Influence of Water and Fertilizer Reduction on Respiratory Metabolism in Sugar Beet Taproot (Beta vulgaris L.)
Abstract
1. Introduction
2. Results
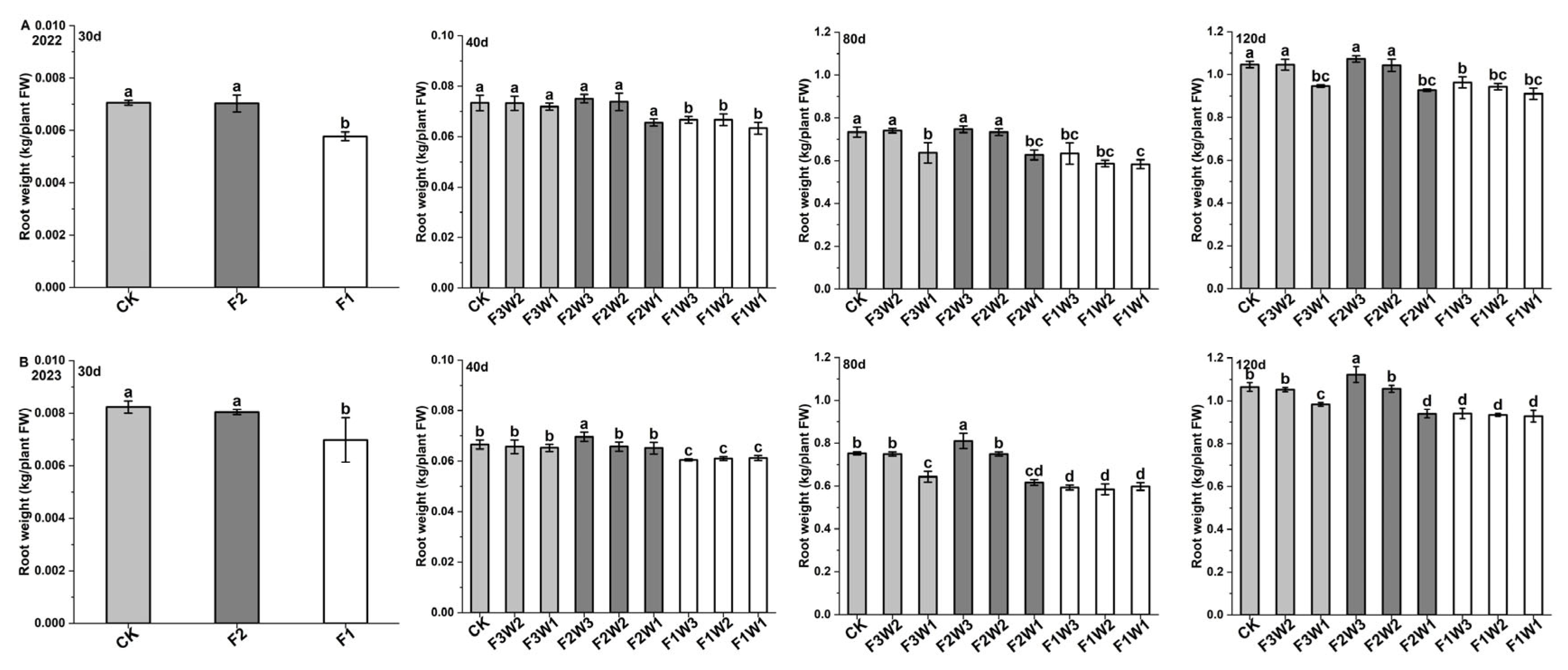
2.1. Sugar Beet Taproot Growth
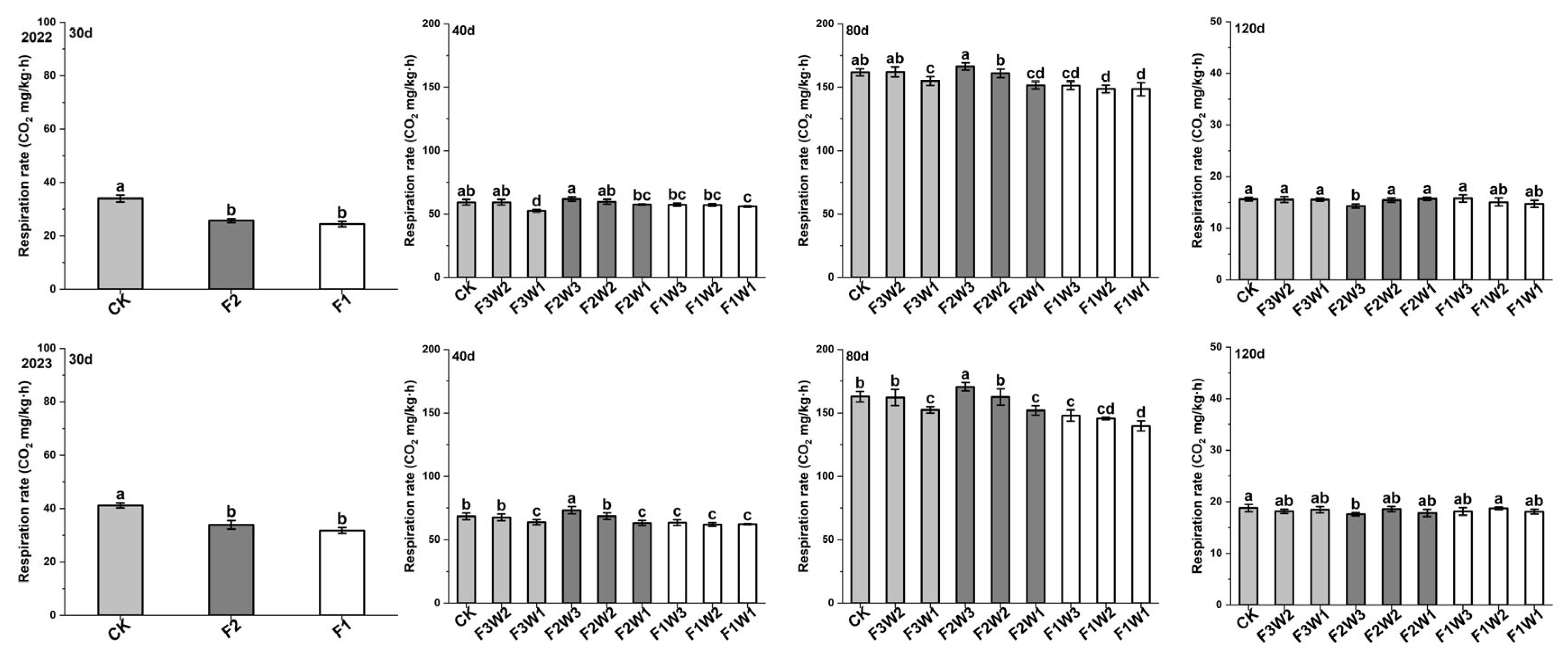
2.2. Respiration Rate of Sugar Beet Taproot
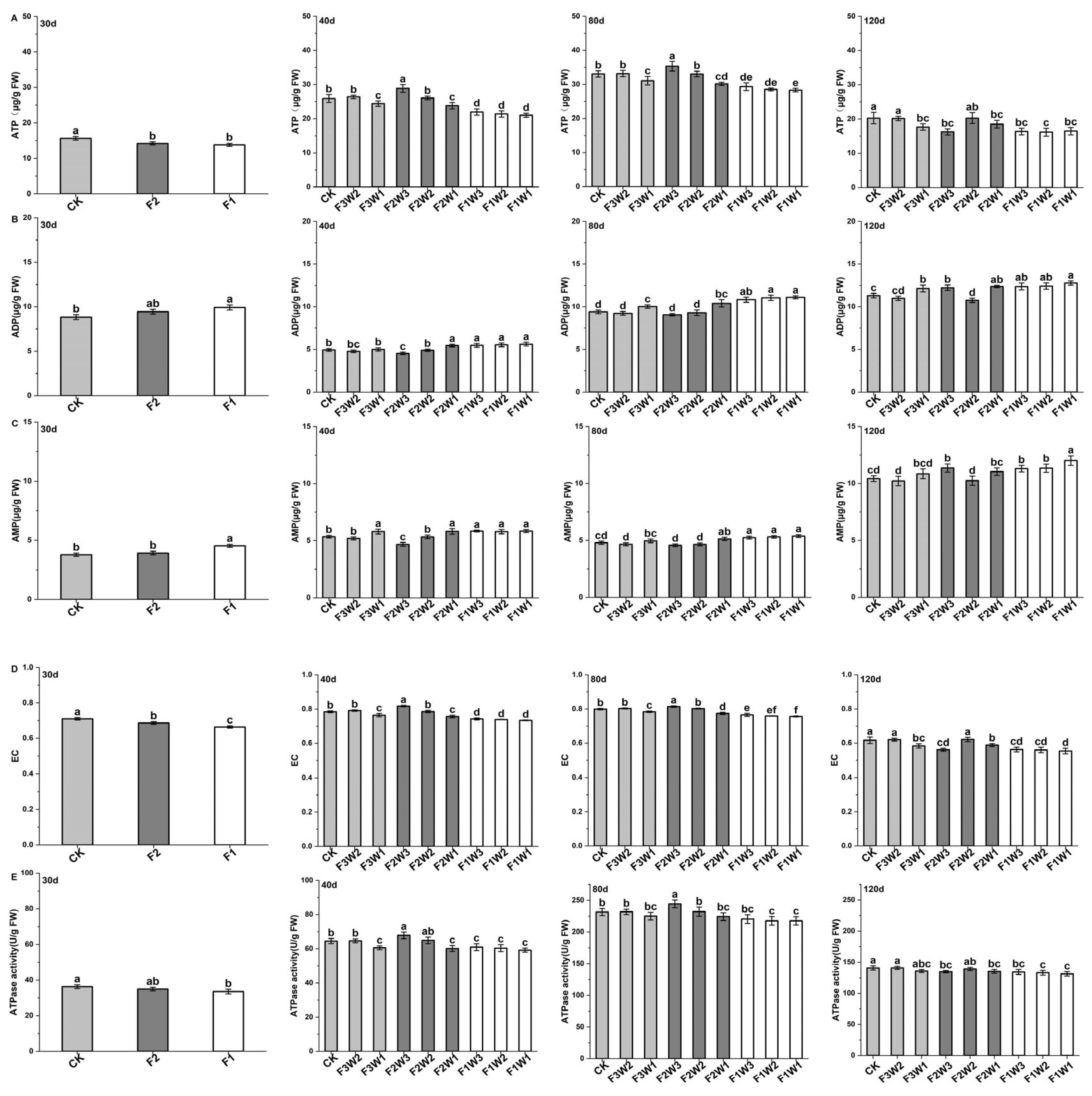
2.3. Energy Metabolism of Sugar Beet Taproot
2.3.1. Contents of ATP, ADP, and AMP
2.3.2. Energy Charge
2.3.3. ATPase
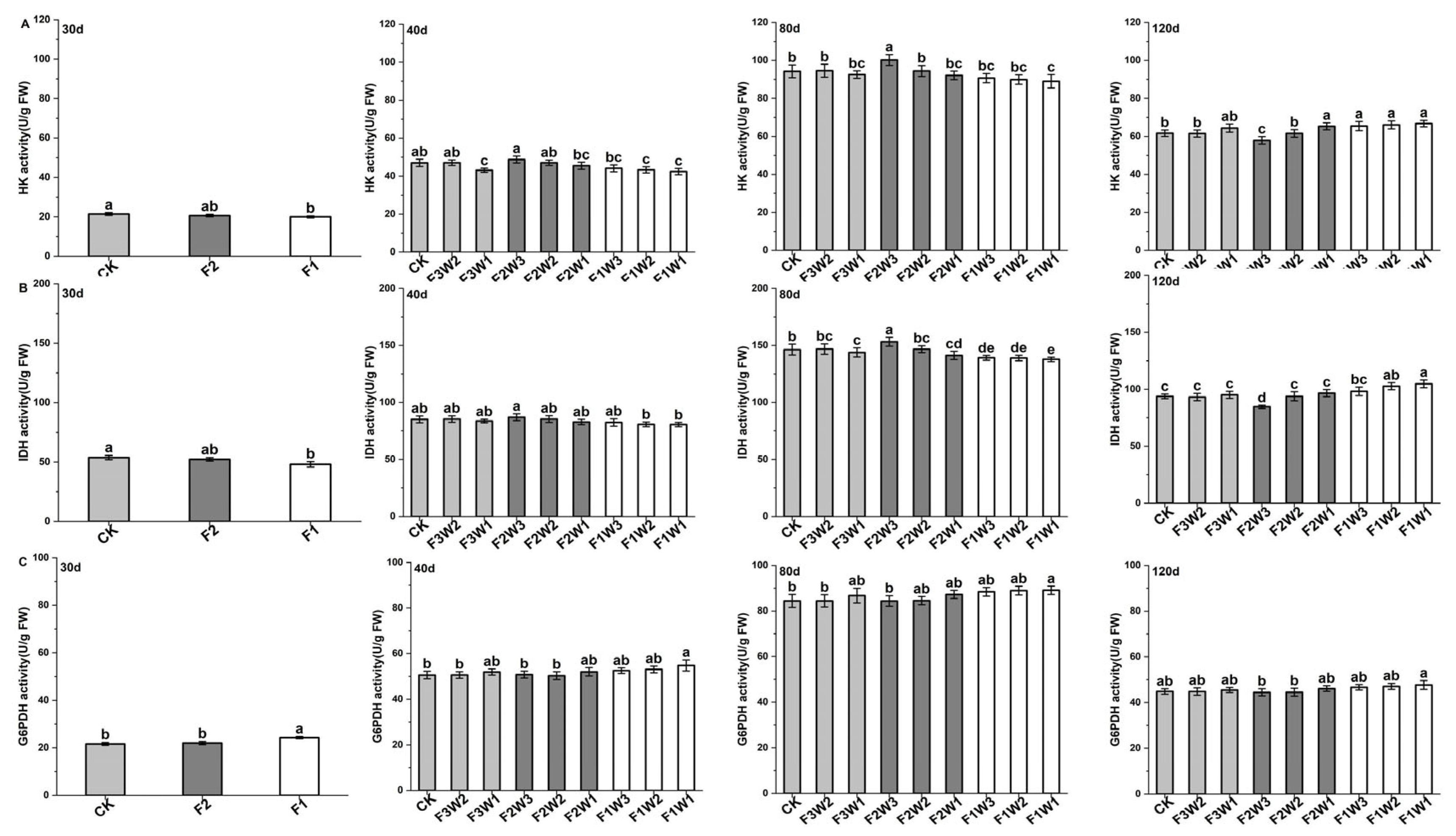
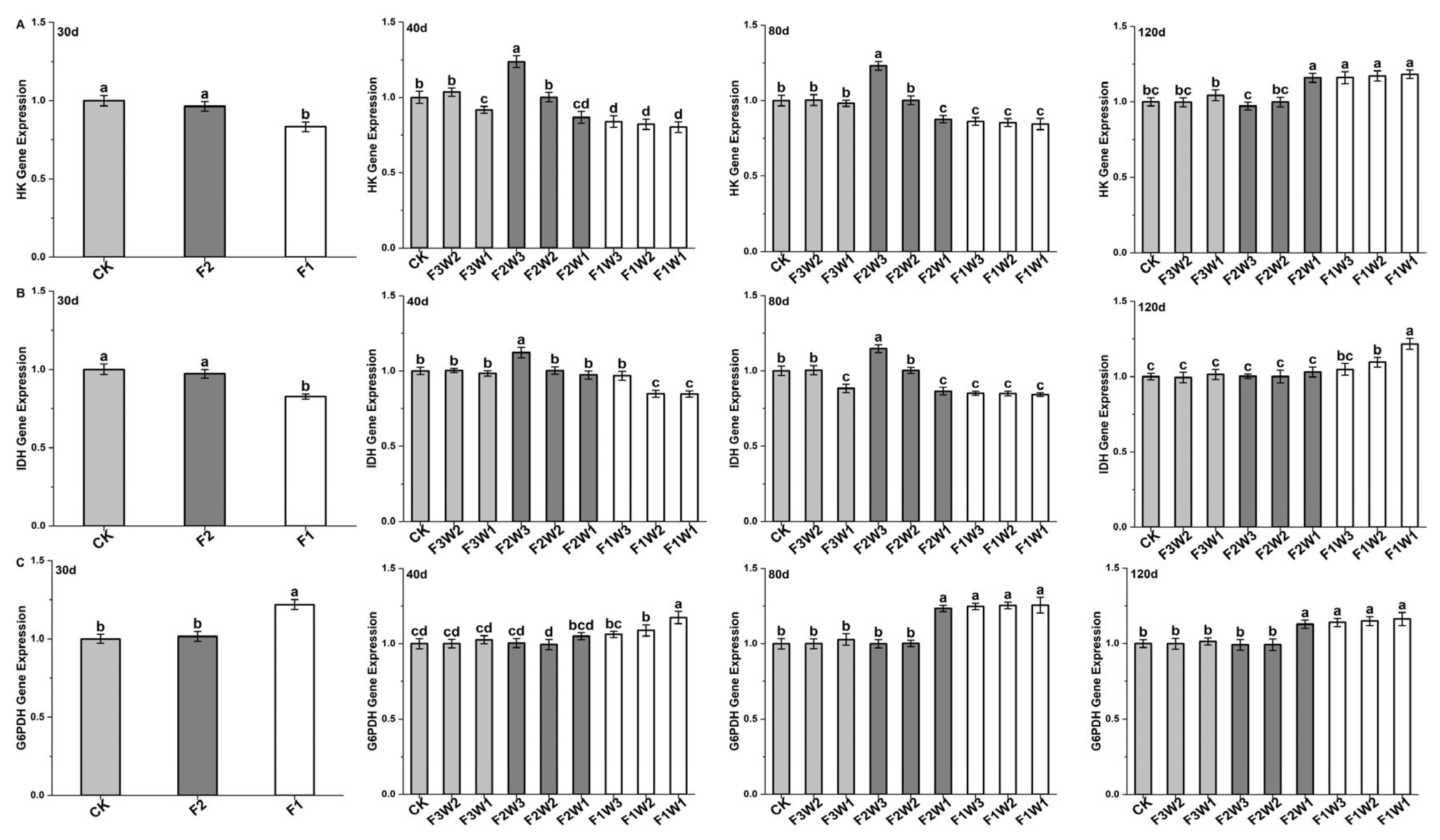
