Nanosized-Selenium-Application-Mediated Cadmium Toxicity in Aromatic Rice at Different Stages
Abstract
:1. Introduction
2. Results
2.1. Effect of NanoSe Application on Cd Content of Aromatic Rice
2.2. Effect of NanoSe Application on Se Content of Aromatic Rice
2.3. Effect of NanoSe Application on the Transfer Coefficients of Cd and Se in Aromatic Rice
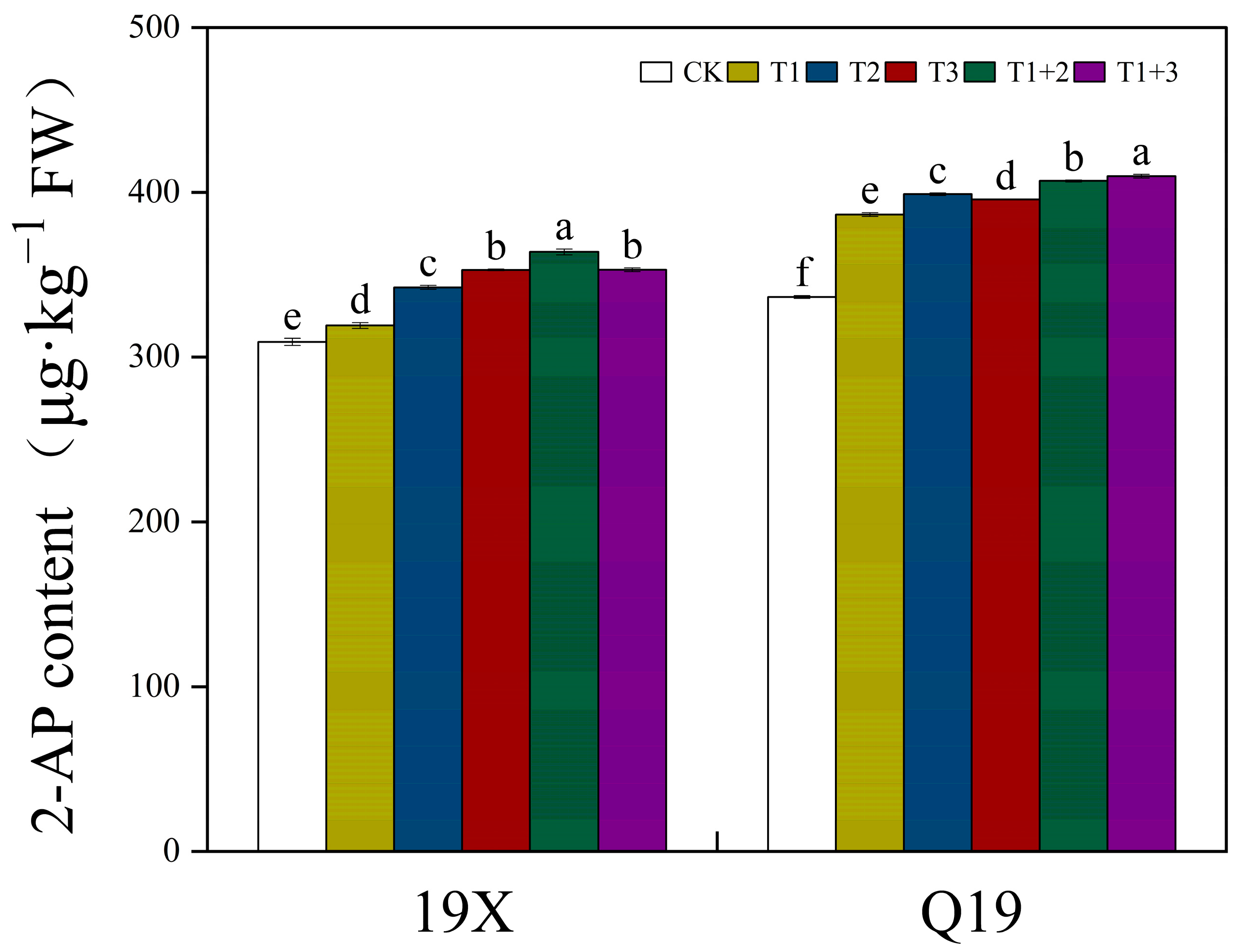
2.4. Effect of NanoSe Application on 2-AP Content of Aromatic Rice
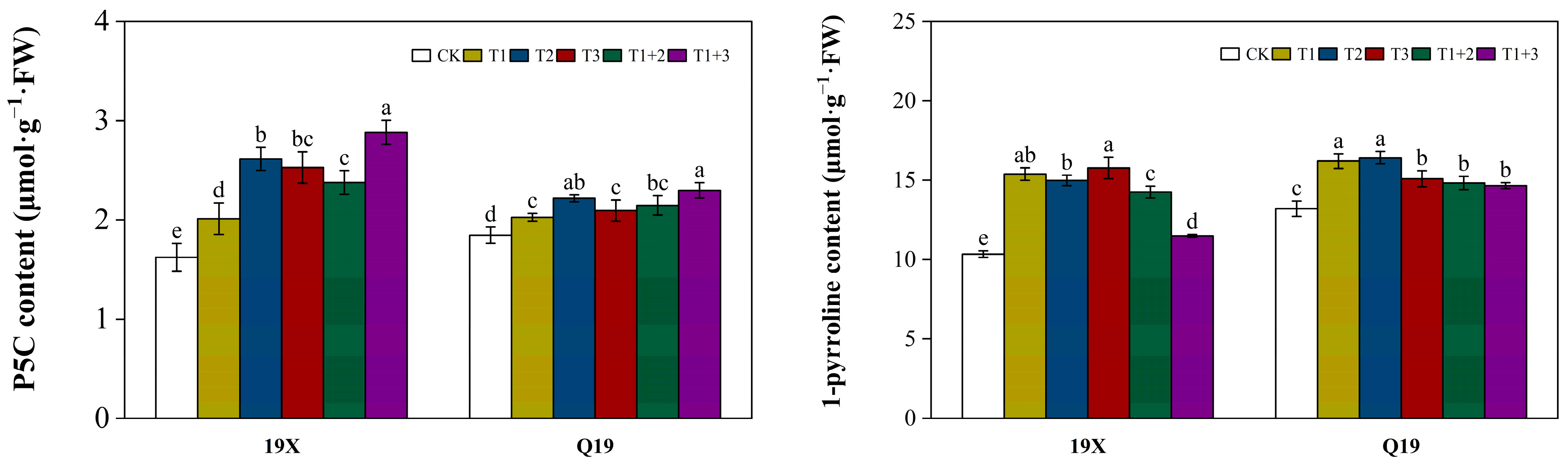
2.5. Effect of NanoSe Application on Precursors of 2-AP Biosynthesis of Aromatic Rice
2.6. Effect of NanoSe Application on Grain Yield and Yield-Related Traits of Aromatic Rice
3. Discussion
3.1. Effects of NanoSe on Cd and Se Content
3.2. Influence of NanoSe on 2-AP Synthesis
3.3. Effects of NanoSe on Yield Formation
4. Materials and Methods
4.1. Plant Materials and Experimental Design
- CK: No NanoSe was applied;
- T1: NanoSe was foliar-applied at the panicle initial stage (45 days after the transplanting);
- T2: NanoSe was foliar-applied at the heading stage (70 days after the transplanting);
- T3: NanoSe was foliar-applied at the grain-filling stage (78 days after the transplanting);
- T1+2: NanoSe was foliar-applied at both panicle initial stage and heading stage;
- T1+3: NanoSe was foliar-applied at both panicle initial stage and grain-filling stage;
- The NanoSe was provided by Green Huinong Biotechnology (Shenzhen) Co., Ltd., Shenzhen, China. The applied Se concentration was 6.67 mg L−1 in each treatment.
