2021. [Google Scholar]
- da Silva, A.L.G.; Pinheiro, M.C.B. Reproductive success of four species of Eugenia L. (Myrtaceae). Acta Bot. Bras. 2009, 23, 526–534. [Google Scholar] [CrossRef]
- Fernandez Winzer, L.; Berthon, K.A.; Carnegie, A.J.; Pegg, G.S.; Leishman, M.R. Austropuccinia psidii on the move: Survey based insights to its geographical distribution, host species, impacts and management in Australia. Biol. Invasions 2019, 21, 1215–1225. [Google Scholar] [CrossRef]
- Amador, T.S.; Barbedo, C.J. Germination inhibits the growth of new roots and seedlings in Eugenia uniflora and Eugenia brasiliensis. J. Seed Sci. 2015, 37, 241–247. [Google Scholar] [CrossRef]
- Amador, T.S.; Barbedo, C.J. Potential for regeneration inhibition of roots and seedlings in germinating Eugenia pyriformis seeds. Pesqui. Agropecuária Bras. 2011, 46, 814–821. [Google Scholar] [CrossRef]
- Hue, T.S.; Abdullah, T.L.; Sinniah, U.R.; Abdullah, N.A.P. Seed traits and germination behaviour of kemunting (Rhodomyrtus tomentosa) populations as affected by different temperatures. Seed Sci. Technol. 2013, 41, 199–213. [Google Scholar] [CrossRef]
- Stuepp, C.A.; Wendling, I.; Xavier, A.; Zuffellato-Ribas, K.C. Vegetative propagation and application of clonal forestry in Brazilian native tree species. Pesqui. Agropecuária Bras. 2018, 53, 985–1002. [Google Scholar] [CrossRef]
- García-Gonzáles, R.; Quiroz, K.; Carrasco, B.; Caligari, P. Plant tissue culture: Current status, opportunities and challenges. Int. J. Agric. Nat. Resour. 2010, 37, 5–30. [Google Scholar] [CrossRef]
- Cassells, A.C. Pathogen and biological contamination management in plant tissue culture: Phytopathogens, vitro pathogens, and vitro pests. In Plant Cell Culture Protocols; Loyola-Vargas, V., Ochoa-Alejo, N., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2006; Volume 877, pp. 35–50. [Google Scholar]
- Read, P.E.; Yang, G. Influencing propagation by stock plant PGR treatments. In Proceedings of the III International Symposium on Growth Regulators in Ornamental Horticulture, Skierniewice, Poland, 5–10 September 1988; pp. 121–128. [Google Scholar]
- Gonzalez, R.G.; Sánchez, D.S.; Campos, J.M.; Vazquez, E.P.; Guerra, Z.Z.; Quesada, A.L.; Valdivia, R.M.; Gonzalez, M.G. Plant regeneration from leaf and stem explants from two sweet potato(Ipomoea batatas L. Lam.) cultivars. Biotecnol. Apl. 1999, 16, 11–14. [Google Scholar]
- de Assis, F.A.; Rodrigues, F.A.; Pasqual, M.; de Assis, G.A.; Luz, J.M.Q.; Janoni, F.; Costa, I.; Costa, B.N.S.; Soares, J.D.R. Antioxidants in the control of microorganism contamination and phenol oxidation in Eugenia pyriformis. Biosci. J. 2018, 34, 49–58. [Google Scholar] [CrossRef]
- Golle, D.P.; Reiniger, L.R.S.; Stefanel, C.M.; Muniz, M.F.B.; da Silva, K.B. Combination of NAA and TDZ for in vitro multiplication of Eugenia involucrata DC. Rev. Árvore 2018, 41, e410509. [Google Scholar] [CrossRef]
- Montalvo, G.; Quiala, E.; Matos, J.; Morffi, H.; Feria, M.d.; Chávez, M.; Balbón, R.; Pérez, M. In vitro establishment and acclimatization of two threatened species of the genus Eugenia (Myrtaceae). In Proceedings of the II International Symposium on Guava and other Myrtaceae, Mérida, Mexico, 10–13 November 2008; Aguascalientes, Mexico, 17–18 November 2008. pp. 235–240. [Google Scholar]
- Idowu, P.E.; Ibitoye, D.O.; Ademoyegun, O.T. Tissue culture as a plant production technique for horticultural crops. Afr. J. Biotechnol. 2009, 8, 3782–3788. [Google Scholar]
- Thorpe, T.A. History of plant tissue culture. Mol. Biotechnol. 2007, 37, 169–180. [Google Scholar] [CrossRef]
- Houllou, L.M.; Bussmeyer, E.C.; Barbosa, M.R.; de Souza, R.A.; de Souza, L.M.; Malafaia, C.B. In vitro germination of black pitanga, Eugenia sulcata Spring ex Mart. for the production of seedlings aimed at the recomposition of Atlantic forest areas. Rev. Ciênc. Agríc. 2021, 19, 193–201. [Google Scholar] [CrossRef]
