Morphological Seed Traits Predict Early Performance of Native Species to Pelletized Seed Enhancement Technologies
Abstract
1. Introduction
- Characterize the amenability of 64 native species subject to pelleting by identifying species with comparable (or higher) total emergence and survival when pelletized relative to bare seeds;
- Quantify the degree to which pelletization modifies emergence speed across species;
- Quantify whether morphological seed traits can be used to predict species performance (amenability and emergence speed) to pelleting across a diverse range of species.

2. Results
2.1. Emergence and Survival
2.2. Emergence Speed
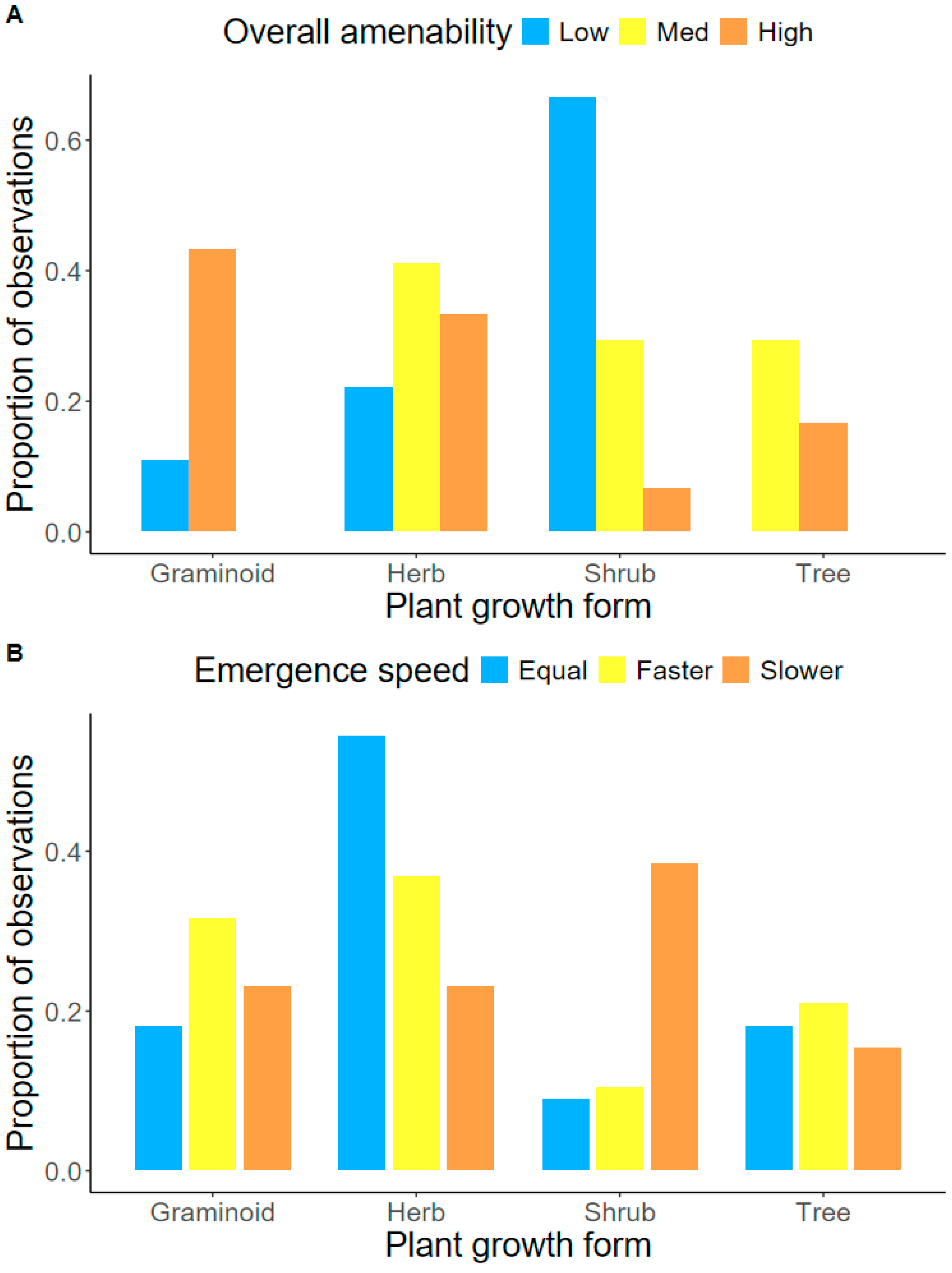
2.3. Ranking Species Performance in Pellets
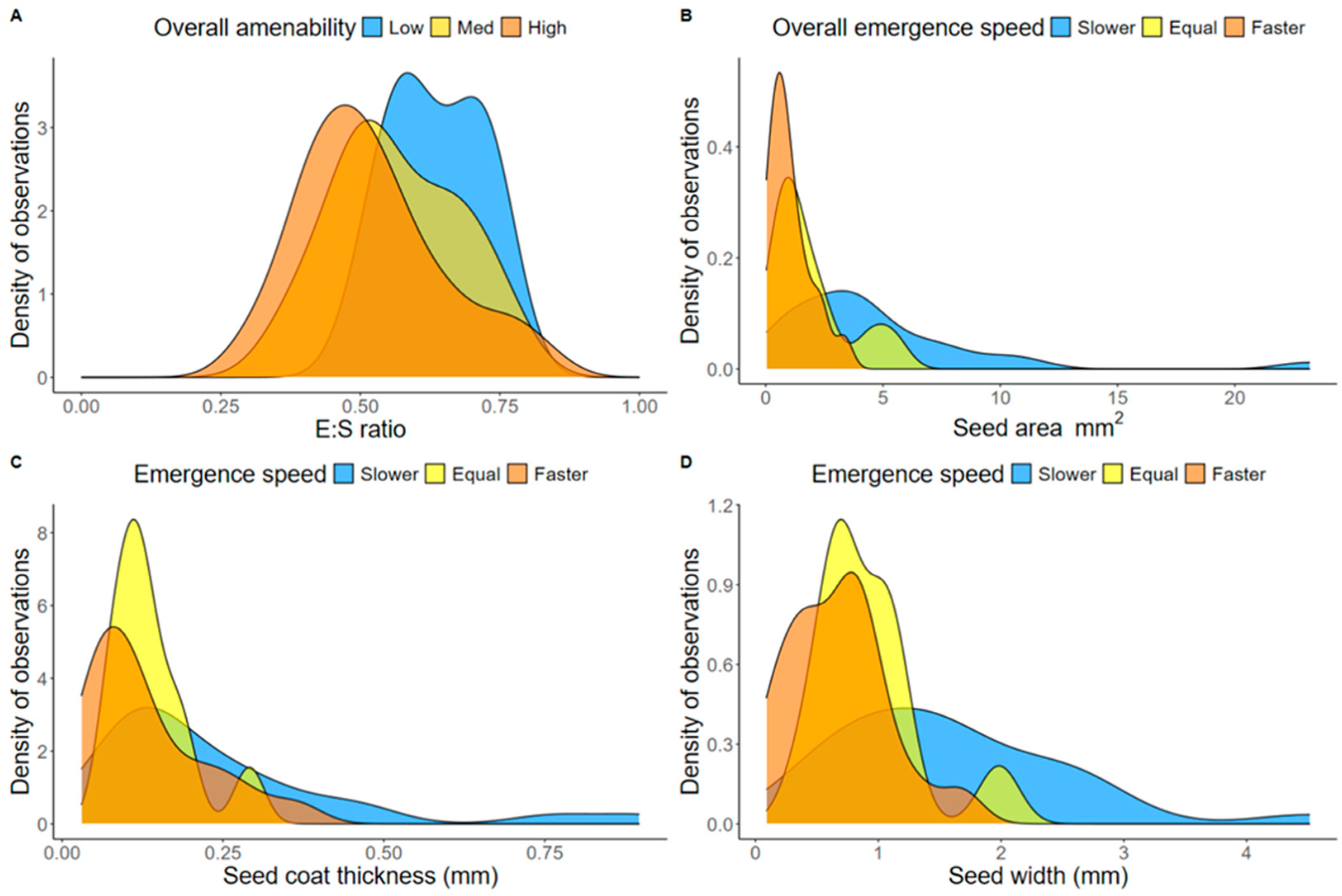
2.4. Morphological Seed Traits and Performance in Pellets
3. Discussion
4. Materials and Methods
4.1. Seed Preparation
4.1.1. Breaking Dormancy
4.1.2. Pellet Preparation
4.2. Greenhouse Experiment
4.3. Data Collection
4.4. Statistical Analyses
4.4.1. Emergence Experiment
4.4.2. Characterizing Performance of Pellets
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ceccon, E.; González, E.J.; Martorell, C. Is Direct Seeding a Biologically Viable Strategy for Restoring Forest Ecosystems? Evidences from a Meta-Analysis. Land. Degrad. Dev. 2016, 27, 511–520. [Google Scholar] [CrossRef]
- Hallett, L.M.; Standish, R.J.; Jonson, J.; Hobbs, R.J. Seedling Emergence and Summer Survival after Direct Seeding for Woodland Restoration on Old Fields in South-Western Australia. Ecol. Manage Restor. 2014, 15, 140–146. [Google Scholar] [CrossRef]
- Kildisheva, O.A.; Erickson, T.E.; Merritt, D.J.; Dixon, K.W. Setting the scene for dryland recovery: An overview and key findings from a workshop targeting seed-based restoration. Restor. Ecol. 2016, 24, S36–S42. [Google Scholar] [CrossRef]
- Merritt, D.J.; Dixon, K.W. Restoration seed banks—A matter of scale. Science 2011, 332, 424–425. [Google Scholar] [CrossRef]
- Williams, M.I.; Schuman, G.E.; Hild, A.L.; Vicklund, L.E. Wyoming big sagebrush density: Effects of seeding rates and grass competition. Restor. Ecol. 2002, 10, 385–391. [Google Scholar] [CrossRef]
- Applestein, C.; Bakker, J.D.; Delvin, E.G.; Hamman, S.T. Evaluating seeding methods and rates for prairie restoration. Nat. Areas J. 2018, 38, 347–355. [Google Scholar] [CrossRef]
- Barr, S.; Jonas, J.L.; Paschke, M.W. Optimizing seed mixture diversity and seeding rates for grassland restoration. Restor. Ecol. 2017, 25, 396–404. [Google Scholar] [CrossRef]
- Scotton, M. Mountain grassland restoration: Effects of sowing rate, climate and soil on plant density and cover. Sci. Total Environ. 2019, 651, 3090–3098. [Google Scholar] [CrossRef]
