Combined Metabolomics and Network Pharmacology Analysis Reveal the Effect of Rootstocks on Anthocyanins, Lipids, and Potential Pharmacological Ingredients of Tarroco Blood Orange (Citrus sinensis L. Osbeck)
Abstract
1. Introduction
2. Results
2.1. Effect of Grafting on Fruit Flavor, Nutritional Quality, and Antioxidant Capacity
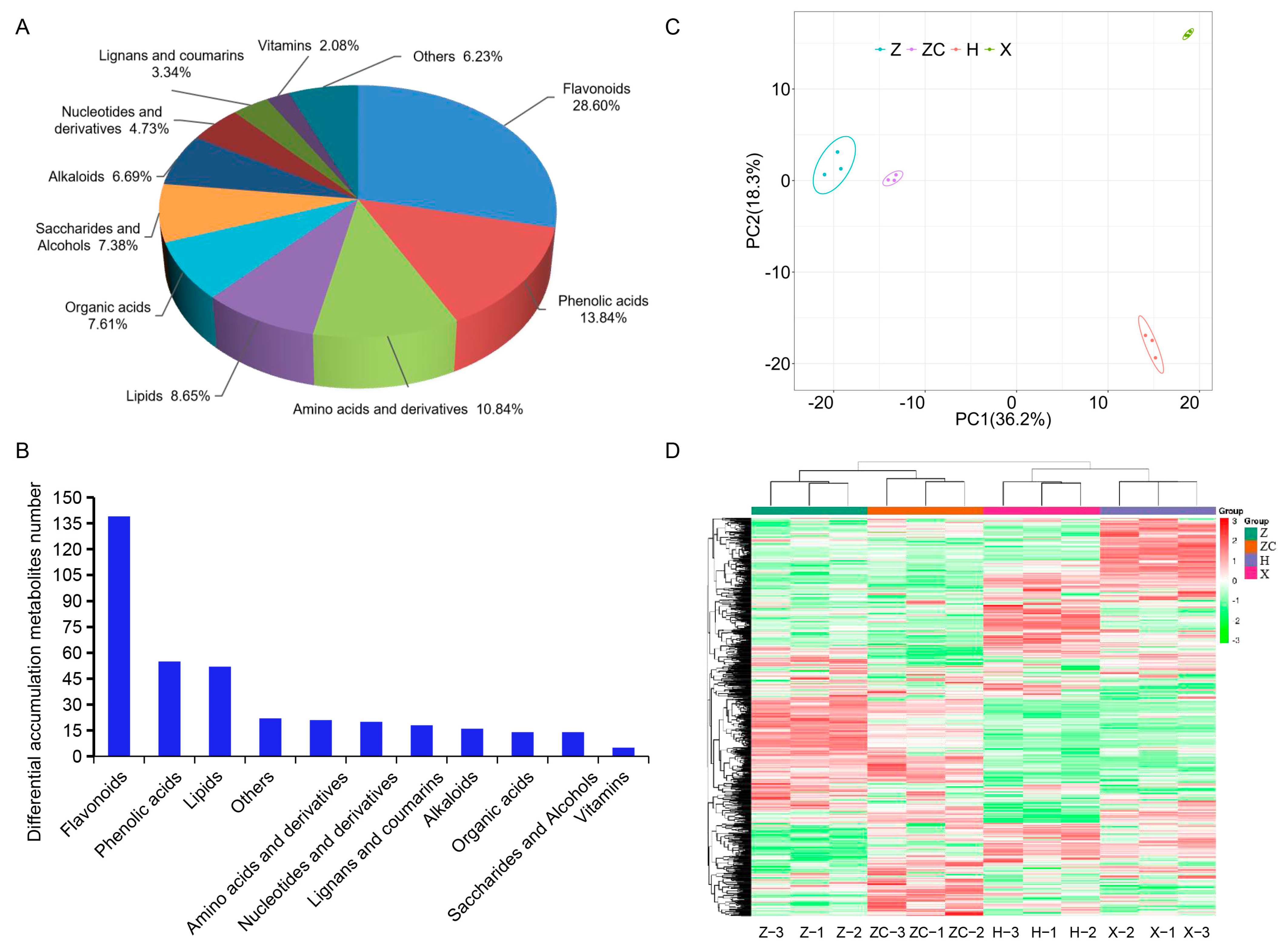
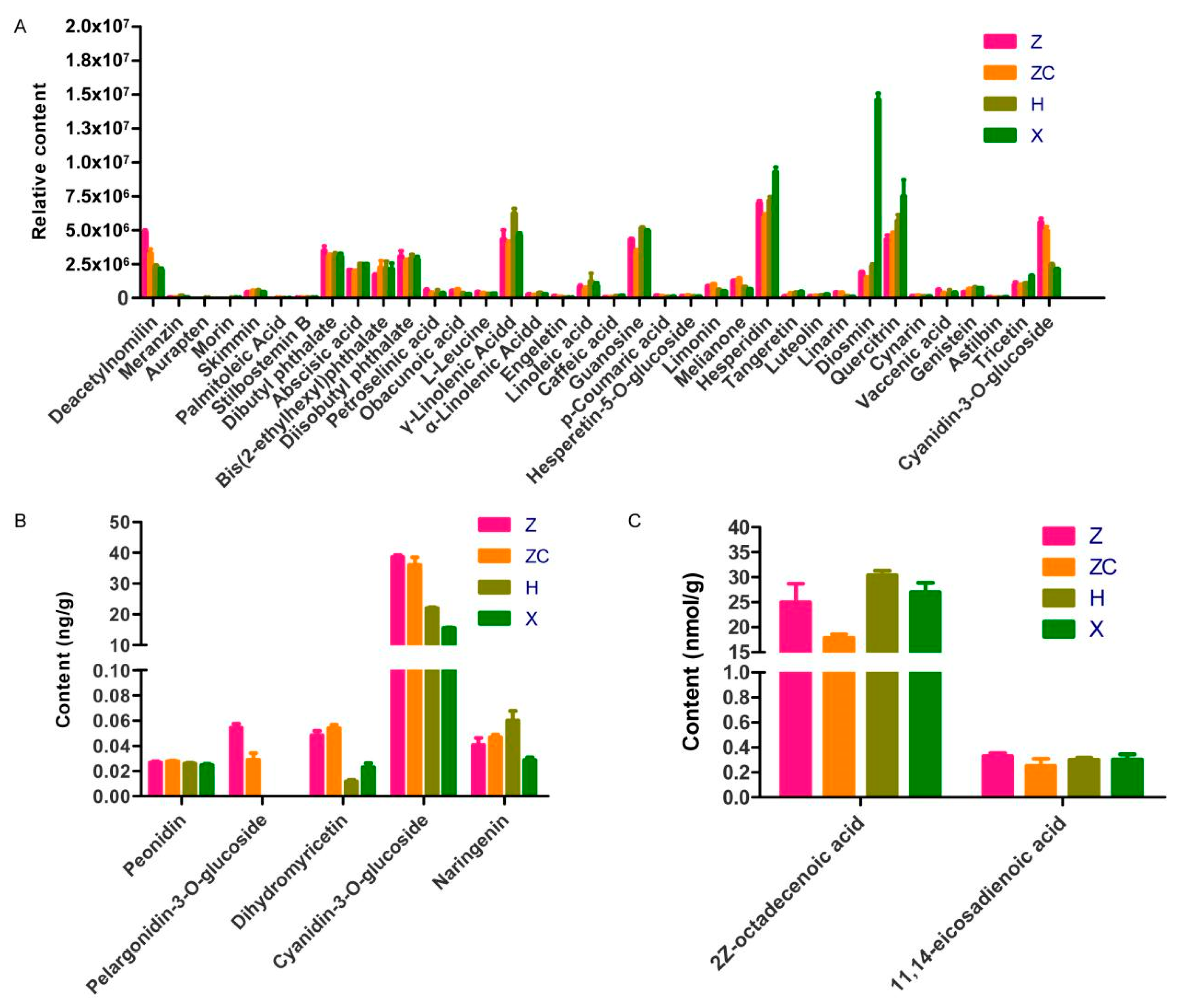
2.2. Secondary Metabolic Profiling of Four Scion–Rootstock Combinations
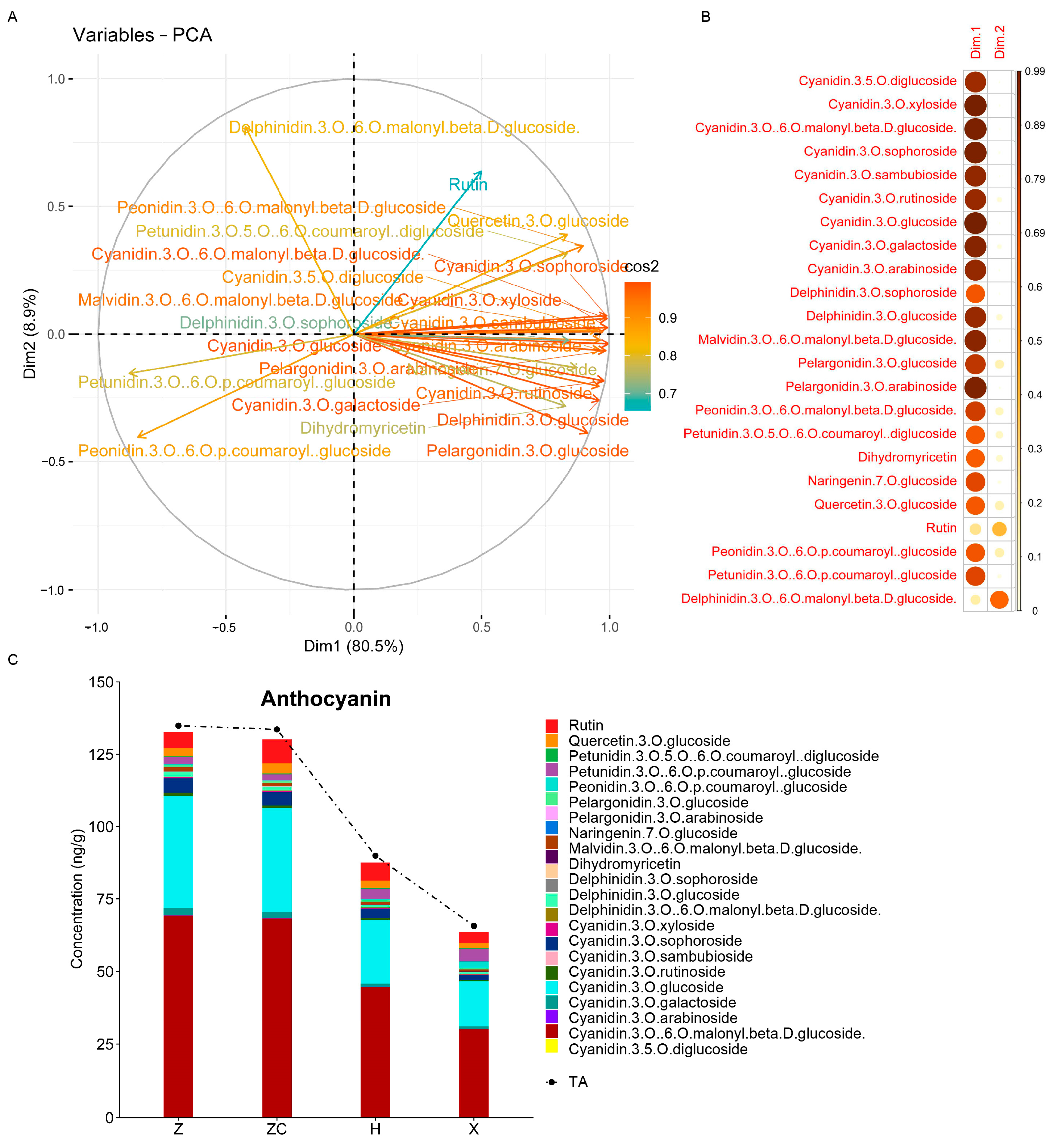
2.3. Targeted Metabolomic Analyses Detected Metabolites Related to Anthocyanin Synthesis
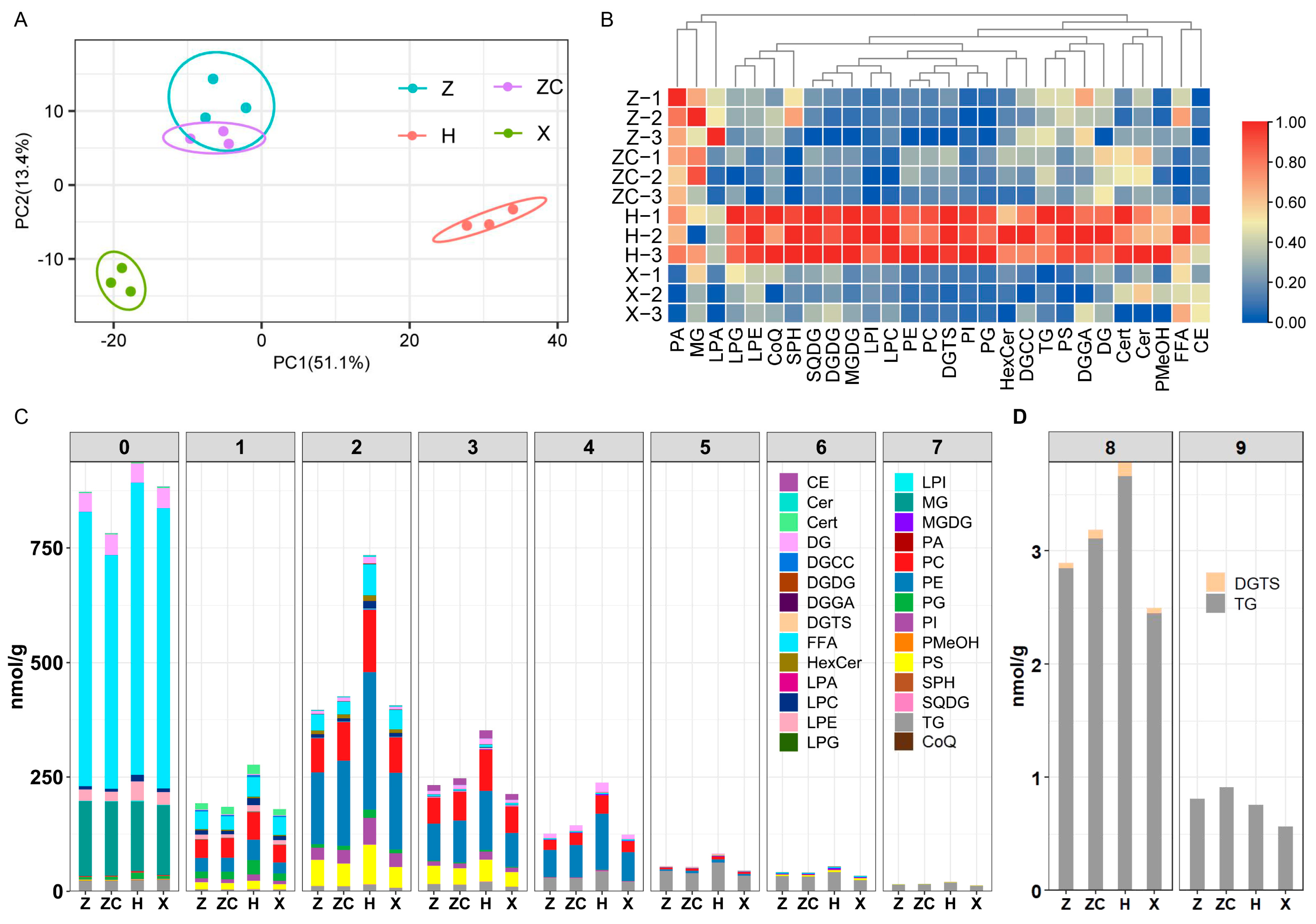
2.4. Targeted Lipidomics Analyses Detected Metabolites Related to Lipid Synthesis
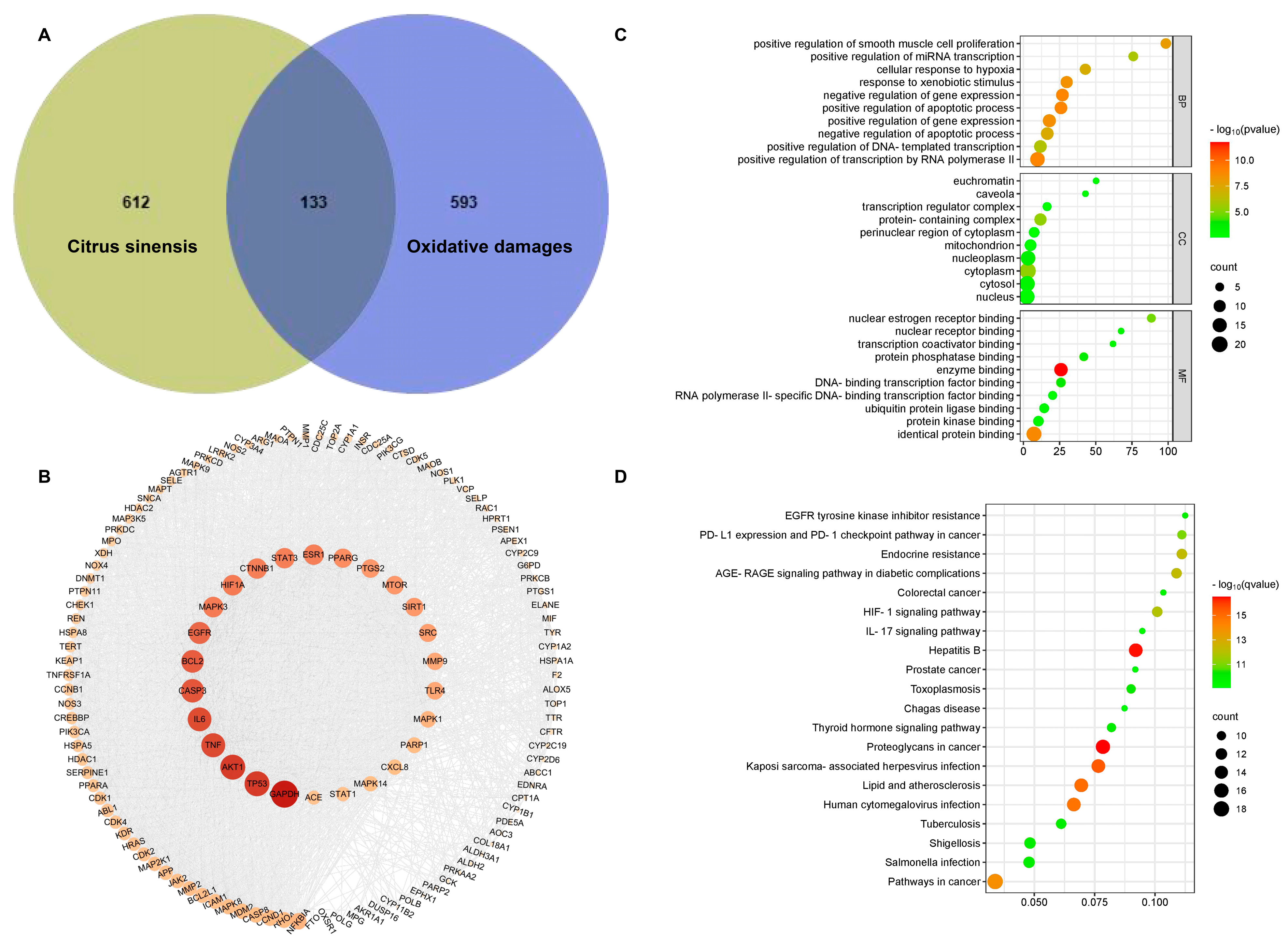
2.5. Network Pharmacology Analysis
2.6. The Effect of Four Rootstocks on Core Metabolites
3. Discussion
3.1. Rootstock Z and ZC Could Promote Anthocyanin Accumulation
3.2. Rootstock H Could Promote UFA Synthesis
3.3. Rootstocks Z and ZC Could Promote Antioxidant Activity by Increasing TPC, TFC, and TAC Content
3.4. Network Pharmacology Analysis Reveals Antioxidant Mechanism of Blood Orange
4. Conclusions
5. Materials and Methods
5.1. Plant Materials and Sample Collection
5.2. Fruit Quality and Major Functional Nutrients Determination
5.3. Metabolomics Profiles
5.4. Determination of Antioxidant Activity
5.5. Prediction of the Potential Active Ingredient
5.6. Prediction of Core Targets of Citrus and Diseases
5.7. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Analysis
5.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huang, L.; Grosser, J.; Gmitter, F.G.; Sims, C.A.; Wang, Y. Effects of scion/rootstock combination on flavor quality of orange juice from huanglongbing (hlb)-affected trees: A two-year study of the targeted metabolomics. J. Agric. Food Chem. 2020, 68, 3286–3296. [Google Scholar] [CrossRef]
- Wang, M.; Chen, Y.; Li, S.; Yu, J.; Yang, L.; Hong, L. Widely Targeted Metabolomic Analysis Provides New Insights into the Effect of Rootstocks on Citrus Fruit Quality. Metabolites 2024, 14, 242. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.A.M.; Borges, F.; Guimaraes, C.; Lima, J.L.F.C.; Matos, C.; Reis, S. Phenolic acids and derivatives: Studies on the relationship among structure, radical scavenging activity, and physicochemical parameters. J. Agric. Food Chem. 2000, 48, 2122–2126. [Google Scholar] [CrossRef]
- Niciforovic, N.; Abramovic, H. Sinapic acid and its derivatives: Natural sources and bioactivity. Compr. Rev. Food Sci. Food Saf. 2014, 13, 34–51. [Google Scholar] [CrossRef]
- Legua, P.; Forner, J.B.; Hernández, F.; Forner-Giner, M.A. Physicochemical properties of orange juice from ten rootstocks using multivariate analysis. Sci. Hortic. 2013, 160, 268–273. [Google Scholar] [CrossRef]