- Wendling, I.; Trueman, S.J.; Xavier, A. Maturation and related aspects in clonal forestry—Part II: Reinvigoration, rejuvenation and juvenility maintenance. New For. 2014, 45, 473–486. [Google Scholar] [CrossRef]
- Wendling, I.; Trueman, S.J.; Xavier, A. Maturation and related aspects in clonal forestry—Part I: Concepts, regulation and consequences of phase change. New For. 2014, 45, 449–471. [Google Scholar] [CrossRef]
- Aleksic, V.; Knezevic, P. Antimicrobial and antioxidative activity of extracts and essential oils of Myrtus communis L. Microbiol. Res. 2014, 169, 240–254. [Google Scholar] [CrossRef]
- Delgado, L.F.; Mello, J.I.O.; Barbedo, C.J. Potential for regeneration and propagation from cut seeds of Eugenia (Myrtaceae) tropical tree species. Seed Sci. Technol. 2010, 38, 624–634. [Google Scholar] [CrossRef]
- Delgado, L.F.; Barbedo, C.J. Root and seedling regeneration of Eugenia spp. (Myrtaceae) of different maturity stages. Hoehnea 2020, 47, e042020. [Google Scholar] [CrossRef]
- da Silva, J.A.A.; Teixeira, G.H.D.A.; Citadin, I.; Wagner Júnior, A.; Danner, M.A.; Martins, A.B.G. Advances in the propagation of Brazilian cherry tree. Rev. Bras. Frutic. 2019, 41, e971. [Google Scholar] [CrossRef]
- Mitchell, R.G.; Zwolinski, J.; Jones, N.B. A review on the effects of donor maturation on rooting and field performance of conifer cuttings. S. Afr. For. J. 2004, 2004, 53–63. [Google Scholar] [CrossRef]
- von Aderkas, P.; Bonga, J.M. Influencing micropropagation and somatic embryogenesis in mature trees by manipulation of phase change, stress and culture environment. Tree Physiol. 2000, 20, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Sivanesan, I.; Muthu, M.; Gopal, J.; Tasneem, S.; Kim, D.-H.; Oh, J.-W. A fumigation-based surface sterilization approach for plant tissue culture. Int. J. Environ. Res. Public Health 2021, 18, 2282. [Google Scholar] [CrossRef]
- Sahu, P.K.; Tilgam, J.; Mishra, S.; Hamid, S.; Gupta, A.; K, J.; Verma, S.K.; Kharwar, R.N. Surface sterilization for isolation of endophytes: Ensuring what (not) to grow. J. Basic Microbiol. 2022, 62, 647–668. [Google Scholar] [CrossRef]
- Lund, B.-O.; Miller, D.M.; Woods, J.S. Studies on Hg (II)-induced H2O2 formation and oxidative stress in vivo and in vitro in rat kidney mitochondria. Biochem. Pharmacol. 1993, 45, 2017–2024. [Google Scholar] [CrossRef]
- Risher, J.F.; Amler, S.N. Mercury exposure: Evaluation and intervention: The inappropriate use of chelating agents in the diagnosis and treatment of putative mercury poisoning. NeuroToxicology 2005, 26, 691–699. [Google Scholar] [CrossRef] [PubMed]
- De, B.; Mukherjee, A.K. Mercuric chloride induced membrane damage in tomato cultured cells. Biol. Plant. 1996, 38, 469–473. [Google Scholar] [CrossRef]
- Gallon, F.I.; da Silva, P.R.D.; da Silva, D.C.; de Castro, F.B.; Stefenon, V.M. Explants sterilization through metal nanoparticles for in vitro mass propagation of Eugenia involucrata. Plant Cell Cult. Micropropag. 2018, 14, 45–55. [Google Scholar]
- Marino, G.; Altan, A.D.; Biavati, B. The effect of bacterial contamination on the growth and gas evolution of in vitro cultured apricot shoots. Vitr. Cell. Dev. Biol.-Plant 1996, 32, 51–56. [Google Scholar] [CrossRef]
- Liu, T.-H.A.; Hsu, N.-W.; Wu, R.-Y. Control of leaf-tip necrosis of micropropagated ornamental statice by elimination of endophytic bacteria. Vitr. Cell. Dev. Biol.-Plant 2005, 41, 546–549. [Google Scholar] [CrossRef]
- Quambusch, M.; Winkelmann, T. Bacterial endophytes in plant tissue culture: Mode of action, detection, and control. In Plant Cell Culture Protocols; Loyola-Vargas, V.M., Ochoa-Alejo, N., Eds.; Springer: New York, NY, USA, 2018; pp. 69–88. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Lloyd, G.; McCown, B. Commercially-feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot-tip culture. Comb. Proc. Int. Plant Propagators Soc. 1980, 30, 421–426. [Google Scholar]
- Sen, J.; Sharma, A. Micropropagation of Withania somnifera from germinating seeds and shoot tips. Plant Cell Tissue Organ Cult. 1991, 26, 71–73. [Google Scholar] [CrossRef]