- Goodale, U.M.; Antonelli, A.; Nelson, C.R.; Chau, M.M. Seed banks needed to restore ecosystems. Science 2023, 379, 147. [Google Scholar] [CrossRef]
- Nevill, P.G.; Cross, A.T.; Dixon, K.W. Ethical seed sourcing is a key issue in meeting global restoration targets. Curr. Biol. 2018, 28, R1378–R1379. [Google Scholar] [CrossRef] [PubMed]
- Andres, S.E.; Atkinson, J.; Coleman, D.; Boast, J.B.; Allen, S.; Wright, I.; Gallagher, R.V. Constraints of commercially available seed diversity in restoration: Implications for plant functional diversity. Plants People Planet. 2024. [Google Scholar] [CrossRef]
- Cross, A.T.; Pedrini, S.; Dixon, K.W. Foreword: International standards for native seeds in ecological restoration. Restor. Ecol. 2020, 28, S216–S218. [Google Scholar] [CrossRef]
- Pedrini, S.; D’Agui, H.M.; Arya, T.; Turner, S.; Dixon, K.W. Seed quality and the true price of native seed for mine site restoration. Restor. Ecol. 2022, 30, e13638. [Google Scholar] [CrossRef]
- Andres, S.; Mills, C.; Gallagher, R.; Adams, V. A framework for ecological restoration cost accounting across context and scale. Biol. Conserv. 2024, 295, 110671. [Google Scholar] [CrossRef]
- Jamieson, G. New perspectives on seed enhancement. In Proceedings of the IV International Symposium on Seed, Transplant and Stand Establishment of Horticultural Crops, Translating Seed and Seedling, San Antonio, TX, USA, 3 December 2006; pp. 143–150. [Google Scholar]
- Taylor, A.G.; Allen, P.S.; Bennett, M.A.; Bradford, K.J.; Burris, J.S.; Misra, M.K. Seed enhancements. Seed Sci. Res. 1998, 8, 245–256. [Google Scholar] [CrossRef]
- Alfonzetti, M.; Tetu, S.G.; Mills, C.H.; Gallagher, R.V. Assessing the efficacy of extruded seed pellets and microbial amendments for native revegetation. Restor. Ecol. 2023, 31, e13857. [Google Scholar] [CrossRef]
- Madsen, M.D.; Davies, K.W.; Williams, C.J.; Svejcar, T.J. Agglomerating seeds to enhance native seedling emergence and growth. J. Appl. Ecol. 2012, 49, 431–438. [Google Scholar] [CrossRef]
- Madsen, M.D.; Hulet, A.; Phillips, K.; Staley, J.L.; Davies, K.W.; Svejcar, T.J. Extruded seed pellets: A novel approach for enhancing sagebrush seedling emergence. Nativ. Plants J. 2016, 17, 230–243. [Google Scholar] [CrossRef]
- Taylor, J.B.; Cass, K.L.; Armond, D.N.; Madsen, M.D.; Pearson, D.E.; St Clair, S.B. Deterring rodent seed-predation using seed-coating technologies. Restor. Ecol. 2020, 28, 927–936. [Google Scholar] [CrossRef]
- Andres, S.E.; Mills, C.H.; Cuneo, P.; Siemon, J.; Elgey, M.; Kelly, J.; Gallagher, R.V. New approaches with drones for restoring biodiverse native vegetation. Australasian Plant Conservation: J. Aust. Netw. Plant Conserv. 2022, 31, 12–15. [Google Scholar] [CrossRef]