- Hou, J.; Liang, L.; Su, M.; Yang, T.; Mao, X.; Wang, Y. Variations in phenolic acids and antioxidant activity of navel orange at different growth stages. Food Chem. 2021, 360, 129980. [Google Scholar] [CrossRef]
- Ding, S.; Wang, P.; Pang, X.; Zhang, L.; Qian, L.; Jia, X.; Chen, W.; Ruan, S.; Sun, L. The new exploration of pure total flavonoids extracted from Citrus maxima (Burm.) Merr. as a new therapeutic agent to bring health benefits for people. Front. Nutr. 2022, 9, 958329. [Google Scholar] [CrossRef] [PubMed]
- Sdiri, S.; Salvador, A.; Farhat, I.; Navarro, P.; Besada, C. Influence of Postharvest Handling on Antioxidant Compounds of Citrus Fruits. In Citrus: Molecular Phylogeny, Antioxidant Properties and Medicinal; Hayat, K., Ed.; Nova Publisher: Hauppauge, NY, USA, 2014. [Google Scholar]
- Galati, E.M.; Monforte, M.T.; Kirjavainen, S.; Forestieri, A.M.; Tripodo, M.M. Biological effects of hesperidin, a citrus flavonoid. (note i): Antiinflammatory and Analgesic Activity. Farmaco 1994, 40, 709–712. [Google Scholar]
- Adams, M.; Bauer, R.; Efferth, T. Activity-guided isolation of scopoletin and isoscopoletin, the inhibitory active principles towards ccrf-cem leukaemia cells and multi-drug resistant cem/adr5000 cells, from artemisia argyi. Planta Medica 2006, 72, 862–864. [Google Scholar] [CrossRef]
- Chen, J.; Li, G.; Sun, C.; Peng, F.A.O.; Yu, L.; Chen, Y.; Tan, Y.; Cao, X.; Tang, Y.; Xie, X.; et al. Chemistry, pharmacokinetics, pharmacological activities, and toxicity of quercitrin. Phytother. Res. 2022, 36, 1545–1575. [Google Scholar] [CrossRef]
- Habibi, F.; Ramezanian, A.; Guillén, F.; Serrano, M.; Valero, D. Blood oranges maintain bioactive compounds and nutritional quality by postharvest treatments with γ-aminobutyric acid, methyl jasmonate or methyl salicylate during cold storage. Food Chem. 2020, 306, 125634. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.X.; Liu, Z.J.; Zhang, F.C.; Zhou, X.M.; Sun, X.X.; Li, Y.Y.; Liu, W.W.; Xiao, H.; Wang, N.; Lu, H.; et al. Metabolomic and transcriptomic analyses reveal the effects of self-and hetero-grafting on anthocyanin biosynthesis in grapevine. Hortic. Res. 2022, 9, uhac103. [Google Scholar] [CrossRef] [PubMed]
- Winkel-Shirley, B. Flavonoid Biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. 2001, 126, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gao, X.H.; Tong, L.; Liu, M.Z.; Zhou, X.K.; Tahir, M.M.; Xing, L.B.; Ma, J.J.; An, N.; Zhao, C.P.; et al. Multi-omics analyses reveal MdMYB10 hypermethylation being responsible for a bud sport of apple fruit color. Hortic. Res. 2022, 9, uhac179. [Google Scholar] [CrossRef] [PubMed]