4.2. Sample Collection and Analysis
4.2.1. Determination of Se and Cd Content
4.2.2. Calculation of the Transfer Factor of Se and Cd
4.2.3. Determination of 2-Acetyl-1-Pyrroline (2-AP) and Its Biosynthetic Precursor
4.2.4. Determination of Grain Yield
4.3. Data Statistics and Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- di Toppi, L.S.; Gabbrielli, R. Response to cadmium in higher plants. Environ. Exp. Bot. 1999, 41, 105–130. [Google Scholar] [CrossRef]
- Ismael, M.A.; Elyamine, A.M.; Moussa, M.G.; Cai, M.; Zhao, X.; Hu, C. Cadmium in plants: Uptake, toxicity, and its interactions with selenium fertilizers. Metallomics 2019, 11, 255–277. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.; Yang, W.; Chen, Y.; Yang, L.; Zhou, H.; Yang, Y.; Zhao, Z.; Wu, P.; Zia-Ur-Rehman, M. Exploring the mechanism of Cd uptake and translocation in rice: Future perspectives of rice safety. Sci. Total Environ. 2023, 897, 165369. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Tang, Z.; Wang, P.; Zhao, F. Geographical variations of cadmium and arsenic concentrations and arsenic speciation in Chinese rice. Environ. Pollut. 2018, 238, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Cao, C.; Ma, Y.; Su, D.; Li, J. Identification of cadmium bioaccumulation in rice (Oryza sativa L.) By the soil-plant transfer model and species sensitivity distribution. Sci. Total Environ. 2019, 692, 1022–1028. [Google Scholar] [CrossRef] [PubMed]
- Duan, G.; Shao, G.; Tang, Z.; Chen, H.; Wang, B.; Tang, Z.; Yang, Y.; Liu, Y.; Zhao, F. Genotypic and Environmental Variations in Grain Cadmium and Arsenic Concentrations Among a Panel of High Yielding Rice Cultivars. Rice 2017, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Hussain, B.; Yin, X.; Lin, Q.; Hamid, Y.; Usman, M.; Hashmi, M.L.; Lu, M.; Imran, T.M.; He, Z.; Yang, X.E. Mitigating cadmium exposure risk in rice with foliar nano-selenium: Investigations through Caco-2 human cell line in-vivo bioavailability assay. Environ. Pollut. 2024, 356, 124356. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Luo, N.; Li, Y.W.; Cai, Q.Y.; Li, H.Y.; Mo, C.H.; Wong, M.H. Cadmium in rice: Transport mechanisms, influencing factors, and minimizing measures. Environ. Pollut. 2017, 224, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Ran, M.; Wu, J.; Jiao, Y.; Li, J. Biosynthetic selenium nanoparticles (Bio-SeNPs) mitigate the toxicity of antimony (Sb) in rice (Oryza sativa L.) By limiting Sb uptake, improving antioxidant defense system and regulating stress-related gene expression. J. Hazard. Mater. 2024, 470, 134263. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, B.; Deng, K.; Gao, X.; Sun, G.; Zhang, Z.; Li, P.; Wang, W.; Li, H.; Zhang, Z.; et al. NRT1.1B improves selenium concentrations in rice grains by facilitating selenomethinone translocation. Plant Biotechnol. J. 2019, 17, 1058–1068. [Google Scholar] [CrossRef]
- Luca, F.; Donata, M.I.R.C.; Rossella, A.; Michele, M.; Matteo, O.; Alice, L.; Christoph, V.H.; Luciano, P. Advances in selenium supplementation: From selenium-enriched yeast to potential selenium-enriched insects, and selenium nanoparticles. Anim. Nutr. 2023, 14, 193–203. [Google Scholar]