- Gornish, E.; Arnold, H.; Fehmi, J. Review of seed pelletizing strategies for arid land restoration. Restor. Ecol. 2019, 27, 1206–1211. [Google Scholar] [CrossRef]
- Lieurance, P.E.; Mills, C.H.; Tetu, S.G.; Gallagher, R.V. Putting seed traits into pellets: Using seed mass data to improve seed encapsulation technology for native plant revegetation. J. Appl. Ecol. 2024, 61, 847–858. [Google Scholar] [CrossRef]
- James, J.J.; Svejcar, T.J.; Rinella, M.J. Demographic processes limiting seedling recruitment in arid grassland restoration. J. Appl. Ecol. 2011, 48, 961–969. [Google Scholar] [CrossRef]
- Palma, A.C.; Laurance, S.G. A review of the use of direct seeding and seedling plantings in restoration: What do we know and where should we go? Appl. Veg. Sci. 2015, 18, 561–568. [Google Scholar] [CrossRef]
- Copeland, S.M.; Baughman, O.W.; Boyd, C.S.; Davies, K.W.; Kerby, J.; Kildisheva, O.A.; Svejcar, T. Improving restoration success through a precision restoration framework. Restor. Ecol. 2021, 29, e13348. [Google Scholar] [CrossRef]
- Orrock, J.L.; Brudvig, L.A.; Damschen, E.I.; Mattingly, W.B.; Cruz, J.; Veldman, J.W.; Larsen-Gray, A.L. The Past as a Lens for Biodiversity Conservation on a Dynamically Changing Planet: Long-term, large-scale experiment reveals the effects of seed limitation, climate, and anthropogenic disturbance on restoration of plant communities in a biodiversity hotspot. Proc. Natl. Acad. Sci. USA 2023, 120, e2201943119. [Google Scholar] [PubMed]
- Shackelford, N.; Paterno, G.B.; Winkler, D.E.; Erickson, T.E.; Leger, E.A.; Svejcar, L.N.; Breed, M.F.; Faist, A.M.; Harrison, P.A.; Curran, M.F.; et al. Drivers of seedling establishment success in dryland restoration efforts. Nat. Ecol. Evol. 2021, 5, 1283–1290. [Google Scholar] [CrossRef]
- Larson, J.E.; Agneray, A.C.; Boyd, C.S.; Bradford, J.B.; Kildisheva, O.A.; Suding, K.N.; Copeland, S.M. A recruitment niche framework for improving seed-based restoration. Restor. Ecol. 2023, 31, e13959. [Google Scholar] [CrossRef]
- Madsen, M.D.; Davies, K.W.; Boyd, C.S.; Kerby, J.D.; Svejcar, T.J. Emerging seed enhancement technologies for overcoming barriers to restoration. Restor. Ecol. 2016, 24, S77–S84. [Google Scholar] [CrossRef]
- Barak, R.S.; Lichtenberger, T.M.; Wellman-Houde, A.; Kramer, A.T.; Larkin, D.J. Cracking the case: Seed traits and phylogeny predict time to germination in prairie restoration species. Ecol. Evol. 2018, 8, 5551–5562. [Google Scholar] [CrossRef]
- Dalziell, E.L.; Lewandrowski, W.; Commander, L.E.; Elliott, C.P.; Erickson, T.E.; Tudor, E.P.; Merritt, D.J. Seed traits inform the germination niche for biodiverse ecological restoration. Seed. Sci. Technol. 2022, 50, 103–124. [Google Scholar] [CrossRef]