- Bairu, M.W.; Stirk, W.A.; Dolezal, K.; Van Staden, J. Optimizing the micropropagation protocol for the endangered Aloe polyphylla: Can meta-topolin and its derivatives serve as replacement for benzyladenine and zeatin? Plant Cell Tissue Organ Cult. 2007, 90, 15–23. [Google Scholar] [CrossRef]
- Mishra, Y.; Rawat, R.; Nema, B.; Shirin, F. Effect of seed orientation and medium strength on in vitro germination of Pterocarpus marsupium Roxb. Not. Sci. Biol. 2013, 5, 476–479. [Google Scholar] [CrossRef]
- Ikeuchi, M.; Favero, D.S.; Sakamoto, Y.; Iwase, A.; Coleman, D.; Rymen, B.; Sugimoto, K. Molecular mechanisms of plant regeneration. Annu. Rev. Plant Biol. 2019, 70, 377–406. [Google Scholar] [CrossRef]
- Nikolić, R.; Mitić, N.; Miletić, R.; Nešković, M. Effects of cytokinins on in vitro seed germination and early seedling morphogenesis in Lotus corniculatus L. J. Plant Growth Regul. 2006, 25, 187–194. [Google Scholar] [CrossRef]
- Chiwocha, S.D.; Cutler, A.J.; Abrams, S.R.; Ambrose, S.J.; Yang, J.; Ross, A.R.; Kermode, A.R. The etr1-2 mutation in Arabidopsis thaliana affects the abscisic acid, auxin, cytokinin and gibberellin metabolic pathways during maintenance of seed dormancy, moist-chilling and germination. Plant J. 2005, 42, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Miransari, M.; Smith, D.L. Plant hormones and seed germination. Environ. Exp. Bot. 2014, 99, 110–121. [Google Scholar] [CrossRef]
- Khan, M.A.; Gul, B.; Weber, D.J. Action of plant growth regulators and salinity on seed germination of Ceratoides lanata. Can. J. Bot. 2004, 82, 37–42. [Google Scholar] [CrossRef]
- Blando, F.; Onlu, S.; Colella, G.; Konczak, I. Plant regeneration from immature seeds of Eugenia myrtifolia Sims. Vitr. Cell. Dev. Biol.-Plant 2013, 49, 388–395. [Google Scholar] [CrossRef]
- Griffis, J.L. In vitro rooting of Surinam cherry (Eugenia uniflora L.). HortScience 2006, 41, 1025. [Google Scholar] [CrossRef]
- Toussaint, A.N.; Lebrun, A.; Roggemans, J. Cutting and in vitro propagation of Eugenia smithii Poir. In Proceedings of the II International Symposium on Propagation of Ornamental Plants, Herzliya, Israel, 23–28 June 1991; pp. 77–84. [Google Scholar]
- Uematsu, C.; Tsujimoto, M.; Sugimoto, M. In vitro propagation of Eugenia uniflora L. Plant Biotechnol. 1999, 16, 159–162. [Google Scholar] [CrossRef]
- Benson, E.E. Special symposium: In vitro plant recalcitrance do free radicals have a role in plant tissue culture recalcitrance? Vitr. Cell. Dev. Biol.-Plant 2000, 36, 163–170. [Google Scholar] [CrossRef]
- Sui, L.; Kong, L.; Liu, X.; Zhang, Y. Anti-browning in tissue culture of ‘Donghong’ kiwifruit. In Proceedings of the 2nd International Conference on Mechanical, Electrical and Material Application (MEMA), Xi’an, China, 25–27 October 2020; p. 012195. [Google Scholar]
- Longo, L.; Scardino, A.; Vasapollo, G.; Blando, F. Anthocyanins from Eugenia myrtifolia Sims. Innov. Food Sci. Emerg. Technol. 2007, 8, 329–332. [Google Scholar] [CrossRef]
- Amente, G.; Chimdessa, E. Control of browning in plant tissue culture: A review. J. Sci. Agric. 2021, 5, 67–71. [Google Scholar] [CrossRef]
- Ndakidemi, C.F.; Mneney, E.; Ndakidemi, P.A. Effects of ascorbic acid in controlling lethal browning in in vitro culture of Brahylaena huillensis using nodal segments. Am. J. Plant Sci. 2014, 5, 42300. [Google Scholar] [CrossRef]
- Singh, P.; Patel, R. Factors affecting in vitro degree of browning and culture establishment of pomegranate. Afr. J. Plant Sci. 2016, 10, 43–49. [Google Scholar] [CrossRef]
- Abdelwahd, R.; Hakam, N.; Labhilili, M.; Udupa, S.M. Use of an adsorbent and antioxidants to reduce the effects of leached phenolics in in vitro plantlet regeneration of faba bean. Afr. J. Biotechnol. 2008, 7, 997–1002. [Google Scholar]
- Cai, X.; Wei, H.; Liu, C.; Ren, X.; Thi, L.T.; Jeong, B.R. Synergistic effect of NaCl pretreatment and PVP on browning suppression and callus induction from petal explants of Paeonia lactiflora Pall.‘Festival Maxima’. Plants 2020, 9, 346. [Google Scholar] [CrossRef] [PubMed]