- Ruxton, C.H.S.; Derbyshire, E. Latest evidence on omega-3 fatty acids and health. Nutr. Food Sci. 2009, 39, 423–438. [Google Scholar] [CrossRef]
- Wang, Y.L.; Lin, S.X.; Wang, Y.; Liang, T.; Jiang, T.; Liu, P.; Li, X.Y.; Lang, D.Q.; Liu, Q.; Shen, C.Y. p-Synephrine ameliorates alloxan-induced diabetes mellitus through inhibiting oxidative stress and inflammation via suppressing the NF-kappa B and MAPK pathways. Food Funct. 2023, 14, 1971–1988. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M.A.; Imtiaz, M.; Kong, Q.; Cheng, F.; Ahmed, W.; Huang, Y.; Bie, Z. Grafting: A technique to modify ion accumulation in horticultural crops. Front. Plant Sci. 2016, 7, 1457. [Google Scholar] [CrossRef] [PubMed]
- Shivran, M.; Sharma, N.; Dubey, A.K.; Singh, S.K.; Sharma, N.; Sharma, R.M.; Singh, N.; Singh, R. Scion–rootstock relationship: Molecular mechanism and quality fruit production. Agriculture 2022, 12, 2036. [Google Scholar] [CrossRef]
- Morales Alfaro, J.; Bermejo, A.; Navarro, P.; Quiñones, A.; Salvador, A. Effect of rootstock on citrus fruit quality: A review. Food Rev. Int. 2021, 39, 2835–2853. [Google Scholar] [CrossRef]
- Miao, L.; Di, Q.; Sun, T.; Li, Y.; Duan, Y.; Wang, J.; Yan, Y.; He, C.; Wang, C.; Yu, X. Integrated Metabolome and Transcriptome Analysis Provide Insights into the Effects of Grafting on Fruit Flavor of Cucumber with Different Rootstocks. Int. J. Mol. Sci. 2019, 20, 3592. [Google Scholar] [CrossRef]
- Ferrer, V.; Paymal, N.; Quinton, C.; Costantino, G.; Paoli, M.; Froelicher, Y.; Ollitrault, P.; Tomi, F.; Luro, F. Influence of the Rootstock and the Ploidy Level of the Scion and the Rootstock on Sweet Orange (Citrus sinensis) Peel Essential Oil Yield, Composition and Aromatic Properties. Agriculture 2022, 12, 214. [Google Scholar] [CrossRef]
- Abu Glion, H.; Alkalai-Tuvia, S.; Zaaroor-Presman, M.; Chalupowicz, D.; Zanbar, M.; Amichai, M.; Cohen, S.; Shemer, T.; Sarig, S.; Fallik, E. Effects of Rootstock/Scion Combination and Two Irrigation Water Qualities on Cherry Tomato Yield and Postharvest Fruit Quality. Horticulturae 2019, 5, 35. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, C.; Luan, Z.; Zhang, D.; Dong, B. Exploring the potential targets of the Abrus cantoniensis Hance in the treatment of hepatitis E based on network pharmacology. Front. Vet. Sci. 2023, 10, 1155677. [Google Scholar] [CrossRef]
- Cao, X.; Shi, K.; Xu, Y.; Zhang, P.; Zhang, H.; Pan, S. Integrated metabolomics and network pharmacology to reveal antioxidant mechanisms and potential pharmacological ingredients of citrus herbs. Food Res. Int. 2023, 174, 113514. [Google Scholar] [CrossRef] [PubMed]
- Zeng, T.; Xiao, Q.; Zhang, J.; Sun, X.; Guo, B.; Pei, J.; Huang, L.-F. Identification of a secondary Q-marker in high-quality ecotypes of Carthamus tinctorius L. and exploration of the target preference. Food Funct. 2023, 14, 2710–2726. [Google Scholar] [CrossRef]