- Laumann, P.D.; Ferreira, M.C.; da Silva, D.A.; Vieira, D.L.M. Germination traits explain the success of direct seeding restoration in the seasonal tropics of Brazil. For. Ecol. Manage 2023, 529, 120706. [Google Scholar] [CrossRef]
- Arend da Silva, I.; Guido, A.; Müller, S.C. Predicting plant performance for the ecological restoration of grasslands: The role of regenerative traits. Restor. Ecol. 2020, 28, 1183–1191. [Google Scholar] [CrossRef]
- Moles, A.T.; Westoby, M. Seedling survival and seed size: A synthesis of the literature. J. Ecol. 2005, 92, 372–383. [Google Scholar] [CrossRef]
- Murali, K.S. Patterns of seed size, germination and seed viability of tropical tree species in southern India 1. Biotropica 1997, 29, 271–279. [Google Scholar] [CrossRef]
- Tunjai, P.; Elliott, S. Effects of seed traits on the success of direct seeding for restoring southern Thailand’s lowland evergreen forest ecosystem. New For. 2012, 43, 319–333. [Google Scholar] [CrossRef]
- Barton, L.V. Dormancy in seeds imposed by the seed coat. Differ. Dev. 1965, 15, 2374–2392. [Google Scholar]
- Crocker, W. Role of seed coats in delayed germination. Bot. Gaz. 1906, 42, 265–291. [Google Scholar] [CrossRef]
- Saatkamp, A.; Cochrane, A.; Commander, L.; Guja, L.K.; Jimenez-Alfaro, B.; Larson, J.; Walck, J.L. A research agenda for seed-trait functional ecology. New Phytol. 2019, 221, 1764–1775. [Google Scholar] [CrossRef]
- Bu, H.Y.; Wang, X.J.; Zhou, X.H.; Qi, W.; Liu, K.; Ge, W.J.; Zhang, S.T. The ecological and evolutionary significance of seed shape and volume for the germination of 383 species on the eastern Qinghai-Tibet plateau. Folia Geobot. 2016, 51, 333–341. [Google Scholar] [CrossRef]
- Grime, J.P.; Mason, G.; Curtis, A.V.; Rodman, J.; Band, S.R. A comparative study of germination characteristics in a local flora. J. Ecol. 1981, 69, 1017–1059. [Google Scholar] [CrossRef]
- Thompson, K.B.S.R.; Band, S.R.; Hodgson, J.G. Seed size and shape predict persistence in soil. Funct. Ecol. 1993, 7, 236–241. [Google Scholar] [CrossRef]
- Richardson, W.C.; Badrakh, T.; Roundy, B.A.; Aanderud, Z.T.; Petersen, S.L.; Allen, P.S.; Madsen, M.D. Influence of an abscisic acid (ABA) seed coating on seed germination rate and timing of bluebunch wheatgrass. Ecol. Evol. 2019, 9, 7438–7447. [Google Scholar] [CrossRef]
- Pywell, R.F.; Bullock, J.M.; Roy, D.B.; Warman, L.I.Z.; Walker, K.J.; Rothery, P. Plant traits as predictors of performance in ecological restoration. J. Appl. Ecol. 2003, 40, 65–77. [Google Scholar] [CrossRef]
- Chambers, J.C. Seed movements and seedling fates in disturbed sagebrush steppe ecosystems: Implications for restoration. Ecol. Appl. 2000, 10, 1400–1413. [Google Scholar] [CrossRef]