- Di, X.; Jing, R.; Qin, X.; Liang, X.; Wang, L.; Xu, Y.; Sun, Y.; Huang, Q. The role and transcriptomic mechanism of cell wall in the mutual antagonized effects between selenium nanoparticles and cadmium in wheat. J. Hazard. Mater. 2024, 472, 134549. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Li, M.; Rizwan, M.; Dai, Z.; Yuan, Y.; Hossain, M.M.; Cao, M.; Xiong, S.; Tu, S. Synergistic effect of silicon and selenium on the alleviation of cadmium toxicity in rice plants. J. Hazard. Mater. 2021, 401, 123393. [Google Scholar] [CrossRef] [PubMed]
- Hussain, B.; Lin, Q.; Hamid, Y.; Sanaullah, M.; Di, L.; Hashmi, M.L.U.R.; Khan, M.B.; He, Z.; Yang, X. Foliage application of selenium and silicon nanoparticles alleviates Cd and Pb toxicity in rice (Oryza sativa L.). Sci. Total Environ. 2020, 712, 136497. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Thounaojam, T.C.; Chowdhury, D.; Upadhyaya, H. The role of selenium and nano selenium on physiological responses in plant: A review. Plant Growth Regul. 2023, 100, 409–433. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Qin, H.; Jiang, D.; Lu, J.; Zhu, Z.; Huang, X. Bio-nano selenium fertilizer improves the yield, quality, and organic selenium content in rice. J. Food Compos. Anal. 2024, 132, 106348. [Google Scholar] [CrossRef]
- Xiong, Y.; Tian, X.; Qiu, T.; Cong, X.; Zheng, X.; Chen, S.; You, A.; Cheng, S.; Wu, M.; Xu, D. Effects of SeNPs Fertilizer on Se and Microelement Contents, Eating and Cooking Qualities, and Volatile Organic Compounds in Rice Grains. Sustainability 2023, 15, 10553. [Google Scholar] [CrossRef]
- Zeeshan, M.; Hu, Y.X.; Afridi, M.S.; Ahmad, B.; Ahmad, S.; Muhammad, I.; Hale, B.; Iqbal, A.; Wu, H.Y.; Zhou, X.B. Interplay of ZnONPs and/or SeNPs induces arsenic tolerance in soybean by regulation of antioxidants pool, WRKY genes, and expression of arsenic transporters. Environ. Exp. Bot. 2022, 195, 104783. [Google Scholar] [CrossRef]
- Kanu, A.S.; Ashraf, U.; Mo, Z.; Fuseini, I.; Mansaray, L.R.; Duan, M.; Pan, S.; Tang, X. Cadmium Uptake and Distribution in Fragrant Rice Genotypes and Related Consequences on Yield and Grain Quality Traits. J. Chem. 2017, 2017, 1405878. [Google Scholar] [CrossRef]
- Kanu, A.S.; Ashraf, U.; Mo, Z.; Sabir, S.U.; Baggie, I.; Charley, C.S.; Tang, X. Calcium amendment improved the performance of fragrant rice and reduced metal uptake under cadmium toxicity. Environ. Sci. Pollut. Res. 2019, 26, 24748–24757. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, S.; Ma, L.; Kong, L.; Pan, S.; Tang, X.; Tian, H.; Duan, M.; Mo, Z. Effect of Exogenous Melatonin Application on the Grain Yield and Antioxidant Capacity in Aromatic Rice under Combined Lead—Cadmium Stress. Antioxidants 2022, 11, 776. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Hussain, S.; Iqbal, A.; Saleem, M.H.; Rehman, N.U.; Mo, Z.; Chen, X.; Tang, X. Nitric oxide confers cadmium tolerance in fragrant rice by modulating physio-biochemical processes, yield attributes, and grain quality traits. Ecotoxicol. Environ. Saf. 2023, 261, 115078. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Huang, Z.; Li, Y.; Xie, W.; Li, W.; Tang, X.; Ashraf, U.; Kong, L.; Wu, L.; Wang, S.; et al. Selenium-silicon (Se-Si) induced modulations in physio-biochemical responses, grain yield, quality, aroma formation and lodging in fragrant rice. Ecotoxicol. Environ. Saf. 2020, 196, 110525. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Ding, C.; Guo, F.; Zhang, T.; Wang, X. The optimum Se application time for reducing Cd uptake by rice (Oryza sativa L.) And its mechanism. Plant Soil 2018, 431, 231–243. [Google Scholar] [CrossRef]