- Duan, Y.; Zhang, D.; Ye, Y.; Zheng, S.; Huang, P.; Zhang, F.; Mo, G.; Huang, F.; Yin, Q.; Li, J.; et al. Integrated metabolomics and network pharmacology to establish the action mechanism of Qingrekasen granule for treating Nephrotic Syndrome. Front. Pharmacol. 2021, 12, 765563. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Chen, J.; Ge, W.; Liu, Z.; Han, N.; Yin, J. Antichilblain components in eggplant based on network pharmacology and biological evaluation. J. Agric. Food Chem. 2023, 71, 11442–11453. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Li, Y.; Zhang, Y.; Zeng, Q.; Guo, X. Elucidation of the potential antioxidant compound and mechanism of mung bean using network pharmacology and in vitro anti-oxidative activity. Front. Sustain. Food Syst. 2022, 6, 1000916. [Google Scholar] [CrossRef]
- Dadwal, V.; Joshi, R.; Gupta, M. A comparative metabolomic investigation in fruit sections of Citrus medica L. and Citrus maxima L. detecting potential bioactive metabolites using UHPLC-QTOF-IMS. Food Res. Int. 2022, 157, 111486. [Google Scholar] [CrossRef]
- Xiao, Y.; He, C.; Chen, Y.; Ho, C.-T.; Wu, X.; Huang, Y.; Gao, Y.; Hou, A.; Li, Z.; Wang, Y.; et al. UPLC–QQQ–MS/MS-based widely targeted metabolomic analysis reveals the effect of solid-state fermentation with Eurotium cristatum on the dynamic changes in the metabolite profile of dark tea. Food Chem. 2022, 378, 131999. [Google Scholar] [CrossRef]
- Xu, D.; Yuan, H.; Tong, Y.; Zhao, L.; Qiu, L.; Guo, W.; Shen, C.; Liu, H.; Yan, D.; Zheng, B. Comparative proteomic analysis of the graft unions in Hickory (Carya cathayensis) provides insights into response mechanisms to grafting process. Front. Plant Sci. 2017, 8, 676. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Chen, Y.; Wang, M.; Hou, H.; Li, S.; Guan, L.; Yang, H.; Wang, W.; Hong, L. Metabolomic and transcriptomic analyses reveal the effects of grafting on blood orange quality. Front. Plant Sci. 2023, 14, 1169220. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Zhang, H.; Fan, Y.; Yan, L. Anthocyanins accumulation analysis of correlated genes by metabolome and transcriptome in green and purple peppers (Capsicum annuum). BMC Plant Biol. 2022, 22, 358. [Google Scholar] [CrossRef] [PubMed]
- Cebadera-Miranda, L.; Domínguez, L.; Dias, M.I.; Barros, L.; Ferreira, I.C.F.R.; Igual, M.; Martínez-Navarrete, N.; Fernández-Ruiz, V.; Morales, P.; Cámara, M. Sanguinello and Tarocco (Citrus sinensis [L.] Osbeck): Bioactive compounds and colour appearance of blood oranges. Food Chem. 2019, 270, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Morales, J.; Bermejo, A.; Navarro, P.; Forner-Giner, M.