- Krishna, H.; Sairam, R.K.; Singh, S.K.; Patel, V.B.; Sharma, R.R.; Grover, M.; Nain, L.; Sachdev, A. Mango explant browning: Effect of ontogenic age, mycorrhization and pre-treatments. Sci. Hortic. 2008, 118, 132–138. [Google Scholar] [CrossRef]
- Wang, P.J.; Charles, A. Micropropagation through meristem culture. In High-Tech and Micropropagation I; Bajaj, Y.P.S., Ed.; Biotechnology in Agriculture and Forestry; Springer: Berlin/Heidelberg, Germany, 1991; pp. 32–52. [Google Scholar]
- Kataoka, I.; Inoue, H. Micropropagation of java apple (Eugenia javanica Lam.). Jpn. J. Trop. Agric. 1993, 37, 209–213. [Google Scholar] [CrossRef]
- da Silva, P.R.D.; Rispoli, R.G.; Minozzo, M.M.; Jobim, L.H.; Junges, M.; Stefenon, V.M. A regenerative route for Eugenia uniflora L. (Myrtaceae) through in vitro germination and micropropagation. Ann. For. Res. 2014, 57, 39–45. [Google Scholar] [CrossRef]
- da Costa Silveira, A.A.; Gonçalves, L.A.; e Silva, E.C.; da Silva Sales, N.; da Silva, L.C.; Sibov, S.T. Shoot proliferation, leaf anatomy and pigment content of Eugenia dysenterica growing in conventional and natural ventilation systems. Rev. Ceres 2019, 66, 363–371. [Google Scholar] [CrossRef]
- Dore, J. Physiology of regeneration in cormophytes. In Differenzierung und Entwicklung/Differentiation and Development; Lang, A., Ed.; Handbuch der Pflanzenphysiologie Encyclopedia of Plant Physiology; Springer: Berlin/Heidelberg, Germany, 1965; pp. 1648–1738. [Google Scholar]
- Blakesley, D.; Weston, G.D.; Hall, J.F. The role of endogenous auxin in root initiation: Part I: Evidence from studies on auxin application, and analysis of endogenous levels. Plant Growth Regul. 1991, 10, 341–353. [Google Scholar] [CrossRef]
- Metivier, P.S.; Yeung, E.C.; Patel, K.R.; Thorpe, T.A. In vitro rooting of microshoots of Cotinus coggygria Mill, a woody ornamental plant. Vitr. Cell. Dev. Biol.-Plant 2007, 43, 119–123. [Google Scholar] [CrossRef]
- Cardoso-Furlan, F.; Gavilan, N.H.; Zichner-Zorz, A.; Oliveira, L.S.d.; Konzen, E.R.; Ebling-Brondani, G. Active chlorine and charcoal affect the in vitro culture of Bambusa vulgaris. Bosque 2018, 39, 61–70. [Google Scholar] [CrossRef]
- Galán-Ávila, A.; García-Fortea, E.; Prohens, J.; Herraiz, F.J. Development of a direct in vitro plant regeneration protocol from Cannabis sativa L. seedling explants: Developmental morphology of shoot regeneration and ploidy level of regenerated plants. Front. Plant Sci. 2020, 11, 645. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, T.; Kevers, C.; Penel, C.; Greppin, H.; Reid, D.M.; Thorpe, T.A. Plant hormones and plant growth regulators in plant tissue culture. Vitr. Cell. Dev. Biol.-Plant 1996, 32, 272–289. [Google Scholar] [CrossRef]
- Overvoorde, P.; Fukaki, H.; Beeckman, T. Auxin control of root development. Cold Spring Harb. Perspect. Biol. 2010, 2, a001537. [Google Scholar] [CrossRef] [PubMed]
- Pavendan, P.; Rajasekaran, C.S. Effect of different concentrations of plant growth regulators for micropropagation of Eugenia singampattiana Beddome endangered tree species. Res. J. Bot. 2011, 6, 122–127. [Google Scholar] [CrossRef]
- Thomas, P.; Agrawal, M.; Bharathkumar, C. Use of Plant Preservative Mixture™ for establishing in vitro cultures from field plants: Experience with papaya reveals several PPM™ tolerant endophytic bacteria. Plant Cell Rep. 2017, 36, 1717–1730. [Google Scholar] [CrossRef]
- Romadanova, N.V.; Aralbayeva, M.M.; Zemtsova, A.S.; Alexandrova, A.M.; Kazybayeva, S.Z.; Mikhailenko, N.V.; Kushnarenko, S.V.; Bettoni, J.C. In vitro collection for the safe storage of grapevine hybrids and identification of the presence of Plasmopara viticola resistance genes. Plants 2024, 13, 1089. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, E.G.; Degenhardt, J.; Quoirin, M.; da Silva, K. Endophytic bacteria associated with tissue culture and leaves of Plinia peruviana. Pesqui. Agropecuária Bras. 2020, 55, e01844. [Google Scholar] [CrossRef]