- Gao, M.; Zhou, J.; Liu, H.; Zhang, W.; Hu, Y.; Liang, J.; Zhou, J. Foliar spraying with silicon and selenium reduces cadmium uptake and mitigates cadmium toxicity in rice. Sci. Total Environ. 2018, 631–632, 1100–1108. [Google Scholar] [CrossRef] [PubMed]
- Zeeshan, M.; Wang, X.; Salam, A.; Wu, H.; Li, S.; Zhu, S.; Chang, J.; Chen, X.; Zhang, Z.; Zhang, P. Selenium Nanoparticles Boost the Drought Stress Response of Soybean by Enhancing Pigment Accumulation, Oxidative Stress Management and Ultrastructural Integrity. Agronomy 2024, 14, 1372. [Google Scholar] [CrossRef]
- Zeeshan, M.; Hu, Y.X.; Iqbal, A.; Salam, A.; Liu, Y.X.; Muhammad, I.; Ahmad, S.; Khan, A.H.; Hale, B.; Wu, H.Y.; et al. Amelioration of AsV toxicity by concurrent application of ZnO-NPs and Se-NPs is associated with differential regulation of photosynthetic indexes, antioxidant pool and osmolytes content in soybean seedling. Ecotoxicol. Environ. Saf. 2021, 225, 112738. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Mao, K.; Cao, H.; Ali, W.; Lei, D.; Teng, D.; Chang, C.; Yang, X.; Yang, Q.; Niazi, N.K.; et al. Exogenous selenium (cadmium) inhibits the absorption and transportation of cadmium (selenium) in rice. Environ. Pollut. 2021, 268, 115829. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Ali, S.; Adrees, M.; Rizvi, H.; Zia-Ur-Rehman, M.; Hannan, F.; Qayyum, M.F.; Hafeez, F.; Ok, Y.S. Cadmium stress in rice: Toxic effects, tolerance mechanisms, and management: A critical review. Environ. Sci. Pollut. Res. 2016, 23, 17859–17879. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Man, Z.; Li, X.; Zhao, Y.; Chen, R.; Pan, T.; Wang, L.; Dai, X.; Xiao, H.; Liu, F. Multi-phenotype response and cadmium detection of rice stem under toxic cadmium exposure. Sci. Total Environ. 2024, 917, 170585. [Google Scholar] [CrossRef]
- Iqbal, A.; Mo, Z.; Pan, S.G.; Qi, J.Y.; Hua, T.; Imran, M.; Duan, M.; Gu, Q.; Yao, X.B.; Tang, X. Exogenous TiO(2) Nanoparticles Alleviate Cd Toxicity by Reducing Cd Uptake and Regulating Plant Physiological Activity and Antioxidant Defense Systems in Rice (Oryza sativa L.). Metabolites 2023, 13, 765. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Imtiaz, M.; Rizwan, M.; Yuan, Y.; Huang, H.; Tu, S. Dynamics of Selenium uptake, speciation, and antioxidant response in rice at different panicle initiation stages. Sci. Total Environ. 2019, 691, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Xin, J.; Dai, H.; Zhou, W. Effects of Interaction between Cadmium (Cd) and Selenium (Se) on Grain Yield and Cd and Se Accumulation in a Hybrid Rice (Oryza sativa) System. J. Agric. Food. Chem. 2017, 65, 9537–9546. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Liao, G.; Sun, F.; Ma, Y.; Chen, Z.; Chen, H.; Tang, X.; Mo, Z. Foliar spray of La2O3 nanoparticles regulates the growth, antioxidant parameters, and nitrogen metabolism of fragrant rice seedlings in wet and dry nurseries. Environ. Sci. Pollut. Res. 2023, 30, 80349–80363. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Gu, M.; Wei, Y.; He, B.; Wang, X.; Lv, M. Effects of bioorganic selenium on main characters, heavy metal content and selenium absorption of different rice varieties. J. South. Agric. 2021, 52, 2727–2734. [Google Scholar]