- Leger, E.A.; Barga, S.; Agneray, A.C.; Baughman, O.; Burton, R.; Williams, M. Selecting native plants for restoration using rapid screening for adaptive traits: Methods and outcomes in a Great Basin case study. Restor. Ecol. 2021, 29, e13260. [Google Scholar] [CrossRef]
- Tilley, D.; Hulet, A.; Bushman, S.; Goebel, C.; Karl, J.; Love, S.; Wolf, M. When a weed is not a weed: Succession management using early seral natives for Intermountain rangeland restoration. Rangelands 2022, 44, 270–280. [Google Scholar] [CrossRef]
- Weidlich, E.W.; Nelson, C.R.; Maron, J.L.; Callaway, R.M.; Delory, B.M.; Temperton, V.M. Priority effects and ecological restoration. Restor. Ecol. 2021, 29, e13317. [Google Scholar] [CrossRef]
- Baughman, O.W.; Kerby, J.D.; Boyd, C.S.; Madsen, M.D.; Svejcar, T.J. Can delaying germination reduce barriers to successful emergence for early-germinating, fall-sown native bunchgrass seeds in cold deserts? Restor. Ecol. 2023, 31, e13761. [Google Scholar] [CrossRef]
- Vandelook, F.; Verdú, M.; Honnay, O. The role of seed traits in determining the phylogenetic structure of temperate plant communities. Ann. Bot. 2012, 110, 629–636. [Google Scholar] [CrossRef]
- Bond, W.J.; Honig, M.; Maze, K.E. Seed size and seedling emergence: An allometric relationship and some ecological implications. Oecologia 1999, 120, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Pellish, C.A.; Sherrard, M.E.; Leytem, P.A.; Jackson, L.L. Small vertebrate granivores reduce seedling emergence in native tallgrass prairie restoration. Restor. Ecol. 2018, 26, 323–330. [Google Scholar] [CrossRef]
- Shaw, N.; Barak, R.S.; Campbell, R.E.; Kirmer, A.; Pedrini, S.; Dixon, K.; Frischie, S. Seed use in the field: Delivering seeds for restoration success. Restor. Ecol. 2020, 28, S276–S285. [Google Scholar] [CrossRef]
- Mayence, C.E.; Stevens, J.C.; Courtney, P.; Dixon, K.W. Edaphic constraints on seed germination and emergence of three Acacia species for dryland restoration in Saudi Arabia. Plant Ecol. 2018, 218, 55–66. [Google Scholar] [CrossRef]
- Ralph, M. Growing Australian Native Plants from Seed: For Revegetation Tree Planting and Direct Seeding, 2nd ed.; Murray Ralph/Bushland Horticulture and Bloomings Books: Bullarto, Victoria, 2011. [Google Scholar]
- Read, T.R.; Bellairs, S.M. Smoke affects the germination of native grasses of New South Wales. Aust. J. Bot. 1999, 47, 563–576. [Google Scholar] [CrossRef]
- Kader, M.A. A comparison of seed germination calculation formulae and the associated interpretation of resulting data. J. Proceeding R. Soc. New South Wales 2005, 138, 65–75. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Cervantes, E.; Martín, J.J.; Saadaoui, E. Updated Methods for Seed Shape Analysis. Scientifica 2016, 2016, 5691825. [Google Scholar] [CrossRef] [PubMed]