- Vivek, M.; Modgil, M. Elimination of viruses through thermotherapy and meristem culture in apple cultivar ‘Oregon Spur-II’. Virusdisease 2018, 29, 75–82. [Google Scholar] [CrossRef]
- Reed, B.M.; Mentzer, J.; Tanprasert, P.; Yu, X. Internal bacterial contamination of micropropagated hazelnut: Identification and antibiotic treatment. In Pathogen and Microbial Contamination Management in Micropropagation; Cassells, A.C., Ed.; Springer: Dordrecht, The Netherlands, 1997; pp. 169–174. [Google Scholar]
- Franco, L.B.; Hermann, B.R.; Fritsche, Y.; Stefenon, V.M. A four steps protocol for in vitro propagation of Eugenia uniflora L. (Myrtaceae). Genet. Resour. Crop Evol. 2024, 71, 1813–1823. [Google Scholar] [CrossRef]
- Oliveira Junior, M.A.d.; Brogio Colli, B.d.A.; Libório Stipp, L.C.; Latado, R.R.; Stefano Piedade, S.M.D.; Mourão Filho, F.d.A.A. In vitro culture of Rio Grande cherry (Eugenia involucrata DC.). Plant Cell Tissue Organ Cult. 2024, 157, 21. [Google Scholar] [CrossRef]
- Stefanel, C.M.; Reiniger, L.R.S.; Serrote, C.M.L. 6-Benzylaminopurine and 3-Indolebutyric acid on the in vitro multiplication of Eugenia involucrata. Rev. Ceres 2021, 68, 491–497. [Google Scholar] [CrossRef]
- Morel, G.; Wetmore, R.H. Tissue culture of monocotyledons. Am. J. Bot. 1951, 38, 138–140. [Google Scholar] [CrossRef]
- Stefenon, V.M.; Marques Pinheiro, M.V.; de Freitas, F.R.; da Silva, V.J.B.; de Brum Vieira, P.; dos Santos, D.D.; Guerra, M.P. In vitro callogenesis for the induction of somatic embryogenesis and antioxidant production in Eugenia uniflora. Biotecnol. Veg. 2020, 20, 135–146. [Google Scholar]
- Simão, M.J.; Cola, M.P.A.; da Silva, N.C.B. In vitro germination and controlled release fertilizer effects on ex vitro development of Eugenia uniflora L. plantlets. JSFA Rep. 2022, 2, 398–404. [Google Scholar] [CrossRef]
- Engelmann, F.; Engels, J.M.M. Technologies and strategies for ex situ conservation. In Proceedings of the International Conference on Science and Technology for Managing Plant Genetic Diversity in the 21st Century, Kuala Lumpur, Malaysia, 12–16 June 2000; pp. 89–104. [Google Scholar] [CrossRef]
- Pilatti, F.K.; Aguiar, T.; Simões, T.; Benson, E.E.; Viana, A.M. In vitro and cryogenic preservation of plant biodiversity in Brazil. Vitr. Cell. Dev. Biol.-Plant 2011, 47, 82–98. [Google Scholar] [CrossRef]
- Makinson, R.O.; Pegg, G.S.; Carnegie, A.J. Myrtle Rust in Australia—A National Action Plan; Australian Plant Biosecurity Science Foundation: Canberra, Australia, 2020. [Google Scholar]
- O’Donnell, K.; Sharrock, S. Botanic gardens complement agricultural gene bank in collecting and conserving plant genetic diversity. Biopreserv. Biobank. 2018, 16, 384–390. [Google Scholar] [CrossRef]
- Chapman, T.; Miles, S.; Trivedi, C. Capturing, protecting and restoring plant diversity in the UK: RBG Kew and the Millennium Seed Bank. Plant Divers. 2019, 41, 124–131. [Google Scholar] [CrossRef]
- Biffin, E.; Lucas, E.J.; Craven, L.A.; Ribeiro da Costa, I.; Harrington, M.G.; Crisp, M.D. Evolution of exceptional species richness among lineages of fleshy-fruited Myrtaceae. Ann. Bot. 2010, 106, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Mbobo, T.; Richardson, D.M.; Lucas, E.J.; Wilson, J.R.U. Patterns of introduction, naturalisation, invasion, and impact differ between fleshy- and dry-fruited species of Myrtaceae. Perspect. Plant Ecol. Evol. Syst. 2022, 54, 125648. [Google Scholar] [CrossRef]
- Sommerville, K.D.; Errington, G.; Newby, Z.J.; Liyanage, G.S.; Offord, C.A. Assessing the storage potential of Australian rainforest seeds: A decision-making key to aid rapid conservation. Biodivers. Conserv. 2021, 30, 3185–3218. [Google Scholar] [CrossRef]
- Society for Ecological Restoration; International Network for Seed Based Restoration; Royal Botanic Gardens Kew. Seed Information Database (SID). Available online: https://ser-sid.org/ (accessed on 28 June 2024).