- Huang, P.; Yang, W.; Li, Q.; Liao, Q.; Si, M.; Shi, M.; Yang, Z. A novel slow-release selenium approach for cadmium reduction and selenium enrichment in rice (Oryza sativa L.). Chemosphere 2023, 342, 140183. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, M.; Abdalla, M.A.; Hussain, M.A.; Muhling, K.H. Silicon-Selenium Interplay Imparts Cadmium Resistance in Wheat through an Up-Regulating Antioxidant System. Int. J. Mol. Sci. 2023, 25, 387. [Google Scholar] [CrossRef]
- Feng, R.; Wei, C.; Tu, S.; Ding, Y.; Song, Z. A dual role of Se on Cd toxicity: Evidences from the uptake of Cd and some essential elements and the growth responses in paddy rice. Biol. Trace Elem. Res. 2013, 151, 113–121. [Google Scholar] [CrossRef]
- Huang, B.; Zhao, F.; Wang, P. The relative contributions of root uptake and remobilization to the loading of Cd and as into rice grains: Implications in simultaneously controlling grain Cd and as accumulation using a segmented water management strategy *. Environ. Pollut. 2022, 293, 118497. [Google Scholar] [CrossRef]
- Hirose, A.; Tanoi, K.; Nakanishi, T.M.; Kobayashi, N.I. Cadmium accumulation dynamics in the rice endosperm during grain filling revealed by autoradiography. Plant Direct 2024, 8, e562. [Google Scholar]
- Rodda, M.S.; Li, G.; Reid, R.J. The timing of grain Cd accumulation in rice plants: The relative importance of remobilisation within the plant and root Cd uptake post-flowering. Plant Soil 2011, 347, 105–114. [Google Scholar] [CrossRef]
- Wang, F.; Wang, M.; Liu, Z.; Shi, Y.; Han, T.; Ye, Y.; Gong, N.; Sun, J.; Zhu, C. Different responses of low grain-Cd-accumulating and high grain-Cd-accumulating rice cultivars to Cd stress. Plant Physiol. Biochem. 2015, 96, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Roychoudhury, A. Differential levels of metabolites and enzymes related to aroma formation in aromatic indica rice varieties: Comparison with non-aromatic varieties. 3 Biotech 2017, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Shafiq, S.; Ilahi, S.; Ghahramani, A.; Bao, G.; Dessoky, E.S.; Widemann, E.; Pan, S.; Mo, Z.; Tang, X. Post-transcriptional regulation of 2-acetyl-1-pyrroline (2-AP) biosynthesis pathway, silicon, and heavy metal transporters in response to Zn in fragrant rice. Front. Plant Sci. 2022, 13, 948884. [Google Scholar] [CrossRef] [PubMed]
- Kaikavoosi, K.; Kad, T.D.; Zanan, R.L.; Nadaf, A.B. 2-Acetyl-1-Pyrroline Augmentation in Scented indica Rice (Oryza sativa L.) Varieties through Δ1-Pyrroline-5-Carboxylate Synthetase (P5CS) Gene Transformation. Appl. Biochem. Biotechnol. 2015, 177, 1466–1479. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Hussain, S.; Rana, M.S.; Saleem, M.H.; Rasul, F.; Ali, K.H.; Potcho, M.P.; Pan, S.; Duan, M.; Tang, X. Molybdenum improves 2-acetyl-1-pyrroline, grain quality traits and yield attributes in fragrant rice through efficient nitrogen assimilation under cadmium toxicity. Ecotoxicol. Environ. Saf. 2021, 211, 111911. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Tang, X.; Mo, Z.; Wang, R.; Xiong, L. Effect of Selenium Fertilizer on Aroma, Selenium Content in Brown Rice and Grain Yield of Aromatic Rice. Acta Agric. Boreali-Sin. 2016, 31, 213–219. [Google Scholar]