- Kleyer, M.; Bekker, R.M.; Knevel, I.C.; Bakker, J.P.; Thompson, K.; Sonnenschein, M.; Peco, B. The LEDA Traitbase: A database of life-history traits of the Northwest European flora. J. Ecol. 2008, 96, 1266–1274. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing, v.4.3.1; R Foundation for Statistical Computing: Vienna, Austria, 2023. Available online: http://www.r-project.org (accessed on 12 June 2023).
- Wickham, H.; Wickham, H. Getting Started with ggplot2. In Ggplot2: Elegant Graphics for Data Analysis; Springer: Cham, Switzerland, 2016; pp. 11–31. [Google Scholar]
- Fox, J.; Friendly, G.G.; Graves, S.; Heiberger, R.; Monette, G.; Nilsson, H. The car package. R Foundation for Statistical Computing. J. Am. Soc. Nephrol. 2007, 1109, 1431. [Google Scholar]
- Wickham, H.; François, R.; Henry, L.; Müller, K.; Vaughan, D. Dplyr: A Grammar of Data Manipulation. R Package Version 1.1.4. 2023. Available online: https://dplyr.tidyverse.org (accessed on 12 June 2023).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Lenth, R. Emmeans: Estimated Marginal Means, Aka Least-Squares Means. R Package Version 1.10.2. 2024. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 12 June 2023).
- Berto, B.; Erickson, T.E.; Ritchie, A.L. Improving Seed Morphology and Germination Potential in Australian Native Grasses Using Seed Enhancement Technologies. Plants 2023, 12, 2432. [Google Scholar] [CrossRef] [PubMed]

| Amenability Metric | Amenability | Emergence Speed | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Emergence | Survival | Average Emergence | Emergence Rate Index | Time Spread of Emergence | ||||||||
| (%) | (%) | (Days) | (%/Day) | (Days) | ||||||||
| Species | Provenance | Growth Form | Bare Seed | Pellet | Bare Seed | Pellet | Bare Seed | Pellet | Bare Seed | Pellet | Bare Seed | Pellet |
| Acacia decurrens | Shrub | 100 | 70 | 100 | 66 | 14 | 25 | 3.57 | 0.49 | 0 | 16 | |
| Acacia falcata | 1 | Shrub | 94 | 48 | 100 | 80 | 23 | 35 | 0.73 | 0.14 | 16 | 27 |
| Acacia falcata | 2 | Shrub | 90 | 30 | 98 | 96 | 20 | 32 | 0.6 | 0.11 | 24 | 42 |
| Acacia implexa | Shrub | 98 | 66 | 100 | 76 | 15 | 27 | 1.11 | 0.26 | 16 | 42 | |
| Acacia parramattensis | Shrub | 94 | 60 | 100 | 67 | 15 | 22 | 1.59 | 0.46 | 8 | 24 | |
| Ajuga australis | Herb | 66 | 22 | 97 | 100 | 36 | 34 | 0.16 | 0.06 | 94 | 36 | |
| Allocasuarina littoralis | Tree | 88 | 66 | 100 | 100 | 21 | 34 | 0.46 | 0.21 | 43 | 49 | |
| Aristida vagans | Graminoid | 30 | 22 | 73 | 64 | 21 | 27 | 0.2 | 0.13 | 27 | 78 | |
| Arthropodium milleflorum | Herb | 88 | 48 | 100 | 79 | 32 | 48 | 0.22 | 0.08 | 42 | 120 | |
| Bothriochloa macera | Graminoid | 54 | 58 | 93 | 90 | 17 | 21 | 0.53 | 0.39 | 13 | 64 | |
| Bursaria spinosa | Shrub | 58 | 100 | 90 | 100 | 36 | 28 | 0.15 | 0.6 | 89 | 12 | |