2.4. Key Enzymes in Respiratory Metabolism of Sugar Beet Taproot
2.4.1. Hexokinase
2.4.2. Isocitrate Dehydrogenase
2.4.3. Glucose-6-Phosphate Dehydrogenase
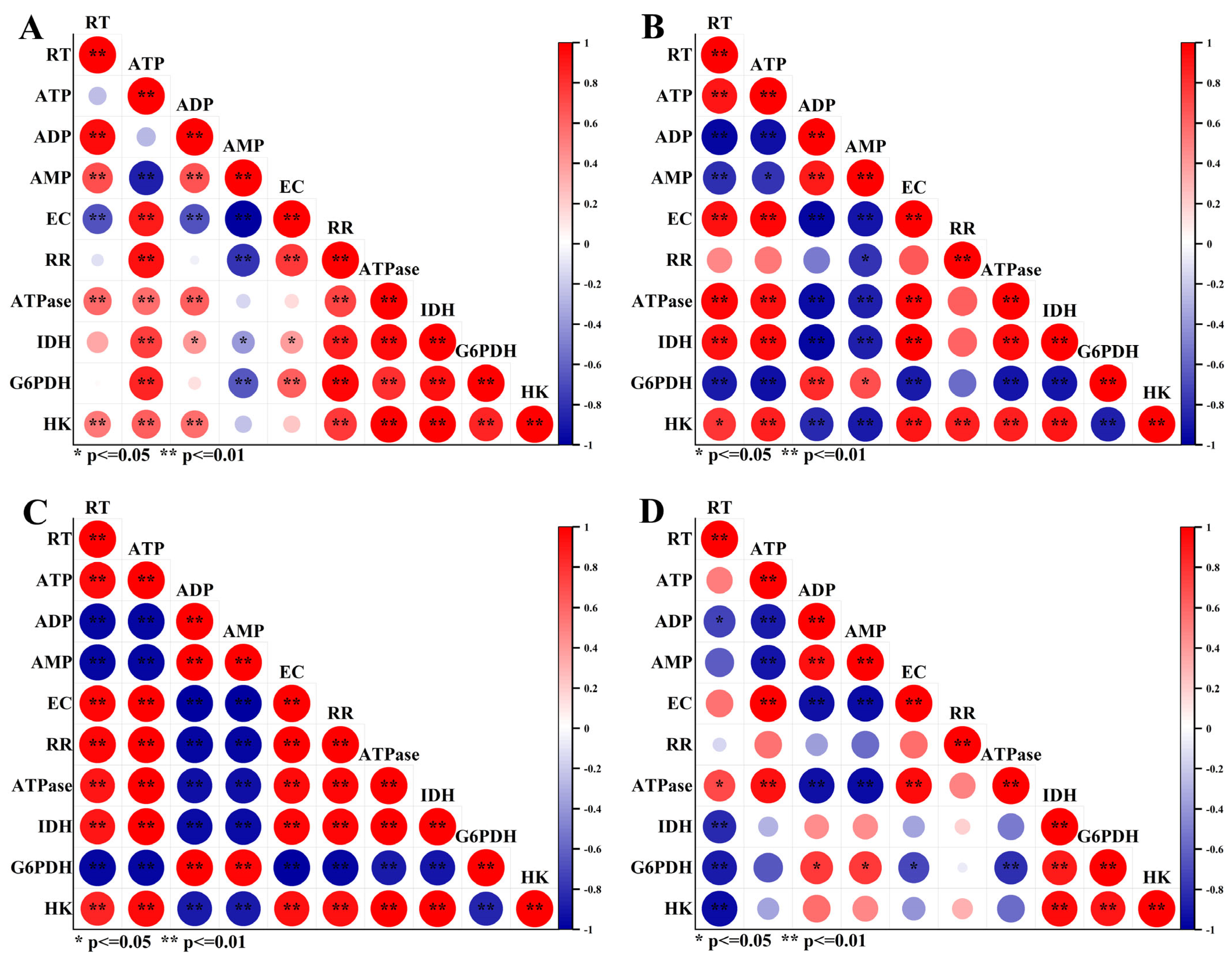
2.5. Correlation Analysis between Taproot Fresh Weight and Respiratory Metabolism Indicators
3. Discussion
4. Materials and Methods
4.1. Experimental Site Description
4.2. Experimental Design
4.3. Collection of Plant Samples
4.4. Respiration Rate
4.5. Respiratory Metabolic Enzyme Activity and Energy Measurement
- (1)