- Yunus, A.G. Introduction to field genebank. In Establishment and Management of Field Genebank, A Training Manual; Saad, M.S., Rao, V.R., Eds.; IPGRI-APO: Serdang, Malaysia, 2001. [Google Scholar]
- International Plant Genetic Resources Institute; Reed, B.M.; Engelmann, F.; Dulloo, M.E.; Engels, J.M.M. Technical Guidelines for the Management of Field and In Vitro Germplasm Collections; Bioversity International: Rome, Italy, 2004; p. 106. [Google Scholar]
- Hawkes, J.G. Genetic conservation of ‘recalcitrant species’—An overview. In Proceedings of the Crop Genetic Resources. The Conservation of Difficult Material, Berkshire, UK, 8–11 September 1980; pp. 83–92. [Google Scholar]
- U.S. Department of Agriculture. USDA-ARS Germplasm Resources Information Network (GRIN). Available online: https://www.ars-grin.gov/ (accessed on 28 June 2024).
- Bini, L.; Gori, M.; Novello, M.A.; Biricolti, S.; Giordani, E.; Lara, M.V.; Niella, F.; Nunziata, A.; Rocha, P.; Filippi, J.M.; et al. Assessing the genetic diversity of wild and commercial Feijoa sellowiana accessions Using AFLPs. Horticulturae 2024, 10, 366. [Google Scholar] [CrossRef]
- Engelmann, F. Use of biotechnologies for the conservation of plant biodiversity. Vitr. Cell. Dev. Biol.-Plant 2011, 47, 5–16. [Google Scholar] [CrossRef]
- Chauhan, R.; Singh, V.; Quraishi, A. In vitro conservation through slow-growth storage. In Synthetic Seeds: Germplasm Regeneration, Preservation and Prospects; Faisal, M., Alatar, A.A., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 397–416. [Google Scholar]
- Huyen, P.X.; Vathany, T.; Cha-um, S.; Kirdmanee, C. Disease-free production and minimal-growth preservation of in vitro banana (Musa spp.). In Proceedings of the XXVII International Horticultural Congress—IHC2006: II International Symposium on Plant Genetic Resources of Horticultural Crops, Seoul, Republic of Korea, 31 July 2006; pp. 233–240. [Google Scholar]
- Reed, B.M.; Bell, R.L. In vitro tissue culture of pear: Advances in techniques for micropropagation and germplasm preservation. In Proceedings of the VIII International Symposium on Pear, Ferrara and Bologna, Italy, 4–9 September 2000; pp. 412–418. [Google Scholar]
- Sarkar, D.; Chakrabarti, S.K.; Naik, P.S. Slow-growth conservation of potato microplants: Efficacy of ancymidol for long-term storage in vitro. Euphytica 2001, 117, 133–142. [Google Scholar] [CrossRef]
- da Silva, R.L.; Ferreira, C.F.; da Silva Ledo, C.A.; de Souza, E.H.; da Silva, P.H.; de Carvalho Costa, M.A.P.; Souza, F.V.D. Viability and genetic stability of pineapple germplasm after 10 years of in vitro conservation. Plant Cell Tissue Organ Cult. 2016, 127, 123–133. [Google Scholar] [CrossRef]
- Watt, M.P.; Thokoane, N.L.; Mycock, D.; Blakeway, F. In vitro storage of Eucalyptus grandis germplasm under minimal growth conditions. Plant Cell Tissue Organ Cult. 2000, 61, 161–164. [Google Scholar] [CrossRef]
- Aitken-Christie, J.; Singh, A.P. Cold storage of tissue cultures. In Cell and Tissue Culture in Forestry; Bonga, J.M., Durzan, D.J., Eds.; Forestry Sciences; Springer: Dordrecht, The Netherlands, 1987; pp. 285–304. [Google Scholar]
- Martínez-Páramo, S.; Horváth, Á.; Labbé, C.; Zhang, T.; Robles, V.; Herráez, P.; Suquet, M.; Adams, S.; Viveiros, A.; Tiersch, T.R. Cryobanking of aquatic species. Aquaculture 2017, 472, 156–177. [Google Scholar] [CrossRef]
- Asghar, W.; El Assal, R.; Shafiee, H.; Anchan, R.M.; Demirci, U. Preserving human cells for regenerative, reproductive, and transfusion medicine. Biotechnol. J. 2014, 9, 895–903. [Google Scholar] [CrossRef]