| Carex inversa | Graminoid | 50 | 98 | 96 | 82 | 48 | 35 | 0.08 | 0.21 | 65 | 41 | |
| Centella asiatica | Herb | 30 | 82 | 80 | 73 | 38 | 34 | 0.08 | 0.27 | 78 | 34 | |
| Chenopodium nutans | Herb | 6 | 42 | 67 | 76 | 17 | 34 | 0.09 | 0.12 | 8 | 113 | |
| Chenopodium trigonon | Herb | 80 | 100 | 35 | 94 | 23 | 14 | 0.39 | 1.86 | 113 | 7 | |
| Chloris truncata | Graminoid | 40 | 62 | 35 | 77 | 24 | 13 | 0.19 | 0.97 | 50 | 15 | |
| Chloris ventricosa | Graminoid | 24 | 20 | 100 | 60 | 13 | 17 | 0.39 | 0.16 | 15 | 31 | |
| Coleus australis | Shrub | 10 | 82 | 100 | 95 | 26 | 24 | 0.1 | 0.32 | 12 | 41 | |
| Commelina cyanea | Herb | 24 | 52 | 92 | 96 | 20 | 24 | 0.21 | 0.28 | 20 | 27 | |
| Convolvulus erubescens | Shrub | 40 | 16 | 90 | 100 | 8 | 15 | 1.37 | 0.51 | 23 | 64 | |
| Corymbia maculata | Tree | 4 | 100 | 69 | 0.02 | 51 | ||||||
| Cymbopogon refractus | Graminoid | 46 | 34 | 74 | 76 | 17 | 27 | 0.46 | 0.17 | 13 | 20 | |
| Daviesia ulicifolia | Shrub | 58 | 62 | 100 | 45 | 31 | 37 | 0.25 | 0.14 | 29 | 105 | |
| Dillwynia sieberi | Shrub | 96 | 72 | 100 | 78 | 27 | 49 | 0.33 | 0.12 | 43 | 105 | |
| Dodonaea viscosa | 1 | Shrub | 62 | 4 | 100 | 100 | 23 | 111 | 0.01 | 0.12 | 33 | 94 |
| Dodonaea viscosa | 2 | Shrub | 28 | 4 | 84 | 100 | 35 | 30 | 0.12 | 0.06 | 94 | 0 |
| Eremophila debilis | Herb | 16 | 32 | 88 | 81 | 40 | 64 | 0.07 | 0.06 | 12 | 56 | |
| Eucalyptus amplifolia | Tree | 84 | 100 | 95 | 100 | 20 | 10 | 0.51 | 2.81 | 105 | 8 | |
| Eucalyptus crebra | 1 | Tree | 78 | 86 | 97 | 88 | 10 | 19 | 1.28 | 0.66 | 36 | 80 |
| Eucalyptus crebra | 2 | Tree | 100 | 100 | 98 | 100 | 16 | 15 | 0.83 | 0.89 | 36 | 23 |
| Eucalyptus eugenioides | Tree | 66 | 88 | 100 | 95 | 18 | 19 | 0.94 | 1.23 | 8 | 8 | |
| Eucalyptus longifolia | Tree | 42 | 92 | 90 | 100 | 27 | 20 | 0.21 | 0.83 | 34 | 41 | |
| Eucalyptus moluccana | 1 | Tree | 86 | 82 | 100 | 95 | 14 | 14 | 0.92 | 0.84 | 57 | 36 |
| Eucalyptus moluccana | 2 | Tree | 100 | 100 | 100 | 92 | 13 | 12 | 1.31 | 1.58 | 50 | 31 |
| Eucalyptus punctata | Tree | 86 | 44 | 93 | 100 | 25 | 34 | 0.58 | 0.25 | 12 | 33 | |
| Eucalyptus tereticornis | Tree | 100 | 82 | 100 | 95 | 10 | 12 | 2.07 | 1.41 | 36 | 15 | |
| Fimbristylis dichotoma | Graminoid | 32 | 98 | 44 | 59 | 36 | 26 | 0.12 | 0.97 | 26 | 12 | |
| Geranium solanderi | Herb | 94 | 88 | 100 | 89 | 20 | 18 | 0.56 | 0.65 | 31 | 24 | |
| Glossocardia bidens | Herb | 34 | 40 | 53 | 50 | 23 | 32 | 0.25 | 0.12 | 13 | 72 | |
| Hardenbergia violacea | Shrub | 84 | 74 | 100 | 81 | 21 | 25 | 0.38 | 0.32 | 43 | 29 | |
| Hypericum gramineum | Herb | 96 | 52 | 81 | 58 | 31 | 23 | 0.34 | 0.58 | 57 | 16 | |
| Indigofera australis | Shrub | 82 | 20 | 71 | 70 | 12 | 16 | 1.05 | 0.19 | 23 | 23 | |