- Luo, H.; He, L.; Du, B.; Pan, S.; Mo, Z.; Duan, M.; Tian, H.; Tang, X. Biofortification with chelating selenium in fragrant rice: Effects on photosynthetic rates, aroma, grain quality and yield formation. Field Crops Res. 2020, 255, 107909. [Google Scholar] [CrossRef]
- Zhang, M.; Tang, S.; Huang, X.; Zhang, F.; Pang, Y.; Huang, Q.; Yi, Q. Selenium uptake, dynamic changes in selenium content and its influence on photosynthesis and chlorophyll fluorescence in rice (Oryza sativa L.). Environ. Exp. Bot. 2014, 107, 39–45. [Google Scholar] [CrossRef]
- Badawy, S.A.; Zayed, B.A.; Bassiouni, S.; Mahdi, A.; Majrashi, A.; Ali, E.F.; Seleiman, M.F. Influence of Nano Silicon and Nano Selenium on Root Characters, Growth, Ion Selectivity, Yield, and Yield Components of Rice (Oryza sativa L.) under Salinity Conditions. Plants 2021, 10, 1657. [Google Scholar] [CrossRef]
- Zhang, L.; Shen, C.; Zhu, S.; Ren, N.; Chen, K.; Xu, J. Effects of Sowing Date and Nitrogen (N) Application Rate on Grain Yield, Nitrogen Use Efficiency and 2-Acetyl-1-Pyrroline Formation in Fragrant Rice. Agronomy 2022, 12, 3035. [Google Scholar] [CrossRef]
- Mo, Z.; Li, W.; Pan, S.; Fitzgerald, T.L.; Xiao, F.; Tang, Y.; Wang, Y.; Duan, M.; Tian, H.; Tang, X. Shading during the grain filling period increases 2-acetyl-1-pyrroline content in fragrant rice. Rice 2015, 8, 9. [Google Scholar] [CrossRef] [PubMed]


| Date | Treatment | 19X | Q19 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| TF Root-Stem (Cd) | TF Stem-Leaf (Cd) | TF Stem-Grain (Cd) | TF Leaf-Grain (Se) | TF Root-Stem (Cd) | TF Stem-Leaf (Cd) | TF Stem-Grain (Cd) | TF Leaf-Grain (Se) | ||
| 7DAL | CK | 4.90 ± 0.16 c | 49.08 ± 1.58 a | 24.14 ± 1.26 a | 27.97 ± 6.61 b | 8.93 ± 0.05 b | 47.68 ± 0.33 b | 22.38 ± 1.25 c | 32.88 ± 0.99 b |
| T1 | 5.12 ± 0.26 c | 29.91 ± 0.69 d | 23.93 ± 1.25 a | 13.07 ± 0.88 d | 7.68 ± 0.07 c | 47.32 ± 1.09 b | 21.24 ± 0.40 c | 14.68 ± 1.55 c | |
| T2 | 7.09 ± 0.49 a | 41.96 ± 0.30 b | 20.44 ± 0.84 c | 20.95 ± 1.44 c | 7.48 ± 0.39 c | 56.68 ± 1.60 a | 30.88 ± 0.40 a | 15.56 ± 0.89 c | |
| T3 | 4.71 ± 0.32 c | 48.41 ± 0.74 a | 24.20 ± 0.89 a | 39.30 ± 1.35 a | 8.97 ± 0.60 b | 46.01 ± 0.61 b | 18.70 ± 0.96 d | 35.02 ± 1.51 b | |
| T4 | 5.75 ± 0.20 b | 38.27 ± 2.26 c | 21.66 ± 0.64 bc | 15.65 ± 0.95 d | 6.61 ± 0.14 d | 46.04 ± 2.51 b | 28.16 ± 0.27 b | 15.65 ± 1.14 c | |
| T5 | 6.30 ± 0.48 b | 29.96 ± 0.11 d | 22.57 ± 1.29 ab | 39.71 ± 0.55 a | 12.92 ± 0.41 a | 40.08 ± 1.65 c | 22.41 ± 1.11 c | 37.87 ± 1.52 a | |
| 14DAL | CK | 13.86 ± 0.31 b | 27.46 ± 2.42 d | 13.66 ± 0.85 c | 28.61 ± 1.25 c | 15.61 ± 0.59 d | 26.40 ± 0.24 c | 11.45 ± 0.20 e | 21.82 ± 1.25 c |
| T1 | 14.52 ± 0.67 b | 32.83 ± 1.88 c | 12.37 ± 0.60 c | 19.88 ± 0.70 d | 17.09 ± 0.35 c | 35.87 ± 0.27 a | 12.05 ± 0.29 d | 19.19 ± 2.21 d | |
| T2 | 16.34 ± 0.41 a | 27.73 ± 1.87 d | 19.62 ± 0.33 b | 20.67 ± 0.76 d | 20.78 ± 0.80 a | 26.78 ± 0.37 c | 13.13 ± 0.14 c | 22.95 ± 0.14 c | |
| T3 | 7.27 ± 0.26 d | 55.94 ± 1.07 a | 19.49 ± 1.17 b | 38.87 ± 2.41 a | 18.91 ± 0.46 b | 33.70 ± 0.86 b | 14.49 ± 0.12 a | 32.56 ± 0.96 a | |
| T4 | 12.21 ± 0.93 c | 41.28 ± 3.41 b | 21.62 ± 1.07 a | 25.33 ± 3.36 c | 13.66 ± 0.67 e | 34.50 ± 1.04 ab | 13.84 ± 0.65 b | 13.41 ± 0.20 e | |