Á.; Salvador, A. Rootstock effect on fruit quality, anthocyanins, sugars, hydroxycinnamic acids and flavanones content during the harvest of blood oranges ‘Moro’ and ‘Tarocco Rosso’ grown in Spain. Food Chem. 2021, 342, 128305. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Deng, H.; Xiong, B.; Li, S.; Yang, L.; Yang, Y.; Huang, S.; Tan, L.; Sun, G.; Wang, Z. Rootstock effects on anthocyanin accumulation and associated biosynthetic gene expression and enzyme activity during fruit development and ripening of blood oranges. Agriculture 2022, 12, 342. [Google Scholar] [CrossRef]
- Tanaka, Y.; Ohmiya, A. Seeing is believing: Engineering anthocyanin and carotenoid biosynthetic pathways. Curr. Opin. Biotechnol. 2008, 19, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Lo Piero, A.R. The state of the art in biosynthesis of anthocyanins and its regulation in pigmented sweet oranges [(Citrus sinensis) L. Osbeck]. J. Agric. Food Chem. 2015, 63, 4031–4041. [Google Scholar] [CrossRef] [PubMed]
- Carmona, L.; Alquézar, B.; Marques, V.V.; Peña, L. Anthocyanin biosynthesis and accumulation in blood oranges during postharvest storage at different low temperatures. Food Chem. 2017, 237, 7–14. [Google Scholar] [CrossRef]
- Flood, V.M.; Webb, K.L.; Rochtchina, E.; Kelly, B.; Mitchell, P. Fatty acid intakes and food sources in a population of older Australians. Asia Pac. J. Clin. Nutr. 2007, 16, 322–330. [Google Scholar] [PubMed]
- Musazadeh, V.; Dehghan, P.; Saleh-Ghadimi, S.; Abbasalizad Farhangi, M. Omega 3-rich camelina sativa oil in the context of a weight loss program improves glucose homeostasis, inflammation and oxidative stress in patients with NAFLD: A randomised placebo-controlled clinical trial. Int. J. Clin. Pract. 2021, 75, e14744. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.D.; Whitley, B.M.; Hoyo, C.; Grant, D.J.; Iraggi, J.D.; Newman, K.A.; Gerber, L.; Taylor, L.A.; McKeever, M.G.; Freedland, S.J. A high ratio of dietary n-6/n-3 polyunsaturated fatty acids is associated with increased risk of prostate cancer. Nutr. Res. 2011, 31, 1–8. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Sailo, B.L.; Banik, K.; Harsha, C.; Prasad, S.; Gupta, S.C.; Bharti, A.C.; Aggarwal, B.B. Chronic diseases, inflammation, and spices: How are they linked? J. Transl. Med. 2018, 16, 14. [Google Scholar] [CrossRef] [PubMed]
- Proteggente, A.R.; Saija, A.; De Pasquale, A.; Rice-Evans, C.A. The compositional characterisation and antioxidant activity of fresh juices from sicilian sweet orange (Citrus sinensisL. Osbeck) varieties. Free Radic. Res. 2009, 37, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Valero, D.; Díaz-Mula, H.M.; Zapata, P.J.; Castillo, S.; Guillén, F.; Martínez-Romero, D.; Serrano, M. Postharvest treatments with salicylic acid, acetylsalicylic acid or oxalic acid delayed ripening and enhanced bioactive compounds and antioxidant capacity in sweet cherry. J. Agric. Food Chem. 2011, 59, 5483–5489. [Google Scholar] [CrossRef] [PubMed]