- Panis, B.; Nagel, M. Challenges and prospects for the conservation of crop genetic resources in field genebanks, in in vitro collections and/or in liquid nitrogen. Plants 2020, 9, 1634. [Google Scholar] [CrossRef] [PubMed]
- Streczynski, R.; Clark, H.; Whelehan, L.M.; Ang, S.-T.; Hardstaff, L.K.; Funnekotter, B.; Bunn, E.; Offord, C.A.; Sommerville, K.D.; Mancera, R.L. Current issues in plant cryopreservation and importance for ex situ conservation of threatened Australian native species. Aust. J. Bot. 2019, 67, 1–15. [Google Scholar] [CrossRef]
- Chang, T.; Zhao, G. Ice inhibition for cryopreservation: Materials, strategies, and challenges. Adv. Sci. 2021, 8, 2002425–2002458. [Google Scholar] [CrossRef]
- Bischof, J.C.; Diller, K.R. From nanowarming to thermoregulation: New multiscale applications of bioheat transfer. Annu. Rev. Biomed. Eng. 2018, 20, 301–327. [Google Scholar] [CrossRef]
- Verhoeven, A.; García-Plazaola, J.I.; Fernández-Marín, B. Shared mechanisms of photoprotection in photosynthetic organisms tolerant to desiccation or to low temperature. Environ. Exp. Bot. 2018, 154, 66–79. [Google Scholar] [CrossRef]
- Kaczmarczyk, A.; Funnekotter, B.; Menon, A.; Phang, P.Y.; Al-Hanbali, A.; Bunn, E.; Mancera, R. Current issues in plant cryopreservation. In Current Frontiers in Cryobiology; Katkov, I., Ed.; InTechOpen: London, UK, 2012; pp. 417–438. [Google Scholar]
- Reed, B.M. Cryopreservation—Practical considerations. In Plant Cryopreservation: A Practical Guide; Reed, B.M., Ed.; Springer: New York, NY, USA, 2008. [Google Scholar]
- Bi, W.-L.; Pan, C.; Hao, X.-Y.; Cui, Z.-H.; Kher, M.M.; Marković, Z.; Wang, Q.-C.; da Silva, J.A.T. Cryopreservation of grapevine (Vitis spp.)—A review. Vitr. Cell. Dev. Biol.-Plant 2017, 53, 449–460. [Google Scholar] [CrossRef]
- Bettoni, J.C.; Bonnart, R.; Volk, G.M. Challenges in implementing plant shoot tip cryopreservation technologies. Plant Cell Tissue Organ Cult. 2021, 144, 21–34. [Google Scholar] [CrossRef]
- Wang, M.-R.; Bi, W.; Shukla, M.R.; Ren, L.; Hamborg, Z.; Blystad, D.-R.; Saxena, P.K.; Wang, Q.-C. Epigenetic and genetic integrity, metabolic stability, and field performance of cryopreserved plants. Plants 2021, 10, 1889. [Google Scholar] [CrossRef] [PubMed]
- Pence, V.C. Tissue cryopreservation for plant conservation: Potential and challenges. Int. J. Plant Sci. 2014, 175, 40–45. [Google Scholar] [CrossRef]
- de Oliveira, K.E.S.d.; de Souza, R.A.V.d.; Carvalho, L.S.O.; Paiva, L.V. Influence of ethylene glycol on Eucalyptus grandis cryopreservation using the V cryo-plate technique. Crop Breed. Appl. Biotechnol. 2022, 22, e378422210. [Google Scholar] [CrossRef]
- Santos, C.A.F.; Corrêa, L.C.; da Costa, S.R. Genetic divergence among Psidium accessions based on biochemical and agronomic variables. Crop Breed. Appl. Biotechnol. 2011, 11, 149–156. [Google Scholar] [CrossRef]
- Zheng, B.; Zhao, Q.; Wu, H.; Wang, S.; Zou, M. A comparative metabolomics analysis of guava (Psidium guajava L.) fruit with different colors. ACS Food Sci. Technol. 2020, 1, 96–106. [Google Scholar] [CrossRef]
- Rodríguez, N.; Valdés-Infante, J.; Becker, D.; Velázquez, B.; González, G.; Sourd, D.; Rodríguez, J.; Billotte, N.; Risterucci, A.-M.; Ritter, E.; et al. Characterization of guava accessions by SSR markers, extension of the molecular linkage map, and mapping of QTLs for vegetative and reproductive characters. In Proceedings of the I International Guava Symposium, Lucknow, India, 5–8 December 2005; pp. 201–216. [Google Scholar]