| T5 | 14.00 ± 0.59 b | 39.03 ± 1.62 b | 18.63 ± 1.25 b | 35.20 ± 0.82 b | 21.75 ± 1.40 a | 33.68 ± 1.60 b | 15.05 ± 0.22 a | 29.77 ± 0.41 b | |
| MA | CK | 10.42 ± 0.09 c | 35.00 ± 2.37 b | 19.30 ± 0.74 c | 16.49 ± 1.40 c | 32.59 ± 2.61 d | 31.78 ± 0.54 bc | 13.53 ± 0.07 b | 23.10 ± 2.88 ab |
| T1 | 8.45 ± 0.18 e | 40.77 ± 1.4 a | 22.95 ± 0.67 a | 17.43 ± 2.09 c | 22.37 ± 0.54 e | 25.84 ± 0.57 d | 12.56 ± 0.23 c | 12.35 ± 3.12 e | |
| T2 | 13.16 ± 0.33 a | 28.40 ± 0.49 c | 15.35 ± 0.64 d | 19.23 ± 0.68 c | 40.37 ± 2.15 c | 23.53 ± 0.84 e | 13.08 ± 0.33 bc | 17.56 ± 0.58 cd | |
| T3 | 9.29 ± 0.27 d | 41.73 ± 2.39 a | 16.59 ± 0.92 d | 37.14 ± 3.86 a | 44.92 ± 1.58 ab | 30.72 ± 1.45 c | 13.45 ± 0.40 b | 20.00 ± 0.16 bc | |
| T4 | 11.19 ± 0.72 b | 28.98 ± 1.09 c | 20.81 ± 0.95 b | 23.82 ± 1.21 b | 46.43 ± 1.79 a | 39.86 ± 1.64 a | 14.96 ± 0.64 a | 16.03 ± 0.72 d | |
| T5 | 12.75 ± 0.71 a | 33.72 ± 0.57 b | 13.54 ± 0.37 e | 36.10 ± 1.03 a | 42.68 ± 2.58 bc | 33.30 ± 0.36 b | 11.53 ± 0.30 d | 24.98 ± 0.79 a | |
| Cultivar | Treatment | Effective Panicle Number per Plant | Grains Number per Panicle | Seed-Setting Rate (%) | 1000-Grain Weight (g) | Grain Yield (g/pot) |
|---|---|---|---|---|---|---|
| 19X | CK | 7.33 ± 0.58 a | 174.67 ± 6.00 b | 61.27 ± 0.02 c | 20.04 ± 1.34 c | 78.40 ± 4.89 c |
| T1 | 7.00 ± 0.00 a | 17506 ± 10.44 b | 62.28 ± 0.01 c | 22.13 ± 1.26 ab | 84.27 ± 1.20 c | |
| T2 | 7.67 ± 0.58 a | 171.27 ± 5.97 b | 67.62 ± 0.02 b | 23.55 ± 1.19 a | 104.41 ± 8.26 b | |
| T3 | 7.33 ± 0.58 a | 215.43 ± 7.42 a | 75.47 ± 0.04 a | 21.32 ± 0.89 bc | 126.65 ± 4.85 a | |
| T1+2 | 7.67 ± 0.58 a | 187.15 ± 6.60 b | 55.36 ± 0.02 d | 21.47 ± 0.42 bc | 85.03 ± 2.46 c | |
| T1+3 | 7.67 ± 0.58 a | 217.65 ± 3.60 a | 66.58 ± 0.01 b | 23.55 ± 0.42 a | 130.35 ± 6.93 a | |
| Q19 | CK | 6.33 ± 0.58 c | 171.17 ± 5.14 b | 55.49 ± 0.06 a | 21.62 ± 0.73 c | 64.77 ± 5.84 d |
| T1 | 7.67 ± 0.58 b | 172.70 ± 5.88 b | 57.01 ± 0.08 a | 23.79 ± 0.63 a | 89.14 ± 8.42 c | |
| T2 | 8.00 ± 0.00 ab | 193.81 ± 7.83 a | 60.39 ± 0.00 a | 22.34 ± 0.15 bc | 104.58 ± 4.20 ab | |
| T3 | 6.33 ± 0.58 c | 192.27 ± 8.47 a | 59.20 ± 0.06 a | 23.27 ± 0.37 ab | 105.23 ± 10.41 ab | |
| T1+2 | 7.67 ± 0.58 b | 156.17 ± 3.34 c | 60.31 ± 0.02 a | 24.14 ± 0.90 a | 94.68 ± 7.59 bc | |
| T1+3 | 8.00 ± 0.00 ab | 177.39 ± 6.91 b | 63.93 ± 0.01 a | 22.42 ± 0.98 bc | 114.30 ± 3.50 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, B.; Luo, H.; Yao, X.; Xing, P.; Deng, S.; Zhang, Q.; Yi, W.; Gu, Q.; Peng, L.; Yu, X.; et al. Nanosized-Selenium-Application-Mediated Cadmium Toxicity in Aromatic Rice at Different Stages. Plants 2024, 13, 2253. https://doi.org/10.3390/plants13162253
Cui B, Luo H, Yao X, Xing P, Deng S, Zhang Q, Yi W, Gu Q, Peng L, Yu X, et al. Nanosized-Selenium-Application-Mediated Cadmium Toxicity in Aromatic Rice at Different Stages. Plants. 2024; 13(16):2253. https://doi.org/10.3390/plants13162253
Chicago/Turabian StyleCui, Baoling, Haowen Luo, Xiangbin Yao, Pipeng Xing, Sicheng Deng, Qianqian Zhang, Wentao Yi, Qichang Gu, Ligong Peng, Xianghai Yu, and et al. 2024. "Nanosized-Selenium-Application-Mediated Cadmium Toxicity in Aromatic Rice at Different Stages" Plants 13, no. 16: 2253. https://doi.org/10.3390/plants13162253