- Kesarkar, S.; Bhandage, A.; Deshmukh, S.; Shevkar, K.; Abhyankar, M. Flavonoids: An overview. J. Pharm. Res. 2009, 2, 1148–1154. [Google Scholar]
- Lo Scalzo, R.; Iannoccari, T.; Summa, C.; Morelli, R.; Rapisarda, P. Effect of thermal treatments on antioxidant and antiradical activity of blood orange juice. Food Chem. 2004, 85, 41–47. [Google Scholar] [CrossRef]
- Kelebek, H.; Canbas, A.; Selli, S. Determination of phenolic composition and antioxidant capacity of blood orange juices obtained from cvs. Moro and Sanguinello (Citrus sinensis (L.) Osbeck) grown in Turkey. Food Chem. 2008, 107, 1710–1716. [Google Scholar] [CrossRef]
- Zou, Z.; Xi, W.; Hu, Y.; Nie, C.; Zhou, Z. Antioxidant activity of Citrus fruits. Food Chem. 2016, 196, 885–896. [Google Scholar] [CrossRef]
- Cömert, E.D.; Gökmen, V. Evolution of food antioxidants as a core topic of food science for a century. Food Res. Int. 2018, 105, 76–93. [Google Scholar] [CrossRef]
- Ordóñez-Díaz, J.L.; Hervalejo, A.; Pereira-Caro, G.; Muñoz-Redondo, J.M.; Romero-Rodríguez, E.; Arenas-Arenas, F.J.; Moreno-Rojas, J.M. Effect of rootstock and harvesting period on the bioactive compounds and antioxidant activity of two orange cultivars (‘Salustiana’ and ‘Sanguinelli’) widely used in juice industry. Processes 2020, 8, 1212. [Google Scholar] [CrossRef]
- Modica, G.; Pannitteri, C.; Di Guardo, M.; La Malfa, S.; Gentile, A.; Ruberto, G.; Pulvirenti, L.; Parafati, L.; Continella, A.; Siracusa, L. Influence of rootstock genotype on individual metabolic responses and antioxidant potential of blood orange cv. Tarocco Scirè. J. Food Compos. Anal. 2022, 105, 104246. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, X.; Gong, Q.; Cao, J.; Shen, W.; Yin, X.; Grierson, D.; Zhang, B.; Xu, C.; Li, X.; et al. Three AP2/ERF family members modulate flavonoid synthesis by regulating type IV chalcone isomerase in citrus. Plant Biotechnol. J. 2020, 19, 671–688. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Huang, F.; Deng, C.; Wang, Y.; Kai, G. Bioactivities, biosynthesis and biotechnological production of phenolic acids in Salvia miltiorrhiza. Crit. Rev. Food Sci. Nutr. 2018, 59, 953–964. [Google Scholar] [CrossRef]