| Laxmannia gracilis | Herb | 60 | 6 | 100 | 100 | 44 | 45 | 0.11 | 0.03 | 78 | 7 | |
| Lomandra longifolia | Graminoid | 30 | 73 | 74 | 0.07 | 0.37 | ||||||
| Melaleuca decora | Shrub | 38 | 100 | 84 | 86 | 39 | 28 | 0.14 | 0.98 | 56 | 56 | |
| Mentha satureioides | Herb | 2 | 4 | 100 | 0 | 57 | 44 | 0.02 | 0.07 | 0 | 72 | |
| Microlaena stipoides | Graminoid | 20 | 60 | 17 | 0.3 | 8 | ||||||
| Ozothamnus diosmifolius | Shrub | 32 | 12 | 69 | 0 | 13 | 8 | 0.51 | 0.75 | 21 | 0 | |
| Panicum simile | Graminoid | 4 | 20 | 50 | 40 | 22 | 31 | 0.09 | 0.09 | 0 | 49 | |
| Pentapogon micranthus | Graminoid | 92 | 100 | 87 | 100 | 21 | 17 | 0.5 | 1 | 41 | 20 | |
| Phyllanthus virgatus | Herb | 8 | 75 | 47 | 0.04 | 7 | ||||||
| Plantago gaudichaudii | Herb | 52 | 94 | 96 | 100 | 21 | 13 | 0.3 | 1.34 | 72 | 16 | |
| Poa labillardierei | Graminoid | 78 | 56 | 90 | 46 | 17 | 14 | 0.62 | 2 | 24 | 0 | |
| Pomax umbellata | Shrub | 12 | 52 | 0 | 69 | 73 | 63 | 0.03 | 0.1 | 37 | 28 | |
| Sarga leioclada | Graminoid | 8 | 4 | 75 | 100 | 19 | 33 | 0.11 | 0.04 | 13 | 34 | |
| Senecio quadridentatus | Herb | 8 | 75 | 33 | 0.04 | 34 | ||||||
| Setaria distans | Graminoid | 38 | 24 | 58 | 83 | 28 | 43 | 0.18 | 0.06 | 26 | 97 | |
| Solanum prinophyllum | Herb | 74 | 80 | 95 | 88 | 19 | 26 | 0.7 | 0.33 | 16 | 29 | |
| Sporobolus creber | Graminoid | 24 | 98 | 83 | 98 | 31 | 20 | 0.09 | 1.28 | 41 | 8 | |
| Syncarpia glomulifera | Tree | 30 | 6 | 80 | 100 | 31 | 36 | 0.14 | 0.04 | 35 | 21 | |
| Themeda triandra | Graminoid | 16 | 12 | 100 | 100 | 10 | 14 | 0.46 | 0.43 | 7 | 0 | |
| Wahlenbergia gracilis | Herb | 86 | 54 | 72 | 37 | 33 | 22 | 0.25 | 0.61 | 57 | 8 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andres, S.E.; Lieurance, P.E.; Mills, C.H.; Tetu, S.G.; Gallagher, R.V. Morphological Seed Traits Predict Early Performance of Native Species to Pelletized Seed Enhancement Technologies. Plants 2024, 13, 2256. https://doi.org/10.3390/plants13162256
Andres SE, Lieurance PE, Mills CH, Tetu SG, Gallagher RV. Morphological Seed Traits Predict Early Performance of Native Species to Pelletized Seed Enhancement Technologies. Plants. 2024; 13(16):2256. https://doi.org/10.3390/plants13162256
Chicago/Turabian StyleAndres, Samantha E., Paige E. Lieurance, Charlotte H. Mills, Sasha G. Tetu, and Rachael V. Gallagher. 2024. "Morphological Seed Traits Predict Early Performance of Native Species to Pelletized Seed Enhancement Technologies" Plants 13, no. 16: 2256. https://doi.org/10.3390/plants13162256
APA StyleAndres, S. E., Lieurance, P. E., Mills, C. H., Tetu, S. G., & Gallagher, R. V. (2024). Morphological Seed Traits Predict Early Performance of Native Species to Pelletized Seed Enhancement Technologies. Plants, 13(16), 2256. https://doi.org/10.3390/plants13162256