- Padilla-Ramirez, J.S.; Gonzalez-Gaona, E. Collection and characterization of Mexican guava (Psidium guajava L.) germplasm. In Proceedings of the II International Symposium on Guava and other Myrtaceae, Mérida, Mexico, 10–13 November 2008; Aguascalientes, Mexico, 17–18 November 2008. pp. 49–54. [Google Scholar]
- Valecillos, C.; Aranguren, Y.; Fermin, G. Natural resources conservation: Guava and other Myrtaceae germplasm ex situ conservation in Mérida, Mexico, Venezuela. In Proceedings of the II International Symposium on Guava and other Myrtaceae, Mérida, Mexico, 10–13 November 2008; Aguascalientes, Mexico, 17–18 November 2008. p. 95. [Google Scholar]
| Genera | No. Native Species | Threatened Species in AU | Conservation Action in AU | Ex Situ Sites |
|---|---|---|---|---|
| Archirhodomyrtus | 1 | LC | n/a | Yes |
| Austromyrtus | 3 | LC | n/a | Yes |
| Decaspermum | 2 | - D. struckoilicum: CR | Conservation advice in effect from 20 March 2023 (conservation advice Decaspermum struckoilicum Struck Oil myrtle) | Yes - D. struckoilicum: 1 |
| Eugenia | 1 | LC | Yes | |
| Gossia | 20 | - G. fragrantissima: EN - G. gonoclada: EN - G. inophloia: CR (QLD) - G. acmenoides: EN population (NSW) | G. fragrantissima: conservation advice in effect from 16 July 2000, Border Ranges Rainforest Biodiversity Management Plan—NSW and Queensland 2010; G. gonoclada: conservation advice Gossia gonoclada angle-stemmed myrtle 2016, Gossia gonoclada recovery plan 2019–2029 | Yes - G. fragrantissima: 6 (four seed accessions in Australian PlantBank) - G. gonoclada: 1 - G. inophloia: 10 - G. acmenoides: 8 |
| Lenwebbia | 2 | - Lenwebbia sp. Main Range: CR - Lenwebbia sp. Blackall Range: EN (QLD) | Lenwebbia sp. Main Range: conservation advice in effect from 22 April 2022 | Yes - Lenwebbia sp. Main Range: 1 |
| Lithomyrtus | 11 (All native to AU) | - L. linariifolia: V (NT) | Yes L. linariifolia:1 | |
| Pilidiostigma | 6 | LC | Yes | |
| Rhodamnia | 20 | - R. angustifolia: CR - R. longisepala: CR - R. maideniana: CR - R. rubescens: CR - R. arenaria: EN (QLD) | Conservation advice in effect (R. angustifolia; R. longisepala; R. maideniana; R. rubescens) | Yes - R. angustifolia: 0 - R. longisepala: 1 - R. maideniana: 8 (one seed accession in PlantBank) - R. rubescens: 10 (three seed accessions in PlantBank and Brisbane Botanic Gardens Conservation Seedbank) |
| Rhodomyrtus | 6 | - R. psidioides: CR | Conservation advice in effect from 12 December 2020 | Yes - R. psidioides: 16 (six seed accessions in PlantBank, Australian National Botanic Gardens Seed Bank, and Brisbane Botanic Gardens Conservation Seedbank) |
| Uromyrtus | 4 | - U. australis: EN | Conservation advice in effect from 23 November 2021 and Border Ranges Rainforest Biodiversity Management Plan—NSW and Queensland 2010 | Yes - U. australis: 9 (one seed accession in PlantBank) |
| Total | 76 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, J.; O’Donohue, B.; Sommerville, K.D.; Mitter, N.; O’Brien, C.; Hayward, A. Tissue Culture Innovations for Propagation and Conservation of Myrteae—A Globally Important Myrtaceae Tribe. Plants 2024, 13, 2244. https://doi.org/10.3390/plants13162244
Bao J, O’Donohue B, Sommerville KD, Mitter N, O’Brien C, Hayward A. Tissue Culture Innovations for Propagation and Conservation of Myrteae—A Globally Important Myrtaceae Tribe. Plants. 2024; 13(16):2244. https://doi.org/10.3390/plants13162244
Chicago/Turabian StyleBao, Jingyin, Billy O’Donohue, Karen D. Sommerville, Neena Mitter, Chris O’Brien, and Alice Hayward. 2024. "Tissue Culture Innovations for Propagation and Conservation of Myrteae—A Globally Important Myrtaceae Tribe" Plants 13, no. 16: 2244. https://doi.org/10.3390/plants13162244