- Ru, J.; Li, P.; Wang, J.; Zhou, W.; Li, B.; Huang, C.; Li, P.; Guo, Z.; Tao, W.; Yang, Y.; et al. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 2014, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.A.; Franco, F.N.; Caldeira, C.A.; de Araújo, G.R.; Vieira, A.; Chaves, M.M.; Lara, R.C. Antioxidant effect of Resveratrol: Change in MAPK cell signaling pathway during the aging process. Arch. Gerontol. Geriatr. 2021, 92, 104266. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Dai, J.; Fai, L.Y.; Yao, H.; Son, Y.-O.; Wang, L.; Pratheeshkumar, P.; Kondo, K.; Shi, X.; Zhang, Z. Constitutive activation of epidermal growth factor receptor promotes tumorigenesis of Cr(VI)-transformed cells through decreased reactive oxygen species and apoptosis resistance development. J. Biol. Chem. 2015, 290, 2213–2224. [Google Scholar] [CrossRef]
- Yang, Z.; Mo, Y.; Cheng, F.; Zhang, H.; Shang, R.; Wang, X.; Liang, J.; Liu, Y.; Hao, B. Antioxidant effects and potential molecular mechanism of action of Limonium aureum extract based on systematic network pharmacology. Front. Vet. Sci. 2022, 8, 775490. [Google Scholar] [CrossRef]
- Nakashima, R.; Goto, Y.; Koyasu, S.; Kobayashi, M.; Morinibu, A.; Yoshimura, M.; Hiraoka, M.; Hammond, E.M.; Harada, H. UCHL1-HIF-1 axis-mediated antioxidant property of cancer cells as a therapeutic target for radiosensitization. Sci. Rep. 2017, 7, 6879. [Google Scholar] [CrossRef]
- Singleton, V.-L.; Rudolf, O.; Lamuela-Ravents, R.-M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Method Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Method Enzymol. 1999, 299, 15–27. [Google Scholar] [CrossRef]
- Zulueta, A.; Esteve, M.J.; Frígola, A. ORAC and TEAC assays comparison to measure the antioxidant capacity of food products. Food Chem. 2009, 114, 310–316. [Google Scholar] [CrossRef]
- Mokhtar, M.; Bouamar, S.; Di Lorenzo, A.; Temporini, C.; Daglia, M.; Riazi, A. The influence of ripeness on the phenolic content, antioxidant and antimicrobial activities of Pumpkins (Cucurbita moschata Duchesne). Molecules 2021, 26, 3623. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2023 update. Nucleic Acids Res. 2023, 51, D1373–D1380. [Google Scholar] [CrossRef]
- Amberger, J.S.; Bocchini, C.A.; Scott, A.F.; Hamosh, A. OMIM.org: Leveraging knowledge across phenotype-gene relationships. Nucleic Acids Res. 2019, 47, D1038–D1043. [Google Scholar] [CrossRef]
- Piñero, J.; Ramírez-Anguita, J.M.; Saüch-Pitarch, J.; Ronzano, F.; Centeno, E.; Sanz, F.; Furlong, L.I. The DisGeNET knowledge platform for disease genomics: 2019 update. Nucleic Acids Res. 2020, 48, D845–D855. [Google Scholar] [CrossRef]
- Stelzer, G.; Rosen, N.; Plaschkes, I.; Zimmerman, S.; Twik, M.; Fishilevich, S.; Stein, T.I.; Nudel, R.; Lieder, I.; Mazor, Y.; et al. The GeneCards Suite: From gene data mining to disease genome sequence analyses. Curr. Protoc. Bioinform. 2016, 54, 1.30.1–1.30.33. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; et al. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. 2018. Available online: https://www.R-project.org/ (accessed on 16 May 2024).