- Standard Addition: Set up wells for the standards and samples. Add 50 μL of standards with different concentrations to each standard well.
- (2)
- Sample Addition: Set up blank wells (i.e., wells without the samples and enzyme reagents, but with all other steps remaining the same) and wells for the test samples. Add 40 μL of sample diluent to each test sample well on the coated microplate, followed by 10 μL of the test sample (resulting in a final dilution of 5 times). Gently mix.
- (3)
- Enzyme Addition: Add 100 μL of enzyme conjugate to each well except the blank wells.
- (4)
- Incubation: Cover the plate with a sealing film and incubate at 37 °C for 60 min.
- (5)
- Washing: Carefully remove the sealing film and discard the liquid. Fill each well with washing solution, let it stand for 30 s, and then discard the washing solution. Repeat this step five times and pat dry.
- (6)
- Color Development: Add 50 μL of chromogen solution A to each well, followed by 50 μL of chromogen solution B. Gently shake to mix, and incubate at 37 °C in the dark for 15 min.
- (7)
- Termination: Add 50 μL of stop solution to each well to terminate the reaction (this will cause the color to change from blue to yellow).
- (8)
- Measurement: Set the blank well to zero, and measure the absorbance (OD value) of each well at 450 nm. This should be completed within 15 min after adding the stop solution.
- (9)
- Calculation: Using the concentration and OD values of the standards, create a standard curve with a linear regression equation. Insert the OD values of the samples into this equation to calculate their concentrations, then multiply by the dilution factor to obtain the actual concentration of the samples.