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]

| TSS (%) | TA (%) | VC (mg/100 mL) | TSS/TA | SC (mg/g) | GC (mg/g) | FC (mg/g) | |
|---|---|---|---|---|---|---|---|
| Z | 11.63 ± 0.32 a ** | 0.98 ± 0.04 a | 59.81 ± 1.14 a | 11.91 ± 0.56 ab ** | 23.94 ± 1.32 ab * | 19.899 ± 0.95 a ** | 13.994 ± 0.872 b ** |
| ZC | 11.23 ± 0.12 a ** | 0.85 ± 0.06 a | 59.68 ± 0.67 a | 13.34 ± 0.73 a ** | 25.37 ± 1.81 a * | 21.776 ± 0.75 a ** | 16.185 ± 1.138 a ** |
| H | 10.5 ± 0.15 b ** | 0.95 ± 0.03 a | 61.72 ± 1.74 a | 11.05 ± 0.21 b ** | 21.64 ± 1.58 b * | 17.215 ± 1.45 b ** | 13.866 ± 1.061 b ** |
| X | 10.07 ± 0.15 b ** | 1.00 ± 0.06 a | 63.49 ± 1.66 a | 10.09 ± 0.63 b ** | 21.42 ± 1.04 b * | 16.543 ± 1.13 b ** | 12.517 ± 0.479 b ** |
| TAA (mg/g) | TPC (mg/g) | TFC (mg/g) | TAC (mg/g) | FRAP (μmol TE/g FW) | ABTS (μmol TE/g FW) | DPPH (%) | |
| Z | 9.92 ± 0.174 a | 0.43 ± 0.02 a ** | 0.09 ± 0.00 b ** | 9.17 ± 0.06 a ** | 6.100 ± 0.138 a ** | 2.272 ± 0.092 a * | 22.396 ± 1.170 a ** |
| ZC | 10.96 ± 0.75 a | 0.46 ± 0.04 a ** | 0.10 ± 0.00 a ** | 8.79 ± 0.37 a ** | 6.381 ± 0.503 a ** | 2.298 ± 0.161 a * | 23.795 ± 1.453 a ** |
| H | 12.08 ± 1.08 a | 0.33 ± 0.02 b ** | 0.08 ± 0.00 c ** | 4.93 ± 0.24 b ** | 5.279 ± 0.234 b ** | 1.762 ± 0.042 b * | 17.784 ± 0.684 b ** |
| X | 11.12 ± 1.63 a | 0.32 ± 0.03 b ** | 0.08 ± 0.01 c ** | 3.67 ± 0.14 c ** | 5.755 ± 0.193 b ** | 1.761 ± 0.100 b * | 15.896 ± 0.698 b ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Li, S.; Chen, Y.; Wang, M.; Yu, J.; Bai, W.; Hong, L. Combined Metabolomics and Network Pharmacology Analysis Reveal the Effect of Rootstocks on Anthocyanins, Lipids, and Potential Pharmacological Ingredients of Tarroco Blood Orange (Citrus sinensis L. Osbeck). Plants 2024, 13, 2259. https://doi.org/10.3390/plants13162259
Yang L, Li S, Chen Y, Wang M, Yu J, Bai W, Hong L. Combined Metabolomics and Network Pharmacology Analysis Reveal the Effect of Rootstocks on Anthocyanins, Lipids, and Potential Pharmacological Ingredients of Tarroco Blood Orange (Citrus sinensis L. Osbeck). Plants. 2024; 13(16):2259. https://doi.org/10.3390/plants13162259
Chicago/Turabian StyleYang, Lei, Shuang Li, Yang Chen, Min Wang, Jianjun Yu, Wenqin Bai, and Lin Hong. 2024. "Combined Metabolomics and Network Pharmacology Analysis Reveal the Effect of Rootstocks on Anthocyanins, Lipids, and Potential Pharmacological Ingredients of Tarroco Blood Orange (Citrus sinensis L. Osbeck)" Plants 13, no. 16: 2259. https://doi.org/10.3390/plants13162259
APA StyleYang, L., Li, S., Chen, Y., Wang, M., Yu, J., Bai, W., & Hong, L. (2024). Combined Metabolomics and Network Pharmacology Analysis Reveal the Effect of Rootstocks on Anthocyanins, Lipids, and Potential Pharmacological Ingredients of Tarroco Blood Orange (Citrus sinensis L. Osbeck). Plants, 13(16), 2259. https://doi.org/10.3390/plants13162259