4.6. Energy Charge Calculation Formula
4.7. Total RNA Extraction and cDNA Synthesis from Sugar Beets
4.8. Real-Time Quantitative PCR
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, Y.W.; Li, Y.C.; Yu, L.Y.; Wang, Y.Q.; Ni, H.T. Analysis of the development of beet sugar industry in China’s three major producing areas. Sugar Crops Chin. 2017, 39, 74–76. [Google Scholar]
- Zhou, Y.L.; Li, X.W.; Liu, N.; Zhang, W.B.; WU, Y.L.; Lu, B.F.; Liu, X.X. Discussion and Analysis on Development of Beet Sugar Industry in Inner Mongolia. Sugar Crops Chin. 2020, 42, 59–64. [Google Scholar]
- Wang, Y.; Zhao, Y.H.; Li, B.; Fan, X.Y. Affecting Factors of Farmers’ Willingness to Adopt Water-saving Technology in Northwest Arid Region of China-Case study of Middle Reaches of Heihe River. Water Sav. Irri. 2012, 11, 50–54. [Google Scholar]
- Li, W.J.; Zhang, F.S. Discussion on Rational Application of Nitrogen Fertilizer in Sugar Beet. Sugar Crops Chin. 2020, 42, 50–56. [Google Scholar]
- Fabeiro, C.; Olalla, F.M.; López, R.; Domínguez, A. Production and quality of the sugar beet (Beta vulgaris L.) cultivated under controlled deficit irrigation conditions in a semi-arid climate. Agric. Water Manag. 2003, 62, 215–227. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Wang, H.Y.; Lei, Q.L.; Zhang, J.Z.; Zhai, L.M.; Ren, T.Z.; Liu, H.B. Recommended Methods for Optimal Nitrogen Application Rate. Scientia. Agric. Sini. 2018, 51, 117–127. [Google Scholar]
- Bai, X.S.; Lin, M.; Yang, H.Z.; Wang, S.L.; Pan, J.H.; Liu, H.J. Demonstration of Sugar beet Integrated Cultivation Technology for Saving Cost, Stable Yield and Increasing Sugar Production in the Northern of Tianshan Mountain. Sugar Crops Chin. 2018, 40, 37–40. [Google Scholar]
- Howell, D.P.; Levin, E.; Springer, A.; Kraemer, S.M.; Phippard, D.; Schief, W.R.; Smith, J.D. Mapping a common interaction site used by Plasmodium falciparum Duffy binding-like domains to bind diverse host receptors. Mol. Microbiol. 2007, 67, 78–87. [Google Scholar] [CrossRef]
- Liu, Y.; Li, P.; Xiao, L.; Wang, W.; Yu, K.X.; Shi, P. Heterogeneity in short-term allocation of carbon to roots of Pinus tabuliformis seedlings and root respiration under drought stress. Plant Soil 2020, 452, 359–378. [Google Scholar] [CrossRef]
- Wang, J.K.; Wang, Y.L.; Chen, H.Z.; Xiang, J.; Zhang, Y.K.; Zhu, D.F.; Zhang, Y.P. Mechanism of High Temperature Affecting Carbon and Nitrogen Metabolism of Rice Grain at the Early Stage of Grain Filling. Chin. J. Agrometeorol. 2020, 41, 32–42. [Google Scholar]
- Ren, X.S.; Wang, Z.M.; Jiao, J.; Tian, L.X.; Liu, Z.Y.; Li, J. Effects of GA3 treatment on respiratory metabolism of maize seeds under low temperature stress. Chin. J. Ecol. 2020, 39, 847–854. [Google Scholar]
- Pu, X.C.; Han, J.G.; Li, M. Metabolic Control of Zoysiagrass Seed When Breaking Dormancy. Grassl. China 1994, 3, 20–24. [Google Scholar]
- Chen, Y.M.; Wang, T.; Zhang, F.J.; Zhuang, W.B.; Shu, X.C.; Wang, Z.; Yang, Q. Comparison of Metabolites in the Needles of Taxus wallichiana var. mairei and Taxus wallichiana var. mairei cv. ‘Jinxishan’. Acta Bot. Bor.-Occid. Sin. 2019, 39, 801–807. [Google Scholar]
- Li, S.H.; Li, Y.M.; Gao, Y.; He, X.R.; Zhang, D.L.; Liu, B.B.; Li, Q.M. Effects of CO2 enrichment on non-structural carbohydrate metab-olism in leaves of cucumber seedlings under salt stress. Sci. Hortic. 2020, 265, 109275. [Google Scholar] [CrossRef]
- Che-Othman, M.H.; Jacoby, R.P.; Millar, A.H.; Taylor, N.L. Wheat mitochondrial respiration shifts from the tricarboxylic acid cycle to the GABA shunt under salt stress. New Phytol. 2020, 225, 1047–1048. [Google Scholar] [CrossRef] [PubMed]
- Bailey-Serres, J.; Tom, J.; Freeling, M. Expression and distribution of cytosolic 6-phosphogluconate dehydrogenase isozymes in maize. Biochem. Genet. 1992, 30, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, R.; Wan, Q.; Xie, G.; Bi, Y. Glucose-6-phosphate dehydrogenase plays a pivotal role in nitric oxide-involved defense against oxidative stress under salt stress in red kidney bean roots. Plant Cell Physiol. 2007, 48, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Hauschild, R.; Von Schaewen, A. Differential regulation of glucose-6-phosphate dehydrogenase isoenzyme activities in potato. Plant Physiol. 2003, 133, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, H.S.; Wang, J.F.; Yang, J.S. Molecular cloning and characterization of rice 6-phosphogluconate dehydrogenase gene that is up-regulated by salt stressa. Mol. Biol. Rep. 2003, 30, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.S.; Li, F.M.; Xu, H. Deficiency of water can enhance root respiration rate of drought-sensitive but not drought-tolerant spring wheat. Agric. Water Manag. 2004, 64, 41–48. [Google Scholar] [CrossRef]
- Ryan, M.G.; Hubbard, R.M.; Pongracic, S.; Raison, R.J.; McMurtrie, R.E. Foliage, fine-root, woody-tissue and stand respiration in Pinus radiata in relation to nitrogen status. Tree Physiol. 1996, 16, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Levang-Brilz, N.; Biondini, M.E. Growth rate, root development and nutrient uptake of 55 plant species from the Great Plains grasslands. Plant Ecol. 2002, 165, 117–144. [Google Scholar] [CrossRef]
- Vogel, J.G.; Valentine, D.W.; Ruess, R.W. Soil and root respiration in mature Alaskan black spruce forests that vary in soil organic matter decomposition rates. Can. J. For. Res. 2005, 35, 161–174. [Google Scholar] [CrossRef]
- Li, C.P.; Wang, S.W.; Ding, J.J.; Pan, C.D.; Ma, B.; Nurxat, k.; Mihirdin, A.; Zulpikar, T. Effects of Nitrogen Application Levels on Respiration Rate and Related Enzyme Activity of Walnut Root. For. Res. 2019, 32, 56–62. [Google Scholar]
- Lu, Y.E. Functional Study of the Glutamine Synthetase Gene and Cytosolic Isocitrate Dehydrogenase Gene in Rice, and Analysis of the Amino Acid Transporter Gene Family. Ph.D. Dissertation, Huazhong Agricultural University, Wuhan, China, 2014. [Google Scholar]
- Jesús, P.; Fernando, G.; Maria, F.S.; Francisco, M.C. Purification and characterization of NADP+-linked isocitrate dehydrogenase from scots pine: Evidence for different physiological roles of the enzyme in primary development. Plant Physiol. 1998, 118, 617–626. [Google Scholar]
- Stitt, M. Nitrate regulation of metabolism and growth. Curr. Opin. Plant Biol. 1999, 2, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Tomomi, A.; Mitsuhiro, O.; Akiko, U.; Toshihiko, H.; Michael, H.; Tomoyuki, Y. Localization of NAD-isocitrate dehydrogenase and glutamate dehydrogenase in rice roots: Candidates for providing carbon skeletons to NADH-glutamate synthase. Plant Cell Physiol. 2005, 46, 1724–1734. [Google Scholar]
- Tomohiro, K.; Masataka, W.; Tomomi, A.; Shuichi, Y.; Naohiro, A.; Ryu, O. Introduction of the ZmDof1 gene into rice enhances carbon and nitrogen assimilation under low-nitrogen conditions. Plant Biotechnol. J. 2011, 9, 826–837. [Google Scholar]
- Yin, H.; Xiao, W.; Sun, G.R.; Li, Y.B.; Li, F.Q. The effect of phosphorus nutrition on respiration metabolism of puccinellia tenuiflora seedlingsunder salinity stress. Nat. Sci. J. Harbin Norm. Univ. 1998, 5, 94–97. [Google Scholar]
- Chen, K.; Liu, W.X.; Feng, D.; Zhu, H.Y.; Zhang, J.M.; Sang, X.M.; Ding, F.Q.; Sun, X.A. Effects of Soil Water Regulation on Growth and Yield of Fruit Radish under Drip Irrigation in Greenhouse. J. Irrig. Drain. 2023, 42, 22–27. [Google Scholar]
- Wei, Q.S.; Guo, D.S.; Wang, L.; Guan, Y.X.; Yu, C.T.; Zhou, Y. Impacts of rhizobia inoculating on root nodule and yield of vegetable soybean under different fertilizer treatments. Jiangsu Agric. Sci. 2021, 49, 77–82. [Google Scholar]
- Guo, M.; Xing, X.M.; Du, C.X.; Wei, L.; Yi, L.X.; Shi, S.D.; Gao, J.; Xiao, Q. Study on Variance of Nitrogen Efficiency Among Sugar Beet Varieties and Its Effect on Yield and Quality. Sugar Crops Chin. 2024, 46, 57–69. [Google Scholar]
- Chen, X.J.; XU, Z.S.; Zhao, B.P.; Mi, J.Z.; Yan, W.K.; Liu, J.H. Effects of salt stress on root respiratory metabolism, antioxidant enzyme activities, and yield of oats. Chin. J. Ecol. 2021, 40, 2773–2782. [Google Scholar]
- Li, Z.X.; Qin, S.J.; Lui, D.G.; Nie, J.Y. Research Progress in Root Respiratory Metabolism of Plant and the Environmental Influencing Factors. Acta Physiol. Sin. 2021, 47, 957–966. [Google Scholar]
- Yu, C.; Li, G.L.; Sun, Y.Q.; LI, N.N.; Zhang, S.Y. Characteristics of respiratory metabolism in growth and development of sugar beet taproot. Acta Agron. Sin. 2023, 49, 3377–3386. [Google Scholar]
- Xu, G.; Song, K.; Lu, D.; Wang, H.; Chen, M. Influence of Water Management and Nitrogen Application on Rice Root and Shoot Traits. Agron. J. 2019, 188, 104449. [Google Scholar] [CrossRef]
- Luo, L.; Lin, S.Z.; Zheng, H.Q.; Lei, Y.; Zhang, Q.; Zhang, Z.Y. The role of antioxidant system in freezing acclimation-induced freezing resistance of Populus suaveolens cuttings. For. Stud. Chin. 2007, 9, 107–113. [Google Scholar] [CrossRef]
- Bo, S.D. Soil and Agricultural Chemistry Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Liu, Y.; Li, P.; Wang, T.; Liu, Q.; Wang, W. Root respiration and belowground carbon allocation respond to drought stress in a perennial grass (Bothriochloa ischaemum). Catena 2020, 188, 104449. [Google Scholar] [CrossRef]
- Guo, X.; Li, Y.; Li, N.N.; Li, G.L.; Sun, Y.Q.; Zhang, S.Y. BvCPD promotes parenchyma cell and vascular bundle development in sugar beet (Beta vulgaris L.) taproot. Front. Plant Sci. 2023, 14, 1271329. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
| Year | Total N (g·kg−1) | Total P (g·kg−1) | Total K (g·kg−1) | Available N (mg·kg−1) | Available P (mg·kg−1) | Available K (mg·kg−1) | pH Value | Organic Matter (g·kg−1) |
|---|---|---|---|---|---|---|---|---|
| 2022 | 2.07 | 0.97 | 22.11 | 134.91 | 10.31 | 197.5 | 7.98 | 32.02 |
| 2023 | 1.71 | 0.46 | 16.31 | 111.07 | 9.23 | 153.01 | 7.71 | 18.21 |
| Treatment | Amount of Fertilizer Applied (kg/ha) | Total Irrigation Volume (m3/ha) | ||
|---|---|---|---|---|
| Pure N | Pure P | Pure K | ||
| F3W3(CK) | 120 | 150 | 150 | 1350 |
| F3W2 | 1147.5 | |||
| F3W1 | 945 | |||
| F2W3 | 108 | 135 | 135 | 1350 |
| F2W2 | 1147.5 | |||
| F2W1 | 945 | |||
| F1W3 | 96 | 120 | 120 | 1350 |
| F1W2 | 1147.5 | |||
| F1W1 | 945 | |||
| Primer | R 5′ to 3′ | F 5′ to 3′ |
|---|---|---|
| ACT | TGCTTGACTCTGGTGATGGT | AGCAAGATCCAAACGGAGAATG |
| HK | GCGACGTTATGGTTGTAT | ACTCTTCCAACTCCCTCAACAC |
| IDH | GCAACCATCACACCAGATGAA | ACTCTTCCAACTCCCTCAACAC |
| G6PDH | ATGGATCTCCCTACCAGTCG | CTCATCAAAATAGCCACCTCT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.; Li, G.; Jian, C.; Zhang, B.; Sun, Y.; Li, N.; Zhang, S. Influence of Water and Fertilizer Reduction on Respiratory Metabolism in Sugar Beet Taproot (Beta vulgaris L.). Plants 2024, 13, 2282. https://doi.org/10.3390/plants13162282
Chang Y, Li G, Jian C, Zhang B, Sun Y, Li N, Zhang S. Influence of Water and Fertilizer Reduction on Respiratory Metabolism in Sugar Beet Taproot (Beta vulgaris L.). Plants. 2024; 13(16):2282. https://doi.org/10.3390/plants13162282
Chicago/Turabian StyleChang, Yuxin, Guolong Li, Caiyuan Jian, Bowen Zhang, Yaqing Sun, Ningning Li, and Shaoying Zhang. 2024. "Influence of Water and Fertilizer Reduction on Respiratory Metabolism in Sugar Beet Taproot (Beta vulgaris L.)" Plants 13, no. 16: 2282. https://doi.org/10.3390/plants13162282
APA StyleChang, Y., Li, G., Jian, C., Zhang, B., Sun, Y., Li, N., & Zhang, S. (2024). Influence of Water and Fertilizer Reduction on Respiratory Metabolism in Sugar Beet Taproot (Beta vulgaris L.). Plants, 13(16), 2282. https://doi.org/10.3390/plants13162282







